A Polyphasic Characterisation of Tetradesmus almeriensis sp. nov. (Chlorophyta: Scenedesmaceae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microscopy Examination
2.2. Sequence Data and Phylogenetic Analysis
3. Results and Discussion
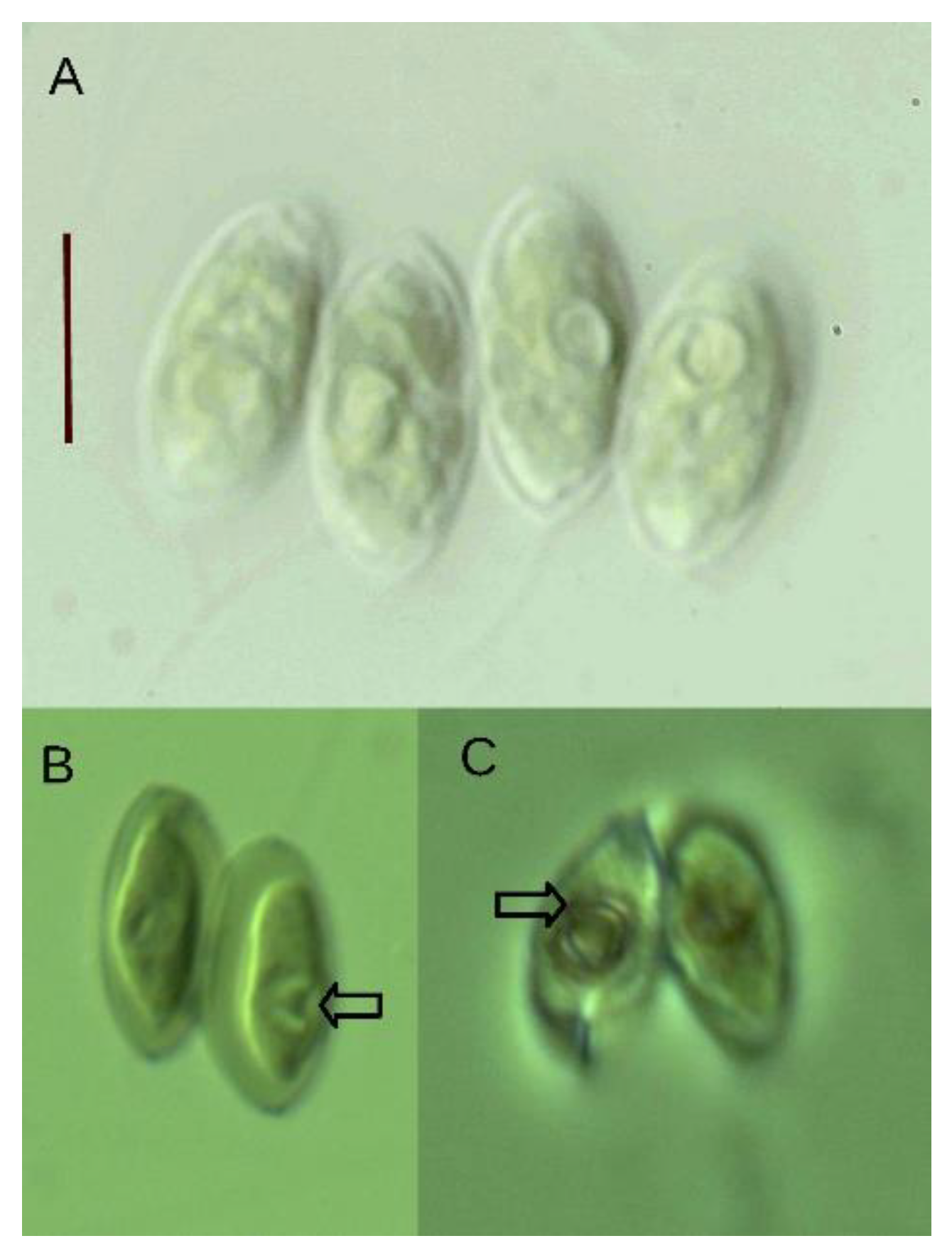
3.1. Taxonomy
3.2. Ecology
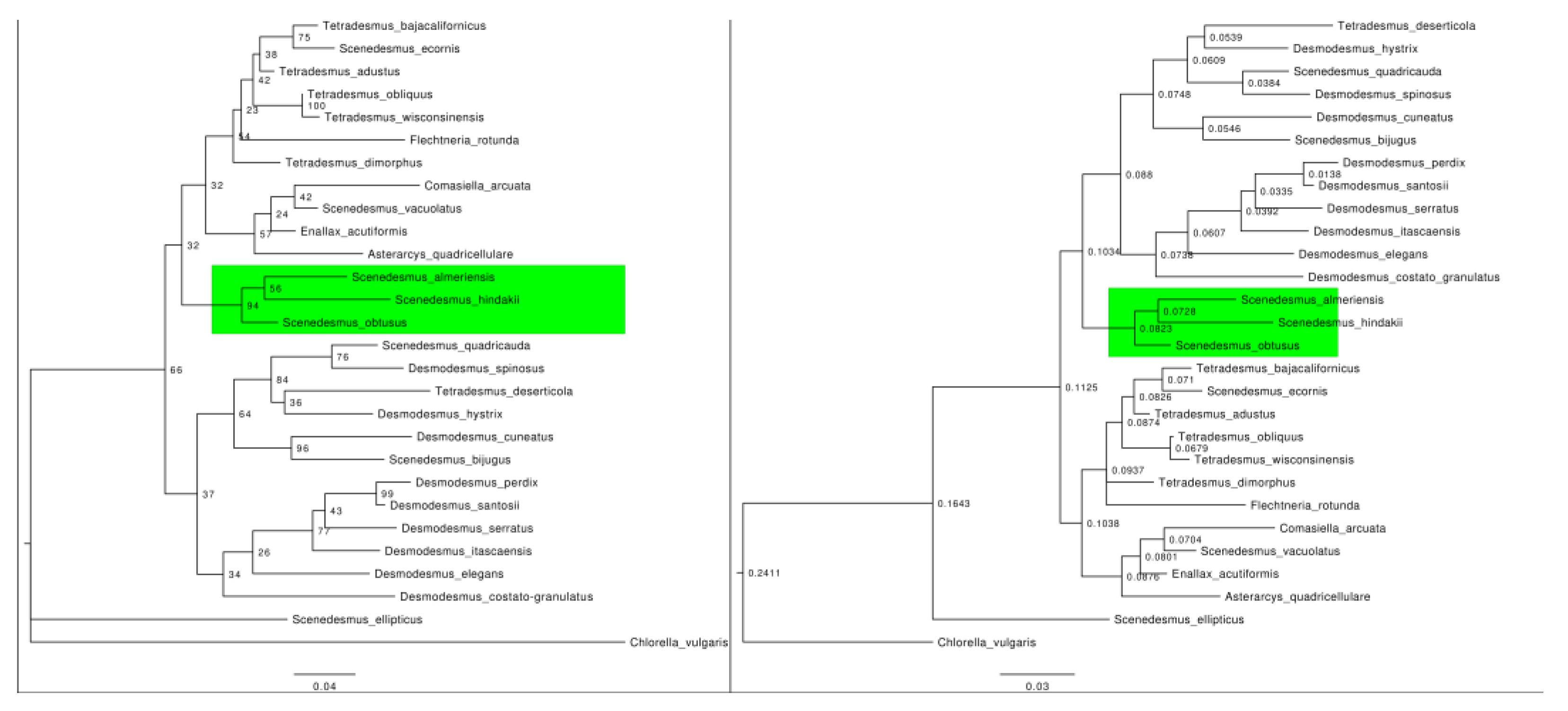
3.3. Phylogenetic Analysis and Systematic Position
3.4. Culture Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hegewald, E.; Wolf, M.; Keller, A.; Friedl, T.; Krienitz, L. ITS2 sequence-structure phylogeny in the Scenedesmaceae with special reference to Coelastrum (Chlorophyta, Chlorophyceae), including the new genera Comasiella and Pectinodesmus. Phycologia 2010, 49, 325–335. [Google Scholar] [CrossRef]
- Meyen, F.J.F. Beobachtungen über einige niedere Algenformen. Nova Acta Phys.-Med. Acad. Caesareae Leopold.-Carol. Nat. 1830, 14, 768–778. [Google Scholar]
- Hegewald, E.; Silva, P.C. Annotated Catalogue of Scenedesmus and Nomenclaturally Related Genera; J. Cramer: Berlin, Germany, 1988. [Google Scholar]
- Komárek, J.; Fott, B.; Huber-Pestalozzi, G. Das Phytoplankton des Süßwassers; Systematik und Biologie-Teil 7, 1. Hälfte: Stuttgart, Germany, 1983. [Google Scholar]
- Trainor, F.R.; Cain, J.R.; Shubert, L.E. Morphology and nutrition of the colonial green alga Scenedesmus: 80 years later. Bot. Rev. 1976, 42, 5–25. [Google Scholar] [CrossRef]
