Simultaneously Tuning Charge Separation and Surface Reaction Kinetics on ZnIn2S4 Photoanode by P-Doping for Highly Efficient Photoelectrochemical Water Splitting and Urea Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Instruments
2.2. Materials Preparation
2.2.1. Preparation of ZIS Films
2.2.2. Preparation of ZIS-Px
2.3. Structural Characterization
2.4. Electrochemical Measurements
2.5. Photoelectrochemical (PEC) Measurements
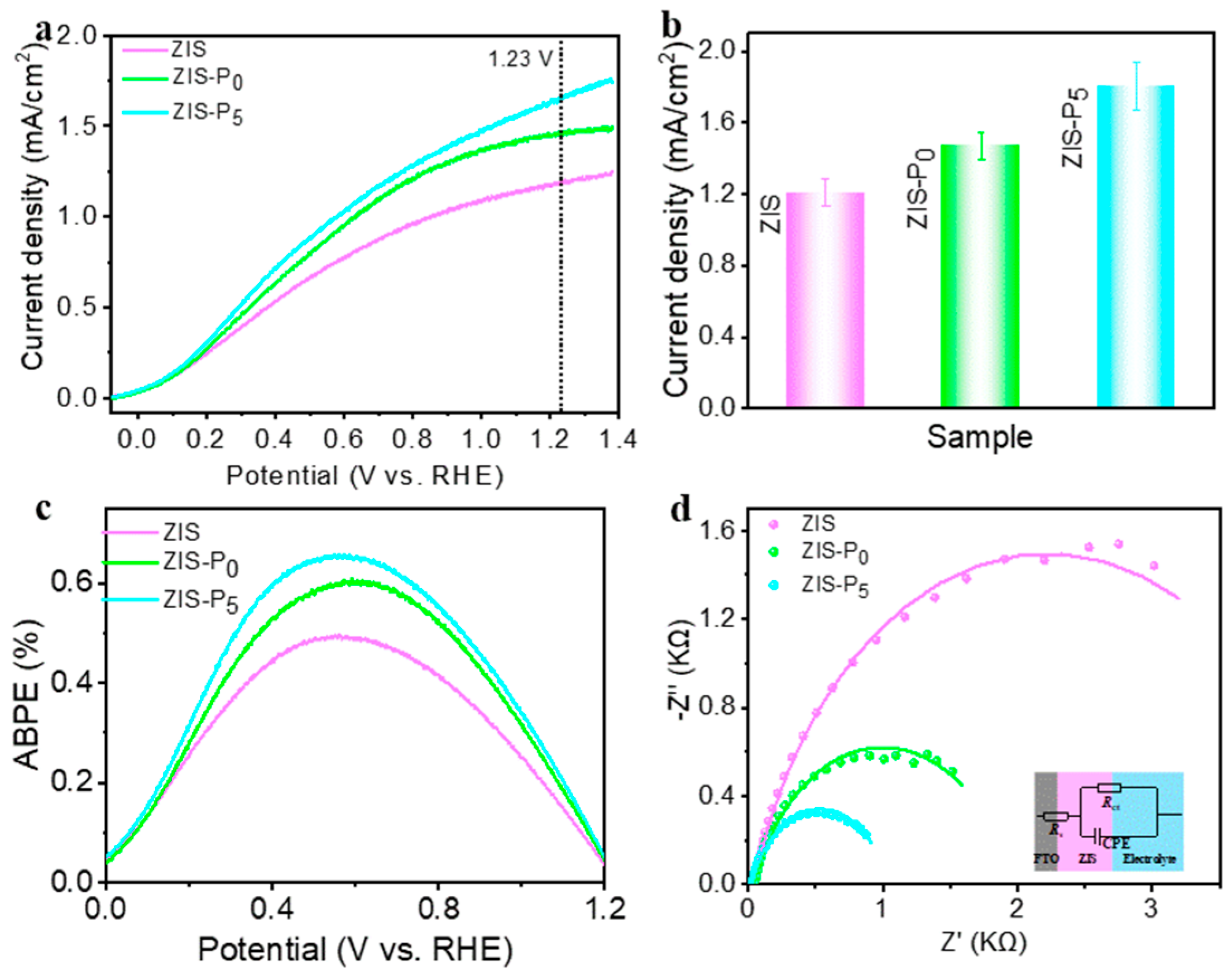
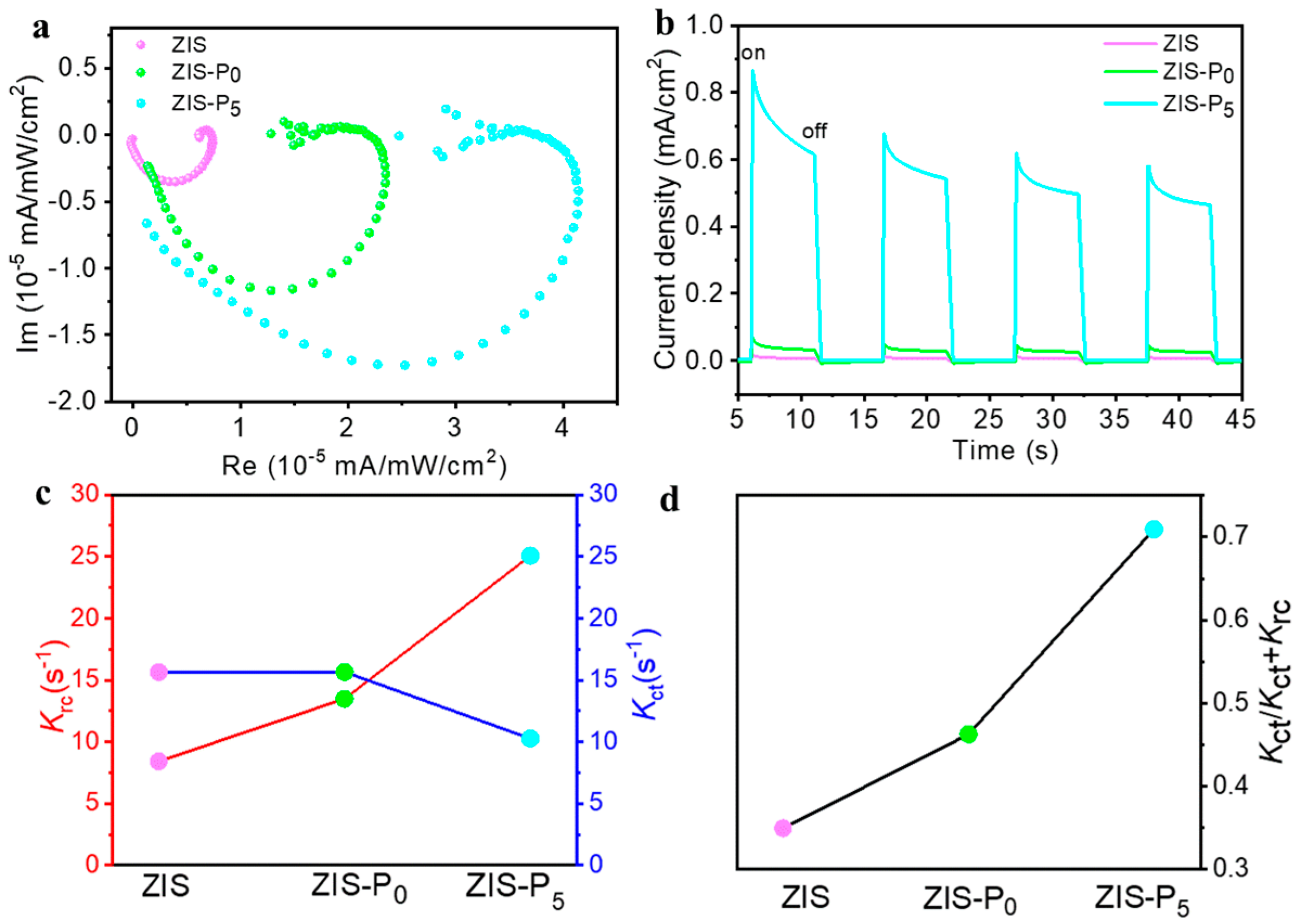
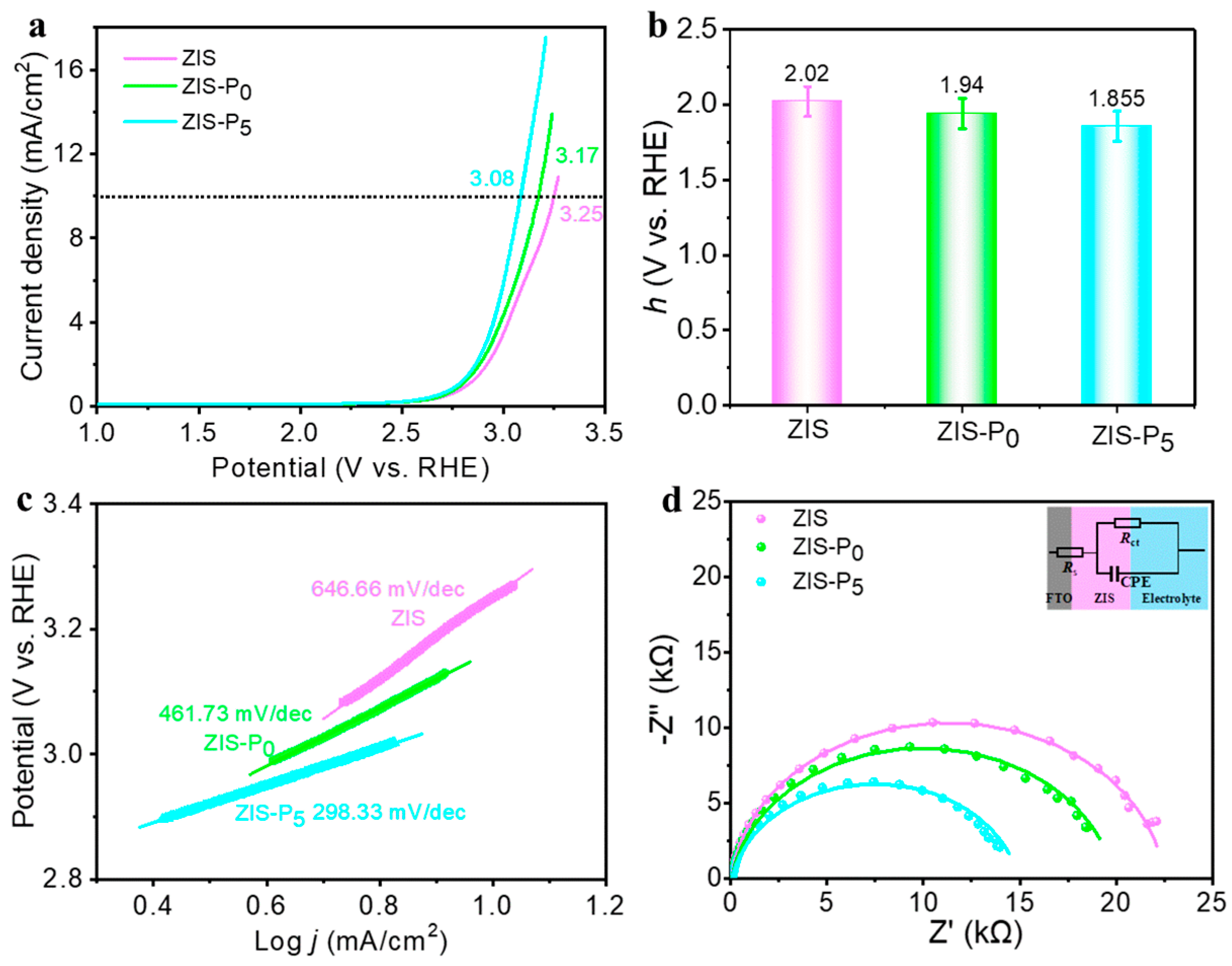
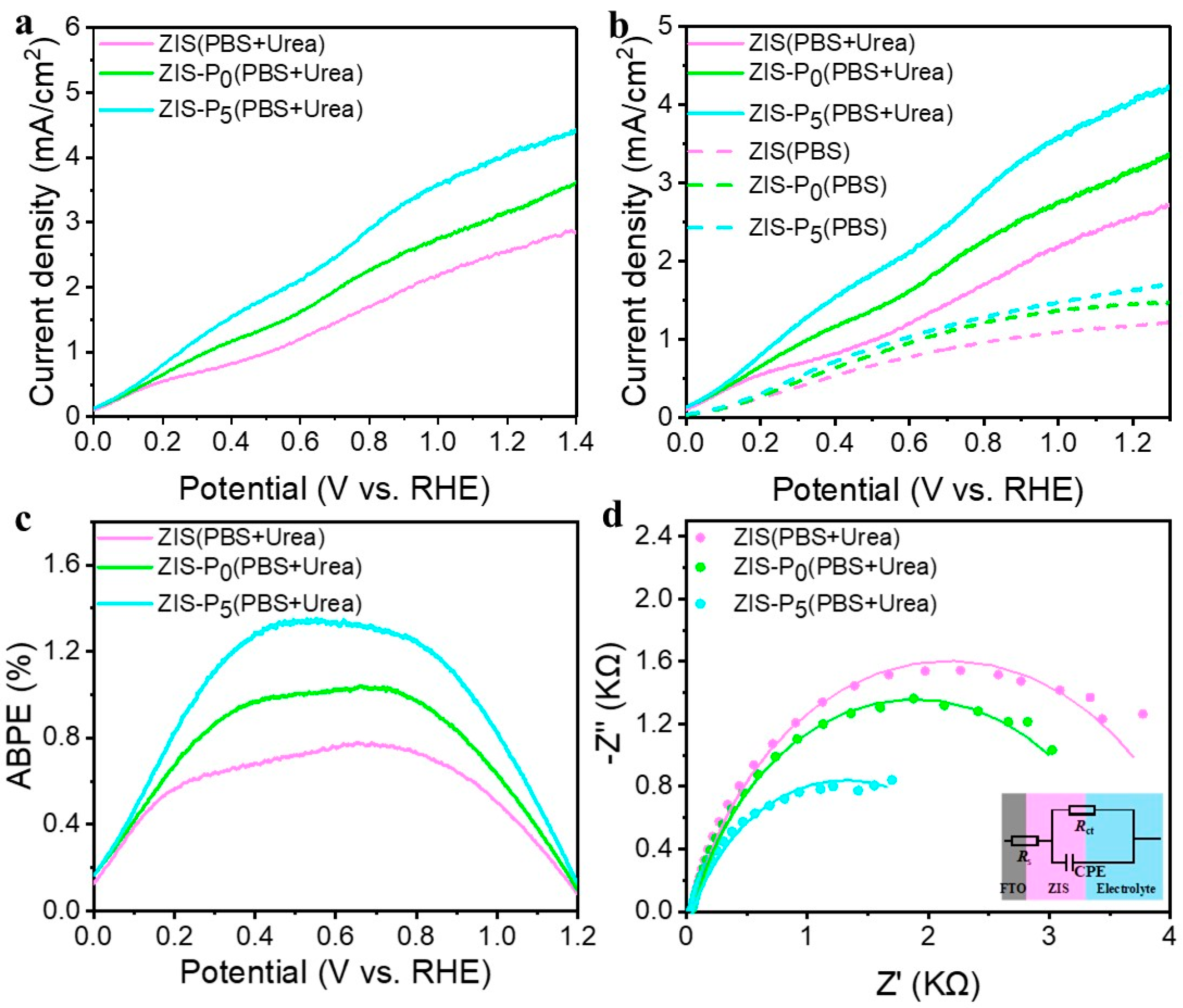
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Tan, M.; Ma, Y.; Yu, C.; Luan, Q.; Li, J.; Liu, C.; Dong, W.; Su, Y.; Qiao, L.; Gao, L.; et al. Boosting photocatalytic hydrogen production via interfacial engineering on 2D ultrathin Z-scheme ZnIn2S4/g-C3N4 heterojunction. Adv. Funct. Mater. 2022, 32, 2111740. [Google Scholar] [CrossRef]
- Han, J.; Liu, Z.; Guo, K.; Wang, B.; Zhang, X.; Hong, T. High-efficiency photoelectrochemical electrodes based on ZnIn2S4 sensitized ZnO nanotube arrays. Appl. Catal. B Environ. 2015, 163, 179–188. [Google Scholar] [CrossRef]
- Zhang, S.; Du, P.; Xiao, H.; Wang, Z.; Zhang, R.; Luo, W.; An, J.; Gao, Y.; Lu, B.Z. Fast Interfacial Carrier Dynamics Modulated by Bidirectional Charge Transport Channels in ZnIn2S4-based Composite Photoanodes Probed by Scanning Photoelectrochemical Microscopy. Angew. Chem. Int. Ed. 2024, 63, e202315763. [Google Scholar] [CrossRef]
- Lee, D.; Wang, W.; Zhou, C.; Tong, X.; Liu, M.; Galli, G.; Choi, K.S. The impact of surface composition on the interfacial energetics and photoelectrochemical properties of BiVO4. Nat. Energy 2021, 6, 287–294. