Semi-Batch Gasification of Refuse-Derived Fuel (RDF)
Abstract
:1. Introduction
2. Materials and Methods
2.1. RDF Characterization
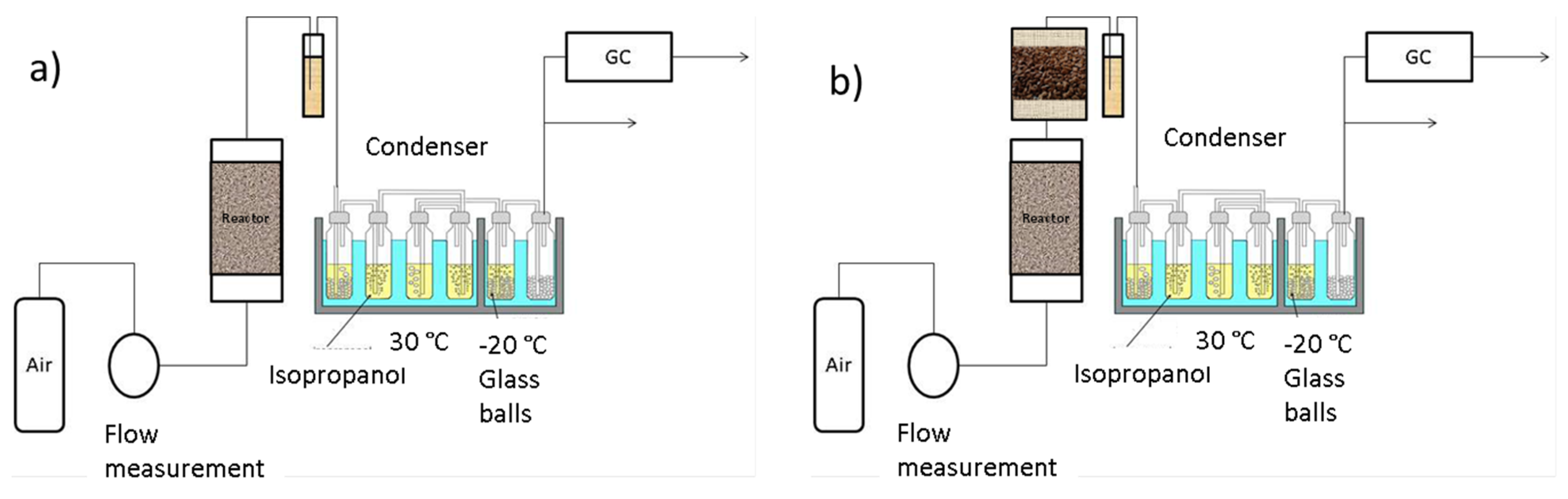
2.2. RDF Gasification Experiments
2.3. Tar and Wax Yield Characterization
3. Results and discussion
3.1. Results of RDF Characterization
3.2. Single Stage RDF Gasification
3.3. Tar and Wax Yield Characterization
3.4. Effect of Secondary Catalysis on the Tar and Wax Yield
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Parliament. DIRECTIVE (EU) 2018/850 of the European parliament and of the council of 30 May 2018, amending Directive 1999/31/EC on the landfill of waste. Off. J. Eur. Union I 2018, 150, 100–108. [Google Scholar]
- Rudra, S.; Tesfagaber, Y.K. Future district heating plant integrated with municipal solid waste (MSW) gasification for hydrogen production. Energy 2019, 180, 881–892. [Google Scholar] [CrossRef]
- Pio, D.T.; Tarelho, L.A.C.; Tavares, A.M.A.; Silva, V. Co-gasification of refused derived fuel and biomass in a pilot-scale bubbling fluidized bed reactor. Energy Convers. Manag. 2020, 206, 112476. [Google Scholar] [CrossRef]
- Antoniou, A.; Stravropoulus, G.; Zabaniotou, A. Activation of end of life tyres pyrolytic char for enhancing viabiliy of pyrolysis—Critical review, analysis and recommendations for a hybrid dual system. Renew. Sustain. Energy Rev. 2014, 39, 1053–1073. [Google Scholar] [CrossRef]
- Haydary, J. Modelling of two stage gasification of waste biomass. Chem. Eng. Trans. 2017, 61, 1465–1470. [Google Scholar] [CrossRef]
- Holmgren, K.M.; Berntsson, T.; Andersson, E.; Rydberg, T. System aspects of biomass gasification with methanol synthesis—Process concepts and energy analysis. Energy 2012, 45, 817–828. [Google Scholar] [CrossRef]
- Tsubouchi, N.; Mochizuki, Y.; Wang, Y.; Ohtsuka, Y. Fate of the Chlorine in Coal in the Heating Process. ISIJ Int. 2018, 58, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Mangena, S.; Bunt, J.; Waanders, F. Physical property behaviour of North Dakota lignite in an oxygen/steam blown moving bed gasifier. Fuel Process. Technol. 2013, 106, 326–331. [Google Scholar] [CrossRef]
- Li, G.; Zhang, K.; Yang, B.; Liu, F.; Weng, Y.; Liu, Z.; Fang, Y. Life cycle analysis of a coal to hydrogen process based on ash agglomerating fluidized bed gasification. Energy 2019, 174, 638–646. [Google Scholar] [CrossRef]
- Abaimov, N.A.; Butakov, E.B.; Burdukov, A.P.; Osipov, P.V.; Ryzhkov, A.F. Investigation of air-blown two-stage entrained-flow gasification of micronized coal. Fuel 2020, 271, 117487. [Google Scholar] [CrossRef]
- Beenackers, A.A.C.M. Biomass gasification in moving beds, a review of European technologies. Renew. Energy 1999, 16, 1–4. [Google Scholar] [CrossRef]
- Campoy, M.; Gómez-Barea, A.; Ollera, P.; Nilsson, S. Gasification of wastes in a polit fluidized bed gasifier. Fuel Process. Technol. 2014, 121, 63–69. [Google Scholar] [CrossRef]
