Abstract
Spent coffee grounds (SCGs) generated in coffee processing for beverages and other products are a very significant organic residue that needs to be properly treated. Waste valorization via oil extraction has the potential to obtain compounds that can be used for producing biodiesel or other high-value products, such as polymers. This work focuses on the ultrasound-assisted extraction of SCG oil using n-hexane as a solvent. Three key process parameters are analyzed: temperature, extraction time, and liquid/solid (L/S) rate of solvent, using a central composite rotatable design (CCRD), an analysis that, to the author’s knowledge, is not yet available in the literature. The data were analyzed using the software StatSoft STATISTICA 13.1 (TIBCO Software Inc., Palo Alto, CA, USA). Results show that all parameters have a statistical influence on the process performance (p < 0.05), being the L/S ratio the most significant, followed by extraction time and temperature. An analysis of variance (ANOVA) showed that the empirical model is a good fit to the experimental data at a 95% confidence level. For the range of conditions considered in this work, the optimal operating conditions for obtaining an oil extraction yield in the range of 12 to 13%wt are a solvent L/S ratio of around 16 mL g−1, for a temperature in the range of 50 to 60 °C, and the longest contact time, limited by the process economics and health and safety issues and also, by the n-hexane boiling temperature.
1. Introduction
Coffee is currently one of the most important agricultural commodities and the most popular beverage worldwide [1,2], with a daily consumption of over 2 billion cups [3]. In the past decade, global consumption has risen steadily at an average rate of more than 1% annually, reaching an expected production of more than 10.53 megatonnes in 2020/21, being the European Union the largest importer and consumer [2,4].
In all life cycle stages of coffee production, significant amounts of solid waste are generated, which must be treated and/or disposed of properly. These can be categorized into two streams: (i) pre-roasting waste, defined as coffee husks from dry processing and coffee pulp produced from wet processing, and (ii) post-roasting residues, such as coffee silver skin and spent coffee grounds (SCGs) that result from coffee brewing [5]. Among these waste streams, the brewing coffee residues are the most significant, since 1 ton of green coffee beans results in about 650 kg of SCGs [6]. On the other hand, to produce 1 kg of soluble coffee, about 2 kg of wet SCGs are generated [7]. This work will focus on the SCG solid residue that remains after the preparation of the coffee beverage.
About 50% of SCGs are generated by the industry for producing soluble coffee, and by the coffee shops chains, like Starbucks, with the remainder corresponding to domestic consumption. The annual amount of SCGs generated by the industry is about six million tons [6]. The current policies for solid residues are based on the waste management hierarchy [8] and a Life Cycle Thinking (LCT) perspective [9]. Although prevention and minimization are at the top of the waste management hierarchy, the generation of waste is inevitable in most situations. Thus, it follows the waste recovery, namely, for energy generation, when recycling is not feasible, or due to the low value of the materials, or because their contamination is high, and it is intended to reduce the amount of waste deposited in landfills. Thus, in a first approach, an attempt should be made to recover as much as possible the components with commercial value and that can be used as raw material for obtaining other products [6,10], such on a SCG biorefinery approach as proposed by Caetano et al. [11].
Different potential applications have been suggested for the SCG residues [7]. For example, the SCG lipids can be used for high-value applications in cosmetics [12], or for polymers and thermosetting resins [13], or for biodiesel production [14]. The remaining biomass, after the oil extraction, can be used for bioethanol by hydrolysis of cellulose and hemicelluloses, followed by sugars fermentation [15,16]. SCGs are also a source of phenolic antioxidants [17] and galactomannan polysaccharides [18]. The whole SCG biomass can be used as a substrate for high commercial value mushrooms cultivation [19] or as a bioremediation material for absorbing water pollutants [20], or for the production of pellets, as fuel for industrial boilers, due to its high heating value of 19.0–26.9 MJ/kg [10]. Additionally, SCGs can be used for the production of biochar or bio-oil through the pyrolysis process [21], among other applications.
Of the various SCG constituents, this work focuses on lipids, which content can reach 15 wt% [6], depending on the coffee type (Arabica or Robusta), brewing methods (e.g., percolating or boiling), moisture content, particle size, L/S ratio, extraction time and extraction technique used (e.g., Soxhlet, ultrasound, microwave or supercritical fluid). In particular, it intends to study the ultrasound-assisted extraction (UAE) method of SCG oil using n-hexane as a solvent and the influence of three key operating parameters: temperature, extraction time, and the solvent liquid/solid rate.
To find a sustainable, economical, and efficient extraction method, it is crucial to scale up the process, ensuring a more environmentally friendly production and economic viability. In this regard, cavitation-based extraction techniques are being widely used as promising tools for natural products extraction, owing to their potential implementation in large-scale and lower capital investment [22]. In this field, UAE has captured much attention, being applied in different chemical processes. This technique has numerous advantages: it is simple to apply, has faster kinetics, greater energy efficiency, higher yield and quality of the extract [23]. Also, the UAE methods are advantageous by comparison with the supercritical fluid extraction (SFE) method, which, although fast and efficient, requires high pressure and temperature with the consequent high operating costs and environmental impacts [24,25]. In contrast, the UAE method requires lower pressure and temperature, and it is simple and easy to implement the technique, decreasing the operating costs. Also, UAE can improve the efficiency and rate of extraction by comparison with the Soxhlet method [26]. The theory behind UAE deals with the acoustic cavitation phenomena, which refers to the formation, growth, and implosion collapse of microbubbles in the liquid media [27]. The collapse of these bubbles generates physical effects, including micro-turbulence, high-velocity inter-particle collisions, and perturbation in the micro-porous particles of the solid matrix, resulting in diffusion acceleration. Ultrasound irradiations enhance the extraction rate through an effective increase in mass transfer between the solvent and substrate, and physical cell wall disruption caused by the formation of micro-cavities [28].
Many studies [23,29,30,31] unveiled the efficient performance of ultrasonic baths in extracting oils from different oleaginous plant seeds or waste materials [31] under mild conditions and in a shorter time when compared to standard methods, together with higher extraction yields and greater purity of the extracts. For example, according to Abdullah and Koc [32], the UAE method allows extracting 98% of the available oil from SCGs in 30 min, reducing the amount of hexane needed to successfully perform the extraction. These authors explain that the main effect of ultrasound waves on oil extraction is the ultrasonic cavitation that helps the penetration of the solvent into the biomass cells. The collapse of bubbles near the surface of the particles creates micro-explosions that increase the penetration of the solvent, thus facilitating the release of the intracellular oil. Also, the ultrasound waves work on disrupting the biological cell walls to enhance the penetration of solvent into the cells and promoting the release of the desired components [33]. Moreover, Rocha et al. [34] reported the positive effect of increasing the solvent-to-solid ratio, on oil yield from Arabica SCGs, using an indirect sonication approach. Likewise, Le et al. [35] remarked a noticeable increase in oil yield after increasing the n-hexane amount, when using a supersonic bath operating at ambient temperature. Ahangari and Sargolzaei [36] studied different methods for extracting oil from SCGs, such as microwave-assisted extraction (MAE), UAE and SFE, and focused on the optimization of SFE.
