Numerical Analysis of the Spontaneous Combustion Accidents of Oil Storage Tanks Containing Sulfur
Abstract
:1. Introduction
2. Materials and Methods
2.1. Geometric Model and Material Parameters
2.1.1. Geometric Model
2.1.2. Material Parameters
2.2. Governing Equations
- Heat transfer modes are heat conduction and heat convection, ignoring heat radiation.
- Air is a continuous medium.
- The physical parameters of air and solid substances are constant and do not change with temperature.
- Air is an incompressible fluid.
- Air is a Newtonian fluid, following the Newtonian formula.
- Ignoring heat dissipation caused by viscous dissipation.
2.2.1. Heat Conduction Energy Equation
- λ—thermal conductivity (W·m−1·°C−1), constant;
- cp—specific heat capacity of the tank wall (J·kg−1·°C−1);
- ρ—the surface density of the storage tank (kg·m−3);
- x, y—two-dimensional coordinate axis (-);
- T—the temperature of the tank wall (°C);
- τ—heat transfer time (s);
- qv—the internal heat source (W·m−3).
2.2.2. Convective Heat Transfer Energy Equation
- u—air velocity in x direction (m·s−1);
- v—air velocity in y direction (m·s−1).
2.3. Boundary Conditions and Initial Conditions
- tw—the temperature of the outer wall of the storage tank (°C);
