Fabrication of a Potential Electrodeposited Nanocomposite for Dental Applications
Abstract
:1. Introduction
2. Results
2.1. Electrodeposition of the Ni-Fe-TiO2 Nanocomposites
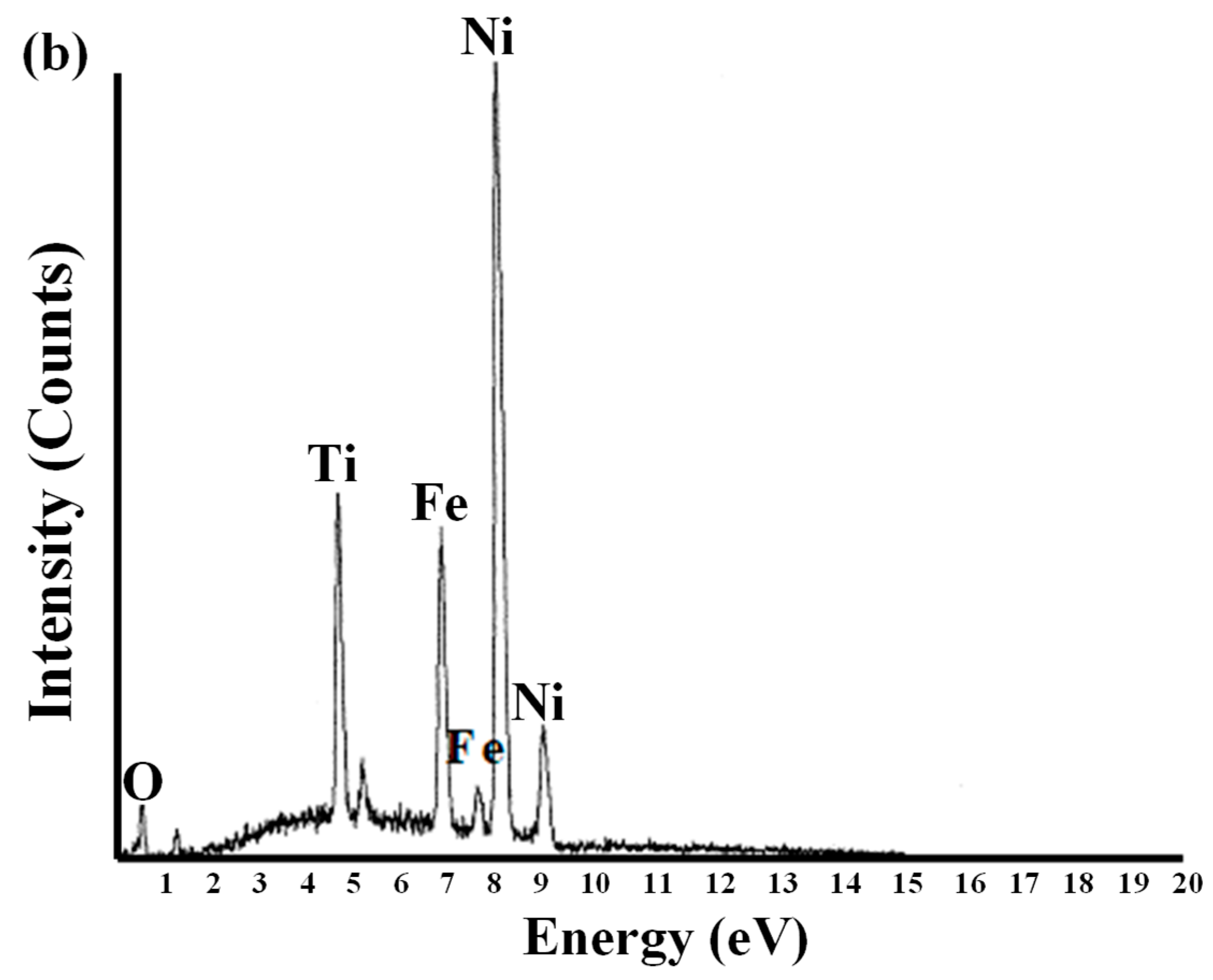
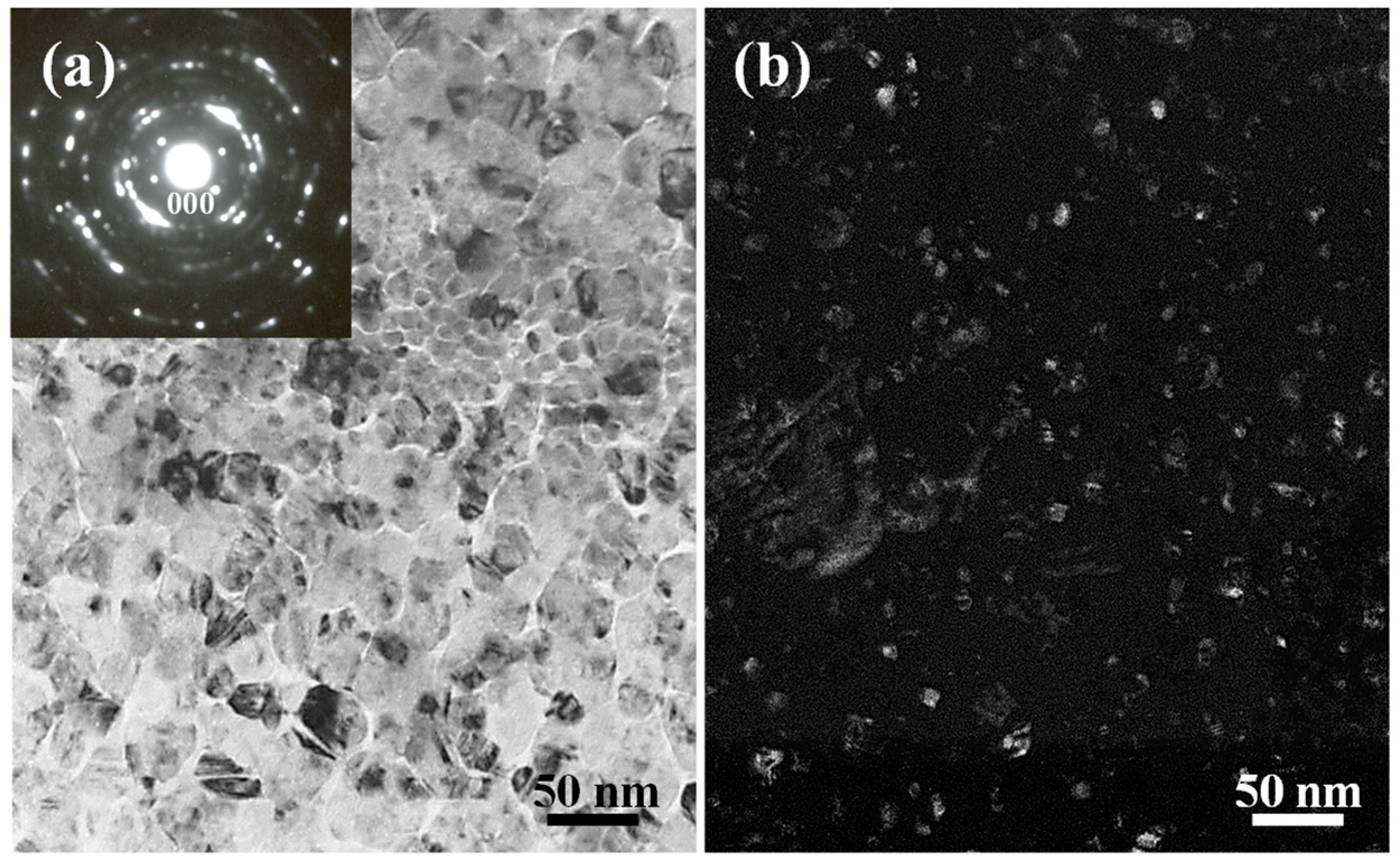
2.2. Microstructural Characterization of the Ni-Fe-TiO2 Nanocomposites
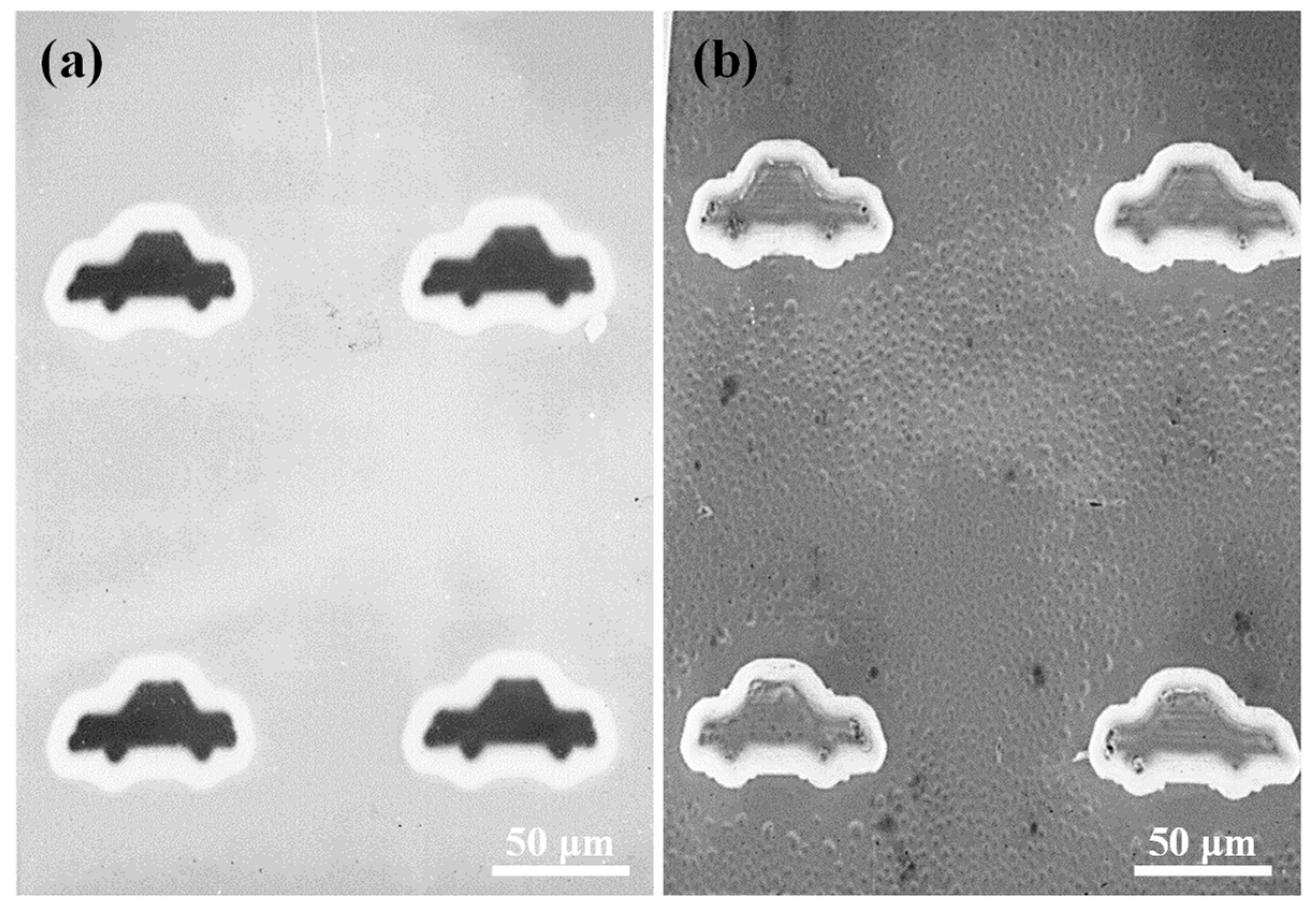
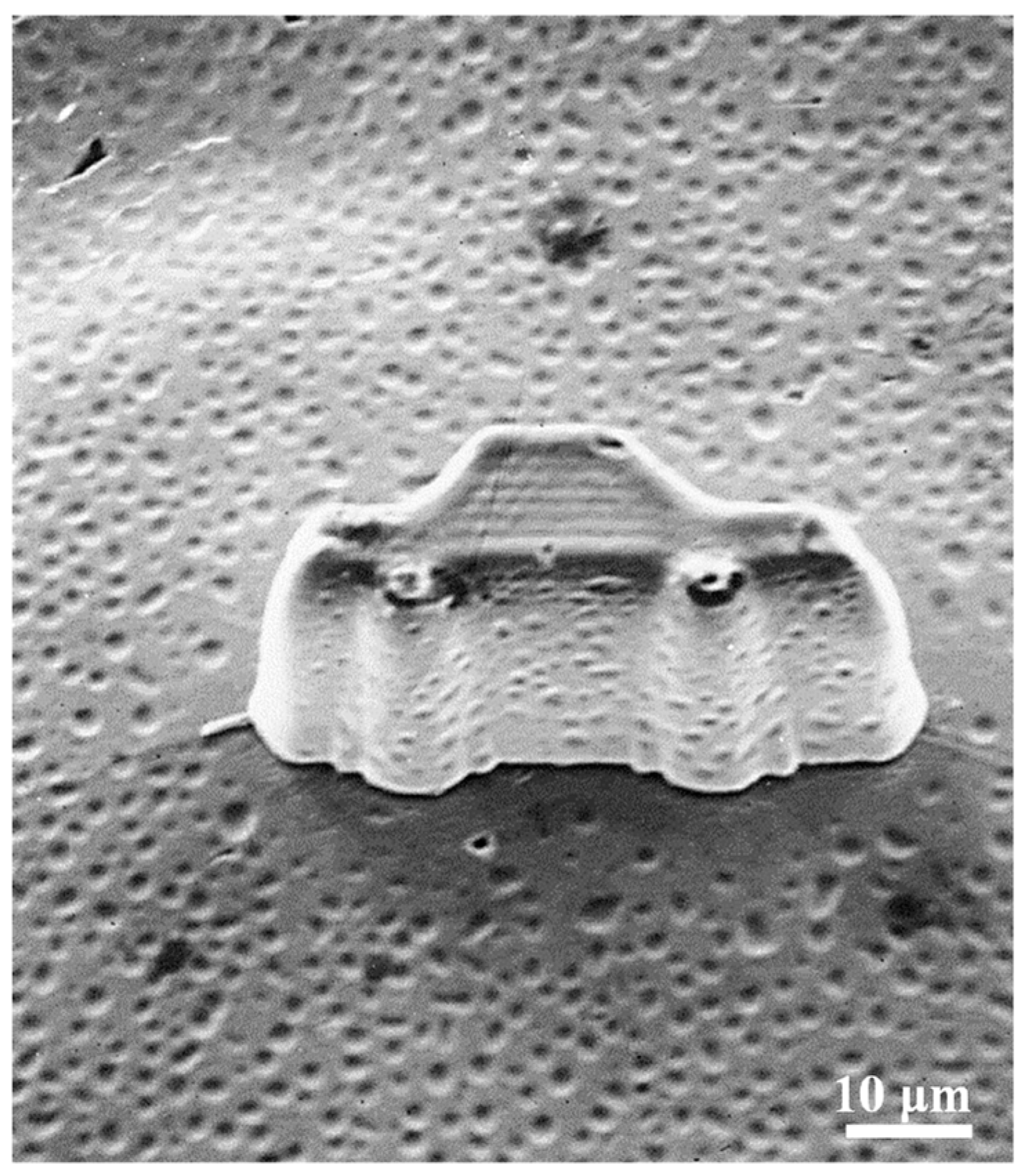
2.3. Cell Viability and Adhesion Behavior of the Ni-Fe-TiO2 Nanocomposites
3. Discussion
4. Materials and Methods
4.1. Materials Preparation
4.2. Surface Characterization
4.3. Microstructure Identification
4.4. Cytotoxicity Assay
4.5. Cell Morphology Observation
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhagyashree, B.; Rao, D.; Panwar, S.; Kothari, N.; Gupta, S. An in vitro comparative evaluation of dentinal crack formation caused by three different nickel-titanium rotary file systems in primary anterior teeth. J. Indian Soc. Pedod. Prev. Dent. 2022, 40, 188–194. [Google Scholar]
- Dhaimy, S.; Kim, H.C.; Bedida, L.; Benkiran, I. Efficacy of reciprocating and rotary retreatment nickel-titanium file systems for removing filling materials with a complementary cleaning method in oval canals. Restor. Dent. Endod. 2021, 46, e13. [Google Scholar] [CrossRef] [PubMed]
- Hasheminia, S.M.; Farhad, A.; Davoudi, H.; Sarfaraz, D. Microleakage of five separated nickel-titanium rotary file systems in the apical portion of the root canal. Dent. Res. J. 2022, 19, 46. [Google Scholar]