The optimization of the main process parameters or variables (sonication time, temperature, type and L/S ratio of solvent) is decisive for the economics and efficiency of the UAE process. Previous studies have highlighted the crucial role of process optimization aided by response surface methodology (RSM) tools for defining the ideal process conditions with minimum experimental runs [37]. The RSM allows one to monitor the process variables and analyze their combined effect on the final output. However, as far as the authors’ knowledge goes, there is no exhaustive study in the literature that analyzes those key operational issues in an integrated and simultaneous way, in order to identify which ones most influence the SCG oil extraction and how the process can be optimized.
Hence, this work aims to fulfill that gap by studying and optimizing the UAE method for SCG oil extraction, using n-hexane, a non-polar solvent with a boiling point of 68 °C. A statistical factorial design was carried out, followed by a Central Composite Rotatable Design (CCRD) to determine the optimum operating conditions to perform the extraction in terms of the L/S ratio of solvent, extraction temperature and solvent contact time. No other studies were found in the literature in which these three key operational factors were studied simultaneously, confirming the novelty of the present work.
2. Materials and Methods
2.1. Spent Coffee Grounds (SCG) Samples
The SCGs used in this work are a commercial mix of Arabica and Robusta coffee collected from a vending coffee machine drawer (of the coffee brand “JURADO”, Orihuela-Alicante, Spain). Immediately after the SCG sample collection, they were dried at 105 ± 5 °C in an air forced oven (WTC Binder; Tuttlingen, Germany) until a constant weight was reached, aiming to remove the water content in the sample. The dehydration process is crucial to eliminate the moisture, avoiding spoilage and microbial growth [32]. The residual moisture content was 12%. The moisture content was determined according to the AOAC method 930.04 [38].
The dried SCG samples were ground in an electric stainless steel grinder (150 W Aromatic Taurus grinder; Barcelona, Spain), and then sieved to get 50-mesh fine particles, to increase the surface area and thus enhance the contact between the solvent and the solid. Finally, SCG samples were stored at −20 °C in non-sterile polyethylene bottles (Kartell S.p.A.—Labware Division, Noviglio, MI, Italy) before they are used.
2.2. Statistical Design
Oil extraction from SCG is a multi-parameter process that depends on several experimental factors, such as moisture content, particle size, L/S ratio, solvent type, extraction method, and contact time. Thus, it is essential to determine the optimal operational conditions, even more, if considering the industrial implementation of the oil extraction process. Process simulation is a good option, but the complexity of interactions between solvent and SCGs and the lack of information for some process key aspects, e.g., phase equilibria, thwart the application of this approach. A univariate sequential experimental procedure can be used, fixing all variables and optimizing only one, which results in the understanding of the effect of only one variable and, thus, the impact of the others and their interactions are neglected. Furthermore, this procedure can make the process optimization cumbersome, as it may require a large number of experiments, which increase rapidly with the number of variables considered.
An adequate and efficient optimization procedure should take into account the influence of all parameters, or at least those considered more relevant, and their interactions, in order to achieve the optimum extraction conditions. In this context, statistical factorial planning, such as a Central Composite Rotatable Design (CCRD), is considered an efficient tool to study and optimize processes with various factors interacting with each other, allowing the identification of the main variables that influence the process response [39,40].
Of the various factors that influence oil extraction from SCGs, the following key process paramenters were selected for the analysis:
- temperature (X1),
- extraction time (X2), and
- liquid/solid (L/S) rate of solvent (X3).
Temperature is a significant factor, as it influences the mass transfer between phases and the phase equilibria. The extraction time is also very important, as an adequate time should be defined to ensure that most of the oil is extracted from SCGs, yet not so large as to lead to a final extraction period, in which no significant amount of oil is produced. A suitable value of L/S rate of solvent should be found, which ensures that the extraction is not limited by the quantity of solvent available.
Therefore, the CCRD will be a 23 experimental design. The range of values considered by the CCRD depends on the system under studied, and may be determined from preliminary experiments or from the literature. In this study both options were used, allowing the definition of the base conditions of temperature, extraction time, and L/S ratio: 42.5 °C, 52.5 min, and 12.91 mg/L respectively [34,41], that corresponds to the central point of the CCRD, for which three experiments were done, in order to evaluate the experimental error. The conditions for the remaining experiments were defined considering the system expected behavior, and the specific details of the methodology [40].
Table 1 presents the experimental conditions chosen, where 0) represents the center point, (+1, −1) represents the maximum and minimum points, and (+1.68, −1.68) represents the axial points. The various combinations of experimental conditions used are presented in Table 2, in which the central point experiments correspond to the assays 1, 7, and 15. A total of 17 experiments were carried out, in which the extraction oil yield was obatined.

Table 1.
Independent variables values for CCRD.

Table 2.
Central composite rotatable design (CCRD) matrix.
The experimental data obtained was analyzed using STATISTICA 13.1 StatSoft software (TIBCO Software Inc., Palo Alto, CA, USA). A second order model was considered to model the experimental data, as shown in Equation (1):
where Y is the corresponding response; are the linear, quadratic and interaction effects; and are the regression coefficients of linear, quadratic and interaction effects, and is the error. The statistical significance analysis of the various factors, either linear or non-linear, was done using analysis of variance (ANOVA), for which a significance level of 95% was considered (equivalent to a p-value < 0.05), and the response surface method (3D and 2D). A comparison between the experimental results and published data will be made whenever possible.