- tf—the air temperature (°C);
- ∂t/∂n—the rate of temperature change in the normal direction of an isothermal surface (-);
- h—air convection heat transfer coefficient (W·m−2·°C−1).
2.4. Meshing
3. Results and Discussion
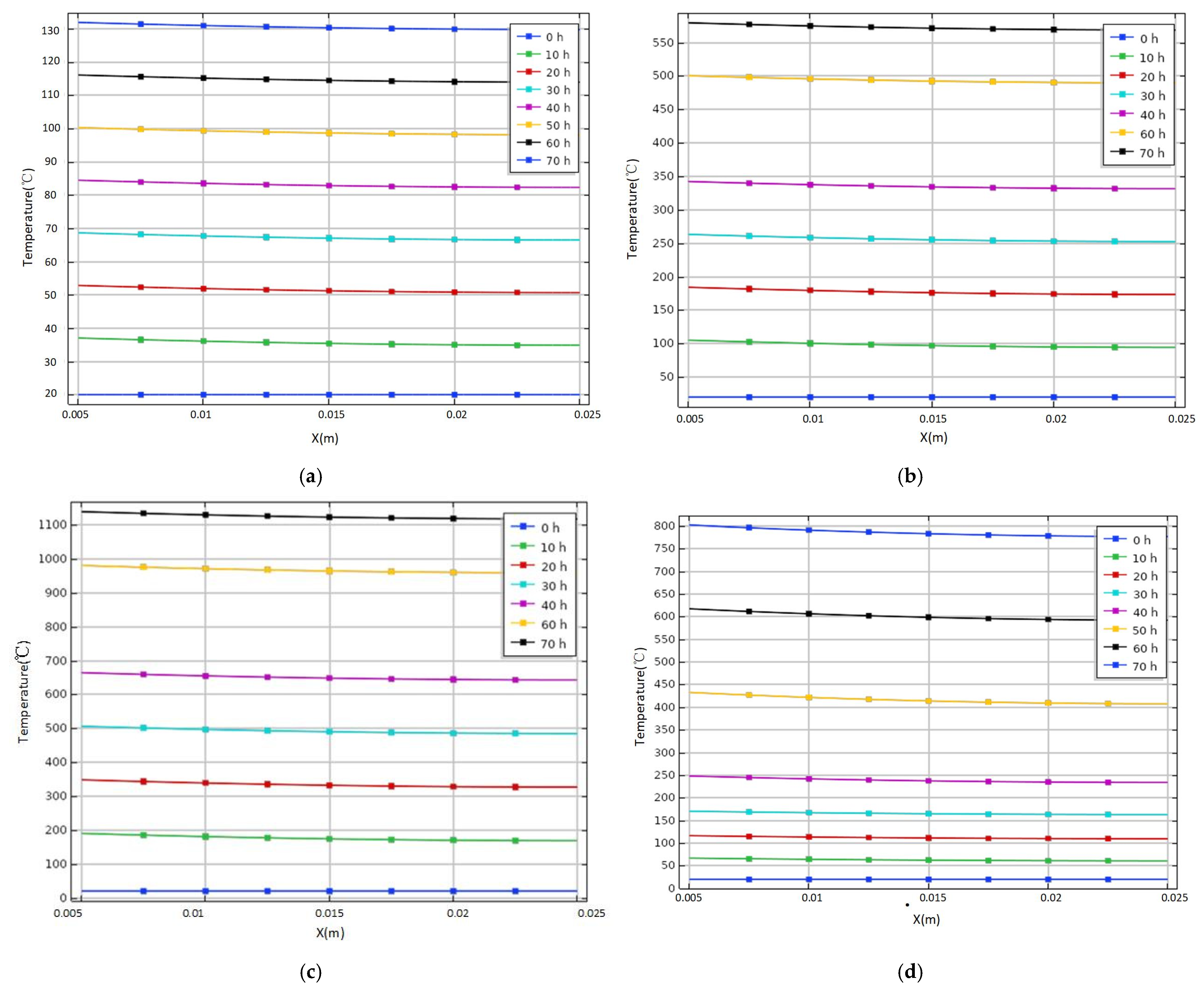
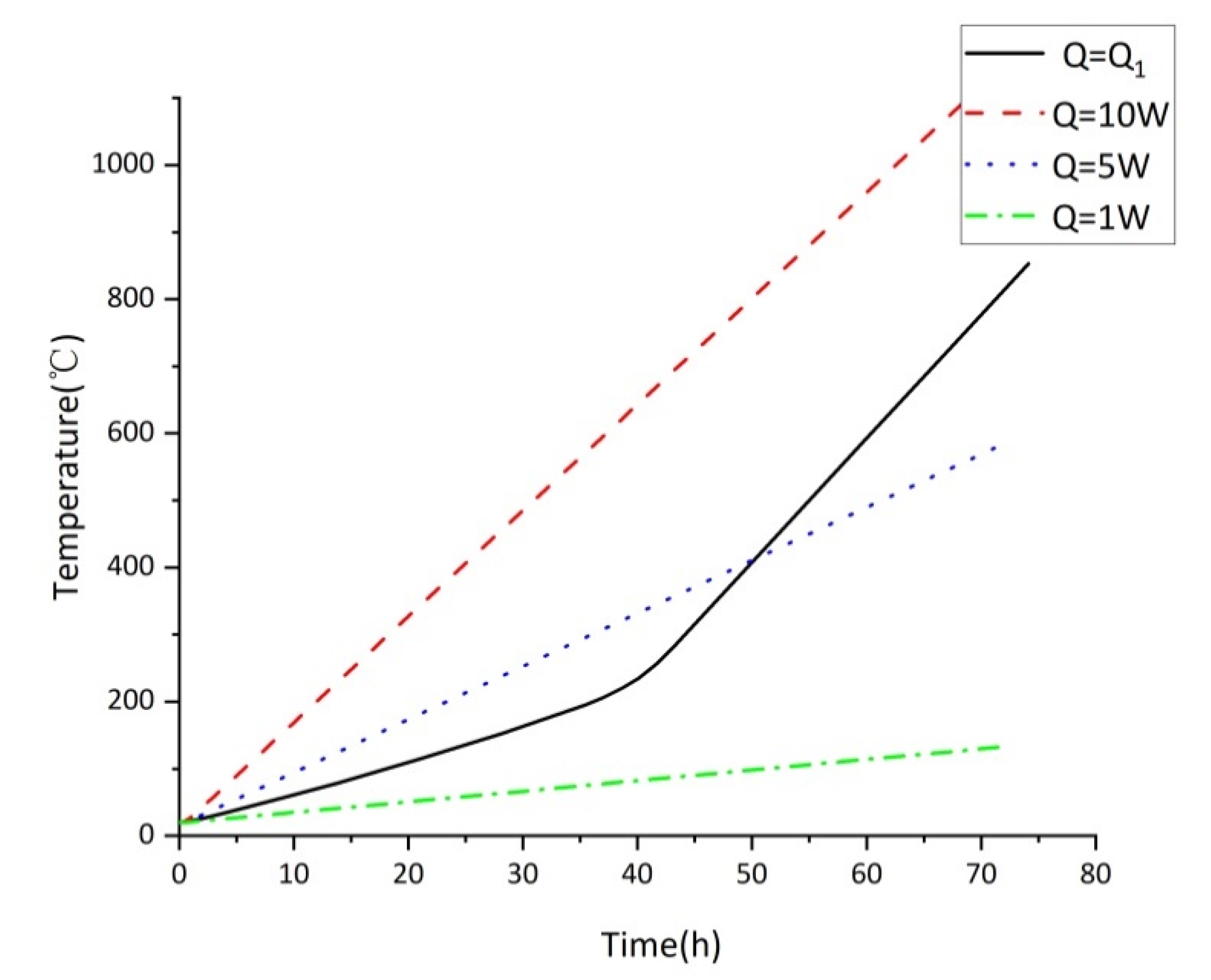
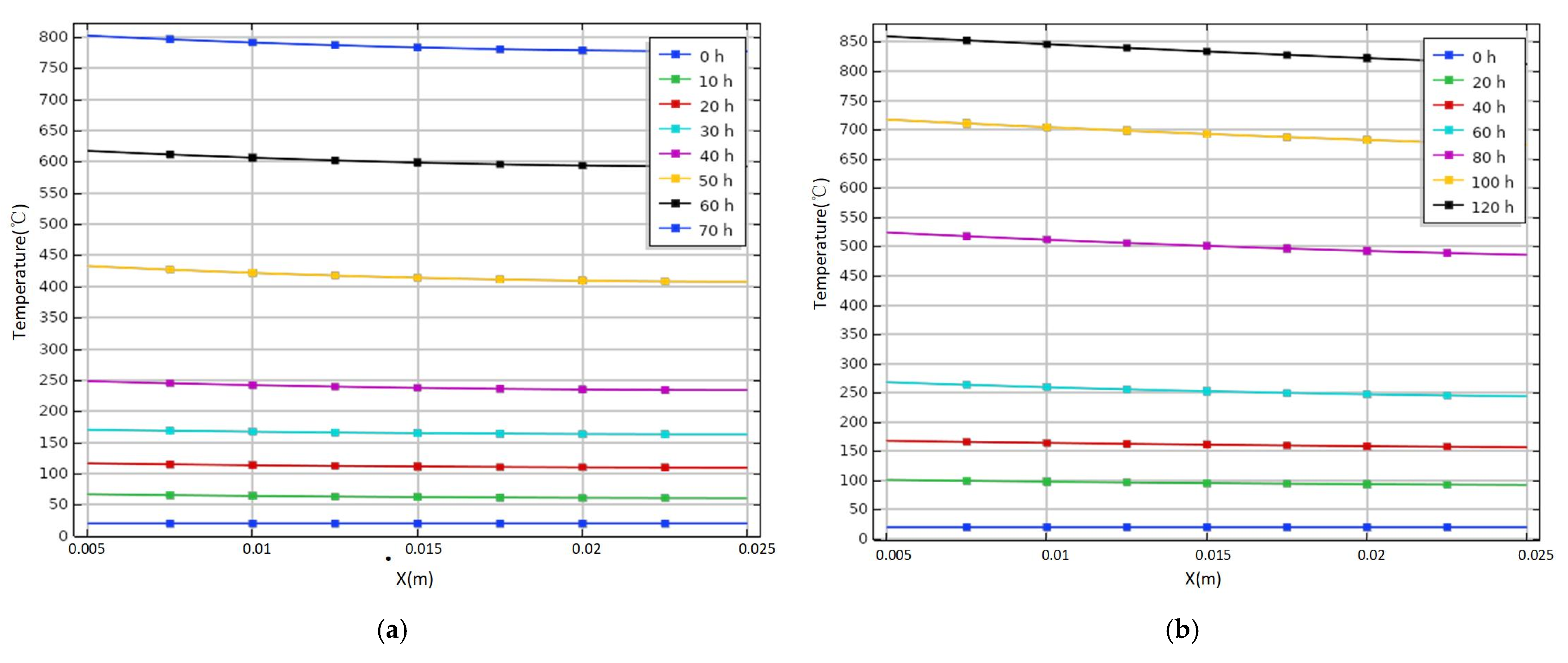
3.1. Variation of Temperature of Tank under Different Heat Source Intensity
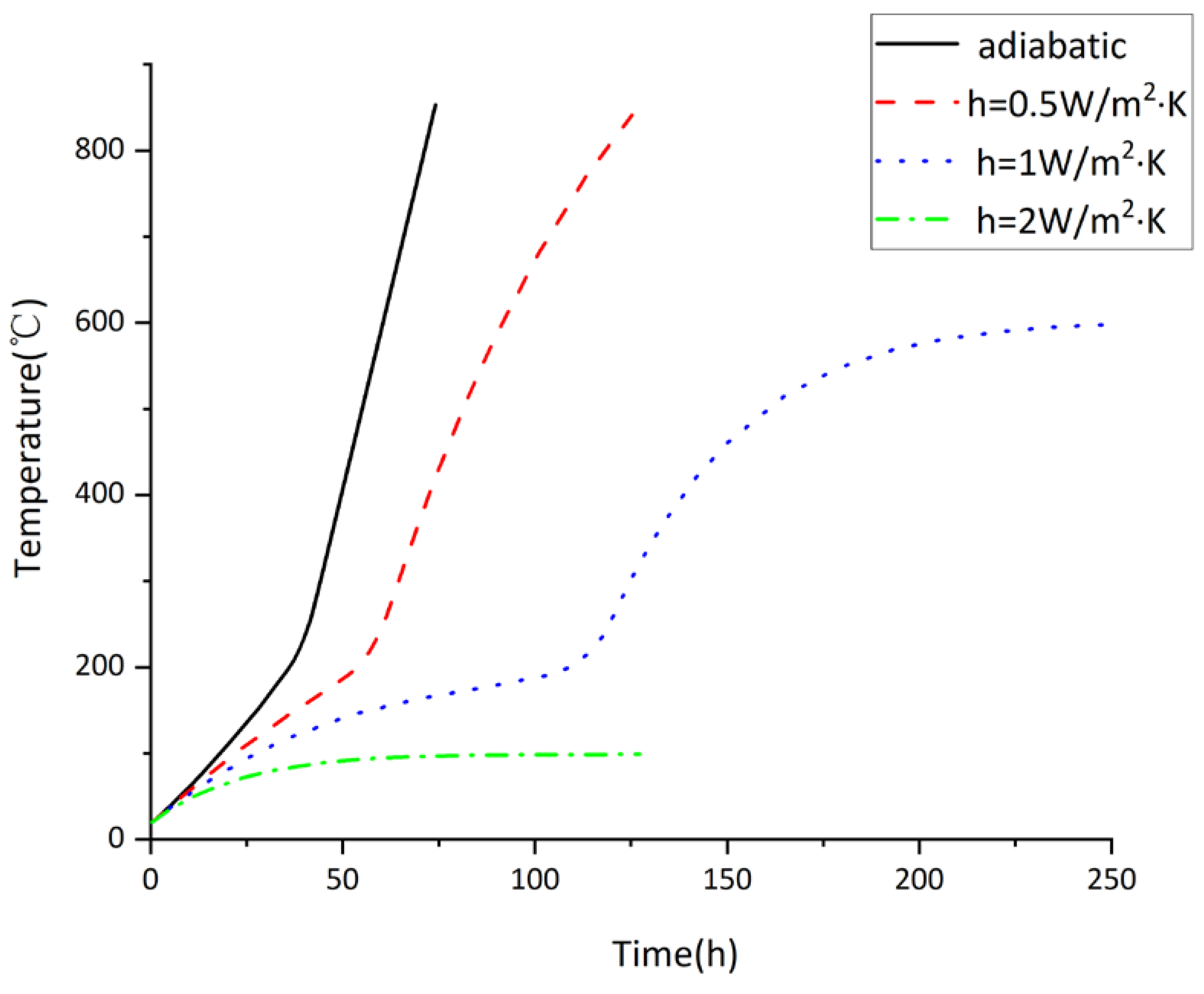
3.2. Variation of Temperature of Tank under Different Air Convective Heat Transfer Coefficients
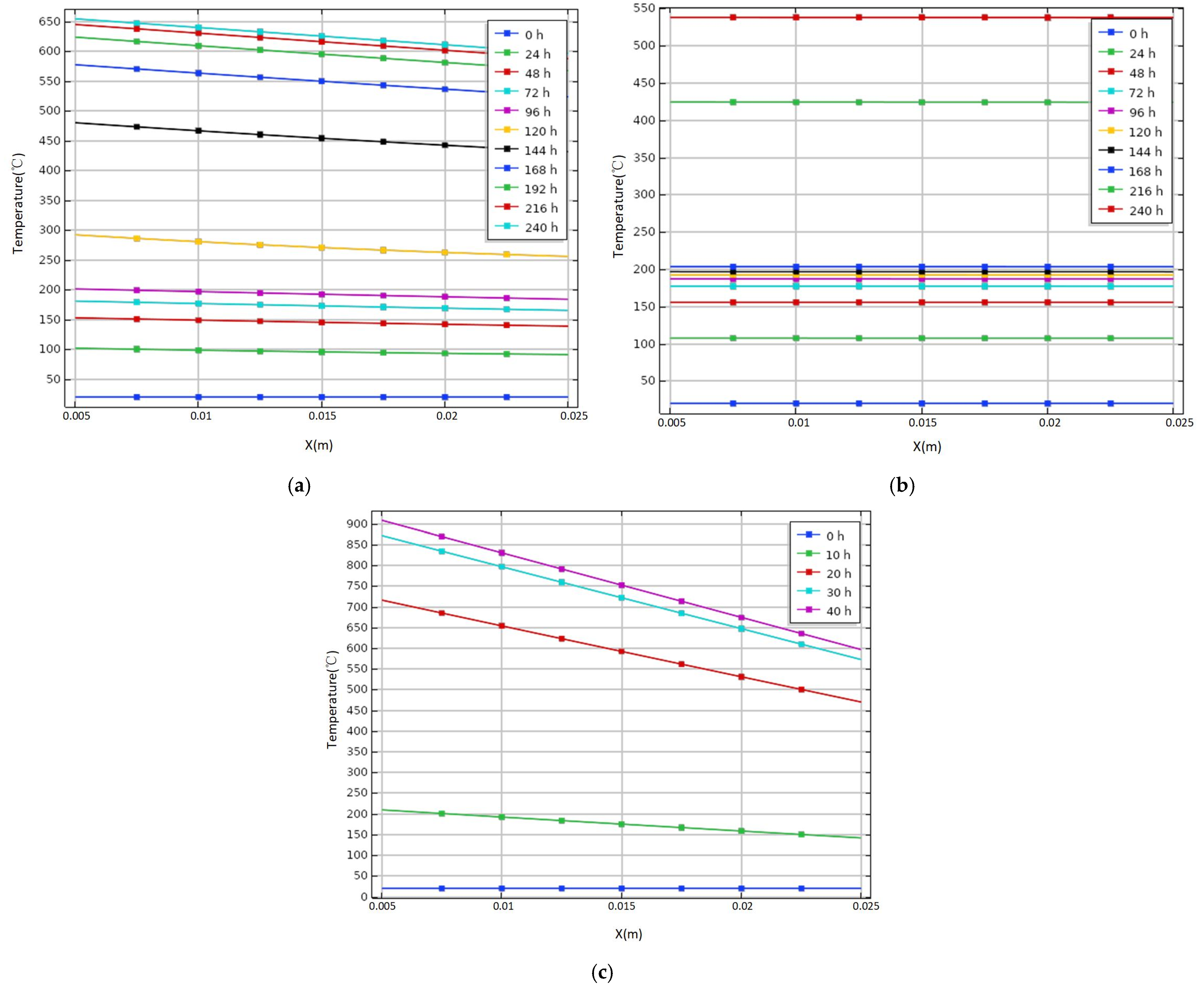
3.3. Variation of Temperature of Tank under Different Wall Materials
4. Conclusions
- The sulfur corrosion products do not release heat as a constant heat source. The oxidation exothermic reaction stage can be determined by the heat transfer on the wall when the heat release changes with time. The rising trend of temperature of the storage tank is different under various exothermic intensities, which has discrepant effects on the spontaneous combustion of the storage tank. Therefore, the different stages and corresponding exothermic trends of sulfur corrosion products should be considered in the actual process.