- Huang, Z.; Quan, J.; Liu, J.; Zhang, W.; Zhang, X.; Hu, X. A microcomputed tomography evaluation of the shaping ability of three thermally-treated nickel-titanium rotary file systems in curved canals. J. Int. Med. Res. 2019, 47, 325–334. [Google Scholar] [CrossRef]
- Tabassum, S.; Zafar, K.; Umer, F. Nickel-titanium rotary file systems: What’s new? Eur. Endod. J. 2019, 4, 111–117. [Google Scholar]
- Ruiz-Sanchez, C.; Faus-Llacer, V.; Faus-Matoses, I.; Zubizarreta-Macho, A.; Sauro, S.; Faus-Matoses, V. The influence of niti alloy on the cyclic fatigue resistance of endodontic files. J. Clin. Med. 2020, 9, 3755. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.; Junior, C.S.; Colombelli, M.F.; Picolli, A.P.; Junior, J.S.; Cosme-Silva, L.; Garcia, L.; Alberton, L.R. Incidence of protaper universal system instrument fractures—A retrospective clinical study. Eur. Endod. J. 2018, 3, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Patnana, A.K.; Chugh, A.; Chugh, V.K.; Kumar, P. The incidence of nickel-titanium endodontic hand file fractures: A 7-year retrospective study in a tertiary care hospital. J. Conserv. Dent. 2020, 23, 21–25. [Google Scholar] [CrossRef]
- Han, Q.G.; Zhang, H.Z.; Fang, L.; Wang, Y.Q. Tribological properties of nanocrystalline cu coatings produced by electroless plating under various lubrication conditions. Rare Met. Mater. Eng. 2011, 40, 356–359. [Google Scholar]
- Gorka, J.; Czuprynski, A.; Zuk, M.; Adamiak, M.; Kopysc, A. Properties and structure of deposited nanocrystalline coatings in relation to selected construction materials resistant to abrasive wear. Materials 2018, 11, 1184. [Google Scholar] [CrossRef] [Green Version]
