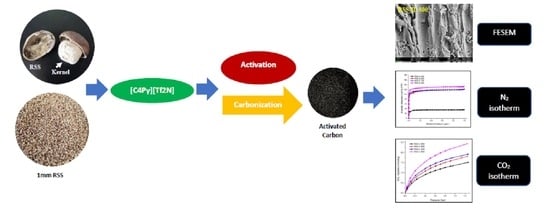
Development of Rubber Seed Shell–Activated Carbon Using Impregnated Pyridinium-Based Ionic Liquid for Enhanced CO2 Adsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
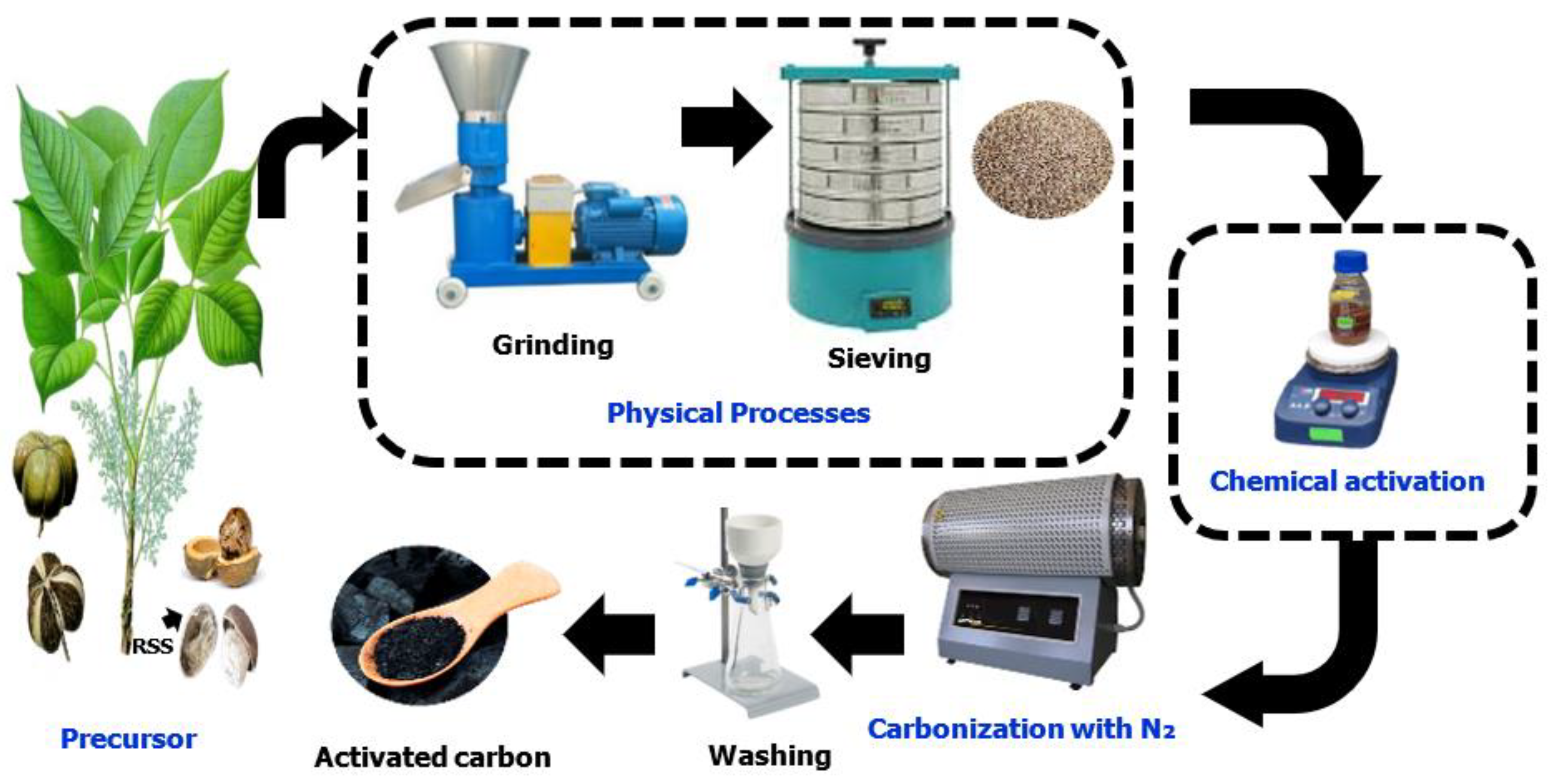
2.2. Preparation and Carbonization of RSS
2.3. Sample Characterization
Activated Carbon Characterization
2.4. CO2 Adsorption and Isotherm Study
3. Results and Discussion
3.1. Characterization of Materials
3.1.1. Proximate and Ultimate Analysis of Raw RSS
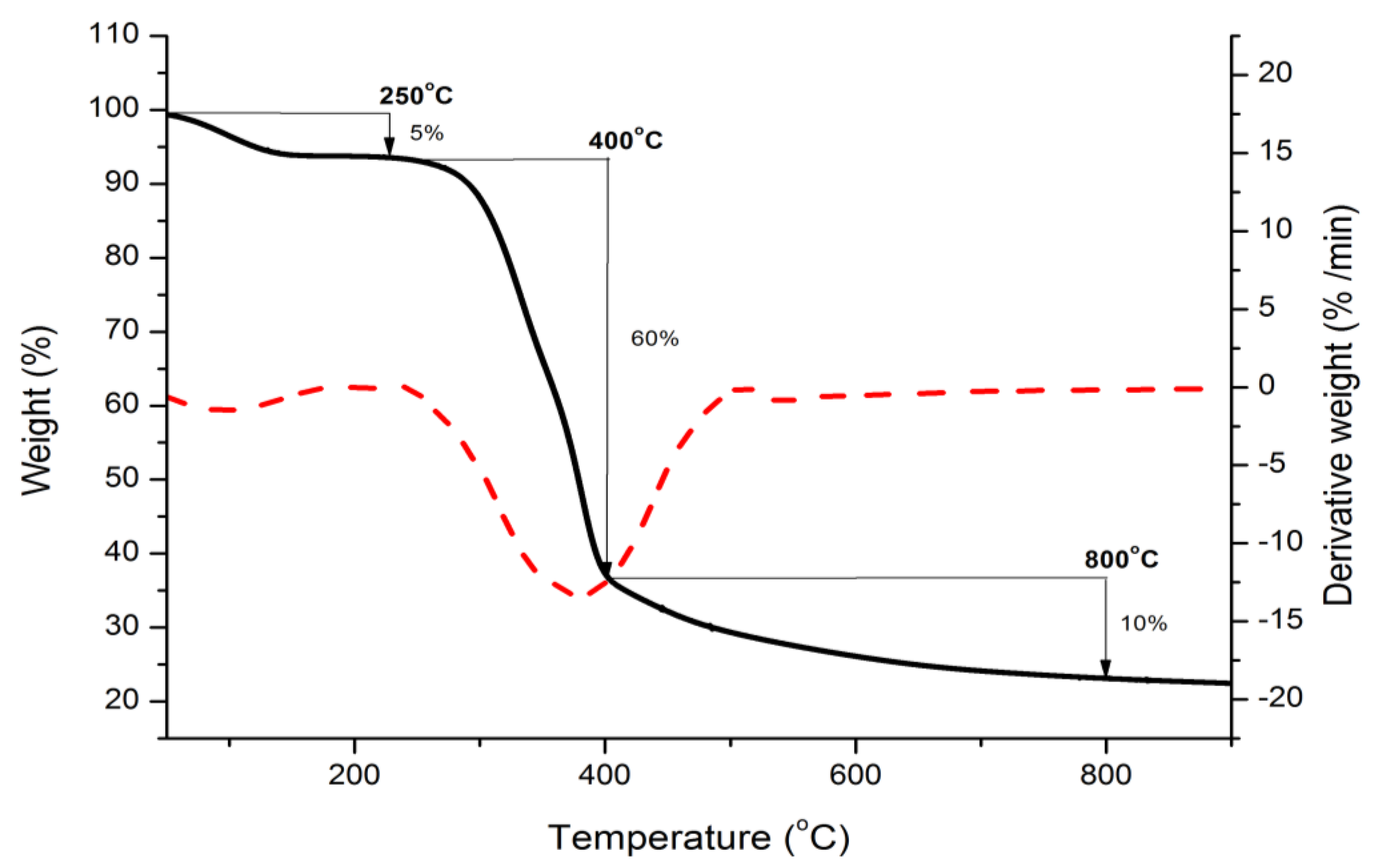
3.1.2. TGA Analysis
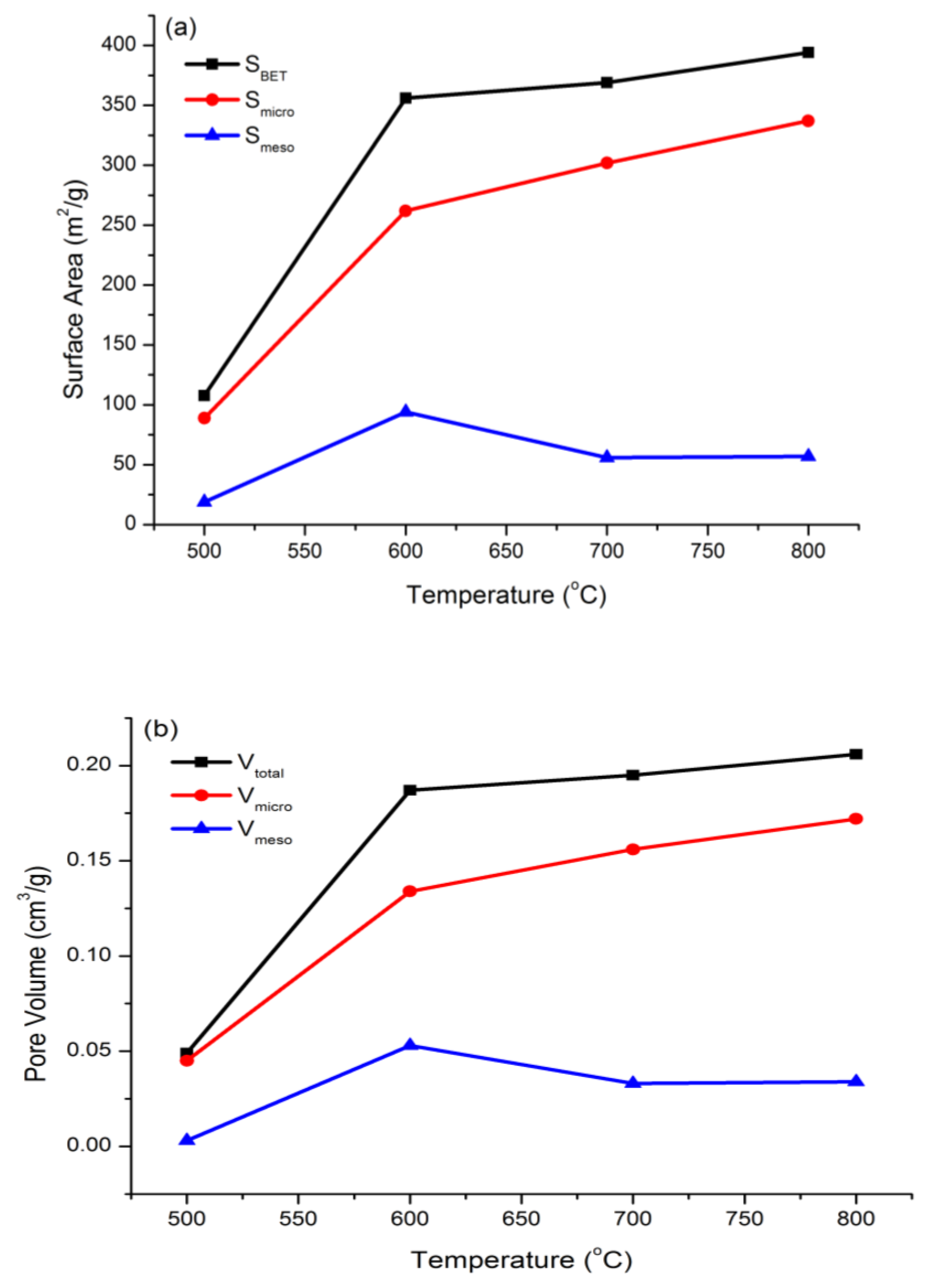
3.1.3. Surface Area and Pore Size Analysis
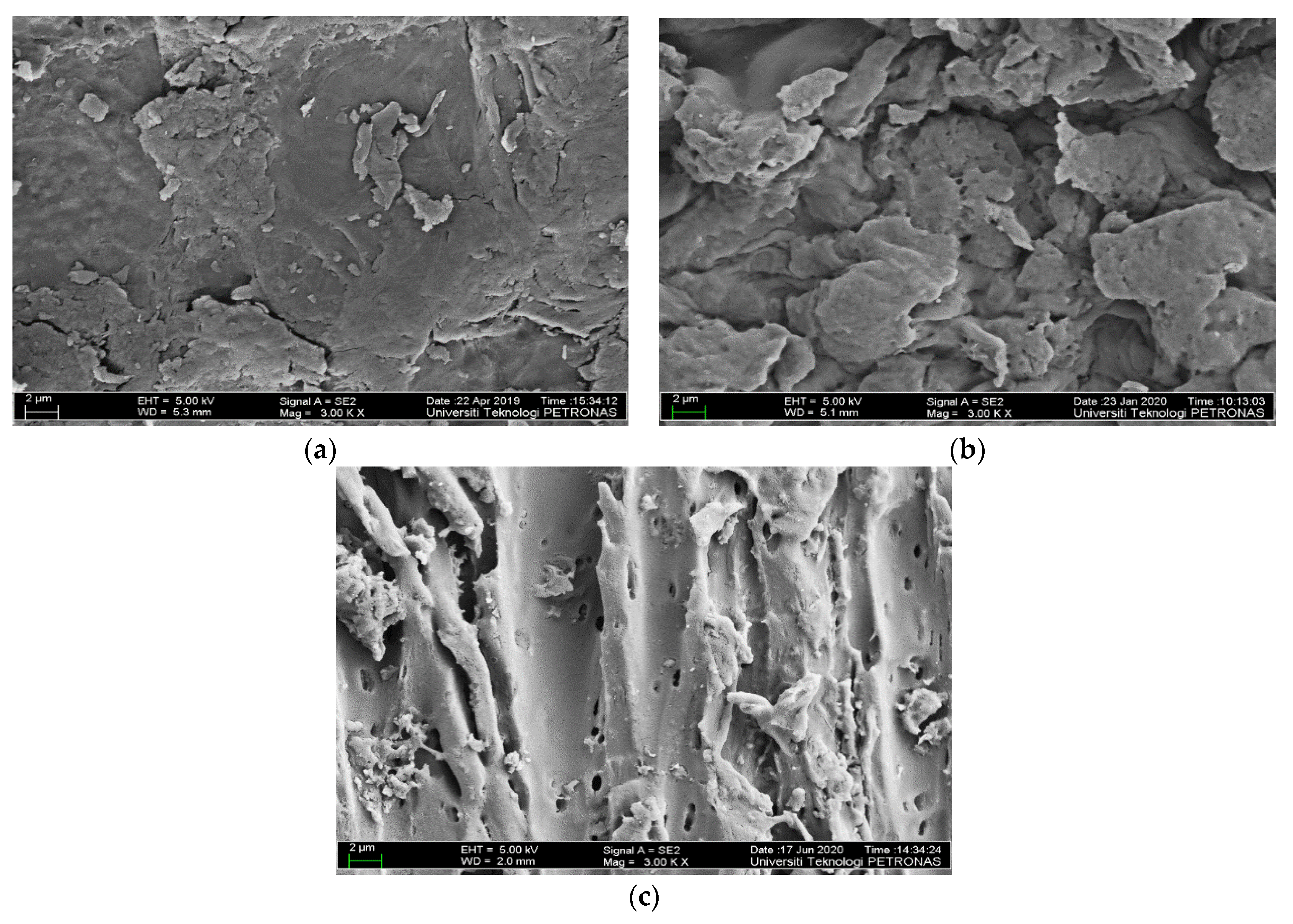
3.1.4. Surface Morphology
3.1.5. Chemical Properties of Activated Carbon
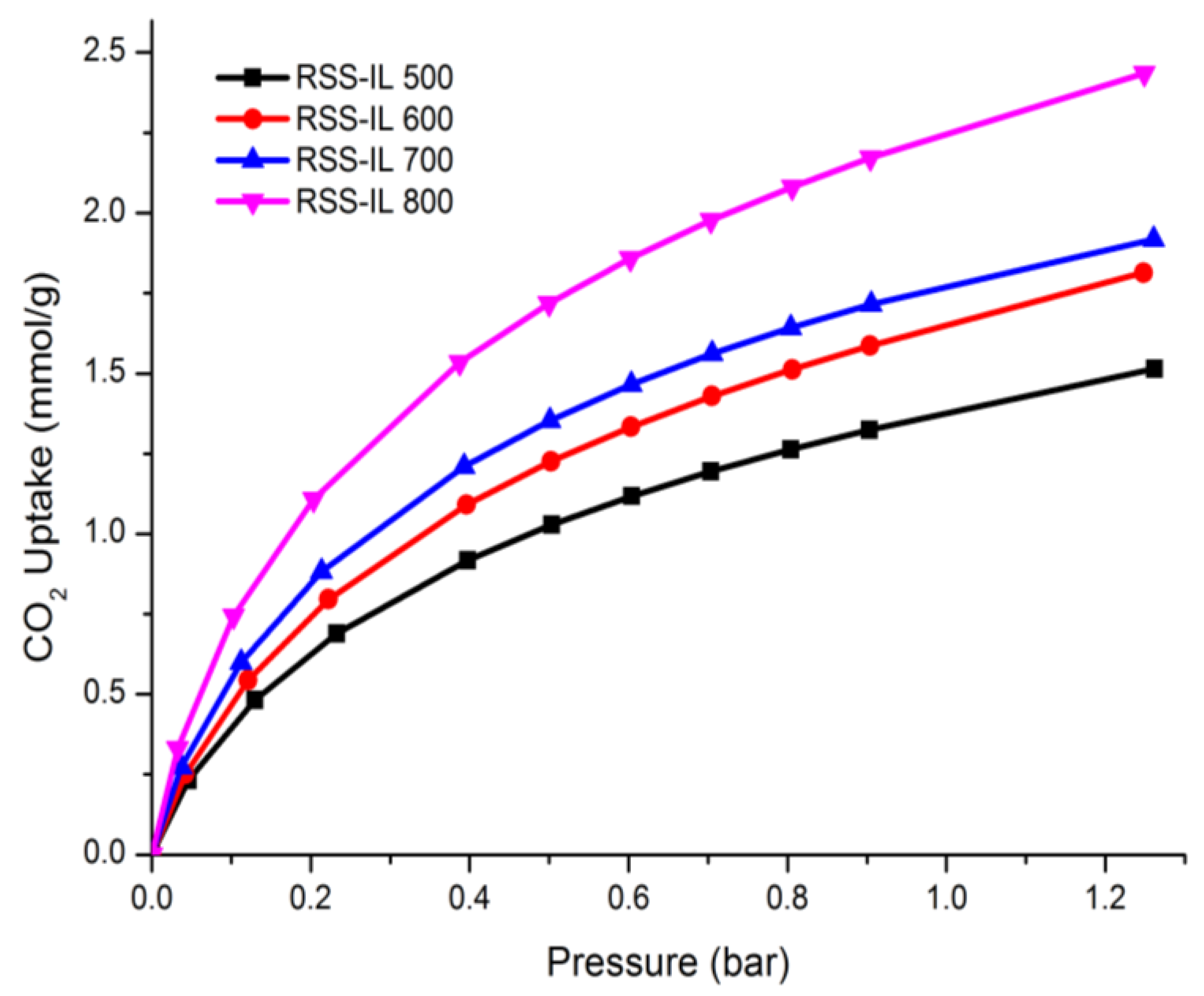
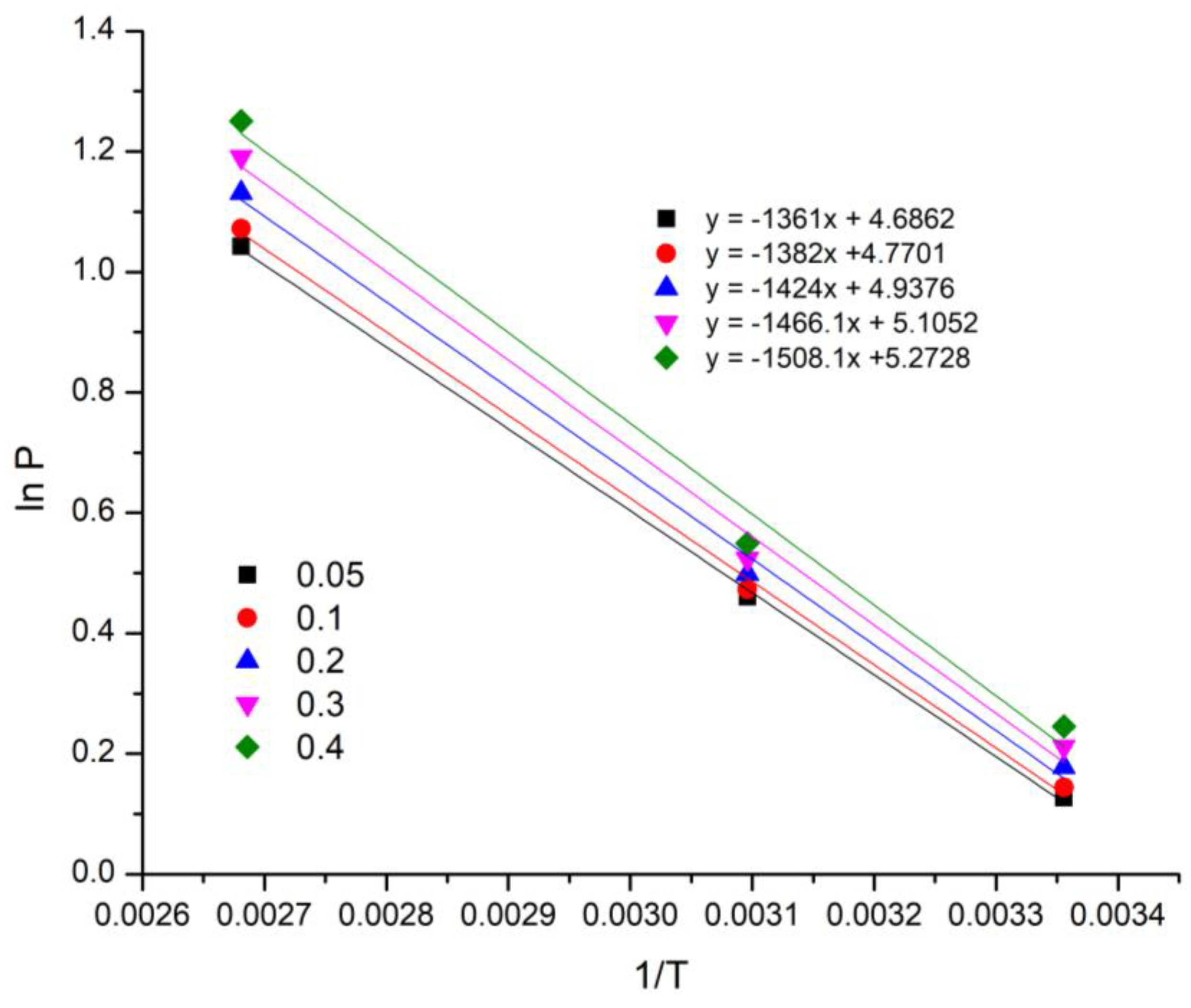
3.2. CO2 Adsorption and Isotherm Study
3.3. Comparison Studies with Other Adsorbent Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaumi, L.A.; Bakar, M.Z.A.; Hameed, B.H. Recent advances in functionalized composite solid materials for carbon dioxide capture. Energy 2017, 124, 461–480. [Google Scholar] [CrossRef]
- Rashidi, A.N.; Yusup, S. An overview of activated carbons utilization for the post-combustion carbon dioxide capture. J. CO2 Util. 2016, 13, 1–16. [Google Scholar] [CrossRef]
- Li, M.; Xiao, R. Preparation of a dual Pore Structure Activated Carbon from Rice Husk Char as an Adsorbent for CO2 Capture. Fuel Process. Technol. 2019, 186, 35–39. [Google Scholar] [CrossRef]
- Hussin, F.; Aroua, M.K. Recent trends in the development of adsorption technologies for carbon dioxide capture: A brief literature and patent reviews (2014–2018). J. Clean. Prod. 2020, 253, 119707. [Google Scholar] [CrossRef]
- Mistar, M.E.; Alfatah, T.; Supardan, M.D. Synthesis and characterization of activated carbon from Bambusa vulgaris striata using two-step KOH activation. J. Mater. Res. Technol. 2020, 9, 6278–6286. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T. A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew. Sustain. Energy Rev. 2018, 87, 1–21. [Google Scholar] [CrossRef]
