1. Introduction
The separation and recovery of noble metals from industrial wastewater has recently received significant attention as a component of sustainable chemistry. Especially, recovery of metals from electronic waste and other sources may be preferable to standard mining practices on a per mass basis, because discarded electronics contain greater relative proportions of metals compared with ores [
1]. Meanwhile, it is also important to note that electronic waste tends to contain noble metals at relatively low levels mixed with other metals [
2]. In order to separate metal ions of interest, liquid–liquid extraction is a common technique where metals such as gold and palladium can be extracted from an aqueous phase by employing specific organic solvents [
3,
4,
5,
6,
7]. This technique is commonly used but is labor intensive and requires organic solvents that can have environmental impacts. In recent years, new approaches to liquid-based separation of noble metals have been demonstrated. These include the use of ionic liquids supported on silica to extract platinum group metals [
8] and the selective separation of gold by adsorption on polyhedral oligomeric silsesquioxanes tethered to imidazole thiones [
9]. Metals can also be recovered via selective precipitation using electrowinning based on tuning electrode redox potentials, although this requires special instrumentation and produces hydrogen as a by-product under low metal ion concentrations [
10,
11]. An alternative approach for selective gold recovery is the use of sulfur atom-containing polymeric sorbents. For instance, 2,2′-thiobisethanol dimethacrylate was designed, synthesized, and demonstrated for selective gold separation under dilute conditions [
12,
13,
14]. Selenium-containing polymers (polyselenoureas) were also synthesized and demonstrated for selective gold recovery [
15]. Although there are now several excellent methods to selectively recover noble metals, especially gold, remaining requirements include (i) improvement of selectivity for specific metal elements, (ii) development of a simple one-pot processing method, (iii) development of a simple separation method for metals of interest, and (iv) development of an extraction method for homogenous, dilute water-based solutions.
Our own group has studied the synthesis of gold nanocrystals because this material exhibits special chemical and physical properties and is also biocompatible. Gold nanoparticles have applications as components of sensing or imaging devices, photonic or plasmonic systems, catalysts, and photothermal or drug delivery processes [
16,
17,
18,
19,
20,
21,
22,
23,
24]. Previously, we demonstrated the formation of a nonapeptide capable of forming β-sheets (RU006: Ac-Ala-Ile-Ala-Lys-Ala-X-Lys-Ile-Ala-NH
2, where X = L-2-naphthylalanine (Nal),
Figure 1A). Combining HAuCl
4 with RU006 in water was found to fabricate gold nanoribbons having widths in the range of 50–100 nm, heights of several nanometers, and lengths below 1 μm [
25,
26]. This process does not need additional reducing agents because the naphthalene moiety in the peptide can reduce HAuCl
4. The formation of these nanoribbons is believed to proceed in several stages. Firstly, AuCl
4− ions are captured in the internal spaces of the peptide network based on electrostatic interactions with the L-lysine (Lys) side chains that form during self-assembly of the peptide. Following this, there is a transfer of electrons from the naphthalene groups to the Au(III) ions such that crystalline gold is gradually produced and gold nanoribbons are generated along the framework of the peptide by self-assembly. The obtained metallic gold-peptide composites are collected by simple centrifugation. Therefore, RU006 can be considered to act as a combination of an extracting agent, a reducing agent, and a precipitating agent during gold nanoribbon synthesis. Replacing the Nal group (which has a peak oxidation potential of 1.50 V) at the 6th position in the RU006 with an anthracene group (which has a stronger reducing effect and an oxidation potential of 1.05 V) to obtain [Ant
6]-RU006 (X = L-2-anthrylalanine (Ant), referred to herein as RU065 (
a),
Figure 1B) has been found to form β-sheet conformation and modify the morphology of the resulting nanocrystals to generate spheres rather than ribbons [
25,
26].