- The disturbance of external environment is expressed by air convection heat transfer coefficient h, and the heat transfer on the tank wall changes with different values of h. The temperature rises fastest under the adiabatic condition compared with other cases. With the increase of h, the heating rate slows down gradually. Due to the continuous heat exchange between the external circumstance and the wall, it is difficult for the temperature of the outer wall to continue ascending. Thus, it can be inferred that the air convection heat transfer coefficient will affect the temperature field of tank spontaneous combustion. The heat exchange between the external circumstance and the wall increases with the increment of the value of h, making it difficult for the temperature of spontaneous combustion to continue to rise, which can play a certain role in heat dissipation and prevention of heat accumulation. However, if the internal heat source continues to heat up, the temperature difference between the external wall temperature and the internal heat source is too large to detect in time, delaying the early warning time and missing the abnormal internal high temperature. Therefore, the appropriate air convection heat transfer coefficient has a great influence on the prevention and control of tank spontaneous combustion accidents.
- The temperature change of tank spontaneous combustion will be affected by the heat preservation and heat dissipation of wall materials. The wall heat transfer rate is slow when epoxy glass flake and low-carbon steel are used as storage tank materials. The former temperature rises slowly within 120 h, and the latter rises slowly within 168 h, so it is easy to prevent and control spontaneous combustion in these two periods. The heat transfer process on the wall is very speedy when rock wool is used as the material of the storage tank, so the outer wall can reach a high temperature of 600 °C in 40 h. Moreover, it is adverse to the detection of internal abnormal conditions because of the large temperature difference between inside and outside of the tank wall, so it is necessary to consider the difference of temperature conduction between different materials in temperature monitoring.
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, S.P.; Jiang, J.C.; Zheng, J. Oxidative characteristics of FeS by thermal analysis. J. Chongqing Univ. 2011, 34, 140–144. [Google Scholar]
- Jiang, X.W.; Zhou, L.F.; Zhou, L. Investigation and analysis of fire cases and safety status of oil storage tanks. Safety Health Environ. 2016, 16, 29–32, 50. [Google Scholar]
