Abstract
Soybean, maize, and bean are crops of great economic importance, but in recent years have suffered with infestations of the caterpillar Helicoverpa armigera, with the main reason being the resistance of this pest to most pesticides. Avermectin emamectin benzoate was recently released to control this pest. Other avermectins, like abamectin, doramectin, eprinomectin, and ivermectin are used in large scale because they potent acaricidal, anthelmintic, and insecticidal activities. Thus, a simple and fast method for the determination of avermectins in these crops based on a quick, easy, cheap, effective, rugged, and safe (QuEChERS) extraction procedure and ultra-high performance liquid chromatography with tandem mass spectrometry (UHPLC-MS/MS) analysis was developed and validated. For extraction, water followed by acetonitrile:isopropanol and a partition step with salts was stablished. With the clean-up step using activated EMR-Lipid, limits of detection of 1.2 μg kg−1 for abamectin, doramectin, emamectin benzoate, and ivermectin, and of 2.4 μg kg−1 for eprinomectin were achieved. The validation showed satisfactory results and the method was successfully applied to commercial samples, indicating that it is suitable for routine analysis.
1. Introduction
Soybean (Glycine max) is among the most important crops in the world and their demand increases every year, as long as soybean may be applied for animal feed and biodiesel production to raw material for cosmetics [1]. Maize (Zea mays) also has its economic importance related to various forms of use, from animal feed to high technology industry. Common bean (Phaseolus vulgaris), an oleaginous crop such as soybean, is an important source of protein.
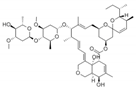
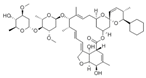
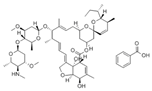
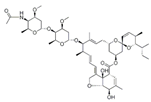
Avermectins are a family of natural products with a large macrocyclic lactone ring consisting of four major components (A1a, A2a, B1a, and B2a) and four minor components (A1b, A2b, B1b, and B2b) isolated from the fermentation broth of Streptomyces avermectinius. Abamectin, doramectin, emamectin benzoate, eprinomectin, and ivermectin are avermectins used in a large scale because they present potent acaricidal, anthelmintic, and insecticidal activities [2,3]. As an example, abamectin is a blend of avermectin B1a (≥80%) and B1b (≤20%), and emamectin benzoate, a novel avermectin derivative developed as a pesticide, is a mixture of B1a (≥90%) and emamectin B1b (≤10%).
In 2013, the first attacks of Helicoverpa armigera were reported on soybean and bean crops [4]. This caterpillar affects several crops, such as soybean, bean, cotton, chickpea, tomato, eggplant, canola, and sunflower. In general, this pest is controlled by the use of insecticides. However, due to extensive use of pesticides, the caterpillar developed resistance against them, causing great damages in the crops [5]. Thus, a phytosanitary emergency was declared in Brazil, and the use of emamectin benzoate was released on an emergency basis. From the class of avermectins, only the use of abamectin as a pesticide was initially allowed in soybean, bean, and maize crops [6]. The need for using emamectin benzoate led to the inclusion of this compound in the list of priority analysis pesticide for registration being officially authorized in Brazil in 2017 for use in soybean, bean, maize, and cotton.
The maximum residue limits (MRL) values for abamectin and emamectin benzoate in soybean, maize, and bean established in Brazil, European Union, USA, and by the Codex Alimentarius are, in general, in the range of 5 to 15 µg kg−1, with the exception of abamectin in beans (80 µg kg−1) established by the Codex Alimentarius.
In recent years, ultra-high performance liquid chromatography coupled with mass spectrometry has been a preferred technique for the determination of pesticides and veterinary drugs in food samples [7,8]. However, the determination in complex matrices with high fat, sugar, and protein content requires special attention in the sample preparation stage, in order to obtain extracts with low concentration of interferents and containing the analytes of interest [9].
As extraction techniques for determination of pesticide residues in cereals and oleaginous crops we can mention the QuEChERS method, which stands for quick, easy, cheap, effective, rugged, and safe [10] and the matrix solid-phase dispersion (MSPD) [11]. Huang et al. [12] analyzed abamectin and ivermectin in olive, soybean, maize, and peanut oils and in lard after liquid–liquid extraction (LLE). Macedo et al. [13] analyzed abamectin, doramectin, and ivermectin in butter using LLE.