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Tan, R.; Sivanantham, A.; Rani, B.J.; Jeong, Y.J.; Cho, I.S. Recent advances in surface regulation and engineering strategies of photoelectrodes toward enhanced photoelectrochemical water splitting. Coord. Chem. Rev. 2023, 494, 215362. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, W.; Bi, Y.; Liang, Y.; Fu, J.; Wang, L.; Zhou, Q. Accelerating Surface Reaction Kinetics and Enhancing Bulk as well as Surface Charge Carrier Dynamics in Al/Al2O3-Coated BiVO4 Photoanode. Adv. Funct. Mater. 2024, 2403396. [Google Scholar] [CrossRef]
- Bai, Z.; Yan, X.; Kang, Z.; Hu, Y.; Zhang, X.; Zhang, Y. Photoelectrochemical performance enhancement of ZnO photoanodes from ZnIn2S4 nanosheets coating. Nano Energy 2015, 14, 392–400. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, X.; Li, H.; Sun, Z.; Cao, M.; Li, Z.; Fang, C.; Zhou, J.; Cao, C.; Dong, J. A semiconductor-electrocatalyst nano interface constructed for successive photoelectrochemical water oxidation. Nat. Commun. 2023, 14, 2574. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, F.A.; Oener, S.Z.; Nellist, M.R.; Gordon, A.M.; Bain, D.C.; Fehrs, J.L.; Boettcher, S.W. Nanoscale semiconductor/catalyst interfaces in photoelectrochemistry. Nat. Mater. 2020, 19, 69–76. [Google Scholar] [CrossRef]
- Gao, R.T.; Zhang, J.; Nakajima, T.; He, J.; Liu, X.; Zhang, X.; Wang, L.; Wu, L. Single-atomic-site platinum steers photogenerated charge carrier lifetime of hematite nanoflakes for photoelectrochemical water splitting. Nat. Commun. 2023, 14, 2640. [Google Scholar] [CrossRef]
- Ma, Z.; Song, K.; Wang, L.; Gao, F.; Tang, B.; Hou, H.; Yang, W. WO3/BiVO4 type-II heterojunction arrays decorated with oxygen-deficient ZnO passivation layer: A highly efficient and stable photoanode. Am. Chem. Soc. Appl. Mater. Interfaces 2018, 11, 889–897. [Google Scholar] [CrossRef]