Based on our prior results on the synthesis of gold nanocrystals using peptides containing aromatic side chains [
25,
26], we investigated the selective recovery of gold from homogenous aqueous (monophasic) solutions containing a mixture of dilute HAuCl
4 and H
2PtCl
6 (5.0 × 10
−5 M each) using peptides (2.0 × 10
−4 M each) [
27]. Much higher selectivity for gold (meaning Au-to-Pt (Au/Pt) atomic ratios of approximately 7.5) was obtained using the anthracene-containing peptide RU065 (
a) than using the naphthalene-containing peptide RU006. The recovery of gold was confirmed by elemental analysis of precipitates obtained from the reaction mixtures after simple centrifugation. These precipitates likely resulted from the rapid reduction of the HAuCl
4 with the anthracene rings inhibiting electron reception by H
2PtCl
6 [
28,
29]. On this basis, we concluded that anthracene-containing peptides could potentially be used to develop a selective gold precipitation/recovery process meeting the four criteria described above.
However, peptide molecules are generally costly to synthesize and so it is important to determine the minimum requirements for the structural features of RU065 (
a) for low-cost, selective gold recovery. This would contribute to the design of low-cost non-peptidyl anthracene derivatives. Therefore, the present study designed and synthesized 24 different anthracene-containing peptides, comprising the original RU065 (
a) [
27] and 23 of its fragment peptides (
b–
x,
Figure 1C). These fragments were obtained by deleting amino acid residues one-by-one from both N- and C-termini of the original RU065 (
a). Each of these compounds was applied to the selective precipitation/recovery of metallic gold from a mixture of dilute HAuCl
4 and H
2PtCl
6 (5.0 × 10
−5 M each), using a peptide (
a) or peptide fragment (
b–
x) at a concentration of 2.0 × 10
−4 M. The selectivity obtained from peptides (
a–
x) was examined by analysis of the precipitates using energy dispersive X-ray spectroscopy-field emission scanning electron microscopy (EDS-FESEM) after samples were collected by centrifugation to determine elemental contents and distributions. Based on the data from EDS-FESEM, we determined the most active miniaturized molecular structure derived from the original RU065 (
a) and characterized its secondary structure using attenuated total reflection Fourier transform infrared (ATR-FTIR) spectroscopy. Inductively coupled plasma-optical emission spectroscopy (ICP-OES) was also employed to compare the residual metal ion concentrations of supernatants after the removal of metallic precipitates by centrifugation, in trials using the original RU065 (
a) and the miniaturized peptides. We also examined the mechanism by which this peptide-based gold recovery system functioned, employing matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOFMS). The findings of this study are expected to assist in the future design of non-peptidyl anthracene derivatives for low-cost, environmentally friendly, and selective recovery of gold.
3. Results and Discussion
Design, Synthesis, and Characterization of the Peptides. As described in the introduction, in prior work, our group synthesized [Ant
6]-RU006, a nonapeptide forming amphiphilic β-sheets (referred to herein as RU065 (
a)) and having the structure Ac-Ala-Ile-Ala-Lys-Ala-Ant-Lys-Ile-Ala-NH
2 (
Figure 1B) [
25,
26]. The L-isoleucine (Ile) residues at the second and eighth positions in this peptide along with the Ant at the sixth position were all situated on the same side and so generated a hydrophobic face. A hydrophilic side was also produced based on the L-alanine (Ala) residues at the first, third, fifth, and ninth positions and the Lys at the seventh position, all of which were either less hydrophobic or, in the case of the Lys, were hydrophilic (see
Figure 1B and
Figure S1). A Lys residue was also present at the fourth position in proximity to the Ant on the hydrophobic side to allow interactions with AuCl
4− and PtCl
62− ions, so as to both concentrate and reduce the metal ions in the self-assembled peptides to generate metallic precipitates. As noted, 23 different peptides having smaller structures than the original RU065 (that is, specimens
b–
x) were obtained by sequentially removing amino acid residues from both N- and C- termini to the Ant group at the sixth position of the original molecule. These fragment peptides are referred to herein as RU065
x-y, where
x and
y are the positions on the original RU065 (
a) at which these fragments begin and end, respectively (
Figure 1C and
Figure S1). As an example, RU065
2–9 (
b) had the structure Ac-Ile-Ala-Lys-Ala-Ant-Lys-Ile-Ala-NH
2, and so spanned the second to ninth positions of the original peptide, based on removing the Ala at the first position.