2.3. Ultrasonic Extraction Process
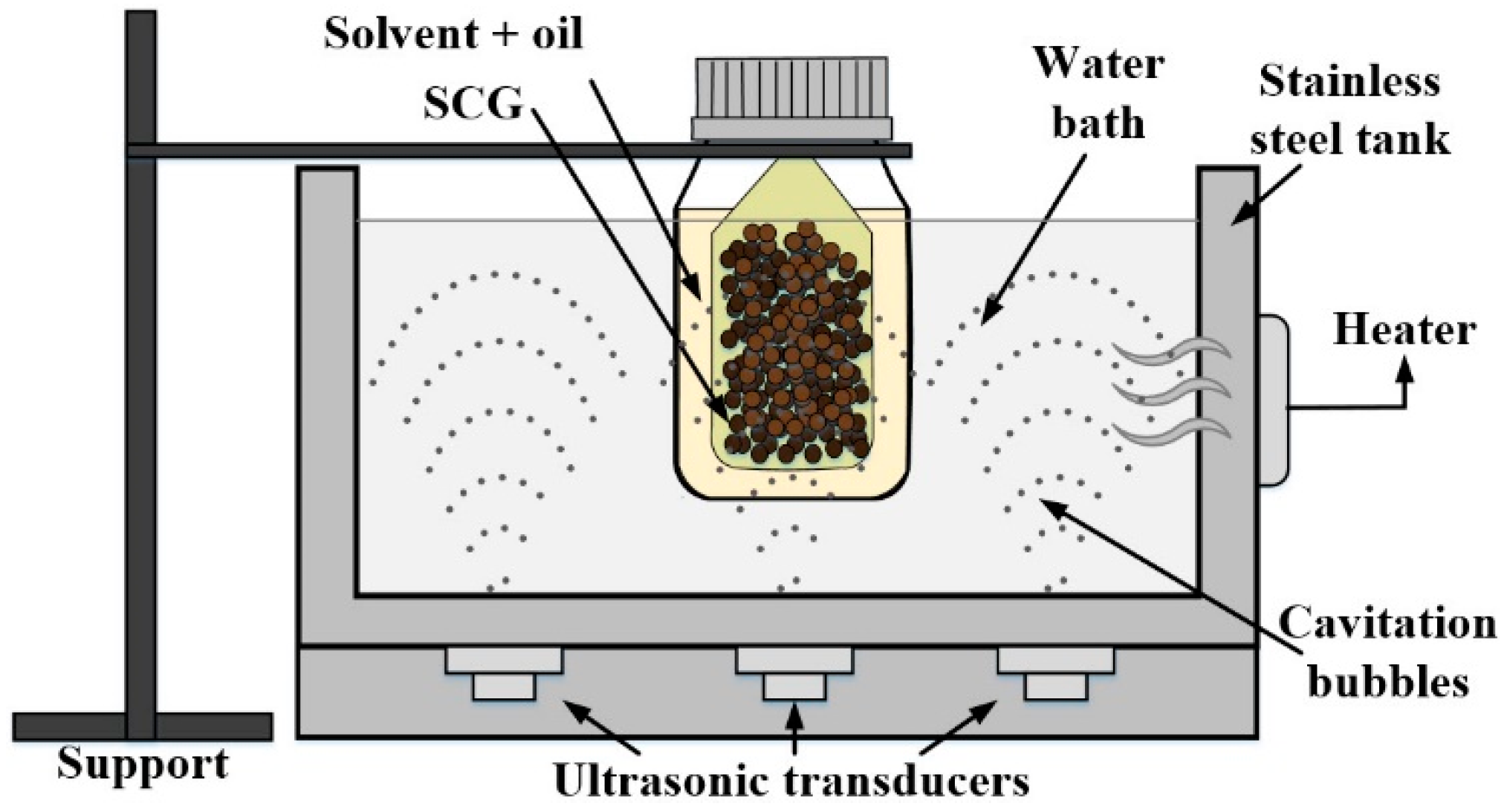
The oil extraction process was performed using an ultrasonic water bath with a 2.7 L maximum capacity (Ultrasonic cleaner, Toctech), as shown in Figure 1, which provides 140 W for the bathtub. SCG samples of 5 g were packed in individually sealed filter paper sachets and immersed in a flask with the n-hexane solvent (99% purity, Sigma-Aldrich, Saint Louis, MO, USA). It was chosen due to its ability to extract oils and being also the most widely used organic solvent in the industry [36,42,43]. To separate the solvent from the oil, a rotary evaporator (Heidolph WB 2001, Schwabach, Germany) was used with maintaining the temperature above the boiling point of the reagent at 69 °C and a speed of 200 rpm during a period (10–15 min). Then, the oil samples were placed in an oven (WTC Binder; Tuttlingen, Germany) at 104 °C, for 6 h, ensuring complete separation of n-hexane.
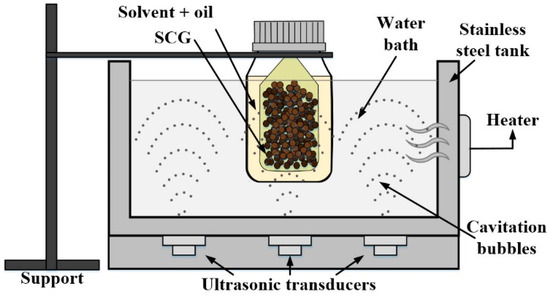
Figure 1.
Experimental setup for the ultrasound-assisted extraction of SCG oil (own authorship).
The experimental conditions were defined according to the statistical factorial planning, described in the previous section. The separation performance is evaluated by the extraction oil yield, calculated according to Equation (2):
where is the SCG oil yield, in dry matter percentage (DM %), is the weight of extracted oil in grams, is the dry weight of SCG in grams, and 100 is the factor to express the oil yield in percentage.
Following its weighting, the collected SCG oils were flushed with nitrogen and kept at −20 °C until further use. Further analysis of the extracted oil, such as the characterization of its fatty acid profile, is beyond the scope of this work, as it aims to study the extraction process and not the oil applications depending on its quality.
3. Results and Discussion
3.1. Factorial Design for SCG Oil Extraction
Results of the experimental design are presented in Table 3. It can be seen that for the set of experimental conditions considered the extracted oil yield is in the range between 0.69 to 12.34% of oil per SCG dry weight. For the central level conditions (42.5 °C, 52.2 min, and 12.91 mL g−1), corresponding to the assays 1, 7 and 15; the oil yields obtained were quite similar (±9%), attesting a good experimental reproducibility and small experimental error. Also, results show that n-hexane is a suitable solvent to extract oil from SCGs. As the extraction process is mainly dependent on Van der Waals type interactions, the non-polar nature of n-hexane results in an easier penetration of the low polar matrix of the SCGs and oil solubilization, also non-polar compounds themselves [42].

Table 3.
Experimental assays conditions and oil yield for the n-hexane extraction of SCG oil.
Table S1, presented in the Supplementary Information, compares the results of different studies for SCG oil extraction using common extraction technologies. Generally, the results obtained in this study are in agreement with the values reported in the literature [32,34,44]. The maximum experimental oil yield value obtained in this study (12.34%) is higher than the ones reported by El Hajjaji et al. [45] (10.9 wt%) and by Rocha et al. [34] (12%), using an ultrasonic bath, but lower than the values reported by Abdullah and Koc [32] (13%) and by Goh et al. [44] (14.52%), using ultrasound assisted-two phase oil extraction and an ultrasonic probe as a direct extraction tool.
In Table S1 the variations in oil yield among different authors may be explained by the different brewing methods of fresh ground coffee beans that can be used (boiling, dip-filtering, percolating, etc.) and affect the lipids content, and also the composition of the coffee blends used, as Coffea arabica (Arabica) and Coffea canephora (Robusta) have different lipid contents and profiles [46]. Moreover, the higher yields achieved when ultrasonic probes instead of ultrasonic bath are used can be explained due to the greater ultrasonic power of the probe, which is up to about 100 times greater than that of the bath [47].
