The Complexity of Lignin Thermal Degradation in the Isothermal Context
Abstract
:1. Introduction
2. Materials and Methods
- –
- Linear heating. A constant 10 °C/min heating rate was applied through the experiment.
- –
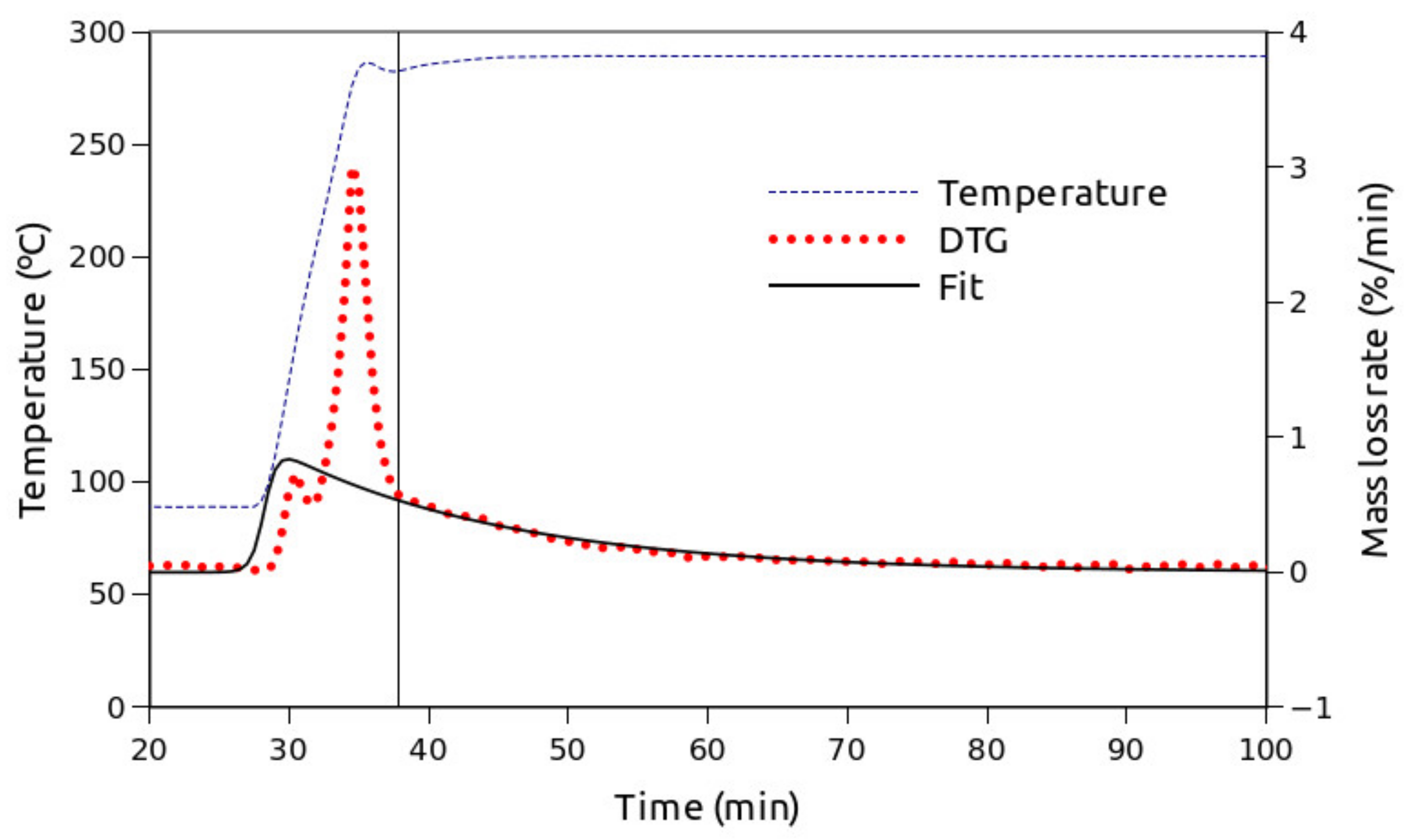
- Two-step isothermal experiments. An isothermal step at the desired temperature was preceded by a linear 10 °C/min heating ramp from room temperature to 90 °C, followed by a 20 min isothermal step and a linear 30 °C/min heating step up to the targeted temperature. The experiment was then allowed to continue until a clear trend of the mass evolution with time was observed.