- Cui, C.; Heggen, M.; Zabka, W.D.; Cui, W.; Osterwalder, J.; Probst, B.; Alberto, R. Atomically dispersed hybrid nickel-iridium sites for photoelectrocatalysis. Nat. Commun. 2017, 8, 1341. [Google Scholar] [CrossRef]
- He, D.; Song, X.; Ke, Z.; Xiao, X.; Jiang, C. Construct Fe2+ species and Au particles for significantly enhanced photoelectrochemical performance of α-Fe2O3 by ion implantation. Sci. China Mater. 2017, 61, 878–886. [Google Scholar] [CrossRef]
- Wang, L.; Nguyen, N.T.; Huang, X.; Schmuki, P.; Bi, Y. Hematite photoanodes: Synergetic enhancement of light harvesting and charge management by sandwiched with Fe2TiO5/Fe2O3/Pt structures. Adv. Funct. Mater. 2017, 27, 1703527. [Google Scholar] [CrossRef]
- Shao, C.; Chen, R.; Zhao, Y.; Li, Z.; Zong, X.; Li, C. Reducing the surface defects of Ta3N5 photoanode towards enhanced photoelectrochemical water oxidation. J. Mater. Chem. A 2020, 8, 23274–23283. [Google Scholar] [CrossRef]
- Fu, J.; Fan, Z.; Nakabayashi, M.; Ju, H.; Pastukhova, N.; Xiao, Y.; Feng, C.; Shibata, N.; Domen, K.; Li, Y. Interface engineering of Ta3N5 thin film photoanode for highly efficient photoelectrochemical water splitting. Nat. Commun. 2022, 13, 729. [Google Scholar] [CrossRef]
- Ning, X.; Lu, B.; Zhang, Z.; Du, P.; Ren, H.; Shan, D.; Chen, J.; Gao, Y.; Lu, X. An efficient strategy for boosting photogenerated charge separation by using porphyrins as interfacial charge mediators. Angew. Chem. Int. Ed. 2019, 58, 16800–16805. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.; Wang, J.; Hai, K.; Ning, X.; Chen, X. Modulation of charge-transfer behavior via adaptive interface treatment for efficient photoelectrochemical water splitting. J. Mater. Chem. A 2024, 12, 6405–6411. [Google Scholar] [CrossRef]
