Assessment of Advanced Oxidation Processes Using Zebrafish in a Non-Forced Exposure System: A Proof of Concept
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Synthetic Waters
2.2. Preparation of Supported g-C3N4 (g-C3N4 onto Calcium Carbonate)
2.3. Physical–Chemical and Chemical Characterization of Water
2.4. Advanced Oxidation Processes
2.5. Assay Organisms
2.6. Free-Choice Non-Forced Exposure System
2.7. Statistical Analysis
3. Results
3.1. Characterization of the Dechlorinated Tap Water
3.2. Advanced Oxidation Processes
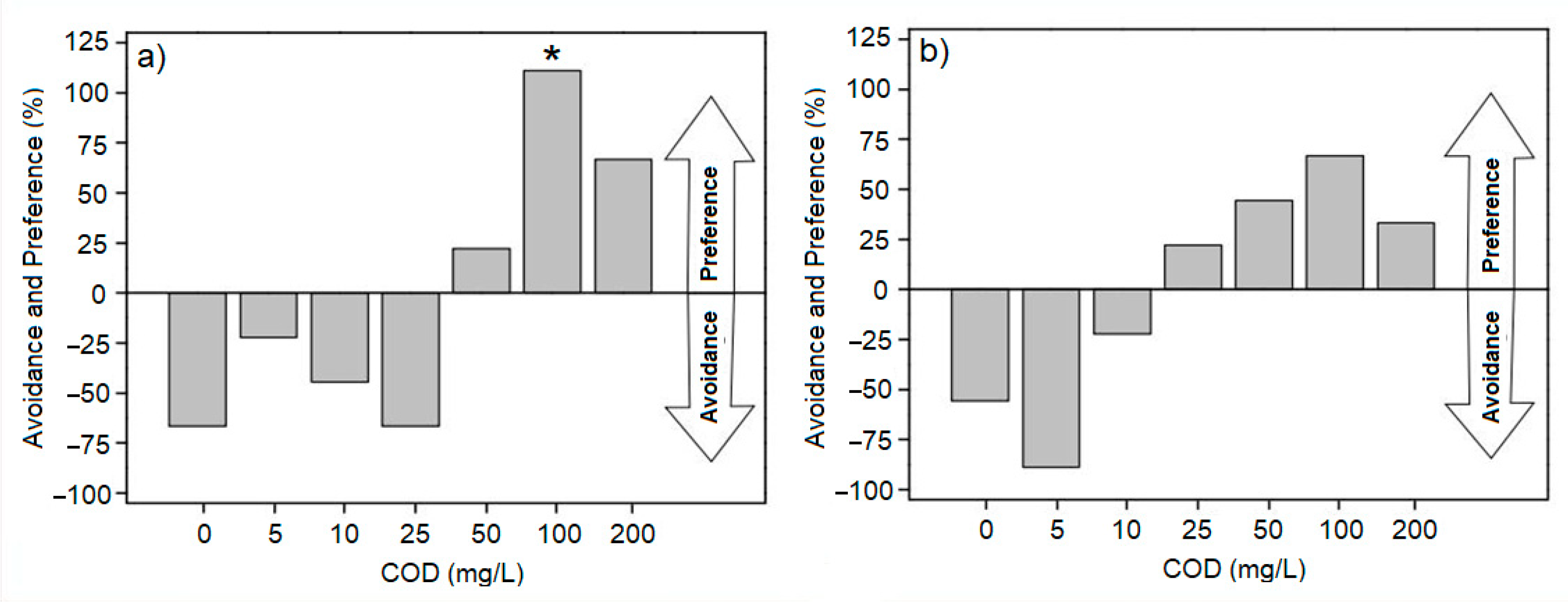
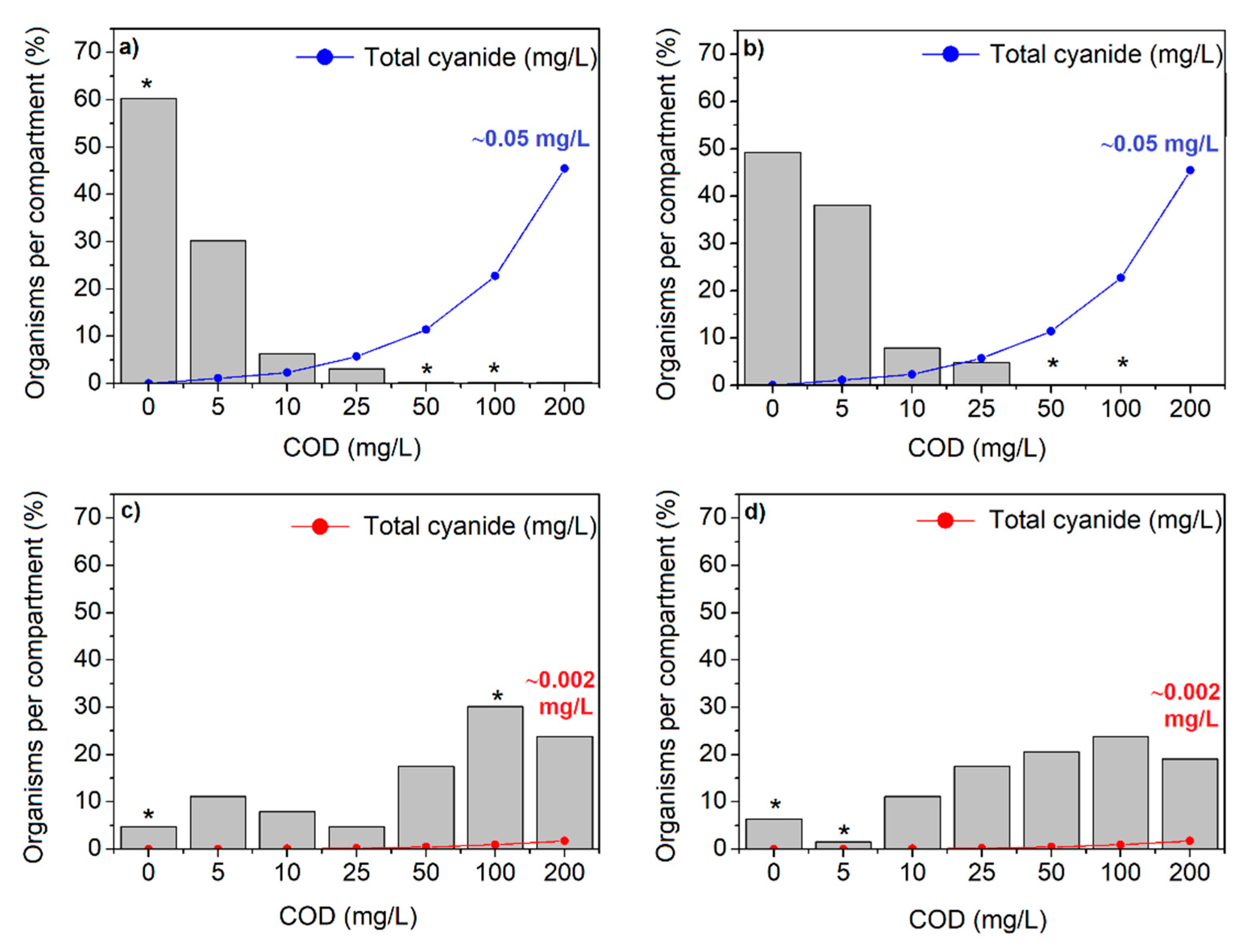
3.3. Water Selection by the Zebrafish
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gheewala, S.H.; Silalertruksa, T.; Nilsalab, P.; Mungkung, R.; Perret, S.R.; Chaiyawannakarn, N. Water footprint and impact of water consumption for food, feed, fuel crops production in Thailand. Water 2014, 6, 1698–1718. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef]
- Yotova, G.; Lazarova, S.; Kudłak, B.; Zlateva, B.; Mihaylova, V.; Wieczerzak, M.; Veneli-nov, T.; Tsakovski, S. Assessment of the Bulgarian wastewater treatment plants’ impact on the receiving water bodies. Molecules 2019, 24, 2274. [Google Scholar] [CrossRef] [PubMed]
- Pereda, O.; Solagaistua, L.; Atristain, M.; de Guzmán, I.; Larrañaga, A.; von Schiller, D.; Elosegi, A. Impact of wastewater effluent pollution on stream functioning: A whole-ecosystem manipulation experiment. Environ. Pollut. 2020, 258, 113719. [Google Scholar] [CrossRef] [PubMed]
- von Sperling, M. Biological Wastewater Treatment Series; IWA Publishing: London, UK, 2007; Volume 1, p. 78. [Google Scholar]
- Ostroumov, S.A. On some issues of maintaining water quality and self-purification. Water Resour. 2005, 32, 337–346. [Google Scholar] [CrossRef]
- Ostroumov, S.A. Water quality and conditioning in natural ecosystems: Biomachinery theory of self-purification of water. Russ. J. Gen. Chem. 2017, 87, 3199–3204. [Google Scholar] [CrossRef]
- Hashemi Monfared, S.A.; Dehghani Darmian, M.; Snyder, S.A.; Azizyan, G.; Pirzadeh, B.; Moghaddam, M.A. Water quality planning in rivers: Assimilative capacity and dilution flow. Bull. Environ. Contam. Toxicol. 2017, 99, 531–541. [Google Scholar] [CrossRef]
- Bolinches, A.; De Stefano, L.; Paredes-Arquiola, J. Adjusting wastewater treatment effluent standards to protect the receiving wa-ters: The case of low-flow rivers in central Spain. Environ. Earth Sci. 2020, 79, 446. [Google Scholar] [CrossRef]