López-Blanco et al. [14] used a modified QuEChERS method for the multiresidue analysis of abamectin and other pesticides in avocado and olive oil. Several sorbents were tested for the clean-up step. Du et al. [15] established a QuEChERS method with a clean-up step using primary secondary amine (PSA) to analyze residues of avermectins, pyriproxyfen, and diflubenzuron in mushrooms. Liu et al. [16] developed a QuEChERS method for multiresidue determination of pesticides, including abamectin, in grains. Wang et al. [17] analyzed abamectin, emamectin benzoate, and other pesticides in soybean applying a modified QuEChERS acetate method. Thus, giving the importance of avermectins residue analysis in soybean, bean, and maize crops, the aim of this work was to develop a suitable method for determination of residues of the avermectins abamectin, doramectin, emamectin benzoate, eprinomectin, and ivermectin in soybean, maize, and bean crops using the same sample preparation step in order to simplify the execution in routine analyses. To the best of our knowledge, there are no methods available in the literature for the determination of avermectins residues in more than one cereal or legumes matrix.
2. Materials and Methods
2.1. Standards, Chemicals, and Materials
Analytical standards of abamectin, doramectin, emamectin benzoate, eprinomectin, and ivermectin B1a with purities from 95.0 to 98.3% were from Dr. Ehrenstorfer (Augsburg, Germany). Sulfadimetoxina-d6, used as surrogate standard (SS) and dimetridazol-d3 used as internal standard (IS) were purchased from Witega Laboratorien Berlin-Adlershof GmbH (Berlin, Germany). Individual stock standard solutions (1000 mg L−1) of the analytes were prepared in acetonitrile HPLC grade and from these, individual solutions at 10 mg L−1 were prepared. Methanol, acetonitrile, and magnesium sulfate were from JT Baker (Phillipsburg, NJ, USA). Isopropanol, ammonium formate, formic acid, trisodium citrate dihydrate, sodium hydrogencitrate sesquihydrate, and sodium chloride were from Sigma-Aldrich (St. Louis, MI, USA). Ultrapurified water (18 MΩ cm) was obtained from a Millipore Milli-Q® system (Molsheim, France). Nylon filters (0.22 µm), silica, Florisil®, Primary Secondary Amine (PSA), and EMR-Lipid were from Agilent Technologies (Santa Clara, CA, USA). The sorbents C18 and Z-Sep+ were from Supelco (Bellefonte, KS, USA).
For method development, soybean, bean, and maize blank samples were obtained from organic production and none of the evaluated pesticides were detected. Sample were processed for 1 min in a IKA A11 Basic (Staufen, Germany) analytical mill.
2.2. UHPLC-MS/MS Parameters
Analysis were performed in a UHPLC-MS/MS system from Waters (Milford, PA, USA) consisting in an Acquity UPLC™ binary pump liquid chromatograph with Xevo TQTM MS/MS triple quadrupole detector, autosampler, and column temperature controller. The QuanpediaTM library of UHPLC-MS/MS was used to select the respective precursor and product ions, as well as energies applied to cone and collision. The mobile phase A, with 10 mmol L−1 of aqueous ammonium formate, was maintained in all tests. Two organic mobile phase B were evaluated: methanol 1% (v/v) formic acid and acetonitrile 0.1% (v/v) formic acid. Three chromatographic columns were tested: Acquity UPLC™ BEH C18 (50 × 2.1 mm, 1.7 μm) and Acquity UPLC™ HSST3 (100 × 2.1 mm i.d., 1.8 μm) from Waters (Wexford, Ireland), and Zorbax Eclipse Plus® C18 (100 × 2.1 mm, 1.8 μm) from Agilent Technologies (Santa Clara, CA, USA). The mobile phase operated in gradient mode, started at 50% B and remained constant for 1 min, increasing for 2 min to reach 80% B and then increases to 100% B, remaining for 0.5 min, and returning to 50% B maintaining constant until the end of the analysis. The flow rate was set at 0.225 mL min−1 and the injection volume at 10 μL. Electrospray ionization in positive mode (ESI+) was used for all compounds and precursor-product ion transitions were monitored in selected reaction monitoring (SRM) mode. The transition with the highest intensity was selected for quantification, and the transition with the second highest intensity was used for identification. The following conditions were used for the ESI source: desolvation and cone gas flow rate were set at 500 and 60 L N2 h−1, respectively; the capillary voltage at 2.5 V; desolvation temperature at 350 °C and source temperature at 150 °C. The collision gas was argon used at 0.15 mL min−1. Partition coefficients of avermectins and UHPLC parameters for the determination of avermectins residues in the selected crops are presented in Table 1.