- Matsui, I.; Mori, H.; Kawakatsu, T.; Takigawa, Y.; Uesugi, T.; Higashi, K. Mechanical behavior of electrodeposited bulk nanocrystalline fe-ni alloys. Mater. Res. Ibero Am. J. 2015, 18, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Latha, N.; Raj, V.; Selvam, M. Effect of plating time on growth of nanocrystalline ni-p from sulphate/glycine bath by electroless deposition method. Bull. Mater. Sci. 2013, 36, 719–727. [Google Scholar] [CrossRef] [Green Version]
- Byun, M.H.; Cho, J.W.; Han, B.S.; Kim, Y.K.; Song, Y.S. Material characterization of electroplated-nanocrystalline nickel-iron alloys for micro electronic mechanical-system. Jpn. J. Appl. Phys. 2006, 45, 7084–7090. [Google Scholar] [CrossRef]
- See, S.H.; Seet, H.L.; Li, X.P.; Lee, J.Y.; Lee, K.Y.T.; Teoh, S.H.; Lim, C.T. Effect of nanocrystalline electroplating of nife on the material permeability. Mater. Sci. Forum 2003, 437–438, 53–56. [Google Scholar] [CrossRef]
- Hu, J.J.; Chai, L.J.; Xu, H.B.; Ma, C.P.; Deng, S.B. Microstructural modification of brush-plated nanocrystalline cr by high current pulsed electron beam irradiation. J. Nano Res. Sw. 2016, 41, 87–95. [Google Scholar] [CrossRef]
- Kosta, I.; Vicenzo, A.; Muller, C.; Sarret, M. Mixed amorphous-nanocrystalline cobalt phosphorous by pulse plating. Surf. Coat. Tech. 2012, 207, 443–449. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Danilov, F.I.; Gordiienko, V.O.; Baskevich, A.S.; Artemchuk, V.V. Improving hardness and tribological characteristics of nanocrystalline cr-c films obtained from cr(iii) plating bath using pulsed electrodeposition. Int. J. Refract. Met. Hard Mater. 