- Poikane, S.; Johnson, R.K.; Sandin, L.; Schartau, A.K.; Solimini, A.G.; Urbanič, G.; Arbačiauskas, K.; Aroviita, J.; Gabriels, W.; Miler, O.; et al. Benthic macroinvertebrates in lake ecological assessment: A review of methods, intercalibration and practical recommendations. Sci. Total Environ. 2016, 543, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Tierney, K.B.; Baldwin, D.H.; Hara, T.J.; Ross, P.S.; Scholz, N.L.; Kennedy, C.J. Olfactory toxicity in fishes. Aquat. Toxicol. 2010, 96, 2–26. [Google Scholar] [CrossRef]
- Lari, E.; Bogart, S.J.; Pyle, G.G. Fish can smell trace metals at environmentally relevant concentrations in freshwater. Chemosphere 2018, 203, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Tawari-Fufeyin, P.; Ekaye, S.A. Fish species diversity as indicator of pollution in Ikpoba River, Benin City, Nigeria. Rev. Fish Biol. Fish. 2007, 17, 21–30. [Google Scholar] [CrossRef]
- Kruk, A.; Penczak, T. Natural regeneration of fish assemblages in the Pilica river after a reduction of point-source pollution. River Res. Appl. 2013, 29, 502–511. [Google Scholar] [CrossRef]
- Moreira-Santos, M.; Donato, C.; Lopes, I.; Ribeiro, R. Avoidance tests with small fish: Determination of the median avoidance concentration and of the lowest-observed-effect gradient. Environ. Toxicol. Chem. 2008, 27, 1576–1582. [Google Scholar] [CrossRef]
- Araújo, C.V.M.; Shinn, C.; Moreira-Santos, M.; Lopes, I.; Espíndola, E.L.G.; Ribeiro, R. Copper-driven avoidance and mortality in temperate and tropical tadpoles. Aquat. Toxicol. 2014, 146, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Vera-Vera, V.C.; Guerrero, F.; Blasco, J.; Araújo, C.V.M. Habitat selection response of the freshwater shrimp Atyaephyra desmarestii experimentally exposed to heterogeneous copper contamination scenarios. Sci. Total Environ. 2019, 662, 816–823. [Google Scholar] [CrossRef]
- Lopes, I.; Baird, D.J.; Ribeiro, R. Avoidance of copper contamination by field populations of Daphnia longispina. Environ. Toxicol. Chem. 2004, 23, 1702–1708. [Google Scholar] [CrossRef]
- Araújo, C.V.M.; Griffith, D.M.; Vera-Vera, V.; Vargas Jentzsch, P.; Cervera, L.; Nieto-Ariza, B.; Salvatierra, D.; Erazo, S.; Jaramillo, R.; Ramos, L.A.; et al. A novel approach to assessing environmental disturbance based on habitat selection by zebra fish as a model organism. Sci. Total Environ. 2018, 619–620, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Clements, W.H.; Hickey, C.W.; Kidd, K.A. How do aquatic communities respond to contaminants? It depends on the ecological context. Environ. Toxicol. Chem. 2012, 31, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Araújo, C.V.M.; Laissaoui, A.; Silva, D.C.V.R.; Ramos-Rodríguez, E.; González-Ortegón, E.; Espíndola, E.L.G.; Baldó, F.; Mena, F.; Parra, G.; Blasco, J.; et al. Not only toxic but repellent: What can organisms’ responses tell us about contamination and what are the ecological consequences when they flee from an environment. Toxics 2020, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Ikehata, K.; Naghashkar, N.J.; El-Din, M.G. Degradation of Aqueous Pharmaceuticals by Ozonation and Advanced Oxidation Processes: A Review. Ozone Sci. Eng. 2006, 28, 353–414. [Google Scholar] [CrossRef]
