Impact of Various Essential Oils and Plant Extracts on the Characterization of the Composite Seaweed Hydrocolloid and Gac Pulp (Momordica cochinchinensis) Edible Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Gac Pulp-Based Films with Plant Essential Oils and Extracts
2.3. Determination of Edible Film Characteristics
2.3.1. Physical Properties
Film Thickness
Moisture Content
Opacity
Colour
2.3.2. Barrier Properties
Water Vapour Permeability
- : was determined by the slope of the straight line (gs−1)
- A: Surface area (m2)
- T: thickness (mm)
- ΔP: the water vapour pressure difference inside and outside of the film (Pa)
2.3.3. Mechanical Properties
2.3.4. Structural Characterization
Scanning Electron Microscopy
X-ray Diffraction
2.4. Statistical Analysis
3. Results and Discussion
3.1. Impact of Essential Oils and Plant Extracts on Thickness, Moisture Content, and Opacity of the Seaweed Hydrocolloid and Gac Pulp Edible Films
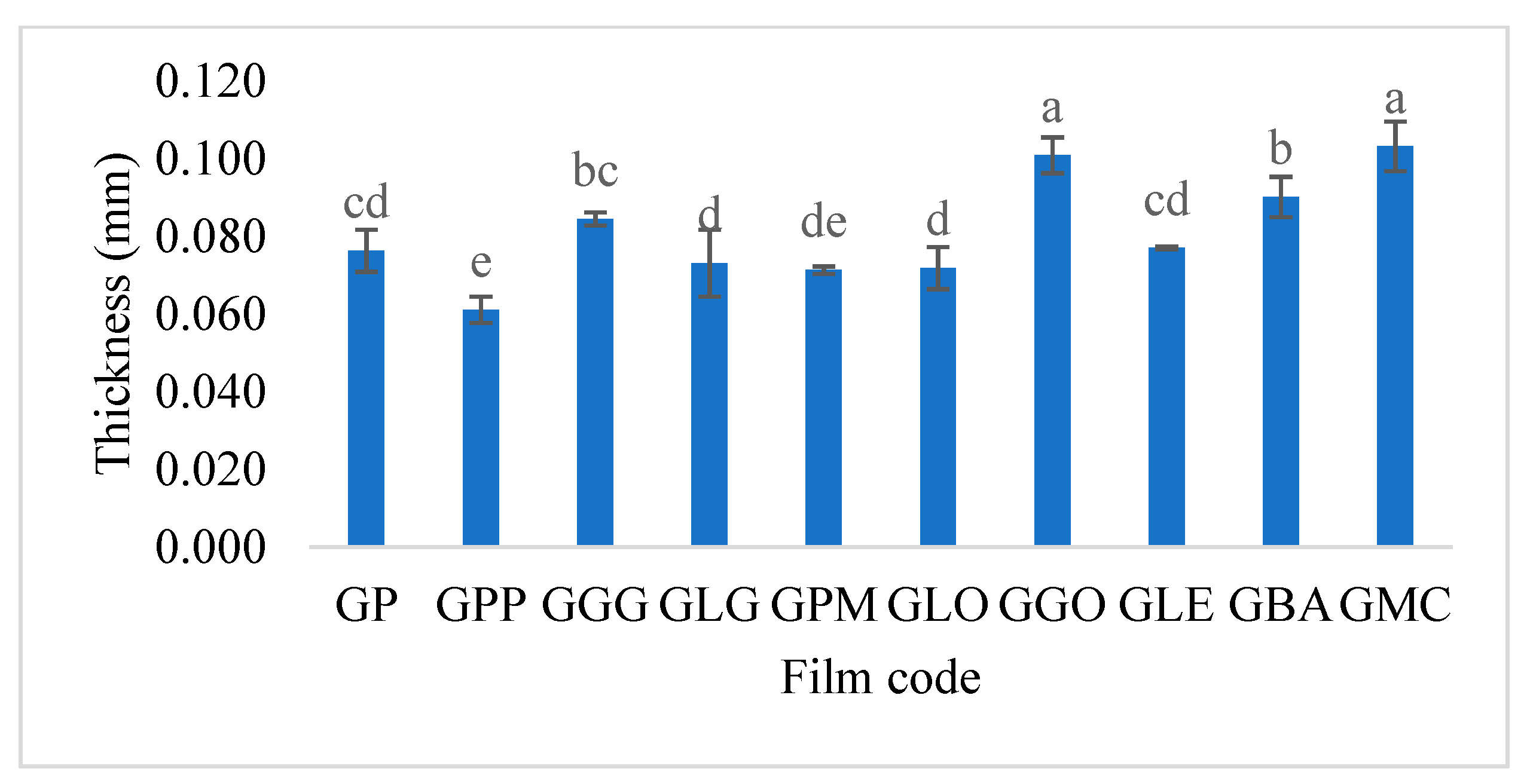
3.1.1. Film Thickness
3.1.2. Moisture Content
3.1.3. Opacity
3.1.4. Colour of the Films
3.2. Impact of Essential Oils and Plant Extracts on Barrier and Mechanical Properties of the Seaweed Hydrocolloid and Gac Pulp Edible Films
3.2.1. Water Vapour Permeability
3.2.2. Mechanical Properties
3.3. Impact of Essential Oils and Plant Extracts on Structure of the Seaweed Hydrocolloid and Gac Pulp Edible Films
3.3.1. Surface and Cross-Section Microstructure
3.3.2. Crystalline Structure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salgado, P.R.; Ortiz, C.M.; Musso, Y.S.; Di Giorgio, L.; Mauri, A.N. Edible films and coatings containing bioactives. Curr. Opin. Food Sci. 2015, 5, 86–92. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Du, W.-X.; Avena-Bustillos, R.J.; Hua, S.S.T.; McHugh, T.H. Antimicrobial volatile essential oils in edible films for food safety. Sci. Against Microb. Pathog. Commun. Curr. Res. Technol. Adv. 2011, 2, 1124–1134. [Google Scholar]
- Nieto, M.B. Structure and function of polysaccharide gum-based edible films and coatings. In Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009; pp. 57–112. [Google Scholar] [CrossRef]
- Moreira, M.R.; Cassani, L.; Martín-Belloso, O.; Soliva-Fortuny, R. Effects of polysaccharide-based edible coatings enriched with dietary fiber on quality attributes of fresh-cut apples. J. Food Sci. Technol. 2015, 52, 7795–7805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimoch-Korzycka, A.; Jarmoluk, A. Polysaccharide-Based Edible Coatings Containing Cellulase for Improved Preservation of Meat Quality during Storage. Molecules 2017, 22, 390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyfzadeh, M.; Motalebi, A.A.; Kakoolaki, S.; Gholipour, H. Chemical, microbiological and sensory evaluation of gutted kilka coated with whey protein based edible film incorporated with sodium alginate during frozen storage. Iran. J. Fish. Sci. 2013, 12, 140–153. [Google Scholar]