- Yin, D.; Ning, X.; Zhang, Q.; Du, P.; Lu, X. Dual modification of BiVO4 photoanode for synergistically boosting photoelectrochemical water splitting. J. Colloid Interface Sci. 2023, 646, 238–244. [Google Scholar] [CrossRef]
- Xu, L.; Quan, J.; Xu, L.; Li, M.; Li, C.; Mujtaba, S.; Ning, X.; Chen, P.; Weng, Q.; An, Z.; et al. Modulating Interfacial Charge Transfer Behavior through the Construction of a Hetero-Interface for Efficient Photoelectrochemical Water Splitting. Separations 2024, 11, 109. [Google Scholar] [CrossRef]
- Li, M.; Saqib, M.; Xu, L.; Li, C.; Quan, J.; Ning, X.; Chen, P.; Weng, Q.; An, Z.; Chen, X. Engineering the transition metal hydroxide–photoanode interface with a highly crystalline mediator for efficient photoelectrochemical water splitting. J. Mater. Chem. A 2024, 12, 19259–19267. [Google Scholar] [CrossRef]
- Xin, X.; Li, Y.; Zhang, Y.; Wang, Y.; Chi, X.; Wei, Y.; Diao, C.; Wang, R.; Guo, P.; Yu, J. Large electronegativity differences between adjacent atomic sites activate and stabilize ZnIn2S4 for efficient photocatalytic overall water splitting. Nat. Commun. 2024, 15, 337. [Google Scholar] [CrossRef]
- Su, T.; Men, C.; Chen, L.; Chu, B.; Luo, X.; Ji, H.; Chen, J.; Qin, Z. Sulfur vacancy and Ti3C2Tx cocatalyst synergistically boosting interfacial charge transfer in 2D/2D Ti3C2Tx/ZnIn2S4 heterostructure for enhanced photocatalytic hydrogen evolution. Adv. Sci. 2022, 9, 2103715. [Google Scholar] [CrossRef]
- Xu, W.; Gao, W.; Meng, L.; Tian, W.; Li, L. Incorporation of sulfate anions and sulfur vacancies in ZnIn2S4 photoanode for enhanced photoelectrochemical water splitting. Adv. Energy Mater. 2021, 11, 2101181. [Google Scholar] [CrossRef]