Table 1.
Partition coefficients of avermectins and UHPLC parameters for the determination of their residues in soybean, bean, and maize.
2.3. Optimized Sample Preparation Procedure
The proposed sample preparation procedure is based on the citrate QuEChERS procedure using 5 g of sample weighed in a 50-mL polypropylene (PP) tube followed by addition of 10 mL of water and homogenization for 1 min in ultra-turrax IKA T25 Digital (Staufen, Germany) at high speed to produce a slurry. Extraction was done with 10 mL of a mixture of acetonitrile:isopropanol 9:1 (v/v) vortexed for 1 min. Partition and salting-out effect were achieved by adding 2 g of MgSO4, 0.5 g of NaCl, 0.5 g of C6H5Na3O7·2 H2O and 0.25 g of C6H6Na2O7·1.5 H2O followed by vortex shaking for 1 min. Tubes were then centrifuged at 4850× g for 8 min at 5 °C. Then, 2 mL of supernatant was transferred to a 15-mL PP tube with 400 mg of EMR-Lipid previously activated with 2 mL ultrapure water according to Agilent Technologies Protocols [18], vortexed for 1 min, and centrifuged at 10,200× g for 8 min at 5 °C. After that, a final step to remove water excess the extract was transfered to a tube containing 640 mg of MgSO4 and 160 mg of NaCl, vortexed for 1 min, and centrifuged at 10,200× g for 8 min at 5 °C. The extract was filtered in 0.22-μm nylon filter and diluted 1:1 (v/v) in the initial composition of the mobile phase for UHPLC-MS/MS analysis.
2.4. Evaluation of the Different QuEChERS Procedures
The preliminary tests consisted in the evaluation of the QuEChERS versions: original [19], citrate [20], and acetate [21] using amounts of sample, solvent, and partition salts established in each version. Considering the low percentage of moisture, it was necessary to prepare a slurry using a ultra-turrax. Different ratio matrix:water (m/v) of 1:1; 1:1.5, and 1:2 were evaluated. Blank samples of soybean, bean and maize spiked at 20 μg kg−1 and matrix matched calibration curves at 1, 2, 5, 10, and 20 μg L−1, corresponding to 4, 8, 20, 40, and 80 μg kg−1, were used for evaluation of the different extraction procedures.
After some tests, the QuEChERS citrate was selected for evaluation of different extraction solvents: acetonitrile and acetonitrile:isopropanol in the proportions 9:1, 8:2, and 7:3 (v/v). To perform the evaluations, 5 g of sample were weighed in 50-mL PP tube with 10 mL of ultrapurified water and the content was homogenized in ultra-turrax for 1 min. Then, 10 mL of the different extraction solvents was added and tubes were vortexed for 1 min. After that, 2 g of MgSO4, 0.5 g of NaCl, 0.5 g of C6H5Na3O7·2 H2O, and 0.25 g of C6H6Na2O7·1.5 H2O were added, tubes were vortexed for 1 min and centrifuged at 4850× g for 8 min at 5 °C. The supernatant (2 mL) was submitted to a clean-up by dispersive solid phase extraction (d-SPE) with 250 mg of C18, 50 mg of PSA, and 300 mg of MgSO4 followed by centrifugation at 10,200× g for 8 min at 5 °C.
2.5. Evaluation of Different Clean-Up Conditions
An aliquot of 2 mL of extract was cleaned with different d-SPE conditions using: 250 mg of C18 and 50 mg of PSA; 100 mg Z-Sep+; 400 mg silica; 400 mg Florisil®; 400 mg EMR-Lipid activated with 2 mL ultrapurified water. The extraction tubes were centrifuged at 10,200× g for 8 min at 5 °C. Only for the assay using EMR-Lipid, a complementary step was included where 2 mL of supernatant was transferred to a tube with 640 mg of MgSO4 and 160 mg of NaCl. Extracts were centrifuged again at 10,200× g for 8 min at 5 °C, filtered with a 0.22-µm nylon filter and diluted 1:1 (v/v) in mobile phase for analysis.