3.2. Statistical Analysis
One important feature of experimental statistical designs is the possibility of identifying the factors, either independently or combined. To do that, normally, an empirical polynomial model is assumed, in which its adjustable parameters are fitted to the experimental data. In this work a quadratic model, as shown in Equation (1), was considered. A maximum of 10 parameters may be calculated from it, meaning that the design of experiments done in this work will have six degrees of liberty. In order to reduce the number of parameters, an analysis of literature was done. From other studies of oil extraction from SCG [41,43], it is possible to conclude that the combined effects of different factors, corresponding to the factors in Equation (1) (as for example the combined influence of the temperature and the solvent L/S ratio), can be considered non-significant. Hence, in this work, only linear and quadratics effects of single factors are considered, after fitting the experimental data to the model Equation (3) was obtained:
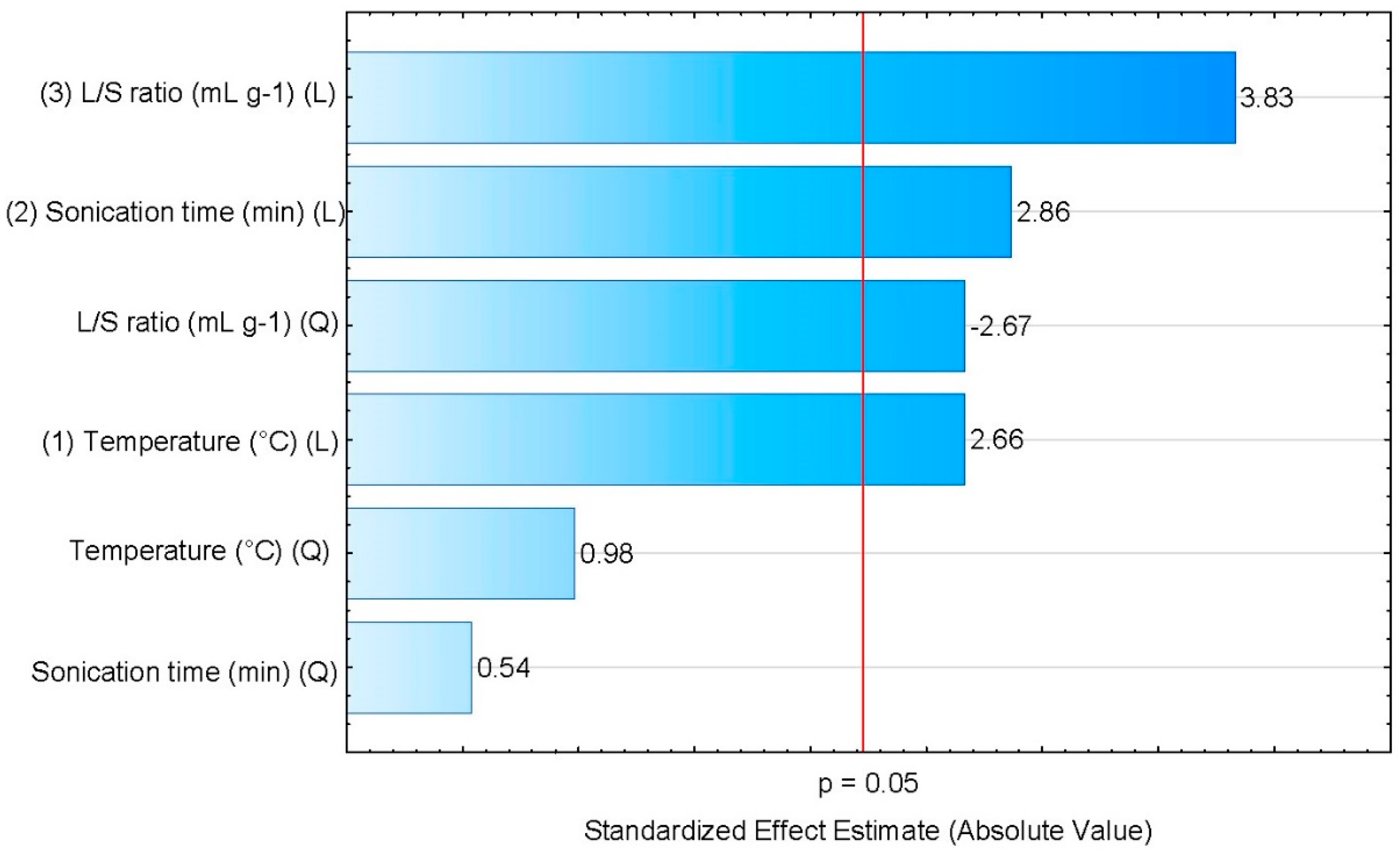
Table 4 and Figure 2 present the results of statistical significance analysis for the simplifeid a model, where , , and are the linear coefficients; ; and are the quadratic coefficients; and the SE are the standardized effects. Please note that in Figure 2, the Pareto diagram, the horizontal bars represent the magnitude of each effect, and the vertical red line represents p = 0.05, allowing the direct identification of which factors are significant as any factor that crosses the red line.

Table 4.
Estimated effects for SCG oil extraction using n-hexane as solvent.
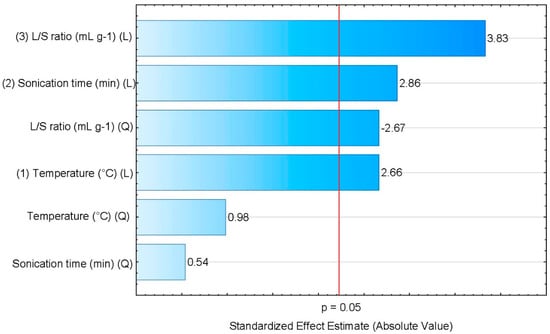
Figure 2.
Pareto diagram of the experimental variables studied for the optimization of oil yield in the SCG extraction, using n-hexane as solvent.
The results of Table 4 show that, except for the quadratic term for the temperature and sonication time effects, all terms are significant for a p-value < 0.05. For the temperature and the sonication time, a positive effect was observed for both linear and quadratic terms, including the non-significant meaning that an increase in those factors leads to a higher oil yield. For the temperature, higher values will lead to faster diffusion of the solvent inside the SCGs, and also a higher solubility of the lipids in the solvent. Also, larger sonication times allow a more complete contact between the SCG and the solvent, facilitating the lipids extraction and consequently resulting in higher oil yields. For the liquid-solid ratio, the linear dependence shows a positive effect, as expected as more solvent available will allow more oil to be solubilized without saturating it. Yet, an inverse dependence is observed for the quadratic term, showing that the influence of the L/S ratio in the extraction process is nonlinear.
A similar conclusion can be drawn from the Pareto plot, Figure 2.
Comparing the relative importance of the various effects, it can be concluded that the L/S ratio is the most influential variable in the SCG oil extraction, followed by sonification time and temperature. Rocha et al. [34] also observed a similar effect, supporting the conclusion that the L/S solvent ratio is the dominant effect in the extraction of oil from SCGs using an ultrasonic bath. To assess if the mathematical model is can adequately represent the experimental data, an analysis of variance (ANOVA) was performed. The results are presented in Table 5, showing that the model is adequate at a 95% confidence level.

Table 5.
Analysis of variance (ANOVA) for SCG oil extraction.