- Wols, B.A.; Hofman-Caris, C.H.M. Review of photochemical reaction constants of organic micropollutants required for UV advanced oxidation processes in water. Water Res. 2012, 46, 2815–2827. [Google Scholar] [CrossRef] [PubMed]
- Asghar, A.; Raman, A.A.A.; Daud, W.M.A.W. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef]
- Gautam, P.; Kumar, S.; Lokhandwala, S. Advanced oxidation processes for treatment of leachate from hazardous waste landfill: A critical review. J. Clean. Prod. 2019, 237, 117639. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced oxidation processes for the removal of antibiotics from water. An overview. Water 2020, 12, 102. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Lindend, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment-A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Bello, M.M.; Raman, A.A.A.; Asghar, A. A review on approaches for addressing the limitations of Fenton oxidation for recalcitrant wastewater treatment. Process. Saf. Environ. Prot. 2019, 126, 119–140. [Google Scholar] [CrossRef]
- Espinoza, I.; Sandoval-Pauker, C.; Ramos Guerrero, L.; Vargas Jentzsch, P.; Muñoz Bisesti, F. Fenton process combined with precipitation for the removal of Direct Blue 1 dye: A new approach. J. Serb. Chem. Soc. 2020, 85, 547–558. [Google Scholar] [CrossRef]
- Sandoval, C.; Molina, G.; Vargas Jentzsch, P.; Pérez, J.; Muñoz, F. Photocatalytic degradation of azo dyes over semiconductors supported on polyethylene terephthalate and polystyrene substrates. J. Adv. Oxid. Technol. 2017, 20, 20170006. [Google Scholar] [CrossRef][Green Version]
- Picho-Chillán, G.; Dante, R.C.; Muñoz-Bisesti, F.; Martín-Ramos, P.; Chamorro-Posada, P.; Vargas-Jentzsch, P.; Sánchez-Arévalo, F.M.; Sandoval-Pauker, C.; Rutto, D. Photodegradation of Direct Blue 1 azo dye by polymeric carbon nitride irradiated with accelerated electrons. Mater. Chem. Phys. 2019, 237, 121878. [Google Scholar] [CrossRef]
- Gangu, K.K.; Maddila, S.; Jonnalagadda, S.B. A review on novel composites of MWCNTs mediated semiconducting materials as photocatalysts in water treatment. Sci. Total Environ. 2019, 646, 1398–1412. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Y.; Zeng, G.; Liu, N.; Liu, S. Graphene and graphene-based nanocomposites used for antibiotics removal in water treatment: A review. Chemosphere 2019, 226, 360–380. [Google Scholar] [CrossRef] [PubMed]