2.6. Validation Procedure
The proposed method was validated according to SANTE guidelines [22] evaluating selectivity, matrix effect, analytical curve and linearity, limits of detection (LOD) and quantification (LOQ), precision (repeatability and intermediate precision) and accuracy. Method selectivity was evaluated by comparing the chromatograms obtained in the UHPLC-MS/MS system for the blank sample extracts and spiked blank samples for each matrix. Calibration curves were prepared in acetonitrile, blank sample extract and blank matrix at 1.0; 2.0; 5.0; 10.0; and 20.0 μg L−1 for each matrix. The LOQ was established as the lowest spike level, which presented signal-to-noise ratio higher than 10 and recoveries between 70–120%, with relative standard deviation (RSD) ≤20%. The LOD was obtained dividing the LOQ by 3.33. Accuracy and precision of the method were evaluated through recovery assays using spiked blank samples of each studied matrix at 4, 8, 20, 40, and 80 μg kg−1 in replicates (n = 5). The matrix effect was evaluated for each matrix comparing the slopes of the analytical curves in solvent and in the blank matrix extract.
2.7. Application in Real Samples
The developed method was applied for the determination of residues of the avermectins under study in 18 soybean samples, 12 bean samples, and 15 maize samples obtained from supermarkets from the Rio Grande do Sul State, Brazil. Each sample of at least 1 kg was collected, milled and stored in a freezer at −20 °C.
3. Results
3.1. UHPLC-MS/MS Analysis
The ionization was performed in an electrospray source operating in positive mode (ESI+). The composition of the mobile phase may significantly influence the analytical signal and the proper separation of the analytes [23]. The mobile phase (A) aqueous solution ammonium formate 10 mmol L−1 and (B) methanol 0.1% formic acid was chosen due to the higher analytical signal obtained for the compounds under study. The selected mobile phase additives favored the formation of [M + NH4]+ adducts used for the detection of abamectin, doramectin, and ivermectin after the chromatographic separation. The formation of the ion [M + H]+, used for emamectin benzoate and eprinomectin, was favored by the addition of formic acid. The addition of acids also reduce the formation of [M + Na]+ adducts. The sodium adducts are avoided due to its high stability and poor fragmentation response [24]. The column Acquity UPLC™ BEH C18 provided high resolution and good peak shape for the avermectins. Still, the chromatographic column chosen is the same used in routine analysis in our laboratory.
3.2. Sample Preparation Evaluations
Soybean and bean are considered complex matrices with high amount of fats and proteins, besides having a low humidity, needing to add water for the extraction. Maize presents a low percentage of water and a particular characteristic that is the presence of starch, which increases its complexity. The preparation of slurry was uniformized for the proportion 1:2 of sample:water, which produced suitable consistence. This proportion of sample:water was reported for low moisture matrices like maize [25], wheat and oat [26], and barley and wheat [27]. Lower proportions of water of 1:1 and 1:1.5 were not effective to have a homogeneous slurry.
Considering the main objective of developing an effective and unified sample preparation procedure for determination of avermectin residues in soybean, bean, and maize, the evaluation of which showed the QuEChERS procedures provide the best results for the evaluated matrices performed. The three most common QuEChERS procedures using acetonitrile as extraction solvent have not been effective for the extraction of the avermectins from the selected matrices presenting recoveries ranging from 35 to 101% and RSD between 3 and 37%. There were no significant differences (p < 0.05) between most of the assays. The QuEChERS citrate was chosen for investigations due to present the best recovery (64%) for eprinomectin, which was the compound with lower recoveries in all cases. This result is closer to the acceptance criterion of 70–120% [22], but an improvement is required. QuEChERS citrate also presented lower RSD values in comparison to the QuEChERS acetate and original.
As avermectins is a class of chemical compounds with high values of Kow, isopropanol was evaluated in different proportions with acetonitrile trying to improve extraction efficiency. Isopropanol may be used as extraction solvent, besides being commonly used as mobile phase in liquid chromatography. Menezes Filho et al. [28] employed isopropanol in a mixture 8:2 (v/v) with water in the extraction of pesticides from mangoes with satisfactory results for most of the analytes. In addition, Seth et al. [29] considered isopropanol an efficient and advantageous extraction solvent for soybean grains and other oilseeds and an attractive alternative to the frequently used solvents. The extraction solvents acetonitrile and acetonitrile:isopropanol 9:1 (v/v) presented similar results for the extraction of abamectin, doramectin, and ivermectin for all matrices, but for emamectin benzoate and eprinomectin the addition of isopropanol improved the extraction efficiency by about 25%. This was very important because emamectin benzoate, which in general presented lower recoveries, with this modification had recoveries above 70% for all matrices. Then, tests with acetonitrile containing different proportions of isopropanol (9:1; 8:2 and 7:3, v/v) were performed to evaluate whether there would be a significant increase in the recoveries of the compounds as the volume of isopropanol increased. No considerable variation in recoveries of avermectins for the three matrices were observed with the different proportions of the extraction solvents. However, it was observed that with higher volumes of isopropanol, the analytical response decreased for abamectin and eprinomectin. Once abamectin and eprinomectin are the two compounds with lowest sensitivity in UHPLC-MS/MS analysis, the ratio 9:1 (v/v) for acetonitrile:isopropanol was selected.