A typical solid-state peptide synthesis technique based on Fmoc chemistry was used to synthesize these peptides [
30]. This synthesis was followed by purification using HPLC (
Figure S2) and characterization by MALDI-TOFMS (
Table S1 and
Figure S2).
Screening of Peptides by UV–Vis Absorption Spectroscopic Measurements. We initially examined the abilities of peptides
a–
x to reduce the metal ions by preparing equivolume mixtures of aqueous peptide solutions (4.0 × 10
−4 M) and aqueous solutions containing HAuCl
4 and H
2PtCl
6 (1.0 × 10
−4 M each) that were assessed using UV–Vis spectroscopy.
Figure S3 shows the spectra of these sample solutions. It is evident that the majority of the peptide solutions (
a–
u) generated a surface plasmonic absorption band at approximately 540 nm, while the short fragments (
v–
x) produced a band at 580 nm. These results suggest the formation of spherical gold nanocrystals via the reduction of Au ions by the anthracene moieties in the former peptides (
a–
u), and also indicate that peptides (
v–
x) caused aggregation of the nanocrystals and/or provided anisotropic gold nanocrystals [
25,
33,
34]. It should be noted that the formation of platinum nanocrystals could not be ascertained from the UV–Vis absorption spectra because this material did not generate characteristic peaks in the visible region of the spectrum [
35].
Screening of Peptides by EDS-FESEM Measurements. We next separated the precipitates recovered from the sample solutions containing peptides (
a–
x), HAuCl
4, and H
2PtCl
6 following centrifugation and analyzed their morphologies and elemental distributions by EDS-FESEM.
Figure S4 presents representative elemental mapping images of the precipitates for all peptides. In these images, the Au and Pt distributions are shown in yellow and purple, respectively, along with the corresponding EDS spectra. The results of replicate trials are summarized in
Figure 2A. The Au/Pt atomic ratio for the precipitates generated using the original RU065 (
a) was 8.4 ± 0.4, while the fragment peptides lacking one, two, and three amino acids from the N-terminus of RU065 (
a) (RU065
x–9 (
x = 2–4,
b–
d)) all provided similar ratios of 8.6 ± 0.2, 8.6 ± 0.6, and 8.8 ± 0.5, respectively. Surprisingly, the deletion of four and five amino acids from the N-terminus of the RU065 (peptides
e and
f) gave much lower ratios of 4.9 ± 0.3 and 5.1 ± 0.6, respectively.
We also examined the series of fragment peptides lacking an Ala residue at the ninth position of the RU065 (a) (RU065x-8 (x = 1–6, g–l)) and found that the deletion of one, two, and three amino acids from the N-terminus of RU0651-8 (g) provided excellent gold selectivity, with ratios of 7.8 ± 0.2 (RU0651–8 (g)), 7.9 ± 0.2 (RU0652–8 (h)), 7.6 ± 0.5 (RU0653–8 (i)) and 7.8 ± 0.4 (RU0654–8 (j)), respectively. These values were comparable to those for peptides a–d. In contrast, greatly decreased Au/Pt atomic ratios of 5.0 ± 0.7 and 3.0 ± 0.8 were determined for RU0655–8 (k) and RU0656–8 (l), respectively. These observations strongly indicate that the Lys residue at the fourth position of the original RU065 (a) was important with regard to obtaining significant gold selectivity.