- –
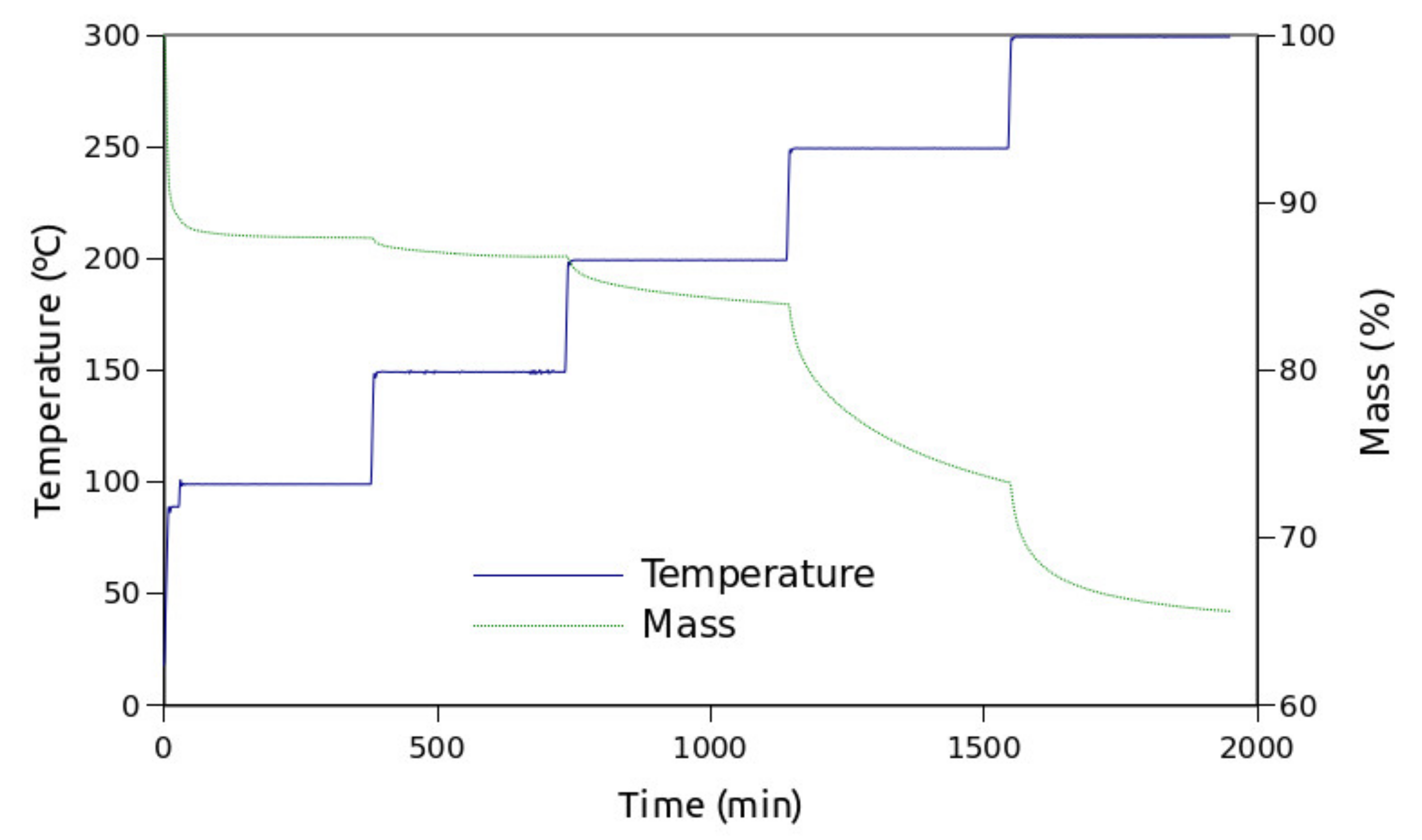
- Stairway heating. Isothermal steps at 90, 100, 150, 200, 250 and 300 °C were chained by 10 °C linear heating steps.