- Altamirano Briones, A.; Cóndor Guevara, I.; Mena, D.; Espinoza, I.; Sandoval-Pauker, C.; Ramos Guerrero, L.; Vargas Jentzsch, P.; Muñoz Bisesti, F. Degradation of meropenem by heterogeneous photocatalysis using TiO2/fiberglass substrates. Catalysts 2020, 10, 344. [Google Scholar] [CrossRef]
- Coronel, S.; Sandoval Pauker, C.; Vargas Jentzsch, P.; de la Torre, E.; Endara, D.; Muñoz-Bisesti, F. Titanium dioxide/copper/carbon composites for the photocatalytic degradation of phenol. Chem. Chem. Technol. 2020, 14, 161–168. [Google Scholar] [CrossRef]
- Galus, M.; Jeyaranjaan, J.; Smith, E.; Li, H.; Metcalfe, C.; Wilson, J.Y. Chronic effects of exposure to a pharmaceutical mixture and municipal wastewater in zebrafish. Aquat. Toxicol. 2013, 132–133, 212–222. [Google Scholar] [CrossRef]
- García-Cambero, J.P.; Beltrán, F.J.; Encinas, A.; Rivas, F.J.; Oropesa, A.L. The added value of a zebrafish embryo-larval model in the assessment of wastewater tertiary treatments. Environ. Sci. Water Res. Technol. 2019, 5, 2269–2279. [Google Scholar] [CrossRef]
- Silva, D.C.V.R.; Queiroz, L.G.; Marassi, R.J.; Araújo, C.V.M.; Bazzan, T.; Cardoso-Silva, S.; Silva, G.C.; Müller, M.; Silva, F.T.; Montagner, C.C.; et al. Predicting zebrafish spatial avoidance triggered by discharges of dairy wastewater: An experimental approach based on self-purification in a model river. Environ. Pollut. 2020, 266, 115325. [Google Scholar] [CrossRef]
- Freeman, E.S.; Carroll, B. The application of thermoanalytical techniques to reaction kinetics. The thermogravimetric evaluation of the kinetics of the decomposition of calcium oxalate monohydrate. J. Phys. Chem. 1958, 62, 394–397. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Garófalo-Villalta, S.; Medina-Espinosa, T.; Sandoval-Pauker, C.; Villacis, W.; Ciobotă, V.; Muñoz Bisesti, F.; Vargas Jentzsch, P. Degradation of Reactive Red 120 dye by a heterogeneous Sono-Fenton process with goethite deposited onto silica and calcite sand. J. Serb. Chem. Soc. 2020, 85, 125–140. [Google Scholar] [CrossRef]
- Laverde-Cerda, E.; Altamirano-Briones, A.; Zárate-Pozo, P.; Sandoval-Pauker, C.; Ramos-Guerrero, L.; Muñoz-Bisesti, F.; Vargas-Jentzsch, P. Iron removal for kinetic studies on Fenton treatments: A new approach based on ferrocyanide. Rev. Mex. Ing. Quim. 2020, 19, 159–164. [Google Scholar] [CrossRef]
- Jiraprasertwong, A.; Maitriwong, K.; Chavadej, S. Production of biogas from cassava wastewater using a three-stage upflow anaerobic sludge blanket (UASB) reactor. Renew. Energy 2019, 130, 191–205. [Google Scholar] [CrossRef]