- Park, J.; Lee, Y.; Ryu, C. Reduction of primary tar vapor from biomass by hot char particles in fixed bed gasification. Biomass Bioenergy 2016, 90, 114–121. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Z.; Li, Z.; Chen, D. Characteristics of tar formation during cellulose, hemicellulose and lignin gasification. Fuel 2014, 118, 250–256. [Google Scholar] [CrossRef]
- Li, C.; Suzuki, K. Tar property, analysis, reforming mechanism and model for biomass gasification—An overview. Renew. Sustain. Energy Rev. 2009, 13, 594–604. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Recent advances in the gasification of waste plastics. A critical overview. Renew. Sustain. Energy Rev. 2018, 82, 576–596. [Google Scholar] [CrossRef]
- Wilk, V.; Hofbauer, H. Conversion of mixed plastic wastes in a dual fluidized bed steam gasifier. Fuel 2013, 107, 787–799. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Yang, Y.; Wang, J.; Leeke, G.A. Pyro-Oil and Wax Recovery from Reclaimed Plastic Waste in a Continuous Auger Pyrolysis Reactor. Energies 2020, 13, 2040. [Google Scholar] [CrossRef] [Green Version]
- Buah, W.; Cunliffe, A.; Williams, P. Characterization of Products from the Pyrolysis of Municipal Solid Waste. Process. Saf. Environ. Prot. 2007, 85, 450–457. [Google Scholar] [CrossRef]
- Arabiourrutia, M.; Elordi, G.; Lopez, G.; Borsella, E.; Bilbao, J.; Olazar, M. Characterization of the waxes obtained by the pyrolysis of polyolefin plastics in a conical spouted bed reactor. J. Anal. Appl. Pyrolysis 2012, 94, 230–237. [Google Scholar] [CrossRef]
- Singh, R.N.; Singh, S.P.; Balwanshi, J.B. Tar removal from producer gas: A review. Res. J. Eng. Sci. 2014, 3, 16–22. [Google Scholar]
- Šuhaj, P.; Haydary, J.; Husár, J.; Steltenpohl, P.; Šupa, I. Catalytic gasification of refuse-derived fuel in a two-stage laboratory scale pyrolysis/gasification unit with catalyst based on clay minerals. Waste Manag. 2019, 85, 1–10. [Google Scholar] [CrossRef]
- Steltenpohl, P.; Husár, J.; Šuhaj, P.; Haydary, J. Performance of Catalysts of Different Nature in Model Tar Component Decomposition. Catalysts 2019, 9, 894. [Google Scholar] [CrossRef] [Green Version]
- Husár, J.; Haydary, J.; Šuhaj, P.; Steltenpohl, P. Potential of tire pyrolysis char as tar-cracking catalyst in solid waste and biomass gasification. Chem. Pap. 2019, 73, 2091–2101. [Google Scholar] [CrossRef]
- Mandal, S.; Bhattacharya, T.K.; Verma, A.K.; Haydary, J. Optimization of process parameters for bio-oil synthesis from pine needles (Pinus roxburghii) using response surface methodology. Chem. Pap. 2018, 72, 603–616. [Google Scholar] [CrossRef]
- Webb, G.A. Modern Magnetic Resonance; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Mittal, K.L. Silanes and Other Coupling Agents; Leiden: Boston, MA, USA, 2009; Volume 5. [Google Scholar]
- Xiang, Z.; Liang, J.; Morgan, H.M., Jr.; Liu, Y.; Mao, H.; Bu, Q. Thermal behavior and kinetic study for co-pyrolysis of lignocellulosic biomass with polyethylene over Cobalt modified ZSM-5 catalyst by thermogravimetric analysis. Bioresour. Technol. 2018, 247, 804–811. [Google Scholar] [CrossRef]