The fragment series RU065x–7 (x = 1–6, m–r), lacking two amino acid residues at the eighth and ninth position of the original RU065 (a), were also assessed by EDS-FESEM. RU0651–7 (m) and RU0652–7 (n) provided suitable levels of selectivity, with ratios of 7.9 ± 0.3 and 7.8 ± 0.4, respectively. However, the deletion of the Ile residue at the second position of RU0651–7 (m) afforded significantly decreased Au/Pt ratios of 3.8 ± 0.3 and 4.3 ± 0.5 in the case of the RU0653–7 (o) and RU0654–7 (p), respectively. The RU065x–7 (x = 5–6, q–r) specimens did not provide large variations in the ratio even though the Lys residue at the fourth position was deleted in both, with RU0655–7 (q) and RU0656–7 (r) giving ratios of 3.2 ± 0.4 and 2.8 ± 0.7, respectively. These data suggest that the presence of a hydrophobic Ile residue at the second position of RU0651–7 (m) was vital to realizing high selectivity for gold. This effect is attributed to the lack of hydrophobic Ile and Ala residues at the eighth and ninth positions of the original RU065 (a) in the RU065x–7 (x = 1–6, m–r) fragments.
Lastly, we examined the selectivity of the RU065
x–6 (
x = 1–5,
s–
w) specimens, which lacked three amino acid residues at the seventh, eighth, and ninth positions of the original RU065 (
a), and of Ac-Ant-NH
2 (RU065
6 (
x)), which comprised only an Ant residue. The selectivity data trend for these peptides was similar to that for the RU065
x–7 (
x = 1–6,
m–
r) series. That is, the ratios for RU065
1–6 (
s) and RU065
2–6 (
t) showed slightly reduced values of 6.2 ± 1.2 and 6.4 ± 1.0, respectively, compared with those for peptides
a–
d,
g–
j,
m, and
n. The removal of the Ile residue at the second position of RU065
1–6 (
s) evidently lowered the ratio, giving values of 2.8 ± 0.3, 4.0 ± 1.2, 2.5 ± 0.4, and 2.6 ± 0.3 for the RU065
x–6 (
x = 3–5,
u–
w) and RU065
6 (
x) fragments, respectively. From these data, it appears that the incorporation of a hydrophobic Ile residue and a positively charged Lys residue is necessary for good selectivity. This is likely because the Ile and Lys residues facilitate the initial self-assembly of the peptide through hydrophobic interactions and also promote the accommodation/enrichment of AuCl
4− ions within the self-assembled peptide nanostructure, based on the proposed mechanism [
25,
26].
Hydropathy Assessment. The EDS-FESEM experiments described above highlighted the importance of characterizing the hydropathy of the original RU065 (
a) and its fragment peptides (
b–
x) by HPLC analysis [
31,
32].
Figure 2B summarizes the
H values for these specimens. Note that more positive values are associated with more hydrophobic compounds while negative values indicate more hydrophilic species. These data demonstrate that the original RU065 (
a) was relatively hydrophobic but a significant transition from hydrophobic to hydrophilic was induced by removing the hydrophobic Ile residue at the second position between RU065
2–9 (
b) and RU065
3–9 (
c). The deletion of the hydrophilic Lys residue at the fourth position led to another significant transition from hydrophilic back to hydrophobic between RU065
4–9 (
d) and RU065
5–9. Similar transitions also occurred between RU065
2–8 (
h) and RU065
3–8 (
i) and between RU065
4–8 (
j) and RU065
5–8 (
k). In the case of the RU065
x–7 (
x = 1–6,
m–
r) series, there was a change from hydrophobic to hydrophilic following the removal of the hydrophobic Ile residue at the second position on going from RU065
2–7 (
n) to RU065
3–7 (
o), resulting in the most hydrophilic peptide lacking two hydrophobic Ile residues at the second and eighth positions. Although the deletion of the Lys at the fourth position to generate RU065
5–7 (
q) decreased the relative hydrophilicity of this peptide (or more accurately increased the relative hydrophobicity), its
H value was still within the hydrophilic range because it did not have two of the Ile resides. The
H values for the RU065
x–6 (x = 1–5,
s–
w) series and RU065
6 (
x) also demonstrated a significant transition from hydrophobic to hydrophilic between RU065
2–6 (
t) and RU065
3–6 (
u) and from hydrophilic to hydrophobic between RU065
4–6 (
v) and RU065
5–6 (
w). These transitions were similar to those that occurred in the other series. The most hydrophobic fragment was RU065
6 (
x), which contained only one Ant residue. The smaller peptide structures afforded higher
H values, likely due to the greater contributions of individual amino acid residues.