- de Souza, L.K.; Gonçalves, A.A.; Queiroz, L.S.; Chaar, J.S.; da Rocha Filho, G.N.; da Costa, C.E. Utilization of acai stone biomass for the sustainable production of nanoporous carbon for CO2 capture. Sustain. Mater. Technol. 2020, 25, e00168. [Google Scholar]
- Sun, K.; Jiang, J.C. Preparation and characterization of activated carbon from rubber-seed shell by physical activation with steam. Biomass Bioenergy 2010, 34, 539–544. [Google Scholar] [CrossRef]
- Borhan, A.; Kamil, A.F. Preparation and Characterization of Activated Carbon from Rubber-seed Shell by Chemical Activation. J. Appl. Sci. 2012, 12, 1124–1129. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Putra, Z.A.; Andika, R.; Bilad, M.R.; Kurniawan, T.; Zulhijah, R.; Hamidah, I. Porous activated carbon particles from rice straw waste and their adsorption properties. J. Eng. Sci. Technol. 2017, 12, 1–11. [Google Scholar]
- Bohli, T.; Ouederni, A.; Fiol, N.; Villaescusa, I. Evaluation of an activated carbon from olive stones used as an adsorbent for heavy metal removal from aqueous phases. C. R. Chim. 2015, 18, 88–99. [Google Scholar] [CrossRef]
- Kemp, K.C.; Baek, S.B.; Lee, W.G.; Meyyappan, M.; Kim, K.S. Activated carbon derived from waste coffee grounds for stable methane storage. Nanotechnology 2015, 26, 385602. [Google Scholar] [CrossRef] [PubMed]
- Furmanski, L.; Costa, P.; Dominguini, L. Production of Activated Carbon from Nutshell as An Alternative Material for Adsorption of Methylene Blue; Universidade do Extremo Sul Catarinense: Santa Catarina, Brazil, 2015. [Google Scholar]
- González-García, P. Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications. Renew. Sustain. Energy Rev. 2018, 82, 1393–1414. [Google Scholar] [CrossRef]
- Yan, K.Z.; Zaini, M.A.A.; Arsad, A.; Nasri, N.S. Rubber Seed Shell Based Activated Carbon by Physical Activation for Phenol Removal. Chem. Eng. Trans. 2019, 72, 151–156. [Google Scholar]
- Eka, D.H.; Aris, Y.T.; Nadiah, W.W. Potential use of Malaysian rubber (Hevea brasiliensis) seed as food, feed and biofuel. Int. food Res. J. 2010, 17, 527–534. [Google Scholar]
- Borhan, A.; Yusup, S.; Mun, Y.S. Surface modification of rubber seed shell activated carbon with malic acid for high CO2 adsorption. IOP Conf. Ser. Earth Environ. Sci. 2020, 460, 012044. [Google Scholar] [CrossRef]