- Fleck, L.; Tavares, M.H.F.; Eyng, E.; de Andrade, M.A.d.M.; Frare, L.M. Optimization of anaerobic treatment of cassava processing wastewater. Eng. Agríc. 2017, 37, 574–590. [Google Scholar] [CrossRef][Green Version]
- Araújo, C.V.M.; Shinn, C.; Mendes, L.B.; Delello-Schneider, D.; Sanchez, A.L.; Espíndola, E.L.G. 2014. Avoidance response of Danio rerio to a fungicide in a linear contamination gradient. Sci. Total Environ. 2014, 484, 36–42. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 203: Fish, Acute Toxicity Test, OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 1992. [Google Scholar] [CrossRef]
- OECD. Test No. 215: Fish, Juvenile Growth Test, OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2000. [Google Scholar] [CrossRef]
- Lawrence, C. The husbandry of zebrafish (Danio rerio): A review. Aquaculture 2007, 269, 1–20. [Google Scholar] [CrossRef]
- Avdesh, A.; Chen, M.; Martin-Iverson, M.T.; Mondal, A.; Ong, D.; Rainey-Smith, S.; Taddei, K.; Lardelli, M.; Groth, D.M.; Verdile, G.; et al. Regular Care and Maintenance of a Zebrafish (Danio rerio) Laboratory: An Introduction. J. Vis. Exp. 2012, 69, e4196. [Google Scholar] [CrossRef]
- Bosch Reig, F.; Gimeno Adelantado, J.V.; Moya Moreno, M.C.M. FTIR quantitative analysis of calcium carbonate (calcite) and silica (quartz) mixtures using the constant ratio method. Application to geological samples. Talanta 2002, 58, 811–821. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Wang, Z.; Dawson, G.; Chen, W. Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. J. Mater. Chem. 2011, 21, 14398–14401. [Google Scholar] [CrossRef]
- Cumbana, A.; Mirione, E.; Cliff, J.; Bradbury, J.H. Reduction of cyanide content of cassava flour in Mozambique by the wetting method. Food Chem. 2007, 101, 894–897. [Google Scholar] [CrossRef]
- Zuk, M.; Pelc, K.; Szperlik, J.; Sawula, A.; Szopa, J. Metabolism of the cyanogenic glucosides in developing flax: Metabolic analysis, and expression pattern of genes. Metabolites 2020, 10, 288. [Google Scholar] [CrossRef]
- Bulgariu, L.; Escudero, L.B.; Bello, O.S.; Iqbal, M.; Nisar, J.; Adegoke, K.A.; Alakhras, F.; Kornaros, M.; Anastopoulo, I. The utilization of leaf-based adsorbents for dyes removal: A review. J. Mol. Liq. 2019, 276, 728–747. [Google Scholar] [CrossRef]
- Hernández-Zamora, M.; Martínez-Jerónimo, F. Congo red dye diversely affects organisms of different trophic levels: A comparative study with microalgae, cladocerans, and zebrafish embryos. Environ. Sci. Pollut. Res. 2019, 26, 11743–11755. [Google Scholar] [CrossRef]