3.3. Clean-Up Procedure Evaluations
In the clean-up step, it was decided to evaluate d-SPE, without considering SPE, in order to keep the QuEChERS advantages. Figure 1 shows recovery results for five sorbents evaluated for soybean. Recoveries above 70% for most of the compounds were obtained. Similar results were observed for bean and maize. The use of C18 + PSA presented the lowest recoveries, with a mean recovery <70% for abamectin (69%), ivermectin (63%), and doramectin (64%), while abamectin presented RSD >20%. Regarding the EMR-Lipid and Florisil®, the five compounds presented adequate recoveries and RSD. Results for Z-Sep+ were satisfactory, except for eprinomectin, which presented recovery of 67%. Silica presented recoveries >70% for all compounds; however, the RSD for abamectin was >20%.
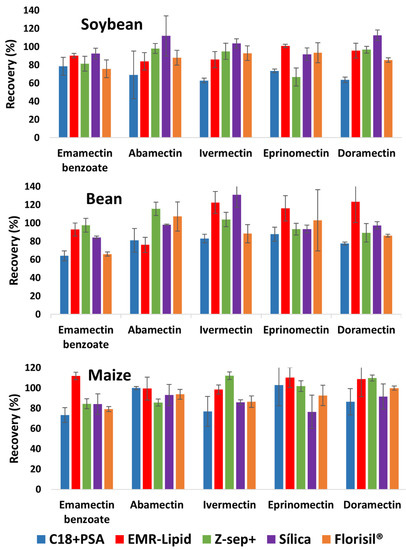
Figure 1.
Recoveries obtained with d-SPE clean-up for soybean, bean, and maize extracts using different sorbents.
The clean-up with C18 + PSA, EMR-Lipid, Silica, and Florisil® presented for some compounds recoveries <70% and >120%, especially for bean. The use of C18 + PSA resulted in the lowest recoveries in comparison to the other sorbents, and emamectin benzoate had only a recovery of 64%. EMR-Lipid showed all recoveries above 70%, with ivermectin and doramectin showing 122 and 123%, respectively. Silica had a mean recovery of 131% for ivermectin and Florisil® achieved a mean recovery of 66% for emamectin benzoate and a RSD of more than 30% for eprinomectin. Z-Sep+ was the only sorbent that presented recoveries between 70–120%. For maize, all sorbents evaluated presented satisfactory recoveries (70–120%). The RSD was satisfactory for all analytes, except for eprinomectin, when using C18 + PSA that presented RSD >20%. As maize obtained recoveries between 70–120% for the five sorbents, the values obtained for soybean and bean were taken into account to select the most suitable sorbent. C18 + PSA was discarded because of the lowest recovery values in comparison to the other sorbents for both matrices. The clean-up that generated the best results for bean was Z-Sep+ and for soybean was EMR-Lipid. In general, EMR-Lipid presented lighter advantage in terms of recovery and RSD, as well the final extract presented less coextractives when evaluated by gas chromatography-mass spectrometry in full scan mode.
The non-activated EMR-Lipid was tested in the clean-up step to verify if it would have better results or similar to the activated one. As a result, the non-activated presented higher recoveries for abamectin, emamectin benzoate, and ivermectin, compared to the activated EMR-Lipid. For eprinomectin the recovery was similar, but doramectin presented a much lower recovery. However, the RSD values for the five compounds increased (RSD >20%) with the use of non-activated EMR-Lipid. With activated EMR-Lipid, the RSD were <10% for all avermectins. Therefore, the activated EMR-Lipid was chosen for the clean-up step of all matrices.