- Zhang, Z.H. Study on Influence Factors for the Formation of Iron Sulfides and Its Pyrophrosity; Northeastern University: Boston, MA, USA, 2009. [Google Scholar]
- Bertani, R.; Biasin, A.; Canu, P.; Della Zassa, M.; Refosco, D.; Simionato, F.; Zerlottin, M. Self-heating of dried industrial tannery wastewater sludge induced by pyrophoric iron sulfides formation. J. Hazard. Mater. 2016, 305, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; Jiang, J.C.; Zhao, S.P.; Zhang, W.; Ni, L.; Zhang, M.; Wang, Z.R. Analysis on oxidation process of sulfurized rust in oil tank. J. Therm. Anal. Calorim. 2017, 128, 125–134. [Google Scholar] [CrossRef]
- Kong, D.; Peng, R.Q.; Sun, X.M. Study of the influence of crude oil on the spontaneous combustion risk of sulfurized rust in crude oil tanks. Fuel 2019, 255, 115816. [Google Scholar] [CrossRef]
- Rickard, D.T. Kinetics and mechanism of pyrite formation at low temperatures. Am. J. Sci. 1975, 275, 636–652. [Google Scholar] [CrossRef]
- Asaki, Z.; Kondo, Y. Oxidation kinetics of iron sulfide in the form of dense plate, pellet and single particle. J. Therm. Anal. 1989, 35, 1751–1759. [Google Scholar] [CrossRef]
- Walker, R.; Steele, A.D.; Morgan, T.D.B. Pyrophoric oxidation of iron sulphide. Surf. Coat. Technol. 1988, 34, 163–175. [Google Scholar] [CrossRef]
- Walker, R.; Steele, A.D.; Morgan, T.D.B. The formation of pyrophoric iron sulphide from rust. Surf. Coat. Technol. 1987, 31, 183–197. [Google Scholar] [CrossRef]
- Walker, R.; Steele, A.D.; Morgan, T.D.B. Deactivation of Pyrophoric Iron Sulfides. Ind. Eng. Chem. Res. 1997, 36, 3662–3667. [Google Scholar] [CrossRef]
- Shukla, A.K.; Singh, R.S. Oxidation of sulphur in pyrites in relation to soil and water regime. J. Indian Soc. Soil Sci. 1992, 40, 848–850. [Google Scholar]
- Somot, S.; Finch, J.A. Possible role of hydrogen sulphide gas in self-heating of pyrrhotite-rich materials. Miner. Eng. 2010, 23, 104–110. [Google Scholar] [CrossRef]
- Mellor, J.W. A Comprehensive Treatise on Inorganic and Theoretical Chemistry. Nature 2014, 117, 249–250. [Google Scholar] [CrossRef] [Green Version]
- Baltrus, J.P.; Diehl, J.R. An investigation of the weathering behaviour of coal-derived pyrite surfaces by X-ray photoelectron spectroscopy. Fuel 1994, 73, 229–235. [Google Scholar] [CrossRef]
- Li, X.; Shang, Y.; Chen, Z.; Chen, X.; Niu, Y.; Yang, M. Study of spontaneous combustion mechanism and heat stability of sulfide minerals powder based on thermal analysis. Powder Technol. 2017, 309, 68–73. [Google Scholar] [CrossRef]