- Feng, Z.; Wu, G.; Liu, C.; Li, D.; Jiang, B.; Zhang, X. Edible coating based on whey protein isolate nanofibrils for antioxidation and inhibition of product browning. Food Hydrocoll. 2018, 79, 179–188. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Yuan, G.; Chen, X.; Li, D. Chitosan films and coatings containing essential oils: The antioxidant and antimicrobial activity, and application in food systems. Food Res. Int. 2016, 89, 117–128. [Google Scholar] [CrossRef]
- Ganiari, S.; Choulitoudi, E.; Oreopoulou, V. Edible and active films and coatings as carriers of natural antioxidants for lipid food. Trends Food Sci. Technol. 2017, 68, 70–82. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Lai, T.K.; Tye, Y.Y.; Rizal, S.; Chong, E.W.N.; Yap, S.W.; Hamzah, A.A.; Nurul Fazita, M.R.; Paridah, M.T. A review of extractions of seaweed hydrocolloids: Properties and applications. Express Polym. Lett. 2018, 12, 296–317. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef]
- Bealer, E.J.; Onissema-Karimu, S.; Rivera-Galletti, A.; Francis, M.; Wilkowski, J.; La Cruz, D.S.-D.; Hu, X. Protein–Polysaccharide Composite Materials: Fabrication and Applications. Polymers 2020, 12, 464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Embuscado, M.E.; Huber, K.C. Edible Films and Coatings for Food Applications; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Bonilla, J.; Vargas, M.; Atarés, L.; Chiralt, A. Effect of Chitosan Essential Oil Films on the Storage-Keeping Quality of Pork Meat Products. Food Bioprocess Technol. 2014, 7, 2443–2450. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Antimicrobial and physical-mechanical properties of agar-based films incorporated with grapefruit seed extract. Carbohydr. Polym. 2014, 102, 708–716. [Google Scholar] [CrossRef]
- Alsaggaf, M.S.; Moussa, S.H.; Tayel, A.A. Application of fungal chitosan incorporated with pomegranate peel extract as edible coating for microbiological, chemical and sensorial quality enhancement of Nile tilapia fillets. Int. J. Biol. Macromol. 2017, 99, 499–505. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Torlak, E.; Akın-Evingür, G.; Özen, I.; Erim, F.B. Antimicrobial and physical properties of chitosan films incorporated with turmeric extract. Int. J. Biol. Macromol. 2017, 101, 882–888. [Google Scholar] [CrossRef]
- Alves, V.L.; Rico, B.P.; Cruz, R.M.; Vicente, A.A.; Khmelinskii, I.; Vieira, M.C. Preparation and characterization of a chitosan film with grape seed extract-carvacrol microcapsules and its effect on the shelf-life of refrigerated Salmon (Salmo salar). LWT 2018, 89, 525–534. [Google Scholar] [CrossRef] [Green Version]
- Castro, F.V.R.; Andrade, M.A.; Silva, A.S.; Vaz, M.F.; Vilarinho, F. The Contribution of a Whey Protein Film Incorporated with Green Tea Extract to Minimize the Lipid Oxidation of Salmon (Salmo salar L.). Foods 2019, 8, 327. [Google Scholar] [CrossRef] [Green Version]