- Han, J.; Zhang, L.; Zhao, B.; Qin, L.; Wang, Y.; Xing, F. The N-doped activated carbon derived from sugarcane bagasse for CO2 adsorption. Ind. Crop. Prod. 2019, 128, 290–297. [Google Scholar] [CrossRef]
- Bergna, D.; Varila, T.; Romar, H.; Lassi, U. Comparison of the Properties of Activated Carbons Produced in One-Stage and Two-Stage Processes. C—J. Carbon Res. 2018, 4, 41. [Google Scholar] [CrossRef]
- Adinata, D.; Daud, W.M.A.W.; Aroua, M.K. Preparation and characterization of activated carbon from palm shell by chemical activation with K2CO3. Bioresour. Technol. 2007, 98, 145–149. [Google Scholar] [CrossRef]
- Krishnan, A.; Gopinath, K.P.; Vo, D.V.N.; Malolan, R.; Nagarajan, V.M.; Arun, J. Ionic liquids, deep eutectic solvents and liquid polymers as green solvents in carbon capture technologies: A review. Environ. Chem. Lett. 2020, 1–24. [Google Scholar] [CrossRef]
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renew. Sustain. Energy Rev. 2018, 96, 502–525. [Google Scholar] [CrossRef]
- Habila, M.A.; AlOthman, Z.A.; Ghfar, A.A.; Al-Zaben, M.I.; Alothman, A.A.; Abdeltawab, A.A.; El-Marghany, A.; Sheikh, M. Phosphonium-based Ionic Liquid Modified Activated Carbon from Mixed Recyclable Waste for Mercury(II) Uptake. Molecules 2019, 24, 570. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, Y.; Zhu, S. Use of Ionic Liquids for Impovement of Cellulosic Ethanol Production. BioResources 2010, 6, 1–11. [Google Scholar] [CrossRef]
- Blanchard, L.A.; Hancu, D.; Beckman, E.J.; Brennecke, J.F. Green processing using ionic liquids and CO2. Nature 1999, 399, 28–29. [Google Scholar] [CrossRef]
- Anthony, J.L.; Anderson, J.L.; Maginn, E.J.; Brennecke, J.F. Anion Effects on Gas Solubility in Ionic Liquids. J. Phys. Chem. B 2005, 109, 6366–6374. [Google Scholar] [CrossRef]
- Yunus, N.M.; Mutalib, M.A.; Man, Z.; Bustam, M.A.; Murugesan, T. Solubility of CO2 in pyridinium based ionic liquids. Chem. Eng. J. 2012, 189, 94–100. [Google Scholar] [CrossRef]