- Li, S.; Meng, L.; Tian, W.; Li, L. Engineering interfacial band bending over ZnIn2S4/SnS2 by interface chemical bond for efficient solar-driven photoelectrochemical water splitting. Adv. Energy Mater. 2022, 12, 2200629. [Google Scholar] [CrossRef]
- Khaselev, O.; Turner, J.A. A monolithic photovoltaic-photoelectrochemical device for hydrogen production via water splitting. Science 1998, 280, 425–427. [Google Scholar] [CrossRef]
- Yan, T.; Wu, T.; Wei, S.; Wang, H.; Sun, M.; Yan, L.; Wei, Q.; Ju, H. Photoelectrochemical competitive immunosensor for 17β-estradiol detection based on ZnIn2S4@ NH2- MIL-125 (Ti) amplified by PDA NS/Mn: ZnCdS. Biosens. Bioelectron. 2020, 148, 111739. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, H.; Lu, J.; Feng, Y.; Meng, F.; Ma, C.; Yan, Y.; Meng, M. Synergy between van der waals heterojunction and vacancy in ZnIn2S4/g-C3N4 2D/2D photocatalysts for enhanced photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 277, 119254. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Z.; Hu, Y.H. Bimetallic cocatalysts for photocatalytic hydrogen production from water. Chem. Eng. J. 2021, 409, 128250. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Tian, W.; Meng, A.; Yang, L. CoNi bimetal cocatalyst modifying a hierarchical ZnIn2S4 nanosheet-based microsphere noble-metal-free photocatalyst for efficient visible-light-driven photocatalytic hydrogen production. Am. Chem. Soc. Sustain. Chem. Eng. 2019, 7, 20190–20201. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, J.; Liao, R.; Deng, W.; Tan, Y. Oxygen-doped ZnIn2S4 as a highly active and etchable photoactive material for chlorpyrifos detection. J. Electroanal. Chem. 2024, 960, 118211. [Google Scholar] [CrossRef]
- Liu, S.; Wu, L.; Zhao, L. A photoelectrochemical platform constructed with C-doped ZnIn2S4/Bi2S3: Excellent for the visual detection of tumor marker. Electrochim. Acta 2024, 491, 144328. [Google Scholar] [CrossRef]