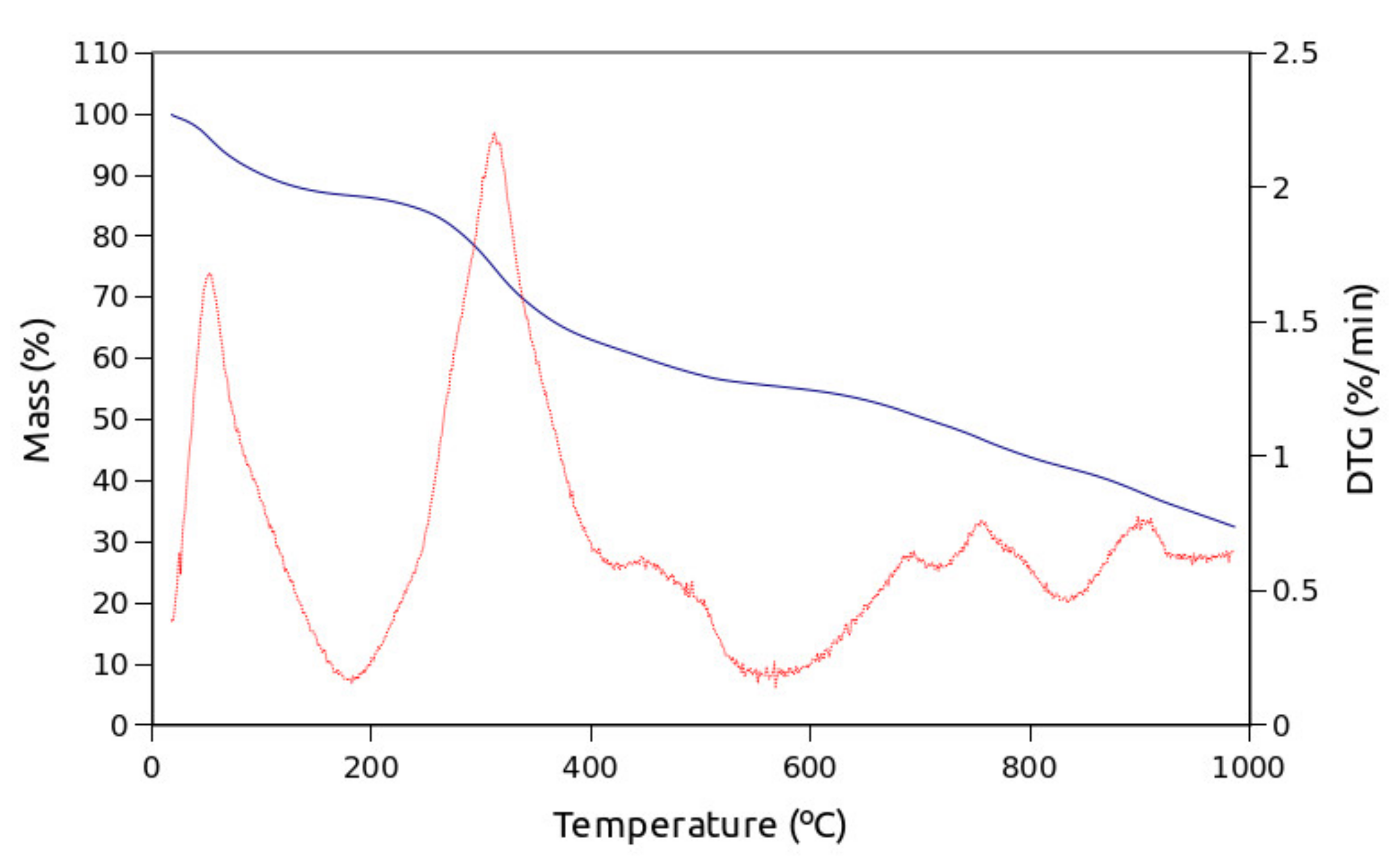
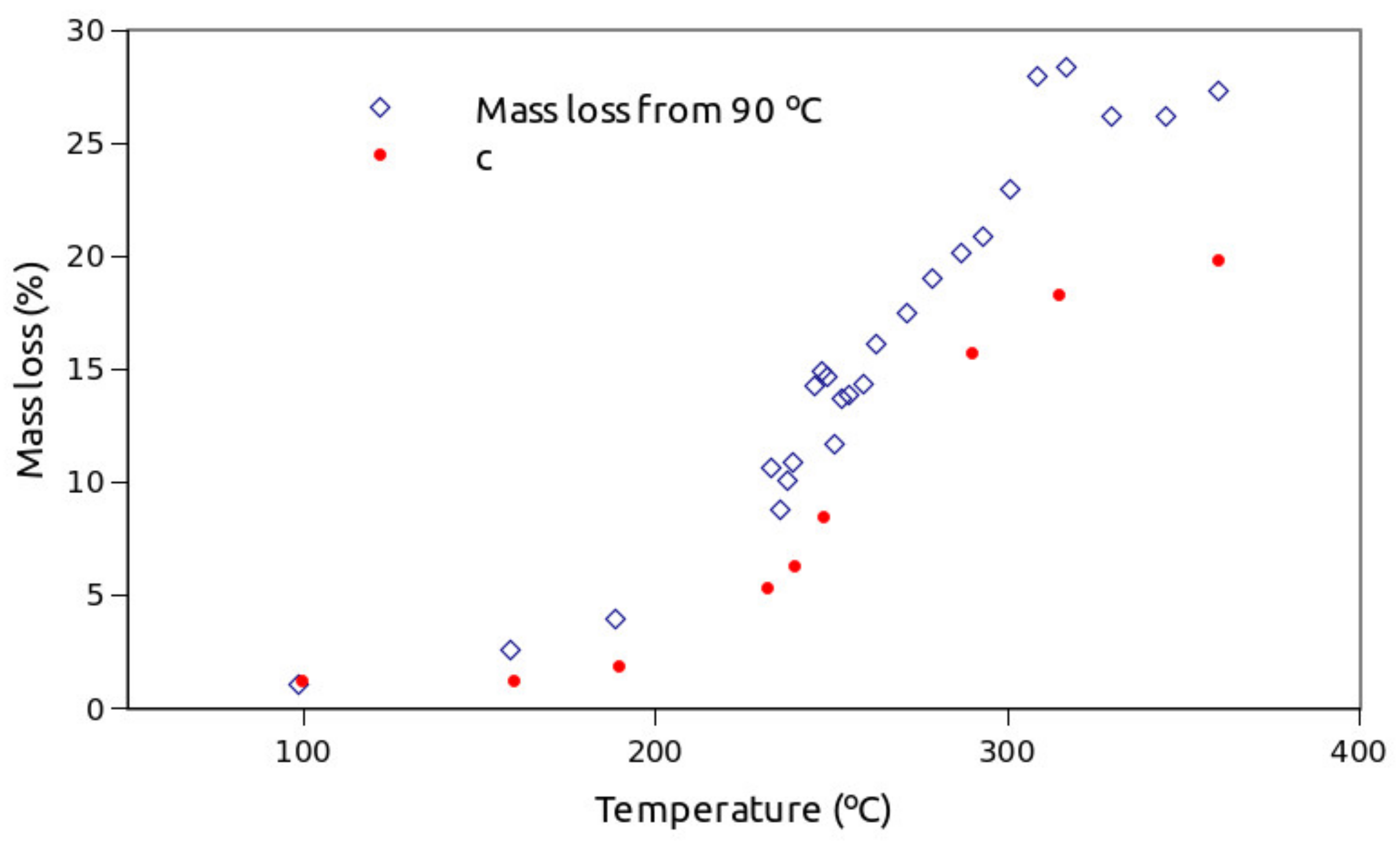
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin structure and its engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [Green Version]
- Olsson, A.-M.; Salmén, L. The effect of lignin composition on the viscoelastic properties of wood. Nord. Pulp Pap. Res. J. 1997, 12, 140–144. [Google Scholar] [CrossRef]
- Bonawitz, N.D.; Chapple, C. The Genetics of Lignin Biosynthesis: Connecting Genotype to Phenotype. Annu. Rev. Genet. 2010, 44, 337–363. [Google Scholar] [CrossRef]
- Amthor, J.S. Efficiency of Lignin Biosynthesis: A Quantitative Analysis. Ann. Bot. 2003, 91, 673–695. [Google Scholar] [CrossRef] [Green Version]
- Boerjan, W.; Ralph, J. Editorial overview: Plant biotechnology-lignin engineering. Curr. Opin. Biotechnol. 2019, 56, 3–5. [Google Scholar] [CrossRef]
- Chabannes, M.; Ruel, K.; Yoshinaga, A.; Chabbert, B.; Jauneau, A.; Joseleau, J.-P.; Boudet, A.-M. In situ analysis of lignins in transgenic tobacco reveals a differential impact of individual transformations on the spatial patterns of lignin deposition at the cellular and subcellular levels: In Situ Analysis of Lignin Deposition. Plant J. 2001, 28, 271–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanholme, R.; Morreel, K.; Darrah, C.; Oyarce, P.; Grabber, J.H.; Ralph, J.; Boerjan, W. Metabolic engineering of novel lignin in biomass crops. N. Phytol. 2012, 196, 978–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lora, J.H.; Glasser, W.G. Recent Industrial Applications of Lignin: A Sustainable Alternative to Nonrenewable Materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Lignin.Org-Dialogue/Newsletters. Available online: https://web.archive.org/web/20071009010219/http://www.lignin.org/01augdialogue.html (accessed on 11 May 2021).