- Uherkovich, G. Die Scenedesmus-Arten Ungarns; Akadémiai Kiadó, Verlag der Ungarisch Akad der Wissenschaften: Budapest, Hungary, 1966. [Google Scholar]
- Hegewald, E. Vergleichende Beobachtungen an Herbarmaterial und Freilandmaterial von Scenedesmus. Algol. Stud. 1979, 24, 264–286. [Google Scholar]
- Lewis, L.A. Diversity and phylogenetic placement of Bracteacoccus Tereg (Chlorophyceae, Chlorophyta) based on 18s ribosomal RNA gene sequence data. J. Phycol. 1997, 33, 279–285. [Google Scholar] [CrossRef]
- Kessler, E.; Schäfer, M.; Hümmer, C.; Kloboucek, A.; Huss, V.A.R. Physiological, biochemical, and molecular characters for the taxonomy of the subgenera of Scenedesmus (Chlorococcales, Chlorophyta). Bot. Acta 1997, 110, 244–250. [Google Scholar] [CrossRef]
- Hegewald, E.; Hangata, N. Phylogenetic studies on Scenedesmaceae (Chlorophyta). Algol. Stud. 2000, 100, 29–49. [Google Scholar] [CrossRef]
- Tsarenko, P.M.; John, D.M. Phylum Chlorophyta (Green Algae) Order Sphaeropleales. Freshw. Algal Flora Br. Isles 2011, 2, 461–465. [Google Scholar]
- Smith, G.M. Tetradesmus, a new four-celled coenobic alga. Bull. Torrey Bot. Club 1913, 40, 75–87. [Google Scholar] [CrossRef]
- Chodat, R. Monographies d’Algues en Culture Pure. Mat. Crypt. Suisse 1913, 4, 234–241. [Google Scholar]
- West, G.S. Algological Notes: XIV-XVII. J. Bot. 1915, 53, 73–84. [Google Scholar]