- Shi, X.; Dai, C.; Wang, X.; Yang, P.; Zheng, L.; Zhao, Z.; Zheng, H. Facile construction TiO2/ZnIn2S4/Zn0.4Ca0.6In2S4 ternary hetero-structure photo-anode with enhanced photo-electrochemical water-splitting performance. Surf. Interfaces 2021, 26, 101323. [Google Scholar] [CrossRef]
- Yang, H.Y.; Wei, J.J.; Zheng, J.Y.; Ai, Q.Y.; Wang, A.J.; Feng, J.J. Integration of CuS/ZnIn2S4 flower-like heterojunctions and (MnCo) Fe2O4 nanozyme for signal amplification and their application to ultrasensitive PEC aptasensing of cancer biomarker. Talanta 2023, 260, 124631. [Google Scholar] [CrossRef]
- Chen, J.; Li, K.; Cai, X.; Zhao, Y.; Gu, X.; Mao, L. Sulfur vacancy-rich ZnIn2S4 nanosheet arrays for visible-light-driven water splitting. Mater. Sci. Semicond. Process. 2022, 143, 106547. [Google Scholar] [CrossRef]
- Yang, R.; Mei, L.; Fan, Y.; Zhang, Q.; Zhu, R.; Amal, R.; Yin, Z.; Zeng, Z. ZnIn2S4-based photocatalysts for energy and environmental applications. Small Methods 2021, 5, 2100887. [Google Scholar] [CrossRef]
- Huang, Y.; He, J.; Xu, W.; Liu, T.; Chen, R.; Meng, L.; Li, L. Converting undesirable defects into activity sites enhances the photoelectrochemical performance of the ZnIn2S4 photoanode. Adv. Energy Mater. 2024, 14, 2304376. [Google Scholar] [CrossRef]
- Liu, W.; Chen, J.; Pan, X.; Wang, T.; Li, Y. Ultrathin Nickel-doped ZnIn2S4 Nanosheets with Sulfur Vacancies for Efficient Photocatalytic Hydrogen Evolution. ChemCatChem 2021, 13, 5148–5155. [Google Scholar] [CrossRef]
- Peng, H.; Yang, H.; Han, J.; Liu, X.; Su, D.; Yang, T.; Liu, S.; Pao, C.-W.; Hu, Z.; Zhang, Q.; et al. Defective ZnIn2S4 nanosheets for visible-light and sacrificial-agent-free H2O2 photosynthesis via O2/H2O redox. J. Am. Chem. Soc. 2023, 145, 27757–27766. [Google Scholar] [CrossRef]