- Xu, M.; Khachatryan, L.; Kibet, J.; Lomnicki, S. Lumped kinetic modeling of isothermal degradation of lignin under conventional pyrolytic and oxidative conditions. J. Anal. Appl. Pyrolysis 2017, 127, 377–384. [Google Scholar] [CrossRef]
- Kawamoto, H. Lignin pyrolysis reactions. J. Wood Sci. 2017, 63, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Grossman, A.; Vermerris, W. Lignin-based polymers and nanomaterials. Curr. Opin. Biotechnol. 2019, 56, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, S.; Mollaabbasi, R.; Patience, G.S. Kinetics of softwood kraft lignin inert and oxidative thermolysis. Biomass Bioenergy 2018, 109, 239–248. [Google Scholar] [CrossRef]
- Mottiar, Y.; Vanholme, R.; Boerjan, W.; Ralph, J.; Mansfield, S. Designer lignins: Harnessing the plasticity of lignification. Curr. Opin. Biotechnol. 2016, 37, 190–200. [Google Scholar] [CrossRef] [Green Version]
- Gupta, C.; Washburn, N.R. Polymer-Grafted Lignin Surfactants Prepared via Reversible Addition–Fragmentation Chain-Transfer Polymerization. Langmuir 2014, 30, 9303–9312. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kadla, J.F. Preparation of a Thermoresponsive Lignin-Based Biomaterial through Atom Transfer Radical Polymerization. Biomacromolecules 2010, 11, 981–988. [Google Scholar] [CrossRef]
- Kai, D.; Zhang, K.; Jiang, L.; Wong, H.Z.; Li, Z.; Zhang, Z.; Loh, X.J. Sustainable and Antioxidant Lignin–Polyester Copolymers and Nanofibers for Potential Healthcare Applications. ACS Sustain. Chem. Eng. 2017, 5, 6016–6025. [Google Scholar] [CrossRef]
- Kouisni, L.; Gagné, A.; Maki, K.; Holt-Hindle, P.; Paleologou, M. LignoForce System for the Recovery of Lignin from Black Liquor: Feedstock Options, Odor Profile, and Product Characterization. ACS Sustain. Chem. Eng. 2016, 4, 5152–5159. [Google Scholar] [CrossRef]
- Zaman, C.Z.; Pal, K.; Yehye, W.A.; Sagadevan, S.; Shah, S.T.; Adebisi, G.A.; Marliana, E.; Rafique, R.F.; Bin Johan, R. Pyrolysis: A Sustainable Way to Generate Energy from Waste. In Pyrolysis; Samer, M., Ed.; InTech: London, UK, 2017; ISBN 978-953-51-3311-7. [Google Scholar]
- Faravelli, T.; Frassoldati, A.; Migliavacca, G.; Ranzi, E. Detailed kinetic modeling of the thermal degradation of lignins. Biomass Bioenergy 2010, 34, 290–301. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J. Materials produced from plant biomass: Part III: Degradation kinetics and hydrogen bonding in lignin. Mater. Res. 2013, 16, 1065–1070. [Google Scholar] [CrossRef] [Green Version]
- Jiang, G.; Nowakowski, D.; Bridgwater, T. A systematic study of the kinetics of lignin pyrolysis. Thermochim. Acta 2010, 498, 61–66. [Google Scholar] [CrossRef]
- Dussan, K.; Dooley, S.; Monaghan, R. A model of the chemical composition and pyrolysis kinetics of lignin. Proc. Combust. Inst. 2019, 37, 2697–2704. [Google Scholar] [CrossRef]
- Cho, J.; Chu, S.; Dauenhauer, P.J.; Huber, G.W. Kinetics and reaction chemistry for slow pyrolysis of enzymatic hydrolysislignin and organosolv extracted lignin derived from maplewood. Green Chem. 2012, 14, 428–439. [Google Scholar] [CrossRef] [Green Version]
- Ferdous, D.; Dalai, A.K.; Bej, S.K.; Thring, R.W. Pyrolysis of Lignins: Experimental and Kinetics Studies. Energy Fuels 2002, 16, 1405–1412. [Google Scholar] [CrossRef]
- Beis, S.; Mukkamala, S.; Hill, N.; Joseph, J.; Baker, C.; Jensen, B.; Stemmler, E.; Wheeler, C.; Frederick, B.G.; Heiningen, A.; et al. Fast Pyrolysis of Lignins. Bioresources 2010, 5, 1408–1424. [Google Scholar]