Additionally, the coefficient of determination (R2) and the adjusted coefficient (adj-R2) was estimated to verify the model adequacy. R2 is a measure of the relationship between response and independent variables, varying between 0 and 1, the closest to 1 its value, the better the model fit to the experimental data. An R2 value of 0.81 was obtained, suggesting that 81% of the dependent variable variations are explained by the model. An adj-R2 value of 0.69 was obtained, which corresponds to a difference R2–Adj-R2 equals 0.12, lower than 0.2. Thus, it can be concluded that the model is an adequate representation of the experimental data [48].
3.3. Response Surface Analysis
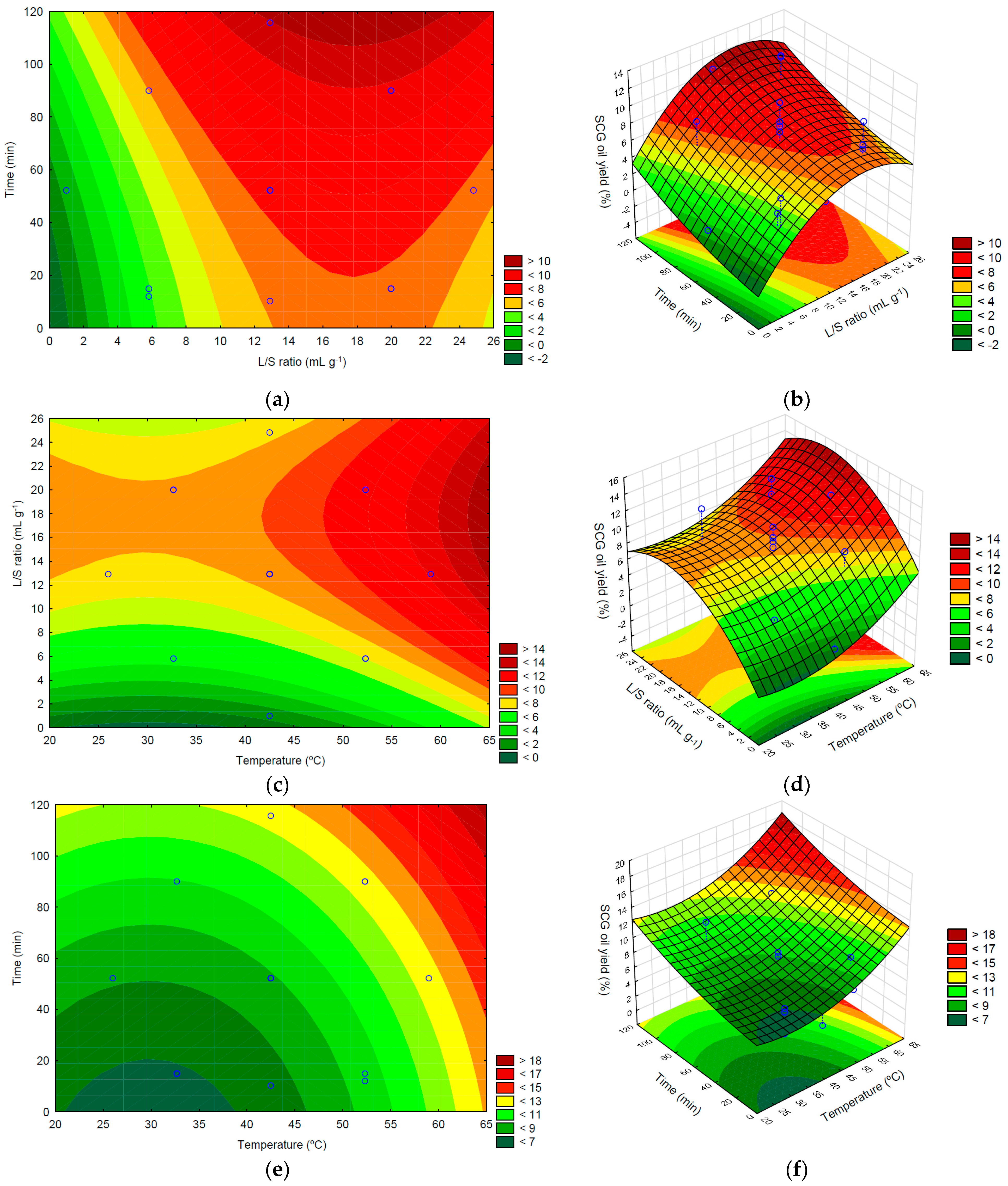
To complement the statistical analysis done in the previous section the experimental data was examined using response-surface plots, which allow an easy evaluation and identification of trends, and how the independent variables influence the oil yield. As 3 factors are considered in this work, the representation of all experimental points and corresponding response surface is not possible, as a four-dimensional representation is needed. Thus, it is necessary to keep one variable constant and vary the others, in order to obtain three dimensional, 3D, plots. Hence, three response surfaces can be obtained, and they are presented in Figure 3, together with the corresponding two dimensional, 2D, contour plots. Figure 3 displays the contour plots for SCG oil extraction for each pair of variables as the other factor was kept constant at its middle level.
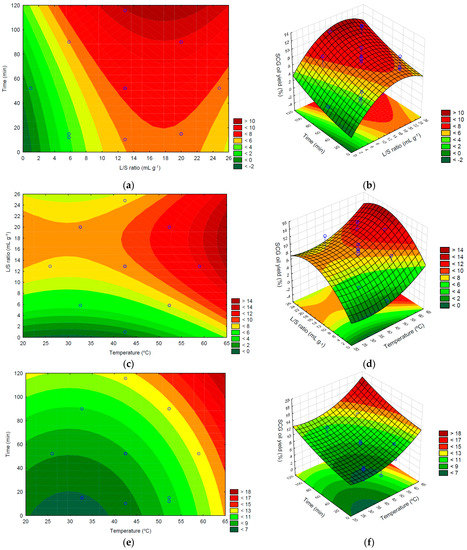
Figure 3.
2D and 3D response surface plots of the SCG oil yield (%), obtained as a function of the combined effect of time (min), temperature (°C) and L/S ratio (mg·g−1) as follows: (a,b) extraction time and L/S ratio; (c,d) L/S ratio and temperature; (e,f) extraction time and temperature.
Figure 3a,b present the combined effect of solvent contact time and L/S ratio on the SCG oil yield. It can be seen that the higher contact times always lead to higher extraction yields, as expected since it will allow more time for the oil to diffuse from the solid matrix to the solvent. The variation is almost linear for the range of values presented in Figure 3a,b, although the variation is slight in the range of the L/S ratio values considered in this work. This behavior agrees with the relative importance of the various effects, as shown in Table 3, where the linear effect is the only relevant factor regarding the influence of the temperature.