- Jia, W.L.; Wu, X.; Liu, Y.; Zhao, J.Y.; Zhang, Y.; Liu, P.F.; Cheng, Q.; Yang, H.G. Porous ZnIn2S4 with confined sulfur vacancies for highly efficient visible-light-driven photocatalytic H2 production. J. Mater. Chem. A 2022, 10, 25586–25594. [Google Scholar] [CrossRef]
- Kang, J.; Yoon, K.Y.; Lee, J.E.; Park, J.; Chaule, S.; Jang, J.H. Meso-pore generating P doing for efficient photoelectrochemical water splitting. Nano Energy 2023, 107, 108090. [Google Scholar] [CrossRef]
- Zhu, C.; Zuo, M.; Yang, Y.; Zhao, N.N.; Wang, X.; Cui, L.; Zhang, C.Y. Construction of a dual-mode biosensor with ferrocene as both a signal enhancer and a signal tracer for electrochemiluminescent and electrochemical enantioselective recognition. Anal. Chem. 2023, 95, 17920–17927. [Google Scholar] [CrossRef]
- Yu, F.; Li, F.; Yao, T.; Du, J.; Liang, Y.; Wang, Y.; Han, H.; Sun, L. Fabrication and kinetic study of a ferrihydrite-modified BiVO4 photoanode. Am. Chem. Soc. Catal. 2017, 7, 1868–1874. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, Z.; Song, Q.; Li, X.; Chen, B.; Liu, Z. Hybrid 0D/2D edamame shaped ZnIn2S4 photoanode modified by Co-Pi and Pt for charge management towards efficient photoelectrochemical water splitting. Appl. Catal. B Environ. 2019, 244, 188–196. [Google Scholar] [CrossRef]
- Qian, H.; Liu, Z.; Guo, Z.; Ruan, M.; Ma, J. Hexagonal phase/cubic phase homogeneous ZnIn2S4 nn junction photoanode for efficient photoelectrochemical water splitting. J. Alloys Compd. 2020, 830, 154639. [Google Scholar] [CrossRef]
- Qian, H.; Liu, Z.; Ya, J.; Xin, Y.; Ma, J.; Wu, X. Construction homojunction and co-catalyst in ZnIn2S4 photoelectrode by Co ion doping for efficient photoelectrochemical water splitting. J. Alloys Compd. 2021, 867, 159028. [Google Scholar] [CrossRef]