- Baron, R.D.; Pérez, L.L.; Salcedo, J.M.; Córdoba, L.J.P.; Sobral, P.J.D.A. Production and characterization of films based on blends of chitosan from blue crab (Callinectes sapidus) waste and pectin from Orange (Citrus sinensis Osbeck) peel. Int. J. Biol. Macromol. 2017, 98, 676–683. [Google Scholar] [CrossRef]
- Torres-León, C.; Vicente, A.A.; Flores-López, M.L.; Rojas, R.; Serna-Cock, L.; Alvarez-Pérez, O.B.; Aguilar, C.N. Edible films and coatings based on mango (var. Ataulfo) by-products to improve gas transfer rate of peach. LWT 2018, 97, 624–631. [Google Scholar] [CrossRef] [Green Version]
- Gheribi, R.; Habibi, Y.; Khwaldia, K. Prickly pear peels as a valuable resource of added-value polysaccharide: Study of structural, functional and film forming properties. Int. J. Biol. Macromol. 2019, 126, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, M.; Salami, M.; Mohammadian, M.; Khodadadi, M.; Emam-Djomeh, Z. Development of antioxidant edible films based on mung bean protein enriched with pomegranate peel. Food Hydrocoll. 2020, 104, 105735. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Gac fruit (Momordica cochinchinensisSpreng.): A rich source of bioactive compounds and its potential health benefits. Int. J. Food Sci. Technol. 2014, 50, 567–577. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S. Phytochemicals and antioxidant activity of different fruit fractions (peel, pulp, aril and seed) of Thai gac (Momordica cochinchinensis Spreng). Food Chem. 2011, 127, 1138–1145. [Google Scholar] [CrossRef]
- Trirattanapikul, W.; Phoungchandang, S. Influence of Different Drying Methods on Drying Characteristics, Carotenoids, Chemical and Physical Properties of Gac Fruit Pulp (Momordica cochinchinensis L.). Int. J. Food Eng. 2016, 12, 395–409. [Google Scholar] [CrossRef]
- Tran, T.T.; Saifullah, M.; Nguyen, N.H.; Nguyen, M.H.; Vuong, Q.V. Comparison of ultrasound-assisted and conventional extraction for recovery of pectin from Gac (Momordica cochinchinensis) pulp. Future Foods 2021, 4, 100074. [Google Scholar] [CrossRef]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Vuong, Q. Effects of different drying methods on extractable phenolic compounds and antioxidant properties from lemon myrtle dried leaves. Heliyon 2019, 5, e03044. [Google Scholar] [CrossRef] [Green Version]
- Vuong, Q.; Pham, H.N.T.; Vu, H.T.; Dang, T.T.; Van Ngo, T.; Chalmers, A.C. Fruit characteristics, phytochemical and antioxidant properties of blueberry ash (Elaeocarpus reticulatus). Heliyon 2018, 4, e00834. [Google Scholar] [CrossRef] [Green Version]
- Dailey, A.; Vuong, Q.V. Optimisation of Ultrasonic Conditions as an Advanced Extraction Technique for Recovery of Phenolic Compounds and Antioxidant Activity from Macadamia (Macadamia tetraphylla) Skin Waste. Technologies 2015, 3, 302–320. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.T.; Roach, P.; Nguyen, M.H.; Pristijono, P.; Vuong, Q.V. Development of biodegradable films based on seaweed polysaccharides and Gac pulp (Momordica cochinchinensis), the waste generated from Gac oil production. Food Hydrocoll. 2020, 99, 105322. [Google Scholar] [CrossRef]