2012, 31, 281–283. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Fang, L.; Zhou, Y.G.; Shi, G.L. Microstructures and corrosion properties of nanocrystalline cu coatings synthesized by electroless plating. Rare Met. Mat. Eng. 2012, 41, 239–242. [Google Scholar]
- Dmitriev, A.I.; Nikonov, A.Y.; Osterle, W. Molecular dynamics sliding simulations of amorphous ni, ni-p and nanocrystalline ni films. Comp. Mater. Sci. 2017, 129, 231–238. [Google Scholar] [CrossRef]
- Tsyntsaru, N.; Kaziukaitis, G.; Yang, C.; Cesiulis, H.; Philipsen, H.G.G.; Lelis, M.; Celis, J.P. Co-w nanocrystalline electrodeposits as barrier for interconnects. J. Solid State Electr. 2014, 18, 3057–3064. [Google Scholar] [CrossRef]
- Umapathy, G.; Senguttuvan, G.; Berchmans, L.J.; Sivakumar, V.; Jegatheesan, P. Influence of cerium substitution on structural, magnetic and dielectric properties of nanocrystalline ni-zn ferrites synthesized by combustion method. J. Mater. Sci. Mater. El. 2017, 28, 17505–17515. [Google Scholar] [CrossRef]
- Farshbaf, P.A.; Bostani, B.; Yaghoobi, M.; Farshbaf, P.A.; Bostani, B.; Yaghoobi, M.; Ahmadi, N.P. Evaluation of corrosion resistance of electrodeposited nanocrystalline ni-fe alloy coatings. Trans. Inst. Met. Finish. 2017, 95, 269–275. [Google Scholar] [CrossRef]
- Bigos, A.; Beltowska-Lehman, E.; Kot, M. Studies on electrochemical deposition and physicochemical properties of nanocrystalline ni-mo alloys. Surf. Coat. Tech. 2017, 317, 103–109. [Google Scholar] [CrossRef]