- Fott, B.; Komárek, J. Die Gattungen Lauterborniella Schmidle und Tetradesmus GM Smith und ihre Stellung in der Familie der Scenedesmaceae (Chlorococcales). Preslia 1974, 46, 198–209. [Google Scholar]
- Hegewald, E.; Wolf, M. Phylogenetic relationships of Scenedesmus and Acutodesmus (Chlorophyta, Chlorophyceae) as inferred from 18S rDNA and ITS-2 sequence comparisons. Plant Syst. Evol. 2003, 241, 185–191. [Google Scholar] [CrossRef]
- Krienitz, L.; Bock, C. Present state of the systematics of planktonic coccoid green algae of inland waters. Hydrobiologia 2012, 698, 295–326. [Google Scholar] [CrossRef]
- Ismagulova, T.; Chekanov, K.; Gorelova, O.; Baulina, O.; Semenova, L.; Selyakh, I.; Chivkunova, O.; Lobakova, E.; Karpova, O.; Solovchenko, A. A new subarctic strain of Tetradesmus obliquus—Part I: Identification and fatty acid profiling. J. Appl. Phycol. 2018, 30, 2737–2750. [Google Scholar] [CrossRef]
- Tsarenko, P.M.; Petlevanny, O.A. Addition to the diversity of algae of Ukraine. Algol. Suppl. 2001, 10, 1–130. [Google Scholar]
- Wynne, M.J.; Hallan, J.K. Reinstatement of Tetradesmus GM Smith (Sphaeropleales, Chlorophyta). Feddes Repert. 2015, 126, 83–86. [Google Scholar] [CrossRef]
- Guiry, M.D.; AlgaeBase. World-WIDE Electronic Publication, National University of Ireland, Galway (Taxonomic Information Republished from AlgaeBase with Permission of MD Guiry). Accessed through World Regist. Mar. Species. 2019. Available online: http//www.algaebase.org (accessed on 11 September 2021).