The influence of the L/S ratio is stronger, and a different behavior is observed. For the lowest values, an increase will lead to higher oil yields, as expected as more oil will be able to solubilize in n-hexane without becoming saturated. However, after reaching a maximum value, the extraction yield diminishes as the ratio L/S increases, showing that the solvent quantity ceases to be the limiting factor, and diffusional effects are significant. Thus, the L/S ratio influence in extraction yield is nonlinear, as expected from the results presented in Table 3, which show that both the linear and quadratic effects are relevant. Also, as the two effects have opposite signals, a maximum extraction is expected and observed experimentally. Thus, for the range of values analyzed in this work large values of contact times, higher than 110 min, and L/S ratio ranging between 13 and 22 mL g−1 is desired to optimize the extraction yield. Similar results were obtained by Moradi et al. [49] for the extraction of sunflower oil, which observed that optimal L/S ratio values were lower than 13 or greater than 23 mL g−1, regardless of the extraction time.
In Figure 3c,d, the influence of the L/S ratio and temperature is shown. Increasing the temperature always leads to higher extraction yields, and the effect is stronger for high-temperature values. This is expected as higher temperatures facilitate oil diffusion in the solid matrix, as the solute (oil) and solvent diffusivities increase, and its solubilization, resulting in high oil yield. Thus, from an operational perspective, higher temperatures are desirable.
For the L/S ratio, a behavior similar to the one shown in Figure 3a,b can be seen. Thus, higher temperatures and L/S ratio ranging between 13 and 22 mL g−1 are desired to have the largest extraction yields possible. Comparing the relative influence of both variables, it can be seen that the L/S ratio has a stronger impact than the temperature, as expected as the absolute values of effects, given in Table 3, are larger for the L/S ratio. This conclusion is in line with that obtained by Rocha et al. [34], in which these authors also verified the greater influence of the solvent/solid ratio compared to the temperature in the extraction of Arabica SCG oil in an ultrasonic bath. This is because as temperature increases, viscosity and surface tension decrease, resulting in increased vapor pressure, which enters cavitation bubbles and reduces its collapse and sonication effects. However, the solvent’s vapor pressure is low at lower temperatures, allowing the violent collapse of cavitation bubbles. Therefore, the sonochemical effect is low temperature-dependent [47].
The 2D and 3D plots for the fixed value of the L/S ratio are given in Figure 3e,f, where the combined effects and interactions between the solvent contact time and temperature on the SCG oil yield can be seen. The results show that increasing the value of both variables, either independently or combined, leads to an increase in the oil yield extraction. This result was expected, as more contact time will result in more oil solubilization and diffusion to the solvent, phenomena that is also enhanced at higher temperatures. The graphs also show that the influence of both variables is somewhat similar, increasing for higher values of the solvent contact time and temperature, as evidenced by the closer contour lines in the graphic. This is in agreement with the effect’s values listed in Table 2 and Figure 2, that although show a smaller influence of the quadratic terms, for larger values of the variables is expected to be seen a nonlinear dependence.
3.4. Optimal Extraction Conditions
Based on the experimental results and the statistical and response surface analysis, it is possible to identify the operational conditions that maximize the oil extraction yield. In particular, regarding the temperature and solvent contact time, the higher the better, as the response surface analysis shows no maximum value for the extraction yield. Yet, there are physical and practical limitations to the range of values possible for both factors. Regarding temperature, in industrial practice is not used pure n-hexane, but a mixture of isomers in which n-hexane is dominant with a boiling point in the range of 67 to 69 °C [50]. Furthermore, the solvent is volatile and inflammable, and processes using it must follow specific health and safety regulations [51]. Hence, the desirable operation should not exceed 60 °C, to avoid excess vaporization of solvent, which may threaten the equipment integrity and increase the fire risk, in agreement with the temperatures used in oil extraction from seeds, which have a range between 50 and 60 °C [51].
For the solvent contact time, a long extraction time is usually necessary for the full recovery of the oil, but economic factors may limit it. The larger the extraction, the more energy is necessary to maintain the mixture at the desired temperature. Moreover, the extraction efficiency is expected to decrease in time, and large extraction times may cause undesirable degradation of the target component, affecting the process economics. Thus, it becomes necessary to optimize the extraction time in order to guarantee both a high quantity and quality of the extract while at the same time reducing the energy consumption of the process. However, more information is needed to determine the most suitable sonication time, as it depends on several factors, such as the type of material, the structure of the cell wall, the resistance to mass transfer for the diffusion of the solvent into the material, and the rate of penetration of the solvent [28]. Also, the experimental results and the surface response analysis do not show any maximum or decrease in the extraction efficiency. Hence, it is not clear what the optimal extraction time is. More studies are needed to determine, in particular an economic analysis.
The L/S ratio and optimal value around 16–18 mL g−1 can be defined based on the surface responses presented in Figure 3b,d. In fact, experimentally it is observed that increasing the L/S ratio while maintaining the other factors fixed may lead to lower extraction yield, as the comparison between experiments 1 and 2 shows (Table 1). No clear maximum is observed, implying that an L/S ratio value within the defined range is adequate. The range of values is lower than 20 (V/W) obtained by Le et al. [35] for the ultrasound-assisted extraction method, and also is lower than 22.5 (g/g) found by Somnuk et al. [43] for the Soxhlet extraction method, but it is higher than 4 (mL g−1) the optimized value determined by Rocha et al. [34].
For optimized operational conditions, an extraction yield between 13 to 14% is achievable. This is in agreement with the experimental results of Abdullah and Koc [32] (13%) and Goh et al. [44] (14.52%), showing that the system analyzed in this work has a similar performance as the best extraction methods presented in the literature.
Although the fatty acid profile of the extracted oil has not been characterized, from the literature, it can be predicted that the oil obtained by UAE will be rich in linoleic and palmitic acids [34,41]. Hence, it can be used as a chemical feedstock to obtain various products, such as biodiesel [11] or alkyd resins [13].
4. Conclusions
Indirect sonication was performed to extract oil from SCG using n-hexane as solvent. The analysis of the effects of principal experimental process parameters was done using the design of experiments. A CCRD was employed with three factors: temperature, time, and L/S ratio, to assess what are the main effects and the interactions/synergies between them. A good agreement was observed between the results and the statistical model considered to represent them. The results show that all three factors have a significant statistical effect on the oil extraction yield, being the L/S ratio the most influential factor.
Moreover, it is concluded that the linear effects are dominant, except for the L/S ratio, in which the quadratic factor is also relevant. Taking into account the surface response analysis, it is possible to conclude that the optimal operating conditions are an L/S ratio of around 16 mL g−1 and the highest possible temperature, in the range of 50 to 60 °C, taking into account the health and safety of using n-hexane as solvent. No clear optimal conditions were obtained for the contact time, although it should be the largest possible, taking into account the process and economic constraints. An oil extraction yield of 12 to 13% is achievable based on the experimental data and statistical analysis performed in this work. Thereby, the current work suggests a simple, cost-effective, eco-friendlier, and efficient indirect ultrasound-assisted extraction of SCG oil.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pr9112085/s1, Table S1: Comparison among studies results using common extraction technologies for SCG oil.