- Wang, C.S.; Su, H.J.; Guo, Y.A.; Guo, J.T.; Zhou, L.Z. Solidification characteristics and segregation behavior of a ni-fe-cr based alloy. Rare Met. Mat. Eng. 2018, 47, 3816–3823. [Google Scholar] [CrossRef]
- Sharma, A.; Bhattacharya, S.; Das, S.; Das, K. A study on the effect of pulse electrodeposition parameters on the morphology of pure tin coatings. Met. Mater. Trans. A 2014, 45, 4610–4622. [Google Scholar] [CrossRef]
- Kolonits, T.; Czigany, Z.; Peter, L.; Bakonyi, I.; Gubicza, J. Influence of bath additives on the thermal stability of the nanostructure and hardness of ni films processed by electrodeposition. Coatings 2019, 9, 644. [Google Scholar] [CrossRef] [Green Version]
- Torabinejad, V.; Aliofkhazraei, M.; Assareh, S.; Allahyarzadeh, M.H.; Rouhaghdam, A.S. Electrodeposition of ni-fe alloys, composites, and nano coatings-a review. J. Alloy. Compd. 2017, 691, 841–859. [Google Scholar] [CrossRef]
- Tripkovic, D.V.; Strmcnik, D.; van der Vliet, D.; Stamenkovic, V.; Markovic, N.M. The role of anions in surface electrochemistry. Faraday Discuss. 2008, 140, 25–40. [Google Scholar] [CrossRef]
- Sun, J.; Du, D.X.; Lv, H.F.; Zhou, L.; Wang, Y.G.; Qi, C.G. Microstructure and corrosion resistance of pulse electrodeposited ni-cr coatings. Surf. Eng. 2015, 31, 406–411. [Google Scholar] [CrossRef]
- Muller, B.; Ferkel, H. Properties of nanocrystalline ni/al2o3 composites. Z. Metallkd. 1999, 90, 868–871. [Google Scholar]