- Hao, Z.; Wang, R.; Zhang, L.; Sheng, H.; Li, Y.; Dong, B.; Cao, L. More comprehensive heterojunction mechanism: Enhanced PEC properties originated from novel ZnIn2S4/Cu2S heterojunction assisted by changed surface states. Chem. Eng. J. 2023, 468, 143568. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Tang, L.; Li, C.; Quan, J.; Xu, L.; Ning, X.; Chen, P.; Weng, Q.; An, Z.; Chen, X. Simultaneously Tuning Charge Separation and Surface Reaction Kinetics on ZnIn2S4 Photoanode by P-Doping for Highly Efficient Photoelectrochemical Water Splitting and Urea Oxidation. Separations 2024, 11, 268. https://doi.org/10.3390/separations11090268
Sun J, Tang L, Li C, Quan J, Xu L, Ning X, Chen P, Weng Q, An Z, Chen X. Simultaneously Tuning Charge Separation and Surface Reaction Kinetics on ZnIn2S4 Photoanode by P-Doping for Highly Efficient Photoelectrochemical Water Splitting and Urea Oxidation. Separations. 2024; 11(9):268. https://doi.org/10.3390/separations11090268
Chicago/Turabian StyleSun, Jiamin, Ling Tang, Chenglong Li, Jingjing Quan, Li Xu, Xingming Ning, Pei Chen, Qiang Weng, Zhongwei An, and Xinbing Chen. 2024. "Simultaneously Tuning Charge Separation and Surface Reaction Kinetics on ZnIn2S4 Photoanode by P-Doping for Highly Efficient Photoelectrochemical Water Splitting and Urea Oxidation" Separations 11, no. 9: 268. https://doi.org/10.3390/separations11090268
APA StyleSun, J., Tang, L., Li, C., Quan, J., Xu, L., Ning, X., Chen, P., Weng, Q., An, Z., & Chen, X. (2024). Simultaneously Tuning Charge Separation and Surface Reaction Kinetics on ZnIn2S4 Photoanode by P-Doping for Highly Efficient Photoelectrochemical Water Splitting and Urea Oxidation. Separations, 11(9), 268. https://doi.org/10.3390/separations11090268