Author Contributions
Conceptualization, M.M., D.N.-G., A.R.-C., T.M.M. and A.A.M.; methodology, M.M., M.V., D.N.-G., T.M.M. and A.A.M.; software, D.N.-G.; validation, D.N.-G., T.M.M. and A.A.M.; formal analysis, M.M., D.N.-G., A.R.-C., T.M.M. and A.A.M.; investigation, M.M., R.R., D.N.-G. and M.P.-I.; resources, A.R.-C. and A.A.M.; writing-original draft preparation, M.M. and D.N.-G.; writing-review and editing, M.M., D.N.-G., T.M.M., A.R.-C. and A.A.M.; supervision, A.R.-C., D.N.-G. and A.A.M.; project administration A.R.-C. and A.A.M.; funding acquisition, A.R.-C. and A.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by base Funding—UIDB/00511/2020 of Laboratory for Process Engineering, Environment, Biotechnology and Energy—LEPABE—funded by National Funds through the FCT/MCTES (PIDDAC);Authors gratefully acknowledge the funding of Project NORTE-06-3559-FSE-000107, cofinanced by Programa Operacional Regional do Norte (NORTE2020), through Fundo Social Europeu (FSE); António Martins thanks FCT (Fundação para a Ciência e Tecnologia) for funding through program DL 57/2016—Norma transitória.
Acknowledgments
The authors would like to express their deepest gratitude and appreciation to the department of vegetal production and microbiology of Miguel Hernández University under the leadership of Pablo Melgarejo for their remarkable technical input.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Girotto, F.; Pivato, A.; Cossu, R.; Nkeng, G.E.; Lavagnolo, M.C. The broad spectrum of possibilities for spent coffee grounds valorisation. J. Mater. Cycles Waste Manag. 2018, 20, 695–701. [Google Scholar] [CrossRef]
- ICO. ICO Trade Statistics Tables; International Coffee Organization (ICO): London, UK, 2021; Available online: http://www.ico.org/trade_statistics.asp (accessed on 22 June 2021).
- Murthy, P.S.; Naidu, M.M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- USDA Foreign Agriculture Service; U.S. Department of Agriculture. USDA Coffee: World Markets and Trade. 2021. Available online: https://www.fas.usda.gov/data/coffee-world-markets-and-trade (accessed on 22 June 2021).
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Dave Oomah, B. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Bio-refinery approach for spent coffee grounds valorization. Bioresour. Technol. 2018, 247, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, M.; Agapiou, A.; Omirou, M.; Vyrides, I.; Ioannides, I.M.; Maratheftis, G.; Fasoula, D. Converting environmental risks to benefits by using spent coffee grounds (SCG) as a valuable resource. Environ. Sci. Pollut. Res. 2018, 25, 35776–35790. [Google Scholar] [CrossRef] [PubMed]
- European Commission. EC The Waste Framework Directive 2008/98/EC; European Commission: Luxembourg, 2008. [Google Scholar]
- Martins, A.A.; Simaria, M.; Barbosa, J.; Barbosa, R.; Silva, D.T.; Rocha, C.S.; Mata, T.M.; Caetano, N.S. Life cycle assessment tool of electricity generation in Portugal. Environ. Dev. Sustain. 2018, 20, 129–143. [Google Scholar] [CrossRef] [Green Version]
- Caetano, N.S.; Silva, V.F.M.; Melo, A.C.; Martins, A.A.; Mata, T.M. Spent coffee grounds for biodiesel production and other applications. Clean Technol. Environ. Policy 2014, 16, 1423–1430. [Google Scholar] [CrossRef] [Green Version]
- Caetano, N.S.; Mata, T.M.; Martins, A.A.; Felgueiras, M.C. New Trends in Energy Production and Utilization. Energy Procedia 2017, 107, 7–14. [Google Scholar] [CrossRef]
- Ribeiro, H.; Marto, J.; Raposo, S.; Agapito, M.; Isaac, V.; Chiari, B.G.; Lisboa, P.F.; Paiva, A.; Barreiros, S.; Simões, P. From coffee industry waste materials to skin-friendly products with improved skin fat levels. Eur. J. Lipid Sci. Technol. 2013, 115, 330–336. [Google Scholar] [CrossRef]
- Saratale, G.D.; Bhosale, R.; Shobana, S.; Banu, J.R.; Pugazhendhi, A.; Mahmoud, E.; Sirohi, R.; Kant Bhatia, S.; Atabani, A.E.; Mulone, V.; et al. A review on valorization of spent coffee grounds (SCG) towards biopolymers and biocatalysts production. Bioresour. Technol. 2020, 314, 123800. [Google Scholar] [CrossRef]
- Caetano, N.S.; Silva, V.F.M.; Melo, A.C.; Mata, T.M. Potential of spent coffee grounds for biodiesel production and other applications. Chem. Eng. Trans. 2013, 35, 1063–1068. [Google Scholar]
- Mussatto, S.I.; Machado, E.M.S.; Carneiro, L.M.; Teixeira, J.A. Sugars metabolism and ethanol production by different yeast strains from coffee industry wastes hydrolysates. Appl. Energy 2012, 92, 763–768. [Google Scholar] [CrossRef] [Green Version]
- Kwon, E.E.; Yi, H.; Jeon, Y.J. Sequential co-production of biodiesel and bioethanol with spent coffee grounds. Bioresour. Technol. 2013, 136, 475–480. [Google Scholar] [CrossRef]
- Panusa, A.; Zuorro, A.; Lavecchia, R.; Marrosu, G.; Petrucci, R. Recovery of natural antioxidants from spent coffee grounds. J. Agric. Food Chem. 2013, 61, 4162–4168. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Pei, W.; Tang, S.; Yan, F.; Peng, Z.; Huang, C.; Yang, J.; Yong, Q. Procuring biologically active galactomannans from spent coffee ground (SCG) by autohydrolysis and enzymatic hydrolysis. Int. J. Biol. Macromol. 2020, 149, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Pandey, A.; Mohan, R.; Soccol, C.R. Use of various coffee industry residues for the cultivation of Pleurotus ostreatus in solid state fermentation. Acta Biotechnol. 2000, 20, 41–52. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Karamesouti, M.; Mitropoulos, A.C.; Kyzas, G.Z. A review for coffee adsorbents. J. Mol. Liq. 2017, 229, 555–565. [Google Scholar] [CrossRef]
- Vardon, D.R.; Moser, B.R.; Zheng, W.; Witkin, K.; Evangelista, R.L.; Strathmann, T.J.; Rajagopalan, K.; Sharma, B.K. Complete utilization of spent coffee grounds to produce biodiesel, bio-oil, and biochar. ACS Sustain. Chem. Eng. 2013, 1, 1286–1294. [Google Scholar] [CrossRef]