- Manyà, J.J.; Velo, A.E.; Puigjaner, L. Kinetics of Biomass Pyrolysis: A Reformulated Three-Parallel-Reactions Model. Ind. Eng. Chem. Res. 2003, 42, 434–441. [Google Scholar] [CrossRef]
- Murugan, P.; Mahinpey, N.; Johnson, K.E.; Wilson, M. Kinetics of the Pyrolysis of Lignin Using Thermogravimetric and Differential Scanning Calorimetry Methods. Energy Fuels 2008, 22, 2720–2724. [Google Scholar] [CrossRef]
- Huang, Y.; Kuan, W.; Chiueh, P.; Lo, S. A sequential method to analyze the kinetics of biomass pyrolysis. Bioresour. Technol. 2011, 102, 9241–9246. [Google Scholar] [CrossRef]
- Yeo, J.Y.; Chin, B.L.F.; Tan, J.K.; Loh, Y.S. Comparative studies on the pyrolysis of cellulose, hemicellulose, and lignin based on combined kinetics. J. Energy Inst. 2019, 92, 27–37. [Google Scholar] [CrossRef]
- Ojha, D.K.; Viju, D.; Vinu, R. Fast pyrolysis kinetics of alkali lignin: Evaluation of apparent rate parameters and product time evolution. Bioresour. Technol. 2017, 241, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Yanez-McKay, A.J.; Natarajan, P.; Li, W.; Mabon, R.; Broadbelt, L.J. Coupled Structural and Kinetic Model of Lignin Fast Pyrolysis. Energy Fuels 2018, 32, 1822–1830. [Google Scholar] [CrossRef]
- Fenner, R.A.; Lephardt, J.O. Examination of the thermal decomposition of kraft pine lignin by Fourier transform infrared evolved gas analysis. J. Agric. Food Chem. 1981, 29, 846–849. [Google Scholar] [CrossRef]
- López-Beceiro, J.; Álvarez-García, A.; Martins, S.; Zaragoza, S.; Menéndez-Valdés, J.; Artiaga, R. Thermal degradation kinetics of two acrylic-based copolymers. J. Therm. Anal. Calorim. 2015, 119, 1981–1993. [Google Scholar] [CrossRef]
- López-Beceiro, J.; Álvarez-García, A.; Sebio-Puñal, T.; Zaragoza, S.; Díaz-Díaz, A.; Janeiro, J.; Artiaga, R.; Álvarez-García, B. Kinetics of Thermal Degradation of Cellulose: Analysis Based on Isothermal and Linear Heating Data. BioResources 2016, 11, 5870–5888. [Google Scholar] [CrossRef] [Green Version]
- López-Beceiro, J.; Díaz-Díaz, A.; Álvarez-García, A.; Tarrío-Saavedra, J.; Naya, S.; Artiaga, R. A Logistic Approach for Kinetics of Isothermal Pyrolysis of Cellulose. Processes 2021, 9, 551. [Google Scholar] [CrossRef]
- Nash, J.C. Spreadsheets in Statistical Practice—Another Look. Am. Stat. 2006, 60, 287–289. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Beceiro, J.; Díaz-Díaz, A.M.; Álvarez-García, A.; Tarrío-Saavedra, J.; Naya, S.; Artiaga, R. The Complexity of Lignin Thermal Degradation in the Isothermal Context. Processes 2021, 9, 1154. https://doi.org/10.3390/pr9071154
López-Beceiro J, Díaz-Díaz AM, Álvarez-García A, Tarrío-Saavedra J, Naya S, Artiaga R. The Complexity of Lignin Thermal Degradation in the Isothermal Context. Processes. 2021; 9(7):1154. https://doi.org/10.3390/pr9071154
Chicago/Turabian StyleLópez-Beceiro, Jorge, Ana María Díaz-Díaz, Ana Álvarez-García, Javier Tarrío-Saavedra, Salvador Naya, and Ramón Artiaga. 2021. "The Complexity of Lignin Thermal Degradation in the Isothermal Context" Processes 9, no. 7: 1154. https://doi.org/10.3390/pr9071154
APA StyleLópez-Beceiro, J., Díaz-Díaz, A. M., Álvarez-García, A., Tarrío-Saavedra, J., Naya, S., & Artiaga, R. (2021). The Complexity of Lignin Thermal Degradation in the Isothermal Context. Processes, 9(7), 1154. https://doi.org/10.3390/pr9071154