- Thakur, R.; Saberi, B.; Pristijono, P.; Stathopoulos, C.; Golding, J.B.; Scarlett, C.J.; Bowyer, M.; Vuong, Q.V. Use of response surface methodology (RSM) to optimize pea starch–chitosan novel edible film formulation. J. Food Sci. Technol. 2017, 54, 2270–2278. [Google Scholar] [CrossRef] [PubMed]
- Saberi, B.; Thakur, R.; Vuong, Q.; Chockchaisawasdee, S.; Golding, J.; Scarlett, C.J.; Stathopoulos, C. Optimization of physical and optical properties of biodegradable edible films based on pea starch and guar gum. Ind. Crop. Prod. 2016, 86, 342–352. [Google Scholar] [CrossRef]
- Abdalrazeq, M.; Giosafatto, C.V.L.; Esposito, M.; Fenderico, M.; Di Pierro, P.; Porta, R. Glycerol-Plasticized Films Obtained from Whey Proteins Denatured at Alkaline pH. Coatings 2019, 9, 322. [Google Scholar] [CrossRef] [Green Version]
- ASTM E96. Standard Test Methods for Water Vapor Transmission of Materials; ASTM International: Conshohocken, PA, USA, 2013. [Google Scholar]
- Tran, T.T.B.; Le Vu, Q.; Pristijono, P.; Kirkman, T.; Nguyen, M.H.; Van Vuong, Q. Optimizing conditions for the development of a composite film from seaweed hydrocolloids and pectin derived from a fruit waste, gac pulp. J. Food Process. Preserv. 2021, e15905. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Q.; Critzer, F.; Davidson, P.M.; Zhong, Q. Physical and antibacterial properties of alginate films containing cinnamon bark oil and soybean oil. LWT 2015, 64, 423–430. [Google Scholar] [CrossRef]
- Arham, R.; Salengke, S.; Metusalach, M.; Mulyati, M. Optimization of agar and glycerol concentration in the manufacture of edible film. Int. Food Res. J. 2018, 25, 1845–1851. [Google Scholar]
- Galus, S.; Lenart, A. Development and characterization of composite edible films based on sodium alginate and pectin. J. Food Eng. 2013, 115, 459–465. [Google Scholar] [CrossRef]
- Nur Hanani, Z.A. Physicochemical characterization of kappa-carrageenan (Euchema cottoni) based films incorporated with various plant oils. Carbohydr. Polym. 2017, 157, 1479–1487. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Y.; Zhang, P.; Ye, L.; Wu, L.; He, S. Physical Characterization and Pork Packaging Application of Chitosan Films Incorporated with Combined Essential Oils of Cinnamon and Ginger. Food Bioprocess Technol. 2017, 10, 503–511. [Google Scholar] [CrossRef]
- Sutrisno, E.; Ningrum, A.; Supriyadi; Munawaroh, H.; Aisyah, S.; Susanto, E. Characterization of tuna (Thunnus albacares) skin gelatin edible film incorporated with clove and ginger essential oils and different surfactants. Food Res. 2021, 5, 440–450. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A. Effect of glycerol and corn oil on physicochemical properties of polysaccharide films—A comparative study. Food Hydrocoll. 