3.4. Validation Results of the Proposed Method
Matrix-matched calibration was used for all matrices, where linearity was obtained with a determination coefficient (r2) > 0.99 for the five compounds and the levels 1, 2, 5, 10, and 20 μg L−1. In order to evaluate results of accuracy and precision, they were evaluated from the recovery tests at the spike levels 4, 8, 20, 40, and 80 μg kg−1, as presented in Table 2. The lowest levels of concentration were chosen taking into account the established MRL values. The proposed method presented adequate accuracy with recovery results between 70–120% for all compounds, matrices, and spike levels, both in repeatability and intermediate precision assays. The precision was also very good, with RSD <20% in all cases. According to SANTE guidelines [22], the matrix effect is considered to have an influence on the analytical performance when the result is not between −20 and 20%. Therefore, for all compounds in soybean and bean the matrix effect was not significant. For maize, doramectin presented a matrix effect of 22%. In order to compensate the matrix effect, matrix-matched calibration was selected for quantification. Considering that all spike levels presented recoveries from 70 to 120% and RSD ≤20% for all matrices, as well signal-to-noise ratio higher than ten, the method LOQ was established at the lowest level evaluated (4 μg kg−1) and the corresponding method LOD at 1.2 μg kg−1.

Table 2.
Maximum Residues Limits, recovery (%), and relative standard deviation (RSD) (%) for repeatability and intermediate precision assays and matrix effects (%) for each compound in the different matrices.
3.5. Application of the Method in Real Samples
From the analysis of 18 soybean samples, one sample presented abamectin at 21.1 μg kg−1, that is two times higher than the MRL (10 μg kg−1) established in Brazil and USA, and three samples presented residues of abamectin < LOQ. Figure 2 demonstrates the determination of abamectin residues in a soybean sample at concentration higher than the method LOQ and the MRL established in Brazil and USA. For the 12 bean samples and 5 maize samples, no residues of the analyzed avermectins were detected. The absence of detectable residues may be due to the reduced number of samples analyzed. However, it is important to monitor these compounds in different crops in order to guarantee the food safety and health of the population.
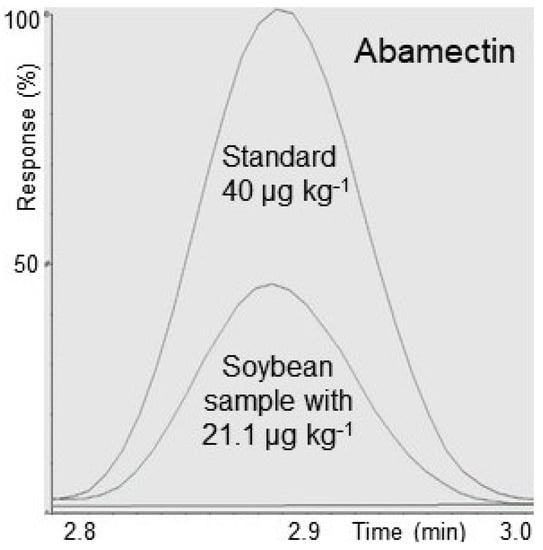
Figure 2.
Overlapping of the signal obtained from a positive sample of soybean for abamectin (21.1 μg kg−1) and a standard in blank matrix corresponding to 40 μg kg−1.
4. Conclusions
The results obtained with the modifications of the QuEChERS citrate method indicate good efficiency of the proposed method for the determination of avermectins residues in soybean, bean, and maize samples. The addition of isopropanol to the acetonitrile improved the extraction efficiency and the clean-up step using EMR-Lipid permitted to obtain clean extracts, avoiding the need of frequent maintenance of the UHPLC-MS/MS system. The use of UHPLC-MS/MS with additives in the mobile phase and specific conditions allowed the determination with method limits of quantification below the MRL values. The validation indicated very good results of accuracy and precision, proving that the proposed method is reliable. The method was efficiently applied to real samples with positive results, including with abamectin in a soybean at concentration higher than the MRL established in Brazil and USA. We emphasize that there are no available studies reporting the development of a method for the determination of avermectins in more than one agricultural crop and that the proposed method allows quantification at adequate levels for monitoring MRL compliance, highlighting the relevance of this work. The proposed method was very effective and is a good alternative for routine laboratories.