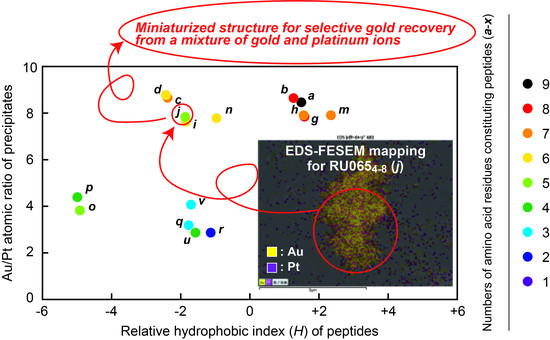
Determination of Miniaturized Active Structures. The relationship between the Au/Pt atomic ratios determined from
Figure 2A and the
H values from
Figure 2B for all peptides, along with the amino acid residues comprising each specimen, are presented in
Figure 3. Those fragments having
H values between −2 and +2 provided the best ratios (in the vicinity of 8). Specifically, these specimens were
a,
b,
c,
d,
g,
h,
i,
j,
m, and
n, among which the smallest peptide was RU065
4–8 (
j), having five amino acid residues including two Lys and one Ile (
Figure 4A). On this basis, we determined that RU065
4–8 (
j) had the minimum structure required to precipitate/recover gold from a mixture of gold and platinum ions.
Next, we characterized the secondary structure of the miniaturized active peptide RU065
4–8 (
j) by measuring the ATR-FTIR spectrum of a peptide film prepared from aqueous solution (
Figure 4B). The amide I region, originating from the amide carbonyl stretching frequencies between 1600 and 1700 cm
−1, is commonly used to determine the amide mode. The ATR-FTIR spectrum showed a strong amide I band around 1630 cm
−1 and a weak band around 1675 cm
−1, indicating that an antiparallel β-sheet conformation was predominant [
25], suggesting that even such a small fragment peptide (
j) preferably formed β-sheet conformation as seen for RU006 and RU065 (
a, [Ant
6]-RU006) in the literature [
25]. This presumably contributed to the enrichment of gold ions during self-assembly and the densification of the obtained metallic gold-peptide composites resulting in easy separation [
27].
Interestingly, peptides RU0654–8 (j) and RU0653–7 (o), each of which comprised five amino acids, showed very different Au/Pt atomic ratios even though the only difference was that the Ile residue in RU0654–8 (j) was replaced with an Ala residue in RU0653–7 (o). Further, peptides RU0654–8 (j) and RU0655–7 (q) had similar hydropathy characteristics but different numbers of amino acid residues, and also showed a significant difference in ratios. These variations can presumably be ascribed to the important role of amide moieties in forming the hydrogen bond networks that facilitated peptide self-assembly and metal precipitation. These results strongly suggest that adequate hydrophobicity and the presence of several amide moieties are important to the self-assembly process that, in turn, accommodates and concentrates gold ions with the subsequent reduction of these ions within the assemblies to provide high Au/Pt atomic ratios.