2012, 27, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Bourbon, A.I.; Pinheiro, A.C.; Cerqueira, M.A.; Rocha, C.M.; Avides, M.C.; Quintas, M.A.; Vicente, A.A. Physico-chemical characterization of chitosan-based edible films incorporating bioactive compounds of different molecular weight. J. Food Eng. 2011, 106, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Shahrampour, D.; Khomeiri, M.; Razavi, S.M.A.; Kashiri, M. Development and characterization of alginate/pectin edible films containing Lactobacillus plantarum KMC 45. LWT 2019, 118, 108758. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Moisture Sensitivity, Optical, Mechanical and Structural Properties of Whey Protein-Based Edible Films Incorporated with Rapeseed Oil. Food Technol. Biotechnol. 2016, 54, 78–89. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Men, H.; Tong, J.; Zhou, J. Preparation and characterization of active films based on chitosan incorporated tea polyphenols. Food Hydrocoll. 2013, 32, 35–41. [Google Scholar] [CrossRef]
- Chana-Thaworn, J.; Chanthachum, S.; Wittaya, T. Properties and antimicrobial activity of edible films incorporated with kiam wood (Cotyleobium lanceotatum) extract. LWT 2011, 44, 284–292. [Google Scholar] [CrossRef]
- Siracusa, V.; Romani, S.; Gigli, M.; Mannozzi, C.; Cecchini, J.P.; Tylewicz, U.; Lotti, N. Characterization of Active Edible Films based on Citral Essential Oil, Alginate and Pectin. Materials 2018, 11, 1980. [Google Scholar] [CrossRef] [Green Version]
- Granato, D.; Masson, M.L. Instrumental color and sensory acceptance of soy-based emulsions: A response surface approach. Food Sci. Technol. 2010, 30, 1090–1096. [Google Scholar] [CrossRef] [Green Version]
- Pereda, M.; Aranguren, M.I.; Marcovich, N.E. Caseinate films modified with tung oil. Food Hydrocoll. 2010, 24, 800–808. [Google Scholar] [CrossRef]
- Vuong, L.T.; Franke, A.A.; Custer, L.J.; Murphy, S.P. Momordica cochinchinensis Spreng. (gac) fruit carotenoids reevaluated. J. Food Compos. Anal. 2006, 19, 664–668. [Google Scholar] [CrossRef]
- Velásquez, P.; Bustos, D.; Montenegro, G.; Giordano, A. Ultrasound-Assisted Extraction of Anthocyanins Using Natural Deep Eutectic Solvents and Their Incorporation in Edible Films. Molecules 2021, 26, 984. [Google Scholar] [CrossRef]
- Rojas-Graü, M.; Tapia, M.; Rodríguez, F.; Carmona, A.; Martin-Belloso, O. Alginate and gellan-based edible coatings as carriers of antibrowning agents applied on fresh-cut Fuji apples. Food Hydrocoll. 2007, 21, 118–127. [Google Scholar] [CrossRef]
- Du, W.-X.; Olsen, C.; Avena-Bustillos, R.; McHugh, T.; Levin, C.; Friedman, M. Effects of Allspice, Cinnamon, and Clove Bud Essential Oils in Edible Apple Films on Physical Properties and Antimicrobial Activities. J. Food Sci. 2009, 74, M372–M378. [Google Scholar] [CrossRef]
- Simsek, M.; Eke, B.; Demir, H. Characterization of carboxymethyl cellulose-based antimicrobial films incorporated with plant essential oils. Int. J. Biol. Macromol. 2020, 163, 2172–2179. [Google Scholar] [CrossRef]