- Dou, Z.; Jiang, J.C.; Zhao, S.P.; Mao, G.B.; Zhang, M.G.; Wang, L.; Wang, Z.R. Experimental investigation on oxidation of sulfurized rust in oil tank. Loss Prev. Process Ind. 2015, 38, 156–162. [Google Scholar] [CrossRef]
- Iliyas, A.; Hawboldt, K.; Khan, F. Advanced kinetics for calorimetric techniques and thermal stability screening of sulfide minerals. Thermochim. Acta 2010, 501, 35–45. [Google Scholar] [CrossRef]
- Kong, D.; Liu, P.; Ping, P.; Chen, G. Evaluation of the pyrophoric risk of sulfide mineral in storage. Loss Prev. Process Ind. 2016, 44, 487–494. [Google Scholar] [CrossRef]
- Man, Y.; Xian, F.C.; Yu, J.S.; Ren, D.B. Particle Size Effect on FeS Spontaneous Combustion Characters in Petroleum. Appl. Mech. Mater. 2014, 608–609, 971–975. [Google Scholar]
- Zhao, S.; Wang, C.; Li, P.; Ding, D.; Wan, X. The Influence of Sulfurization of Rust in Oil Tanks. Energy Sources Part A Recovery Util. Environ. Eff. 2007, 29, 1111–1119. [Google Scholar] [CrossRef]
- Li, P.; Li, J.D.; Zhao, S.P. Prevention of Spontaneous Combustion of Sulfur-Containing Oil Tanks. Pet. Sci. Technol. 2007, 24, 1009–1016. [Google Scholar] [CrossRef]
- Dou, Z.; Mebarki, A.; Ni, L.; Jiang, J.C.; Cai, Z.L.; Zhang, M.G.; Zhao, S.P.; Zhang, W.X.; Pensée, V. SVM application in hazard assessment: Self-heating for sulfurized rust. J. Loss Prev. Process Ind. 2016, 39, 112–120. [Google Scholar] [CrossRef]
- Li, P.; Li, J.; Zhao, S.; Kong, L.; Zhai, Y. Research on the danger of fires in oil tanks with sulfur. Fire Saf. 2005, 40, 331–338. [Google Scholar] [CrossRef]
- Yang, Y.X.; Jiang, J.C.; Zhao, S.P. Natural oxidation experiment of corrosion products of sulfur-containing oil storage tanks. Editor. Board J. Najing Tech Univ. 2010, 3, 67–71. [Google Scholar]
- Dou, Z.; Jiang, J.C.; Zhao, S.P. Experimental study on oxidation and spontaneous combustion of sulfur corrosion products at low temperature in storage tanks. Editor. Board J. Najing Tech Univ. 2014, 36, 118–122. [Google Scholar]
- Yue, D.T. Engineering Thermodynamics and Heat Transfer; Dalian Maritime University Press: Dalian, China, 2002; pp. 152–175. [Google Scholar]
- Fu, Q.S. Thermal Engineering Foundation and Application; Xi’an Jiaotong University Press: Xi’an, China, 2007; pp. 107–163. [Google Scholar]
- Xu, Y.; Li, Z.J.; Liu, H.S.; Zhai, X.W.; Jia, M.T. A model for assessing the compound risk represented by spontaneous coal combustion and methane emission in a gob. J. Clean. Prod. 2020, 273, 122925. [Google Scholar] [CrossRef]
- Li, Z.J.; Jiang, W.J.; Chen, T.F. Kinetic analysis on oxidation and spontaneous combustion of iron sulfides. J. Saf. Sci. Technol. 2018, 14, 24–29. [Google Scholar]