- He, X.; Zhu, J.; Wang, H.; Zhou, M.; Zhang, S. Surface Functionalization of Activated Carbon with Phosphonium Ionic Liquid for CO2 Adsorption. Coatings 2019, 9, 590. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, L.; Zhang, G.; Shu, Z.; Shi, J. Chitosan derived nitrogen-doped microporous carbons for high performance CO2 capture. Carbon 2013, 61, 423–430. [Google Scholar] [CrossRef]
- Mokti, N.; Borhan, A.; Zaine, S.N.A.; Zaid, H.F.M. Synthesis and Characterisation of Pyridinium-Based Ionic Liquid as Activating Agent in Rubber Seed Shell Activated Carbon Production for CO2 Capture. J. Adv. Res. Fluid Mech. Therm. Sci. 2021, 82, 85–95. [Google Scholar] [CrossRef]
- Yunus, N.M.; Mutalib, M.A.; Man, Z.; Bustam, M.A.; Murugesan, T. Thermophysical properties of 1-alkylpyridinum bis(trifluoromethylsulfonyl)imide ionic liquids. J. Chem. Thermodyn. 2010, 42, 491–495. [Google Scholar] [CrossRef]
- Men, Y.; Siebenbürger, M.; Qiu, X.; Antonietti, M.; Yuan, J. Low fractions of ionic liquid or poly(ionic liquid) can activate polysaccharide biomass into shaped, flexible and fire-retardant porous carbons. J. Mater. Chem. A 2013, 1, 11887–11893. [Google Scholar] [CrossRef]
- Rashidi, A.N.; Yusup, S. Potential of palm kernel shell as activated carbon precursors through single stage activation technique for carbon dioxide adsorption. J. Clean. Prod. 2017, 168, 474–486. [Google Scholar] [CrossRef]
- Borhan, A.; Yusup, S.; Lim, J.W.; Show, P.L. Characterization and Modelling Studies of Activated Carbon Produced from Rubber-Seed Shell Using KOH for CO2 Adsorption. Processes 2019, 7, 855. [Google Scholar] [CrossRef]
- Oliveira, G.F.D.; Andrade, R.C.D.; Trindade, M.A.G.; Andrade, H.M.C.; Carvalho, C.T.D. Thermogravimetric and spectroscopic study (TG–DTA/FT–IR) of activated carbon from the renewable biomass source Babassu. Química Nova 2016, 40, 284–292. [Google Scholar] [CrossRef]
- Kaghazchi, T.; Soleimani, M. Effect of Raw Materials on Properties of Activated Carbons. Chem. Eng. Technol. 2006, 29, 1247–1251. [Google Scholar]
- Rashidi, N.A.; Yusup, S.; Ahmad, M.M.; Mohamed, N.M.; Hameed, B.H. Activated Carbon from the Renewable Agricultural Residues Using Single Step Physical Activation: A Preliminary Analysis. APCBEE Procedia 2012, 3, 84–92. [Google Scholar] [CrossRef]