- Oberle, R.R.; Scanlon, M.R.; Cammarata, R.C.; Searson, P.C. Processing and hardness of electrodeposited ni/al2o3 nanocomposites. Appl. Phys. Lett. 1995, 66, 19–21. [Google Scholar] [CrossRef]
- Muller, B.; Ferkel, H. Al2o3-nanoparticle distribution in plated nickel composite films. Nanostructured Mater. 1998, 10, 1285–1288. [Google Scholar] [CrossRef]
- Nicolenco, A.; Mulone, A.; Imaz, N.; Tsyntsaru, N.; Sort, J.; Pellicer, E.; Klement, U.; Cesiulis, H.; Garcia-Lecina, E. Nanocrystalline electrodeposited fe-w/al2o3 composites: Effect of alumina sub-microparticles on the mechanical, tribological, and corrosion properties. Front. Chem. 2019, 7, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arzt, E. Overview no. 130—Size effects in materials due to microstructural and dimensional constraints: A comparative review. Acta Mater. 1998, 46, 5611–5626. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, A.F.; Clark, D.G.; Aust, K.T.; Erb, U. Pulse electrodeposition of ni-sic nanocomposite. Mater. Lett. 2002, 52, 85–90. [Google Scholar] [CrossRef]
- Hsu, H.J.; Abd Waris, R.; Ruslin, M.; Lin, Y.H.; Chen, C.S.; Ou, K.L. An innovative alpha-calcium sulfate hemihydrate bioceramic as a potential bone graft substitute. J. Am. Ceram. Soc. 2018, 101, 419–427. [Google Scholar] [CrossRef]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef] [Green Version]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical applications of tio2 nanostructures: Recent advances. Int. J. Nanomed. 2020, 15, 3447–3470. [Google Scholar] [CrossRef]