Author Contributions
Conceptualization, R.Z. and O.D.P.; Methodology, F.U., N.M.G.B. and O.D.P.; Validation, F.U., N.M.G.B. and L.F.; Formal analysis, F.U., N.M.G.B. and L.F.; Investigation, F.U.; Resources, F.U.; Writing—original draft preparation, F.U., N.M.G.B. and L.F.; Writing—review and editing, R.Z. and M.B.A.; Supervision, O.D.P.; Project administration, R.Z.; Funding acquisition, R.Z. and M.B.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), grant number 312207/2016-6.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Romanato, F.N.; Hamawaki, O.T.; de Sousa, L.B.; Nogueira, A.P.O.; de Neto, D.P.C.; Borges, C.C.R.; Hamawaki, C.D.L.; Hamawaki, R.L. Parametric and non-parametric analysis for determining the adaptability and stability of soybean genotypes in three sowing periods. Biosci. J. 2016, 32, 574–580. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Nan, X.; Yu, H.-T.; Cheng, P.-L.; Zhang, Y.; Liu, Y.-Q.; Zhang, S.-Y.; Hu, G.-F.; Liu, H.; Chen, A.-L. Synthesis, biological activities and structure activity relationships for new avermectin analogues. Eur. J. Med. Chem. 2016, 121, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.S.; Darwesh, D.M. Avermectins: The promising solution to control plant parasitic nematodes. J. Plant Sci. Phytopath. 2019, 3, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Brazil. Brazilian Ministry of Agriculture, Livestock and Supply. Normative Instruction nr. 13. Available online: https://www.infoconsult.com.br/legislacao/instrucao_normativa_mapa/2013/in_mapa_13_2013.htm (accessed on 14 October 2021).
- Bilal, M.; Freed, S.; Ashraf, M.Z.; Zaka, S.M.; Khan, M.B. Activity of acetylcholinesterase and acid and alkaline phosphatases in different insecticide-treated Helicoverpa armigera (Hübner). Environ. Sci. Pollut. Res. Int. 2018, 25, 22903–22910. [Google Scholar] [CrossRef]
- Bird, L.J.; Drynan, L.J.; Walker, P.W. The use of F2 screening for detection of resistance to emamectin benzoate, chlorantraniliprole, and indoxacarb in australian populations of Helicoverpa armígera (Lepidoptera: Noctuidae). J. Econ. Entomol. 2017, 110, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Orso, D.; Floriano, L.; Ribeiro, L.C.; Bandeira, N.M.G.; Prestes, O.D.; Zanella, R. Simultaneous determination of multiclass pesticides and antibiotics in honey samples based on ultra-high performance liquid chromatography-tandem mass spectrometry. Food Anal. Methods 2016, 9, 1638–1653. [Google Scholar] [CrossRef]
- May, M.M.; Ferronato, G.; Bandeira, N.M.G.; Prestes, O.D.; Zanella, R.; Adaime, M.A. Determination of pesticide residues in soy-based beverages using a QuEChERS method with clean-up optimized by central composite design and ultra-high-performance liquid chromatography-tandem mass spectrometry. Food Anal. Methods 2017, 10, 369–378. [Google Scholar] [CrossRef]
- Bandeira, N.M.G.; Ribeiro, L.C.; Rizzetti, T.M.; Martins, M.L.; Adaime, M.B.; Zanella, R.; Prestes, O.D. Evaluation of QuEChERS sample preparation for determination of avermectins residues in ovine muscle by HPLC-FD and UHPLC-MS/MS. J. Braz. Chem. Soc. 2017, 28, 878–886. [Google Scholar] [CrossRef]
- Musarurwa, H.; Chimuka, L.; Pakade, V.E.; Tavengwa, N.T. Recent developments and applications of QuEChERS based techniques on food samples during pesticide analysis. J. Food Compos. Anal. 2019, 84, 103314. [Google Scholar] [CrossRef]
- González-Curbelo, M.Á.; Herrera-Herrera, A.V.; Ravelo-Pérez, L.M.; Hernández-Borges, J. Sample-preparation methods for pesticide-residue analysis in cereals and derivatives. Trends Anal. Chem. 2012, 38, 32–51. [Google Scholar] [CrossRef]
- Huang, J.-X.; Lu, D.-H.; Wan, K.; Wang, F.-H. Low temperature purification method for the determination of abamectin and ivermectin in edible oils by liquid chromatography–tandem mass spectrometry. Chin. Chem. Lett. 2014, 25, 635–639. [Google Scholar] [CrossRef]
- Macedo, F.; Marsico, E.T.; Conte-Júnior, C.A.; de Resende, M.F.; Brasil, T.F.; Netto, A.D.P. Development and validation of a method for the determination of low-ppb levels of macrocyclic lactones in butter, using HPLC-fluorescence. Food Chem. 2015, 179, 239–245. [Google Scholar] [CrossRef]