Transmission Electron Microscopy and X-ray Diffraction. The precipitates generated from mixtures of RU065
4–8 (
j) with HAuCl
4 and H
2PtCl
6 were characterized using TEM (
Figure 5A–C). The image in
Figure 5A demonstrates a spherical particle with a diameter of approximately 30 nm along with numerous other spherical particles with diameters on the order of 2 nm. The image in
Figure 5B presents a magnified view of the nanosphere surrounded by the yellow square in
Figure 5A. This image indicates a
d-spacing of 2.35 Å that corresponds to the Au<111> direction in crystals having a face-centered cubic (fcc) structure [
27,
36]. A magnified view of the region within the blue square is provided in
Figure 5C, with a
d-spacing of 2.21 Å that equates to the Pt<111> direction in an fcc crystal [
27,
36]. These results were verified by acquiring X-ray diffraction (XRD) data. The powder XRD pattern in
Figure 5D contains four broad peaks at 2
θ values of 38.2°, 44.4°, 64.6°, and 77.6° that are attributed to (111), (200), (220), and (311) crystal planes, respectively, in metallic gold having an fcc crystal structure. XRD peaks corresponding to elemental platinum (2
θ = 39.8°, 46.3°, and 67.5°, assigned to (111), (200) and (220) crystal planes, respectively) were not observed, likely because the platinum nanospheres had crystallite sizes smaller than those detectable by powder XRD. These results suggest that the precipitates from the reaction of RU065
4–8 (
j), HAuCl
4, and H
2PtCl
6 were in a metallic state and that metallic platinum formed much smaller particles than metallic gold.
Gold Selectivity Assessment by ICP-OES. ICP-OES was used to better assess the precise selectivity of the peptide-based recovery system for gold by quantifying residual ion concentrations in the supernatants obtained after centrifugation of mixtures comprising the peptides, HAuCl
4 and H
2PtCl
6 (
Figure 6) [
27,
37]. As shown in
Figure 6A, ICP-OES analyses of the supernatants of the reaction mixtures containing the original RU065 (
a) revealed a residual Au(III)/Au(I) concentration of 2.7 × 10
−6 M (equivalent to 95% recovery) and a residual Pt(IV)/Pt(II) concentration of 3.7 × 10
−5 M (26% recovery). These results are comparable to those in our previous work [
27].
ICP-OES analysis of the reaction mixtures containing RU065
4–8 (
j) gave residual concentrations of 1.6 × 10
−6 M (97% recovery) for Au(III)/Au(I) and 3.9 × 10
−5 M (22% recovery) for Pt(IV)/Pt(II) in
Figure 6B. The results for RU065 (
a) and RU065
4–8 (
j) were similar, suggesting that the latter has the same potential to selectively reduce/recover gold ions as RU065 (
a), even though it has four fewer amino acids. Consequently, the minimum requirement for the structure would be Ac-Lys-Ala-Ant-Lys-Ile-NH
2. The high recovery yields for gold with RU065 (
a) and RU065
4–8 (
j) suggest that RU065
4–8 (
j) also concentrated gold ions on the basis of a mechanism involving self-assembly, reduction of gold ions to the metallic state and concentration, enabling easy separation of the metallic solids by simple centrifugation, as RU065 (
a) did in our previous work [
27]. However, the selectivity (that is, the Au recovery %/Pt recovery %) determined by ICP-OES was somewhat lower than the Au/Pt atomic ratio obtained from the EDS-FESEM analyses, as shown in
Figure 2A. These small differences between the selectivity determined by ICP-OES and EDS-FESEM could be attributed to the formation of platinum nanoclusters that were difficult to separate by centrifugation (as shown in
Figure 5A,C), such that different amounts of gold were determined using the two methods.
We next demonstrated selective gold reduction/recovery from a mixture of gold, platinum, and nickel ions using RU065
4–8 (
j) to examine the effects of a transition metal.