- Mohammadi, M.; Hesaraki, S.; Hafezi-Ardakani, M. Investigation of biocompatible nanosized materials for development of strong calcium phosphate bone cement: Comparison of nano-titania, nano-silicon carbide and amorphous nano-silica. Ceram. Int. 2014, 40, 8377–8387. [Google Scholar] [CrossRef]
- Ibrahim, M.A.M. Black nickel electrodeposition from a modified watts bath. J. Appl. Electrochem. 2006, 36, 295–301. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-W.; Tsou, C.-H.; Huang, B.-H.; Hung, K.-S.; Cho, Y.-C.; Saito, T.; Tsai, C.-H.; Hsieh, C.-C.; Liu, C.-M.; Lan, W.-C. Fabrication of a Potential Electrodeposited Nanocomposite for Dental Applications. Inorganics 2022, 10, 165. https://doi.org/10.3390/inorganics10100165
Chang C-W, Tsou C-H, Huang B-H, Hung K-S, Cho Y-C, Saito T, Tsai C-H, Hsieh C-C, Liu C-M, Lan W-C. Fabrication of a Potential Electrodeposited Nanocomposite for Dental Applications. Inorganics. 2022; 10(10):165. https://doi.org/10.3390/inorganics10100165
Chicago/Turabian StyleChang, Chun-Wei, Chen-Han Tsou, Bai-Hung Huang, Kuo-Sheng Hung, Yung-Chieh Cho, Takashi Saito, Chi-Hsun Tsai, Chia-Chien Hsieh, Chung-Ming Liu, and Wen-Chien Lan. 2022. "Fabrication of a Potential Electrodeposited Nanocomposite for Dental Applications" Inorganics 10, no. 10: 165. https://doi.org/10.3390/inorganics10100165
APA StyleChang, C.-W., Tsou, C.-H., Huang, B.-H., Hung, K.-S., Cho, Y.-C., Saito, T., Tsai, C.-H., Hsieh, C.-C., Liu, C.-M., & Lan, W.-C. (2022). Fabrication of a Potential Electrodeposited Nanocomposite for Dental Applications. Inorganics, 10(10), 165. https://doi.org/10.3390/inorganics10100165