- Norajit, K.; Kim, K.M.; Ryu, G.H. Comparative studies on the characterization and antioxidant properties of biodegradable alginate films containing ginseng extract. J. Food Eng. 2010, 98, 377–384. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Du, W.-X.; Avena-Bustillos, R.D.J.; Soares, N.D.F.F.; McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties-A review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar] [CrossRef]
- The, D.P.; Debeaufort, F.; Voilley, A.; Luu, D. Biopolymer interactions affect the functional properties of edible films based on agar, cassava starch and arabinoxylan blends. J. Food Eng. 2009, 90, 548–558. [Google Scholar] [CrossRef]
- Farhan, A.; Hani, N.M. Characterization of edible packaging films based on semi-refined kappa-carrageenan plasticized with glycerol and sorbitol. Food Hydrocoll. 2017, 64, 48–58. [Google Scholar] [CrossRef]
- Chaichi, M.; Hashemi, M.; Badii, F.; Mohammadi, A. Preparation and characterization of a novel bionanocomposite edible film based on pectin and crystalline nanocellulose. Carbohydr. Polym. 2017, 157, 167–175. [Google Scholar] [CrossRef]
- Dhumal, C.V.; Pal, K.; Sarkar, P. Characterization of Tri-Phasic Edible Films from Chitosan, Guar Gum, and Whey Protein Isolate Loaded with Plant-Based Antimicrobial Compounds. Polym. Technol. Mater. 2019, 58, 255–269. [Google Scholar] [CrossRef]
- Xu, T.; Gao, C.; Feng, X.; Yang, Y.; Shen, X.; Tang, X. Structure, physical and antioxidant properties of chitosan-gum arabic edible films incorporated with cinnamon essential oil. Int. J. Biol. Macromol. 2019, 134, 230–236. [Google Scholar] [CrossRef] [PubMed]






| Samples | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Materials % (w/v) | GP | GPP | GGG | GLG | GPM | GLO | GGO | GLE | GBA | GMC | ||
| Film forming materials | Seaweed hydrocolloids | Sodium alginate | 1.03 | 1.28 | 1.03 | 1.03 | 1.03 | 1.03 | 1.03 | 1.03 | 1.03 | 1.03 |
| Kappa-carageenan | 0.65 | 0.58 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 | ||
| Gac pulp | Gac pulp powder | 0.4 | - | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | |
| Gac pulp pectin | - | 0.25 | - | - | - | - | - | - | - | - | ||
| Plasticizer | Glycerol | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | |
| Essential oils | Ginger (Zingiber officinale) oil | - | - | 0.15 | - | - | - | - | - | - | - | |
| Lemongrass (Cymbopogon) oil | - | - | - | 0.15 | - | - | - | - | - | - | ||
| Peppermint (Mentha x piperita) oil | - | - | - | - | 0.15 | - | - | - | - | - | ||
| Lemon myrtle (Backhousia citriodora) oil | - | - | - | - | - | 0.15 | - | - | - | - | ||
| Gac oil | - | - | - | - | - | - | 0.15 | - | - | - | ||
| Natural plant extracts | Lemon myrtle | - | - | - | - | - | - | - | 0.15 | - | - | |
| Blue berries (Vaccinium sect. Cyanococcus) ash | - | - | - | - | - | - | - | - | 0.15 | - | ||
| Macadamia | 0.15 |
| Samples | L | a | b | ΔE | Chroma | Hue Angle |
|---|---|---|---|---|---|---|
| GP | 95.55 ± 0.33 a | −0.33 ± 0.03 d | 4.63 ± 0.09 ef | 2.2 ± 0.90 efg | 4.95 ± 0.99 ef | 94.05 ± 0.3 e |
| GPP | 95.55 ± 0.44 a | −0.34 ± 0.05 d | 4.21 ± 0.32 f | 1.87 ± 0.39 g | 4.22 ± 0.32 f | 94.55 ± 0.38 de |