- Singh, N.B.; Singh, N.P. Formation of CaO from thermal decomposition of calcium carbonate in the presence of carboxylic acids. J. Therm. Anal. Calorim. 2007, 89, 159–162. [Google Scholar] [CrossRef]
- Mehta, A.; Tembe, G.; Parikh, P.; Mehta, G. A study pf ethylene polymerization catalyzed by homogenous and silsesquioxane supported Titanium(IV)complexes. Polymers 2013, 58, 875–882. [Google Scholar] [CrossRef]
- Haydary, J.; Susa, D.; Dudáš, J. Pyrolysis of aseptic packages (tetrapak) in a laboratory screw type reactor and secondary thermal/catalytic tar decomposition. Waste Manag. 2013, 33, 1136–1141. [Google Scholar] [CrossRef]
- Baker, E.G.; Mudge, L.K.; Brown, M.D. Steam gasification of biomass with nickel secondary catalysts. Ind. Eng. Chem. Res. 1987, 26, 1335–1339. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, G.; You, Y.; Xiao, B.; Liu, S.; He, P.; Guo, D.; Guo, X.; Zhang, G. Hydrogen-rich gas production by steam gasification of municipal solid waste (MSW) using NiO supported on modified dolomite. Int. J. Hydrogen Energy 2012, 37, 6503–6510. [Google Scholar] [CrossRef]





| Proximate composition (wt. %): | |
| Moisture | 10.00 |
| Volatile matter | 66.25 |
| Fixed carbon | 9.29 |
| Ash | 14.46 |
| Elemental composition (wt. %): | |
| Carbon | 46.47 |
| Hydrogen | 6.43 |
| Nitrogen | 0.84 |
| Sulfur | 0.35 |
| Chlorine | 0.73 |
| Oxygen * | 30.72 |
| HHV (MJ.kg−1) | 20.81 |
| Temperature (°C) | 700 | 800 | 900 |
| Gas a | 0.46 | 0.63 | 0.67 |
| Tar and Wax | 0.1 | 0.08 | 0.06 |
| Aqueous | 0.25 | 0.13 | 0.10 |
| Ash | 0.19 | 0.16 | 0.17 |
| Temperature (°C) | H2 | N2 | CO | CH4 | CO2 |
|---|---|---|---|---|---|
| 700 | 10.5 | 51.5 | 8.5 | 6.5 | 21.5 |
| 800 | 16 | 45 | 7.5 | 9.5 | 18 |
| 900 | 20 | 36.5 | 16.5 | 9 | 14.5 |
| Undissolved Phase | Dissolved Phase | ||
|---|---|---|---|
| Component | Peak Area (%) | Component | Peak Area (%) |
| Pentene | 15.08 | Isopropanol | 93.87 |
| Hexene | 14.8 | Ethanol | 1.15 |
| Heptene | 11 | Dimethylbenzene | 0.37 |
| Octene | 7.56 | Nonadecene | 0.18 |
| Dimethylbenzene | 6.42 | Octadecene | 0.11 |
| Cyclodecane | 5.64 | Hexadecane | 0.09 |
| Toluene | 4.71 | Tetradecane | 0.09 |
| Hexadiene | 4.15 | Tetradecene | 0.08 |
| Dodecene | 2.91 | Benzene | 0.08 |
| Nonene | 2.59 | Pentdecene | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haydary, J.; Šuhaj, P.; Šoral, M. Semi-Batch Gasification of Refuse-Derived Fuel (RDF). Processes 2021, 9, 343. https://doi.org/10.3390/pr9020343
Haydary J, Šuhaj P, Šoral M. Semi-Batch Gasification of Refuse-Derived Fuel (RDF). Processes. 2021; 9(2):343. https://doi.org/10.3390/pr9020343
Chicago/Turabian StyleHaydary, Juma, Patrik Šuhaj, and Michal Šoral. 2021. "Semi-Batch Gasification of Refuse-Derived Fuel (RDF)" Processes 9, no. 2: 343. https://doi.org/10.3390/pr9020343
APA StyleHaydary, J., Šuhaj, P., & Šoral, M. (2021). Semi-Batch Gasification of Refuse-Derived Fuel (RDF). Processes, 9(2), 343. https://doi.org/10.3390/pr9020343