- Del Campo, J.A.; García-González, M.; Guerrero, M.G. Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Dautor, Y.; Chileh, T.; Úbeda Mínguez, P.; Mañas Fernández, A.; García Maroto, F.; López Alonso, D. Microalgas para biodiesel: Desarrollo de un método de transformación genética para Scenedemus almeriensis, una especie con potencial industrial. Chron. Nat. 2013, 3, 10–17. [Google Scholar]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. JModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. Mrbayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández Sevilla, J.M.; Molina Grima, E.; Pérez Parra, J.; Acién Fernández, F.G.; Magán Cañadas, J.J.; Friedl, T. Nueva Especie de Microalga y su Aplicación Para Consumo Animal, Humano y en la Obtención de Carotenoides. 2016. Available online: https://bit.ly/3kjCy0J (accessed on 9 November 2021).
- Terlova, E.F.; Lewis, L.A. A new species of Tetradesmus (Chlorophyceae, Chlorophyta) isolated from desert soil crust habitats in southwestern North America. Plant Fungal Syst. 2019, 64, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Chodat, R.; Malinesko, O. Sur le polymorphisme de Scenedesmus acutus. Bull. l’Herbier Boissier 1893, 1, 184–190. [Google Scholar]
- Lewis, L.A.; Flechtner, V.R. Cryptic species of Scenedesmus (Chlorophyta) from desert soil communities of western north america. J. Phycol. 2004, 40, 1127–1137. [Google Scholar] [CrossRef]
- He, M.; Yan, Y.; Pei, F.; Wu, M.; Gebreluel, T.; Zou, S.; Wang, C. Improvement on lipid production by Scenedesmus obliquus triggered by low dose exposure to nanoparticles. Sci. Rep. 2017, 7, 15526. [Google Scholar] [CrossRef]
- Sánchez, J.F.; Fernández, J.M.; Acién, F.G.; Rueda, A.; Pérez-Parra, J.; Molina, E. Influence of culture conditions on the productivity and lutein content of the new strain Scenedesmus almeriensis. Process Biochem. 2008, 43, 398–405. [Google Scholar] [CrossRef]
- Suyono, E.A.; Haryadi, W.; Zusron, M.; Nuhamunada, M.; Rahayu, S.; Nugroho, A.P. The effect of salinity on growth, dry weight and lipid content of the mixed microalgae culture isolated from Glagah as biodiesel substrate. J. Life Sci. 2015, 9, 229–233. [Google Scholar]
- Benavente-Valdés, J.R.; Aguilar, C.; Contreras-Esquivel, J.C.; Méndez-Zavala, A.; Montañez, J. Strategies to enhance the production of photosynthetic pigments and lipids in chlorophycae species. Biotechnol. Reports 2016, 10, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Macías-Sánchez, M.D.; Fernandez-Sevilla, J.M.; Fernández, F.G.A.; García, M.C.C.; Grima, E.M. Supercritical fluid extraction of carotenoids from Scenedesmus almeriensis. Food Chem. 2010, 123, 928–935. [Google Scholar] [CrossRef]
- Fernández-Sevilla, J.M.; Molina Grima, E.; Perez Parra, J.; Acien Fernandez, F.G.; Magan Cañadas, J.J.; Friedl, T. Novel Microalgal Species and Use Thereof for Animal and/or Human Consumption and in the Production of Carotenoids. Spanish Patent P200500374. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2006087405 (accessed on 9 November 2021).
- Bessières, D.; Bazile, J.-P.; Tanh, X.N.T.; García-Cuadra, F.; Acien, F.G. Thermophysical behavior of three algal biodiesels over wide ranges of pressure and temperature. Fuel 2018, 233, 497–503. [Google Scholar] [CrossRef]
- Johnsson, N.; Steuer, F. Bioplastic Material from Microalgae: Extraction of Starch and PHA from Microalgae to Create a Bioplastic Material. 2018. Available online: https://www.diva-portal.org/smash/record.jsf?pid=diva2%3A1228894&dswid=-7903 (accessed on 9 November 2021).