| GGG | 95.82 ± 0.06 a | −0.41 ± 0.02 de | 4.61 ± 0.13 ef | 2.09 ± 0.14 fg | 4.63 ± 0.13 ef | 95.13 ± 0.17 cd |
| GLG | 95.86 ± 0.08 a | −0.55 ± 0.03 e | 5.34 ± 0.13 d | 2.78 ± 0.15 de | 5.36 ± 0.13 d | 95.88 ± 0.2 b |
| GPM | 95.77 ± 0.05 a | −0.42 ± 0.01 de | 4.57 ± 0.16 ef | 2.07 ± 0.15 fg | 4.59 ± 0.16 ef | 95.2 ±0.15 cd |
| GLO | 95.7 ± 0.06 a | −0.56 ± 0.01 e | 5.19 ± 0.08 de | 2.69 ± 0.09 def | 5.22 ± 0.08 de | 96.21 ± 0.1 b |
| GGO | 95.8 ± 0.16 a | −0.76 ± 0.03 f | 5.78 ± 5.24 d | 3.26 ± 0.27 d | 5.83 ± 0.24 d | 97.54 ± 0.2 a |
| GLE | 79.87 ± 0.24 d | 2.27 ± 0.18 a | 23.95 ± 0.86 a | 27.11 ± 0.63 a | 24.06 ± 0.87 a | 84.6 ± 0.25 f |
| GBA | 86.55 ± 0.31 b | 1.27 ± 0.04 c | 11.83 ± 0.31 c | 13.6 ± 0.29 c | 11.9 ± 0.3 c | 83.87 ± 0.53 g |
| GMC | 83.55 ± 0.33 c | 2.03 ± 0.08 b | 16.02 ± 0.16 b | 18.72 ± 0.29 b | 16.15 ± 0.16 b | 82.77 ± 0.23 h |
| Samples | WVP (×10−10 g Pa−1 s−1 m−1) | EAB (mm) | TS (N/m) |
|---|---|---|---|
| GP | 1.76 ± 0.08 ab | 15.76 ± 0.26 c | 1364.78 ± 92.10 ab |
| GPP | 1.25 ± 0.011 d | 19.76 ± 1.67 ab | 1372.27 ± 72.72 ab |
| GGG | 1.49 ± 0.08 bcd | 19.17 ± 0.18 b | 1376.46 ± 243.26 ab |
| GLG | 1.68 ± 0.004 ab | 15.69 ± 0.82 c | 1514.59 ± 263 a |
| GPM | 1.81 ± 0.006 ab | 18.10 ± 0.95 b | 1380 ± 147.53 ab |
| GLO | 1.34 ± 0.015 cd | 18.74 ± 1.30 b | 1069.81 ± 180.65 cd |
| GGO | 1.6 ± 0.051 bc | 21.99 ± 0.49 a | 1180.26 ± 97 bc |
| GLE | 1.62 ± 0.32 bc | 19.30 ± 0.54 b | 878.41 ± 187.81 d |
| GBA | 1.67 ± 0.31 ab | 18.76 ± 0.7 b | 1235.41 ± 212.19 abc |
| GMC | 1.98 ± 0.089 a | 17.62 ± 1.7 bc | 1377.48 ± 89.34 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.T.B.; Vu, B.N.; Saifullah, M.; Nguyen, M.H.; Pristijono, P.; Kirkman, T.; Vuong, Q.V. Impact of Various Essential Oils and Plant Extracts on the Characterization of the Composite Seaweed Hydrocolloid and Gac Pulp (Momordica cochinchinensis) Edible Film. Processes 2021, 9, 2038. https://doi.org/10.3390/pr9112038
Tran TTB, Vu BN, Saifullah M, Nguyen MH, Pristijono P, Kirkman T, Vuong QV. Impact of Various Essential Oils and Plant Extracts on the Characterization of the Composite Seaweed Hydrocolloid and Gac Pulp (Momordica cochinchinensis) Edible Film. Processes. 2021; 9(11):2038. https://doi.org/10.3390/pr9112038
Chicago/Turabian StyleTran, Thuy Thi Bich, Boi Ngoc Vu, Md Saifullah, Minh Huu Nguyen, Penta Pristijono, Timothy Kirkman, and Quan Van Vuong. 2021. "Impact of Various Essential Oils and Plant Extracts on the Characterization of the Composite Seaweed Hydrocolloid and Gac Pulp (Momordica cochinchinensis) Edible Film" Processes 9, no. 11: 2038. https://doi.org/10.3390/pr9112038
APA StyleTran, T. T. B., Vu, B. N., Saifullah, M., Nguyen, M. H., Pristijono, P., Kirkman, T., & Vuong, Q. V. (2021). Impact of Various Essential Oils and Plant Extracts on the Characterization of the Composite Seaweed Hydrocolloid and Gac Pulp (Momordica cochinchinensis) Edible Film. Processes, 9(11), 2038. https://doi.org/10.3390/pr9112038