- Hidayu, A.R.; Mohamad, N.F.; Matali, S.; Sharifah, A.S.A.K. Characterization of Activated Carbon Prepared from Oil Palm Empty Fruit Bunch Using BET and FT-IR Techniques. Procedia Eng. 2013, 68, 379–384. [Google Scholar] [CrossRef]
- Schröder, E.; Thomauske, K.; Oechsler, B.; Herberger, S.; Baur, S.; Hornung, A. Activated Carbon from Waste Biomass. Prog. Biomass Bioenergy Prod. 2011, 333–356. [Google Scholar]
- Shamsuddin, S.M.; Yusoff, N.R.N.; Sulaiman, M.A. Synthesis and Characterization of Activated Carbon Produced from Kenaf Core Fiber Using H3PO4 Activation. Procedia Chem. 2016, 19, 558–565. [Google Scholar] [CrossRef]
- Wyasu, G.; Gimba, C.E.; Agbaji, E.B.; Ndukwe, G.I. Thermo-gravimetry(TGA) and DSC of thermal analysis techniques in production of active carbon from lignocellulosic materials. Adv. Appl. Sci. Res. 2016, 7, 109–115. [Google Scholar]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology; Taylor & Francis: London, UK, 2012. [Google Scholar]
- Reshad, S.A.; Tiwari, P.; Goud, V.V. Thermo-chemical conversion of waste rubber seed shell to produce fuel and value-added chemicals. J. Energy Inst. 2018, 91, 940–950. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Kumar, A.; Jena, H.M. Preparation and characterization of high surface area activated carbon from Fox nut (Euryale ferox) shell by chemical activation with H3PO4. Results Phys. 2016, 6, 651–658. [Google Scholar] [CrossRef]
- Daud, W.W.M.A.; Ali, W.S.W.; Sulaiman, M.Z. The effects of carbonization temperature on pore development in palm-shell-based activated carbon. Carbon 2000, 38, 1925–1932. [Google Scholar] [CrossRef]
- Tan, Z.; Zou, J.; Zhang, L.; Huang, Q. Morphology, pore size distribution, and nutrient characteristics in biochars under different pyrolysis temperatures and atmospheres. J. Mater. Cycles Waste Manag. 2018, 20, 1036–1049. [Google Scholar] [CrossRef]
- Lee, J.S.; Wang, X.; Luo, H.; Dai, S. Fluidic Carbon Precursors for Formation of Functional Carbon under Ambient Pressure Based on Ionic Liquids. Adv. Mater. 2010, 22, 1004–1007. [Google Scholar] [CrossRef]
- Rashidi, A.N.; Yusup, S.; Borhan, A. Isotherm and Thermodynamic Analysis of Carbon Dioxide on Activated Carbon. Procedia Eng. 2016, 148, 630–637. [Google Scholar] [CrossRef]