- Panda, D.; Manickam, S. Cavitation Technology—The Future of Greener Extraction Method: A Review on the Extraction of Natural Products and Process Intensification Mechanism and Perspectives. Appl. Sci. 2019, 9, 766. [Google Scholar] [CrossRef] [Green Version]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Couto, R.M.; Fernandes, J.; da Silva, M.D.R.G.; Simões, P.C. Supercritical fluid extraction of lipids from spent coffee grounds. J. Supercrit. Fluids 2009, 51, 159–166. [Google Scholar] [CrossRef]
- Danlami, J.M.; Arsad, A.; Zaini, M.A.A.; Sulaiman, H. A comparative study of various oil extraction techniques from plants. Rev. Chem. Eng. 2014, 30, 605–626. [Google Scholar] [CrossRef]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Sonawane, S.H.; Gogate, P.R. Intensification of extraction of natural products using ultrasonic irradiations-A review of current status. Chem. Eng. Process. Process Intensif. 2012, 53, 10–23. [Google Scholar] [CrossRef]
- Tavares, G.R.; Massa, T.B.; Gonçalves, J.E.; da Silva, C.; dos Santos, W.D. Assessment of ultrasound-assisted extraction of crambe seed oil for biodiesel synthesis by in situ interesterification. Renew. Energy 2017, 111, 659–665. [Google Scholar] [CrossRef]
- Zhang, W.; Pan, Y.G.; Huang, W.; Chen, H.; Yang, H. Optimized ultrasonic-assisted extraction of papaya seed oil from Hainan/Eksotika variety. Food Sci. Nutr. 2019, 7, 2692–2701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanioti, S.; Tzia, C. Optimization of ultrasound-assisted extraction of oil from olive pomace using response surface technology: Oil recovery, unsaponifiable matter, total phenol content and antioxidant activity. LWT-Food Sci. Technol. 2017, 79, 178–189. [Google Scholar] [CrossRef]
- Abdullah, M.; Bulent Koc, A. Oil removal from waste coffee grounds using two-phase solvent extraction enhanced with ultrasonication. Renew. Energy 2013, 50, 965–970. [Google Scholar] [CrossRef]
- Kentish, S.; Ashokkumar, M. The Physical and Chemical Effects of Ultrasound. In Ultrasound Technologies for Food Bioprocessing; Food Engineering Series; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–12. ISBN 9788578110796. [Google Scholar]
- Rocha, M.V.P.; de Matos, L.J.B.L.; de Lima, L.P.; Figueiredo, P.M.d.S.; Lucena, I.L.; Fernandes, F.A.N.; Gonçalves, L.R.B. Ultrasound-assisted production of biodiesel and ethanol from spent coffee grounds. Bioresour. Technol. 2014, 167, 343–348. [Google Scholar] [CrossRef]
- Le, P.T.K.; Vu, Q.T.H.; Nguyen, Q.T.V.; Tran, K.A.; Le, K.A. Extraction and evaluation the biological activities of oil from spent coffee grounds. Chem. Eng. Trans. 2017, 56, 1729–1734. [Google Scholar]
- Ahangari, B.; Sargolzaei, J. Extraction of lipids from spent coffee grounds using organic solvents and supercritical carbon dioxide. J. Food Process. Preserv. 2013, 37, 1014–1021. [Google Scholar] [CrossRef]
- Nde, D.B.; Anuanwen, C.F. Optimization methods for the extraction of vegetable oils: A review. Processes 2020, 8, 209. [Google Scholar] [CrossRef] [Green Version]
- Latimer, G.W.J. Chapter 3: Plants. Official Methods of Analysis of AOAC International, 21st ed.; The Scientific Association of Official Analytical Chemists (AOAC): Rockville, MD, USA, 2019; Volume 3, ISBN 0935584544. [Google Scholar]
- Said, K.A.M.; Amin, M.A.M. Overview on the Response Surface Methodology (RSM) in Extraction Processes. J. Appl. Sci. Process Eng. 2016, 2, 8–17. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons, Inc.: New York, NY, USA, 2017. [Google Scholar]
- Mofijur, M.; Kusumo, F.; Rizwanul Fattah, I.M.; Mahmudul, H.M.; Rasul, M.G.; Shamsuddin, A.H.; Mahlia, T.M.I. Resource recovery from waste coffee grounds using ultrasonic-assisted technology for bioenergy production. Energies 2020, 13, 1770. [Google Scholar] [CrossRef] [Green Version]
- Al-Hamamre, Z.; Foerster, S.; Hartmann, F.; Kröger, M.; Kaltschmitt, M. Oil extracted from spent coffee grounds as a renewable source for fatty acid methyl ester manufacturing. Fuel 2012, 96, 70–76. [Google Scholar] [CrossRef]
- Somnuk, K.; Eawlex, P.; Prateepchaikul, G. Optimization of coffee oil extraction from spent coffee grounds using four solvents and prototype-scale extraction using circulation process. Agric. Nat. Resour. 2017, 51, 181–189. [Google Scholar] [CrossRef]
- Goh, B.H.H.; Ong, H.C.; Chong, C.T.; Chen, W.H.; Leong, K.Y.; Tan, S.X.; Lee, X.J. Ultrasonic assisted oil extraction and biodiesel synthesis of Spent Coffee Ground. Fuel 2020, 261, 116121. [Google Scholar] [CrossRef]
- El Hajjaji, S.; Bouladab, C.; Chergaoui, S.; Fadli, S. Biorefining of Waste Coffee Grounds: Turning an Environmental Problem into an Opportunity. IOP Conf. Ser. Earth Environ. Sci. 2020, 505, 12026. [Google Scholar] [CrossRef]
- Oliveira, L.S.; Franca, A.S.; Camargos, R.R.S.; Ferraz, V.P. Coffee oil as a potential feedstock for biodiesel production. Bioresour. Technol. 2008, 99, 3244–3250. [Google Scholar] [CrossRef]
- Capelo-Martinez, J.L. Microwaves in Organic Synthesis Practical Microwave Synthesis for Organic Chemists Microinstrumentation; Wiley-VCH Verlag GmbH & Co. KGaA: Darmstadt, Germany, 2009; ISBN 9783527314522. [Google Scholar]
- Mäkelä, M. Experimental design and response surface methodology in energy applications: A tutorial review. Energy Convers. Manag. 2017, 151, 630–640. [Google Scholar] [CrossRef]
- Moradi, N.; Rahimi, M.; Moeini, A.; Parsamoghadam, M.A. Impact of ultrasound on oil yield and content of functional food ingredients at the oil extraction from sunflower. Sep. Sci. Technol. 2018, 53, 261–276. [Google Scholar] [CrossRef]
- Anderson, G.E. Solvent Extraction; AOCS Lipid Library: Urbana, IL, USA, 2021; Available online: https://lipidlibrary.aocs.org/edible-oil-processing/solvent-extraction (accessed on 22 June 2021).
- Bart, J.C.J.; Palmeri, N.; Cavalaro, S. Chapter 3—Oleochemical sources: Basic science, processing and applications of oil. In Biodiesel Science and Technology: From Soil to Oil; Woodhead Publishing Series in Energy 7; CRC—Woodhead Publishing Limited: Boca Raton, FL, USA, 2010. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).