Figure 6C shows the residual ion concentrations in the supernatant obtained from a mixture of RU065
4–8 (
j, 2.0 × 10
−4 M), HAuCl
4, H
2PtCl
6, and Ni(NO
3)
2 (metal ion = 5.0 × 10
−5 M each) in water. The data show residual concentrations of 1.8 × 10
−6 M (96% recovery) for Au(III)/Au(I), 4.1 × 10
−5 M (18% recovery) for Pt(IV)/Pt(II) and 5.0 (4.96) × 10
−5 M (approximately 1% recovery) for Ni(II). These results suggest that positively charged transition metal ions such as nickel were more difficult to reduce than noble metals and so primarily remained in solution as a consequence of charge repulsion between the Ni(II) ions and RU065
4–8 (
j). Therefore, the present peptide-based system could be robust for selective gold recovery from a mixture of base metal ions with redox potentials lower than those for noble metal ions.
As reported by Kim et al., a tannin-TiO
2 heterostructure selectively adsorbed gold ions from a solution containing nine different metal ions ([metal ion] = 1.0 × 10
−3 M each) with an Au/Pt ratio of ca. 50 under light irradiation [
38]. Dogan et al. showed that nitrile and amidoxime-containing nanoporous polymer microspheres adsorbed gold ions from a solution containing eleven different metal ions ([metal ion] = 100 ppb each) with an Au/Pt ratio of ca. 3.8 [
39]. These results suggest that for high metal ion concentrations, an excellent Au/Pt ratio can be achieved, whereas for low metal ion concentrations, it is difficult to improve the gold selectivity against platinum. Thus, our results (metal ion = 5.0 × 10
−5 M each) are competitive and it is possible to design low-cost gold-selective recovery agents based on the active short peptide RU065
4–8 (
j).
Mechanistic Study. Our prior work demonstrated that the original RU065 (
a) was able to generate metal nanocrystals after a span of 4 h. This was a more rapid process than was evident using another peptide incorporating a naphthalene moiety in place of an anthracene ring. Specifically, EDS-FESEM indicated that a combination of RU065 (
a), HAuCl
4, and H
2PtCl
6 decreased the Au/Pt ratio from 12.3 to 7.3 after 4 h. On this basis, we suggest that gold nanocrystals were initially generated as the HAuCl
4 was reduced by the anthracene rings, while nanocrystals of platinum were formed more gradually. Consequently, Au(III) was rapidly changed to metallic gold with limited formation of Au(I) as an intermediate, while Pt(IV)/Pt(II) ions received electrons less slowly to produce a platinum precipitate [
27,
28,
29].
The present work also attempted to elucidate the mechanism of the selective reduction/recovery of metallic gold from the mixture of HAuCl
4 and H
2PtCl
6 by RU065
4–8 (
j). This was done by analyzing sample solutions using MALDI-TOFMS.
Figure 7A shows the MALDI-TOFMS profile for the solution containing RU065
4–8 (
j) prepared using the standard conditions described in the Experimental Section but without HAuCl
4 and H
2PtCl
6. A single peak was obtained at
m/
z = 748.5, corresponding to the molecular ion peak (that is, [M + H]
+) along with a less intense peak at
m/
z = 770 likely corresponding to [M + Na]
+. These data indicate that RU065
4–8 (
j) remained intact after a 24 h incubation in water at 40 °C. In contrast, the MALDI-TOFMS profile for the sample solution prepared under the standard conditions contained peaks at
m/
z = 748.7 and 770.9 corresponding to [M + H]
+ and [M + Na]
+ for the intact RU065
4–8 (
j) but also at
m/
z = 764.8, 779.0, 802.9, and 818.1, corresponding to [M +
O + H]
+, [M + 2
O + H]
+, [M + 2
O + Na]
+, and [M + 2
O + K]
+, and peaks around
m/
z = 767 likely attributable to [M + K]
+ and/or [M +
O + Na]
+ (
Figure 7B,C). These results strongly suggest that the anthracene rings were oxidized by gold and/or platinum ions to generate ketone, hydroxide, quinone, and/or hydroquinone-like functional groups during the self-assembly of the peptides and the subsequent reduction of the metal ions to form gold-rich metallic precipitates [
40]. However, since only limited reaction kinetics data were obtained in our previous study [
27], further experiments are required to clarify the reaction mechanisms that lead to good gold selectivity.