- Chu, J.-H.; Kang, J.-K.; Park, S.-J.; Lee, C.-G. Application of magnetic biochar derived from food waste in heterogeneous sono-Fenton-like process for removal of organic dyes from aqueous solution. J. Water Process. Eng. 2020, 37, 101455. [Google Scholar] [CrossRef]
- Engström-Öst, J.; Karjalainen, M.; Viitasalo, M. Feeding and refuge use by small fish in the presence of cyanobacteria blooms. Environ. Biol. Fish. 2006, 76, 109–117. [Google Scholar] [CrossRef]
- Tierney, K.B. Chemical avoidance responses of fish. Aquat. Toxicol. 2016, 174, 228–241. [Google Scholar] [CrossRef]
- Araújo, C.V.M.; Rodríguez, E.N.V.; Salvatierra, D.; Cedeño-Macias, L.A.; Vera-Vera, V.C.; Moreira-Santos, M.; Ribeiro, R. Attractiveness of food and avoidance from contamination as conflicting stimuli to habitat selection by fish. Chemosphere 2016, 163, 177–183. [Google Scholar] [CrossRef]
- Ruuskanen, S.; Rainio, M.J.; Kuosmanen, V.; Laihonen, M.; Saikkonen, K.; Saloniemi, I.; Helander, M. Female preference and adverse developmental effects of glyphosate-based herbicides on ecologically relevant traits in Japanese quails. Environ. Sci. Technol. 2020, 54, 1128–1135. [Google Scholar] [CrossRef]
- Araújo, C.V.M.; Moreira-Santos, M.; Ribeiro, R. Active and passive spatial avoidance by aquatic organisms from environmental stressors: A complementary perspective and a critical review. Environ. Int. 2016, 92–93, 405–415. [Google Scholar] [CrossRef]
- Fleeger, J.W.; Carman, K.R.; Nisbet, R.M. Indirect effects of contaminants in aquatic ecosystems. Sci. Total Environ. 2003, 317, 207–233. [Google Scholar] [CrossRef]
- Moe, S.J.; De Schamphelaere, K.; Clements, W.H.; Sorensen, M.T.; Van Den Brink, P.J.; Liess, M. Combined and interactive effects of global climate change and toxicants on populations and communities. Environ. Toxicol. Chem. 2013, 32, 49–61. [Google Scholar] [CrossRef]
- Hansen, J.A.; Woodward, D.F.; Little, E.E.; DeLonay, A.J.; Bergman, H.L. Behavioral avoidance: Possible mechanism for explaining abundance and distribution of trout species in a metal-impacted river. Environ. Toxicol. Chem. 1999, 18, 313–317. [Google Scholar] [CrossRef]
- Jutfelt, F.; Sundin, J.; Raby, G.D.; Krang, A.-S.; Clark, T.D. Two-current choice flumes for testing avoidance and preference in aquatic animals. Methods Ecol. Evol. 2017, 8, 379–390. [Google Scholar] [CrossRef]
- Moreira-Santos, M.; Ribeiro, R.; Araújo, C.V.M. What if aquatic animals move away from pesticide-contaminated habitats before suffering adverse physiological effects? A critical review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 989–1025. [Google Scholar] [CrossRef]



| Parameter | Units | Theoretical Conditions | Dechlorinated Tap Water *** | |
|---|---|---|---|---|
| Lawrence * | Avbesh et al. ** | |||