- Zhang, Z.H.; Zhao, S.L.; Li, P.; Han, Y. Spontaneous combustion process of hydrogen sulfide corrosion products formed at room temperature. Acta Pet. Sin. (Pet. Process. Sect.) 2012, 1, 122–126. [Google Scholar]
- Hu, Y.F.; Liu, H.; Fu, Y.M.; Xie, Z.W.; Wang, Z.X. Numerical simulation of temperature field combustion disaster sources for oil tanks contaioning sulfur. J. China Univ. Metrol. 2014, 25, 366–371. [Google Scholar]
- Yang, F.Q.; Zhun, W.F.; Liu, X.X. Analysis of factors influencing spontaneous combustion of oil tanks containing sulfur based on ISM and interval-numbe. China Saf. Sci. J. 2016, 26, 62–66. [Google Scholar]
- Yang, R.R.; Wang, Z.R.; Jiang, J.C.; Shen, S.X.; Sun, P.P.; Lu, Y.W. Cause analysis and prevention measures of fire and explosion caused by sulfur corrosion. Eng. Fail. Anal. 2020, 108, 104342. [Google Scholar] [CrossRef]
- Li, H.B.; Fan, Y. Several issues to be considered in the design of super large oil tanks. Pet. Refin. Eng. 2005, 35, 46–48. [Google Scholar]
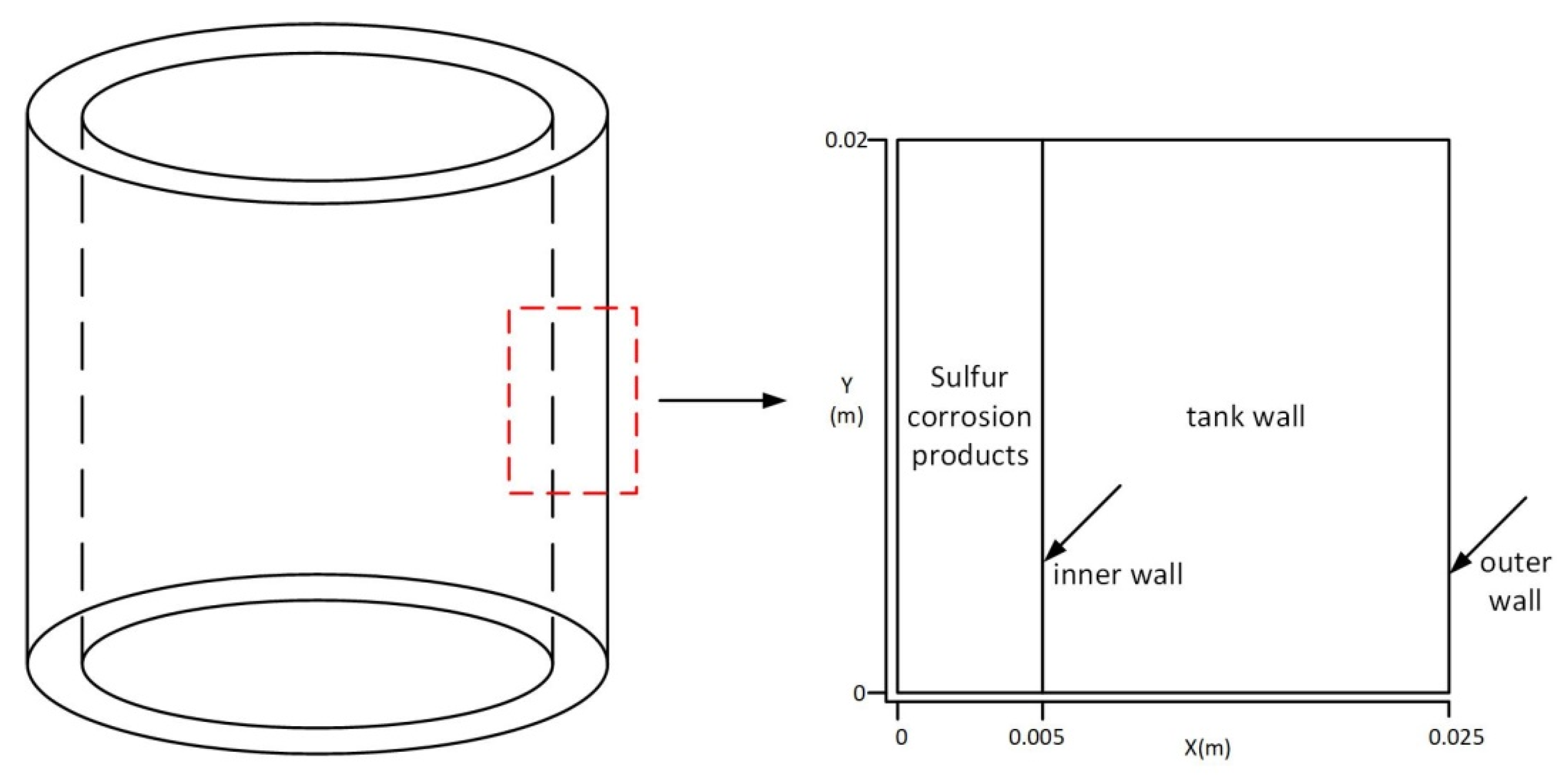

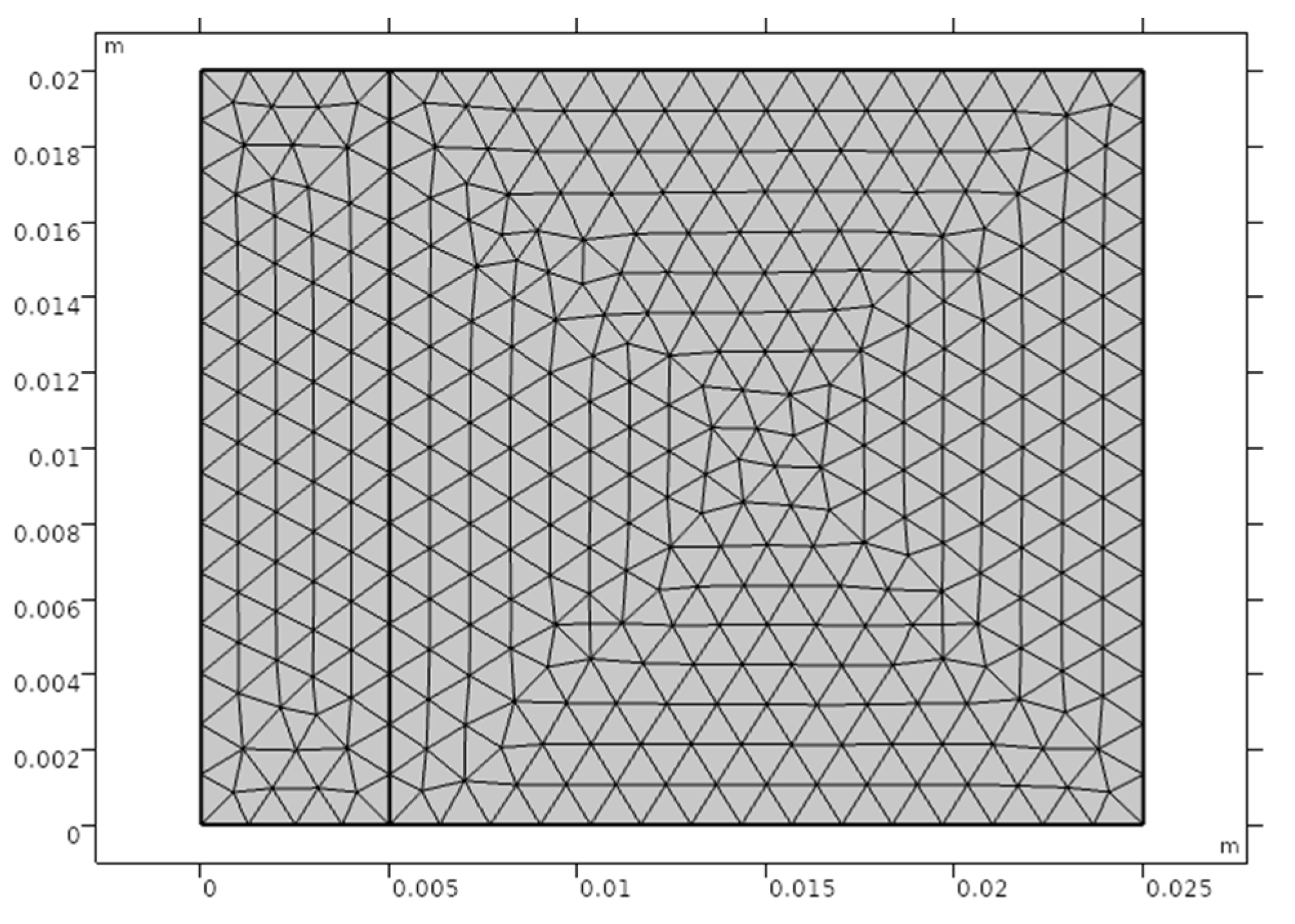


| Materials | Density (kg·m−3) | Specific Heat Capacity (J·kg−1·°C−1) | Thermal Conductivity (W·m−1·°C−1) |
|---|---|---|---|
| Sulfur corrosion products | 4860 | 650 | 0.5 |
| Tank wall (epoxy glass flake) | 3100 | 1581 | 0.2 |
| Physical Quantities | Value |
|---|---|
| Mesh units | 1542 |
| Average element quality | 0.927 |
| Mesh nodes | 822 |
| Material Number | Wall Materials | Density (kg·m−3) | Specific Heat Capacity (J·kg−1·°C−1) | Thermal Conductivity (W·m−1·°C−1) |
|---|---|---|---|---|
| 1 | epoxy glass flake | 3100 | 1581 | 0.2 |
| 2 | low-carbon steel | 7790 | 470 | 43.2 |
| 3 | rock wool | 120 | 750 | 0.037 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Cai, R.; Xu, Y. Numerical Analysis of the Spontaneous Combustion Accidents of Oil Storage Tanks Containing Sulfur. Processes 2021, 9, 626. https://doi.org/10.3390/pr9040626
Li Z, Cai R, Xu Y. Numerical Analysis of the Spontaneous Combustion Accidents of Oil Storage Tanks Containing Sulfur. Processes. 2021; 9(4):626. https://doi.org/10.3390/pr9040626
Chicago/Turabian StyleLi, Zijun, Rongzi Cai, and Yu Xu. 2021. "Numerical Analysis of the Spontaneous Combustion Accidents of Oil Storage Tanks Containing Sulfur" Processes 9, no. 4: 626. https://doi.org/10.3390/pr9040626
APA StyleLi, Z., Cai, R., & Xu, Y. (2021). Numerical Analysis of the Spontaneous Combustion Accidents of Oil Storage Tanks Containing Sulfur. Processes, 9(4), 626. https://doi.org/10.3390/pr9040626