- Beatrice-Lindner, P.; Garrido-Cardenas, J.A.; Sepulveda, C.; Acien-Fernandez, F.G. A new approach for detection and quantification of microalgae in industrial-scale microalgal cultures. Appl. Microbiol. Biotechnol. 2018, 102, 8429–8436. [Google Scholar] [CrossRef]
- Wang, Q.; Song, H.; Liu, X.; Zhu, H.; Hu, Z.; Liu, G. Deep genomic analysis of Coelastrella saipanensis (Scenedesmaceae, Chlorophyta): Comparative chloroplast genomics of Scenedesmaceae. Eur. J. Phycol. 2019, 54, 52–65. [Google Scholar] [CrossRef]
- Harun, R.; Danquah, M.K.; Forde, G.M. Microalgal biomass as a fermentation feedstock for bioethanol production. J. Chem. Technol. Biotechnol. 2010, 85, 199–203. [Google Scholar] [CrossRef]
- Mann, J.E.; Myers, J. On pigments, growth, and photosynthesis of Phaeodactylum tricornutum. J. Phycol. 1968, 4, 349–355. [Google Scholar] [CrossRef]
- Stein, E.D. Isolation and Culture of Algae, Handbook of Phycological Methods; Culture Methods and Growth Measurements; Cambridge University Press: Cambridge, UK, 1973. [Google Scholar]
- Brindley, C.; Jiménez-Ruíz, N.; Acién, F.G.; Fernández-Sevilla, J.M. Light regime optimization in photobioreactors using a dynamic photosynthesis model. Algal Res. 2016, 16, 399–408. [Google Scholar] [CrossRef]
- Zimbro, M.J.; Power, D.A.; Miller, S.M.; Wilson, G.E.; Johnson, J. Manual of Microbiological Culture Media; Becton Dickinson: Sparks, MD, USA, 2009. [Google Scholar]
- Del Mar Morales-Amaral, M.; Gómez-Serrano, C.; Acién, F.G.; Fernández-Sevilla, J.M.; Molina-Grima, E. Outdoor production of Scenedesmus sp. in thin-layer and raceway reactors using centrate from anaerobic digestion as the sole nutrient source. Algal Res. 2015, 12, 99–108. [Google Scholar] [CrossRef]
- Cho, D.-H.; Ramanan, R.; Heo, J.; Kang, Z.; Kim, B.-H.; Ahn, C.-Y.; Oh, H.-M.; Kim, H.-S. Organic carbon, influent microbial diversity and temperature strongly influence algal diversity and biomass in raceway ponds treating raw municipal wastewater. Bioresour. Technol. 2015, 191, 481–487. [Google Scholar] [CrossRef]
- An, S.S.; Friedl, T.; Hegewald, E. Phylogenetic relationships of Scenedesmus and Scenedesmus-like coccoid green algae as inferred from ITS-2 rDNA sequence comparisons. Plant Biol. 1999, 1, 418–428. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turiel, S.; Garrido-Cardenas, J.A.; Gómez-Serrano, C.; Acién, F.G.; Carretero-Paulet, L.; Blanco, S. A Polyphasic Characterisation of Tetradesmus almeriensis sp. nov. (Chlorophyta: Scenedesmaceae). Processes 2021, 9, 2006. https://doi.org/10.3390/pr9112006
Turiel S, Garrido-Cardenas JA, Gómez-Serrano C, Acién FG, Carretero-Paulet L, Blanco S. A Polyphasic Characterisation of Tetradesmus almeriensis sp. nov. (Chlorophyta: Scenedesmaceae). Processes. 2021; 9(11):2006. https://doi.org/10.3390/pr9112006
Chicago/Turabian StyleTuriel, Sara, Jose Antonio Garrido-Cardenas, Cintia Gómez-Serrano, Francisco Gabriel Acién, Lorenzo Carretero-Paulet, and Saúl Blanco. 2021. "A Polyphasic Characterisation of Tetradesmus almeriensis sp. nov. (Chlorophyta: Scenedesmaceae)" Processes 9, no. 11: 2006. https://doi.org/10.3390/pr9112006