| pH | - | 7.0–8.0 | 6.8–7.5 | 7.23 |
| Temperature | °C | 24–30 | 26.0–28.5 | 21.5 |
| Electrical Conductivity | μS/cm | - | 300–1500 | 180.9 |
| Alkalinity | mg CaCO3/L | - | 50–150 | 39.0 |
| Hardness | mg CaCO3/L | 75–200 | 50–100 | 47.0 |
| Dissolved oxygen | mg/L | 7.8 | >6.0 | 6.6 |
| Turbidity | NTU | - | - | 0.31 |
| Biochemical oxygen demand | mg O2/L | - | - | >2 |
| Chemical oxygen demand | mg O2/L | - | - | 43 |
| Parameter | Units | Untreated Synthetic Water | Treated Synthetic Water |
|---|---|---|---|
| pH | - | 5.91 | 7.44 |
| Temperature | °C | 20.0 | 16.6 |
| Electrical conductivity | µS/cm | 64.5 | 1224.0 |
| Turbidity | NTU | 0.61 | 8.32 |
| Chemical oxygen demand | mg O2/L | 53.0 | 16.5 |
| Total organic carbon | mg C/L | 25.6 | 22.5 |
| Concentration of RR-120 | mg/L | 80.00 | 2.38 |
| Parameter | Units | Untreated Synthetic Water | Treated Synthetic Water |
|---|---|---|---|
| pH | - | 6.81 | 8.24 |
| Temperature | °C | 18.6 | 19.4 |
| Electrical conductivity | µS/cm | 1268.5 | 1188.5 |
| Turbidity | NTU | 9.48 | 4.94 |
| Chemical oxygen demand | mg O2/L | 5980.0 | 5510.0 |
| Total organic carbon | mg C/L | 2972.8 | 2731.0 |
| Total cyanide | mg/L | 1.36 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabascango, T.; Ortiz, K.; Sandoval Pauker, C.; Espinoza Pavón, I.; Ramoji, A.; Popp, J.; Pérez, J.; Pinto, C.M.; Rivera-Parra, J.L.; Muñoz-Bisesti, F.; et al. Assessment of Advanced Oxidation Processes Using Zebrafish in a Non-Forced Exposure System: A Proof of Concept. Processes 2021, 9, 734. https://doi.org/10.3390/pr9050734
Cabascango T, Ortiz K, Sandoval Pauker C, Espinoza Pavón I, Ramoji A, Popp J, Pérez J, Pinto CM, Rivera-Parra JL, Muñoz-Bisesti F, et al. Assessment of Advanced Oxidation Processes Using Zebrafish in a Non-Forced Exposure System: A Proof of Concept. Processes. 2021; 9(5):734. https://doi.org/10.3390/pr9050734
Chicago/Turabian StyleCabascango, Tamia, Karol Ortiz, Christian Sandoval Pauker, Isabel Espinoza Pavón, Anuradha Ramoji, Jürgen Popp, Jady Pérez, C. Miguel Pinto, José Luis Rivera-Parra, Florinella Muñoz-Bisesti, and et al. 2021. "Assessment of Advanced Oxidation Processes Using Zebrafish in a Non-Forced Exposure System: A Proof of Concept" Processes 9, no. 5: 734. https://doi.org/10.3390/pr9050734
APA StyleCabascango, T., Ortiz, K., Sandoval Pauker, C., Espinoza Pavón, I., Ramoji, A., Popp, J., Pérez, J., Pinto, C. M., Rivera-Parra, J. L., Muñoz-Bisesti, F., Aldás, M. B., Araújo, C. V. M., & Vargas Jentzsch, P. (2021). Assessment of Advanced Oxidation Processes Using Zebrafish in a Non-Forced Exposure System: A Proof of Concept. Processes, 9(5), 734. https://doi.org/10.3390/pr9050734