- Alabadi, A.; Razzaque, S.; Yang, Y.; Chen, S.; Tan, B. Highly porous activated carbon materials from carbonized biomass with high CO2 capturing capacity. Chem. Eng. J. 2015, 281, 606–612. [Google Scholar] [CrossRef]
- Guo, Y.; Tan, C.; Sun, J.; Li, W.; Zhang, J.; Zhao, C. Porous activated carbons derived from waste sugarcane bagasse for CO2 adsorption. Chem. Eng. J. 2020, 381, 122736. [Google Scholar] [CrossRef]
- Rahman, H.A.; Chin, S.X. Physical and Chemical Properties of the Rice Straw Activated Carbon Produced from Carbonization and KOH Activation Processes. Sains Malaysia. 2019, 48, 385–391. [Google Scholar]
- Tang, Y.-B.; Liu, Q.; Chen, F.-Y. Preparation and characterization of activated carbon from waste ramulus mori. Chem. Eng. J. 2012, 203, 19–24. [Google Scholar] [CrossRef]
- Rashidi, A.N.; Suzana, Y.; Borhan, A. Novel Low-Cost Activated Carbon from Coconut Shell and Its Adsorptive Characteristics for Carbon Dioxide. Key Eng. Mater. 2013, 594, 240–244. [Google Scholar] [CrossRef]
- Yu, C.-H.; Huang, C.-H.; Tan, C.-S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Krishnaiah, D.B.A.; Anisuzzaman, S.M.; Joseph, C.; Khee, T.B. Carbon Dioxide Removal by Adsorption. J. Appl. Sci. 2014, 14, 3142–3148. [Google Scholar] [CrossRef][Green Version]
- Younas, M.; Sohail, M.; Leong, L.K.; Bashir, M.J.; Sumathi, S. Feasibility of CO2 adsorption by solid adsorbents: A review on low-temperature systems. Int. J. Environ. Sci. Technol. 2016, 13, 1839–1860. [Google Scholar] [CrossRef]
- Song, G.; Zhu, X.; Chen, R.; Liao, Q.; Ding, Y.D.; Chen, L. An investigation of CO2 adsorption kinetics on porous magnesium oxide. Chem. Eng. J. 2016, 283, 175–183. [Google Scholar] [CrossRef]
- Khalili, S.; Khoshandam, B.; Jahanshahi, M. Optimization of production conditions for synthesis of chemically activated carbon produced from pine cone using response surface methodology for CO2 adsorption. RSC Adv. 2015, 5, 94115–94129. [Google Scholar] [CrossRef]
- Borhan, A.; Thangamuthu, S.; Taha, M.F.; Ramdan, A.N. Development of activated carbon derived from banana peel for CO2 removal. AIP Conf. 2015, 1674, 020002. [Google Scholar]