- López-Blanco, R.; Nortes-Méndez, R.; Robles-Molina, J.; Moreno-Gonzáles, D.; Gilbert-López, B.; García-Reyes, J.F.; Molina-Díaz, A. Evaluation of different cleanup sorbents for multiresidue pesticide analysis in fatty vegetable matrices by liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1456, 89–104. [Google Scholar] [CrossRef]
- Du, P.; Liu, X.; Gu, X.; Dong, F.; Xu, J.; Kong, Z.; Wu, Y.; Zhu, Y.; Li, Y.; Zheng, Y. Rapid residue analysis of pyriproxyfen, avermectins and diflubenzuron in mushrooms by ultra-performance liquid chromatography coupled with tandem mass spectrometry. Anal. Methods 2013, 5, 6741–6747. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, P.; Wang, X.; Wang, Z.; Xu, X.; Chen, W.; Wu, L.; Zhang, H.; Wang, Q.; Wang, X. Multi-pesticides residue analysis of grains using modified magnetic nanoparticle adsorbent for facile and efficient cleanup. Food Chem. 2017, 230, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheung, W.; Chow, W. Ultra-high performance liquid chromatography/ electrospray ionization-tandem mass spectrometry determination of 151 pesticides in soybeans and pulses. J. AOAC Int. 2013, 96, 1114–1133. [Google Scholar] [CrossRef] [PubMed]
- Agilent Technologies. Enhanced Matrix Removal-Lipid Brochure; 5991-6052EN; Agilent Technologies: Santa Clara, CA, USA, 2016. [Google Scholar]
- Anastassiades, M.; Lehotay, S.; Štajnbaher, D.; Schenck, F. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anastassiades, M.; Scherbaum, E.; Tasdelen, B.; Stajnbaher, D. Recent developments in QuEChERS methodology for pesticide multiresidue analysis. In Pesticide Chemistry: Crop Protection, Public Health, Environmental Safety, 1st ed.; Ohkawa, H.M., Hisashi, L., Philip, W., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 439–458. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Mastovská, K.; Lightfield, A.R. Use of buffering and other means to improve results of problematic pesticides in a fast and easy method for residue analysis of fruits and vegetables. J. AOAC Int. 2005, 88, 615–629. [Google Scholar] [CrossRef] [Green Version]
- European Commission. SANTE/12682/2019. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed. European Commission Directorate-General for Health and Food Safety. (rev.0). 2019. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/AqcGuidance_SANTE_2019_12682.pdf (accessed on 14 October 2021).
- Kemmerich, M.; Rizetti, T.M.; Martins, M.L.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Optimization by central composite design of a modified QuEChERS method for extraction of pesticide multiresidue ins sweet pepper and analysis by ultra-high-performance liquid chromatography–tandem mass spectrometry. Food Anal. Methods 2015, 8, 728–739. [Google Scholar] [CrossRef]
- Moscou, I.C.; Dasenaki, M.E.; Thomaidis, N.S. Ionization study and simultaneous determination of avermectins and milbemycines in fish tissue by LC-ESI-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1104, 134–140. [Google Scholar] [CrossRef]
- Marchis, D.; Ferro, G.L.; Brizio, P.; Squadrone, S.; Abete, M.C. Detection of pesticides in crops: A modified QuEChERS approach. Food Control 2012, 25, 270–273. [Google Scholar] [CrossRef]
- Herrmann, S.S.; Poulsen, M.E. Clean-up of cereal extracts for gas chromatography-tandem quadrupole mass spectrometry pesticide residues analysis using primary secondary amine and C18. J. Chromatogr. A 2015, 1423, 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francesquett, J.Z.; Rizzetti, T.M.; Cadaval, T.R.S.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Simultaneous determination of the quaternary ammonium pesticides paraquat, diquat, chlormequat, and mepiquat in barley and wheat using a modified quick polar pesticides method, diluted standard addition calibration and hydrophilic interaction liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2019, 1592, 101–111. [Google Scholar] [CrossRef]
- Menezes Filho, A.; dos Santos, F.N.; Pereira, P.A.P. Development, validation and application of a methodology based on solid-phase micro extraction followed by gas chromatography coupled to mass spectrometry (SPME/GC-MS) for the determination of pesticide residues in mangoes. Talanta 2010, 81, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Seth, S.; Agrawal, Y.C.; Ghosh, P.K.; Jayas, D.S.; Singh, B.P.N. Oil extraction rates of soya bean using isopropyl alcohol as solvent. Biosys. Eng. 2007, 97, 209–217. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).