- Hao, W.; Björkman, E.; Lilliestråle, M.; Hedin, N. Activated carbons prepared from hydrothermally carbonized waste biomass used as adsorbents for CO2. Appl. Energy 2013, 112, 526–532. [Google Scholar] [CrossRef]
- Yang, H.; Gong, M.; Chen, Y. Preparation of activated carbons and their adsorption properties for greenhouse gases: CH4 and CO2. J. Nat. Gas Chem. 2011, 20, 460–464. [Google Scholar] [CrossRef]
- Caglayan, S.B.; Aksoylu, A.E. CO2 adsorption on chemically modified activated carbon. J. Hazard. Mater. 2013, 252, 19–28. [Google Scholar] [CrossRef]
- Nugent, P.; Belmabkhout, Y.; Burd, S.D.; Cairns, A.J.; Luebke, R.; Forrest, K.; Pham, T.; Ma, S.; Space, B.; Wojtas, L. Porous Materials with Optimal Adsorption Thermodynamics and Kinetics for CO2 Separation. Nature 2013, 495, 80–84. [Google Scholar] [CrossRef]
- Ma, X.; Li, L.; Chen, R.; Wang, C.; Li, H.; Wang, S. Heteroatom-doped nanoporous carbon derived from MOF-5 for CO2 capture. Appl. Surf. Sci. 2018, 435, 494–502. [Google Scholar] [CrossRef]







| Analysis | Raw RSS (%) |
|---|---|
| Proximate | |
| Moisture | 13.9 |
| Volatile Matter | 72.3 |
| Fixed Carbon | 13.6 |
| Ash | 0.2 |
| Ultimate | |
| Carbon | 49.5 |
| Hydrogen | 6.4 |
| Nitrogen | 0.4 |
| Sulfur | 0.3 |
| Oxygen a | 43.4 |
| Samples | SBET (m2/g) | Smicro (m2/g) | Smeso (m2/g) | Vtotal (cm3/g) | Vmicro (cm3/g) | Vmeso (cm3/g) | Micropore Percentage (%) |
|---|---|---|---|---|---|---|---|
| Raw RSS | 19.66 | 17.76 | 1.90 | 0 | 0 | 0 | 90.3 |
| RSS-IL 500 | 107.55 | 88.81 | 18.74 | 0.049 | 0.045 | 0.003 | 82.6 |
| RSS-IL 600 | 355.93 | 261.98 | 93.95 | 0.187 | 0.134 | 0.053 | 73.6 |
| RSS-IL 700 | 357.85 | 301.87 | 55.98 | 0.189 | 0.156 | 0.033 | 84.4 |
| RSS-IL 800 | 393.99 | 337.09 | 56.90 | 0.206 | 0.172 | 0.034 | 85.6 |
| Sample Name | C | H | N | S | O a | C/H |
|---|---|---|---|---|---|---|
| Raw RSS | 49.55 | 6.40 | 0.41 | 0.30 | 43.4 | 7.74 |
| RSS-IL 500 | 77.37 | 3.32 | 0.85 | 0.23 | 18.24 | 23.27 |
| RSS-IL 600 | 81.70 | 2.94 | 0.88 | 0.17 | 14.31 | 27.77 |
| RSS-IL 700 | 84.47 | 2.30 | 0.90 | 0.13 | 12.20 | 36.70 |
| RSS-IL 800 | 85.47 | 1.86 | 1.14 | 0.11 | 11.42 | 45.86 |
| Model | Parameters | Temperature | ||
|---|---|---|---|---|
| 25 °C | 50 °C | 100 °C | ||
| Langmuir Isotherm | qmax (mmol/g) | 2.9542 | 2.2356 | 1.6812 |
| KL (1/bar) | 3.0773 | 1.7466 | 0.5874 | |
| R2 | 0.9904 | 0.9886 | 0.9945 | |
| Freundlich Isotherm | n | 1.8484 | 1.5778 | 1.2309 |
| KF (mmol/g.bar) | 2.3933 | 1.5028 | 1.5517 | |
| R2 | 0.9867 | 0.9920 | 0.9971 | |
| Temkin Isotherm | B | 0.5864 | 0.4347 | 0.5818 |
| KT (mmol/g.bar) | 40.8995 | 23.1806 | 12.9017 | |
| R2 | 0.9784 | 0.9651 | 0.9293 | |
| Adsorbent | Treatment Method | CO2 Adsorption Capacity (mmol/g) | Reference |
|---|---|---|---|
| Banana peel AC | Chemical/KOH | 1.10 | [60] |
| RSS AC | Chemical/KOH | 1.24 | [34] |
| Grass biomass AC | Physical/hydrothermal | 1.45 | [61] |
| Coconut shell AC | Chemical/H3PO4 | 1.70 | [62] |
| Norit® SX2 (commercial AC) | Physical/steam | 1.88 | [33] |
| Norit ROX (commercial AC) | Chemical/Na2CO3 (air oxidation) | 2.02 | [63] |
| Palm Kernel Shell AC | Physical/CO2 | 2.13 | [33] |
| RSS AC | Chemical/malic acid | 2.26 | [17] |
| Zeolite 13X | Physical/hydrothermal | 2.42 | [64] |
| MOF-5 | Chemical/DMF | 2.43 | [65] |
| RSS AC (RSS-IL 800) | Chemical/[C4Py][Tf2N] IL | 2.44 | This study |
| Rice husk AC | Physical/CO2 | 3.10 | [3] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokti, N.; Borhan, A.; Zaine, S.N.A.; Mohd Zaid, H.F. Development of Rubber Seed Shell–Activated Carbon Using Impregnated Pyridinium-Based Ionic Liquid for Enhanced CO2 Adsorption. Processes 2021, 9, 1161. https://doi.org/10.3390/pr9071161
Mokti N, Borhan A, Zaine SNA, Mohd Zaid HF. Development of Rubber Seed Shell–Activated Carbon Using Impregnated Pyridinium-Based Ionic Liquid for Enhanced CO2 Adsorption. Processes. 2021; 9(7):1161. https://doi.org/10.3390/pr9071161
Chicago/Turabian StyleMokti, Nawwarah, Azry Borhan, Siti Nur Azella Zaine, and Hayyiratul Fatimah Mohd Zaid. 2021. "Development of Rubber Seed Shell–Activated Carbon Using Impregnated Pyridinium-Based Ionic Liquid for Enhanced CO2 Adsorption" Processes 9, no. 7: 1161. https://doi.org/10.3390/pr9071161
APA StyleMokti, N., Borhan, A., Zaine, S. N. A., & Mohd Zaid, H. F. (2021). Development of Rubber Seed Shell–Activated Carbon Using Impregnated Pyridinium-Based Ionic Liquid for Enhanced CO2 Adsorption. Processes, 9(7), 1161. https://doi.org/10.3390/pr9071161