Synthesis and Structure of ZnO-Decorated Graphitic Carbon Nitride (g-C3N4) with Improved Photocatalytic Activity under Visible Light
Abstract
:1. Introduction
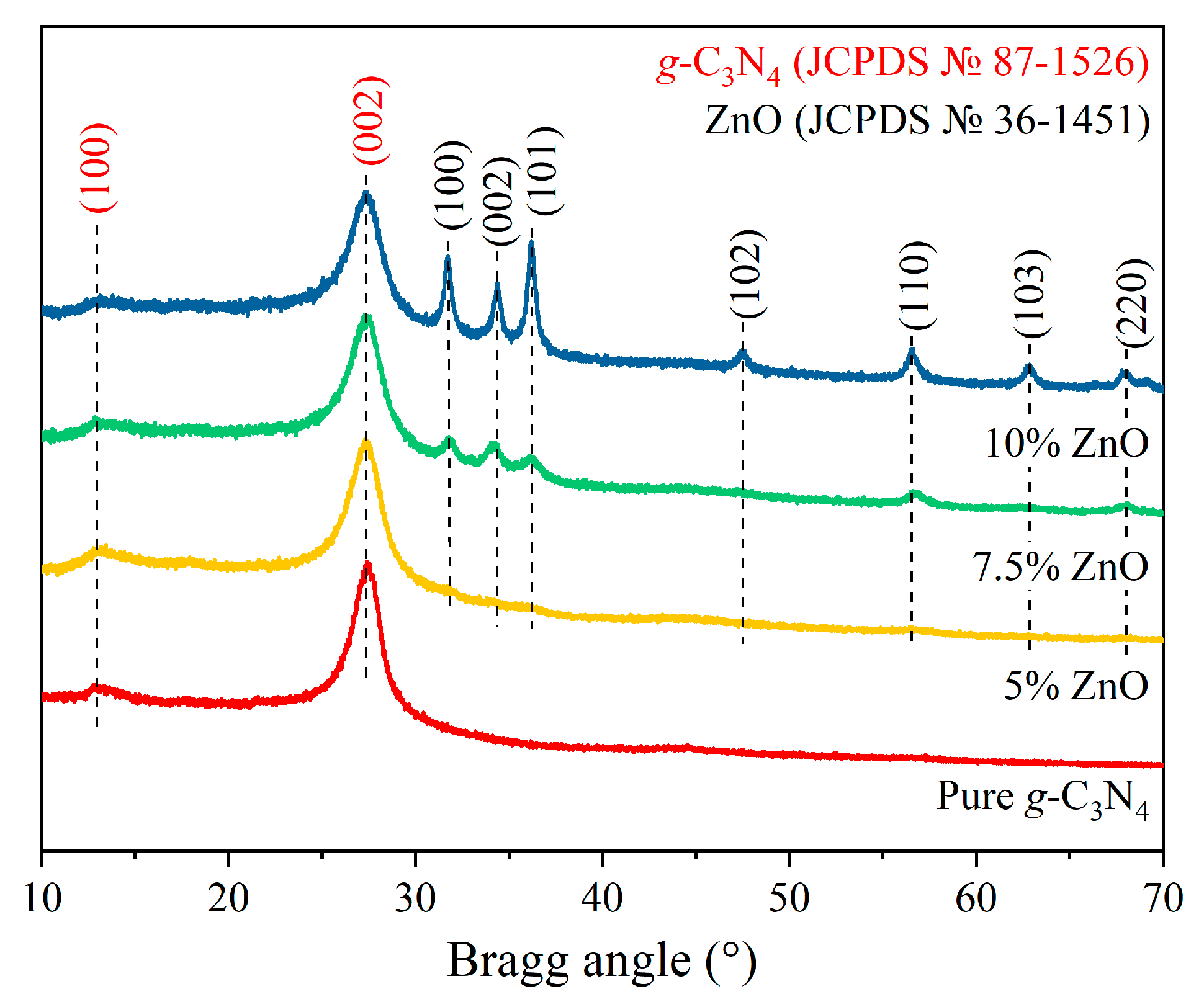
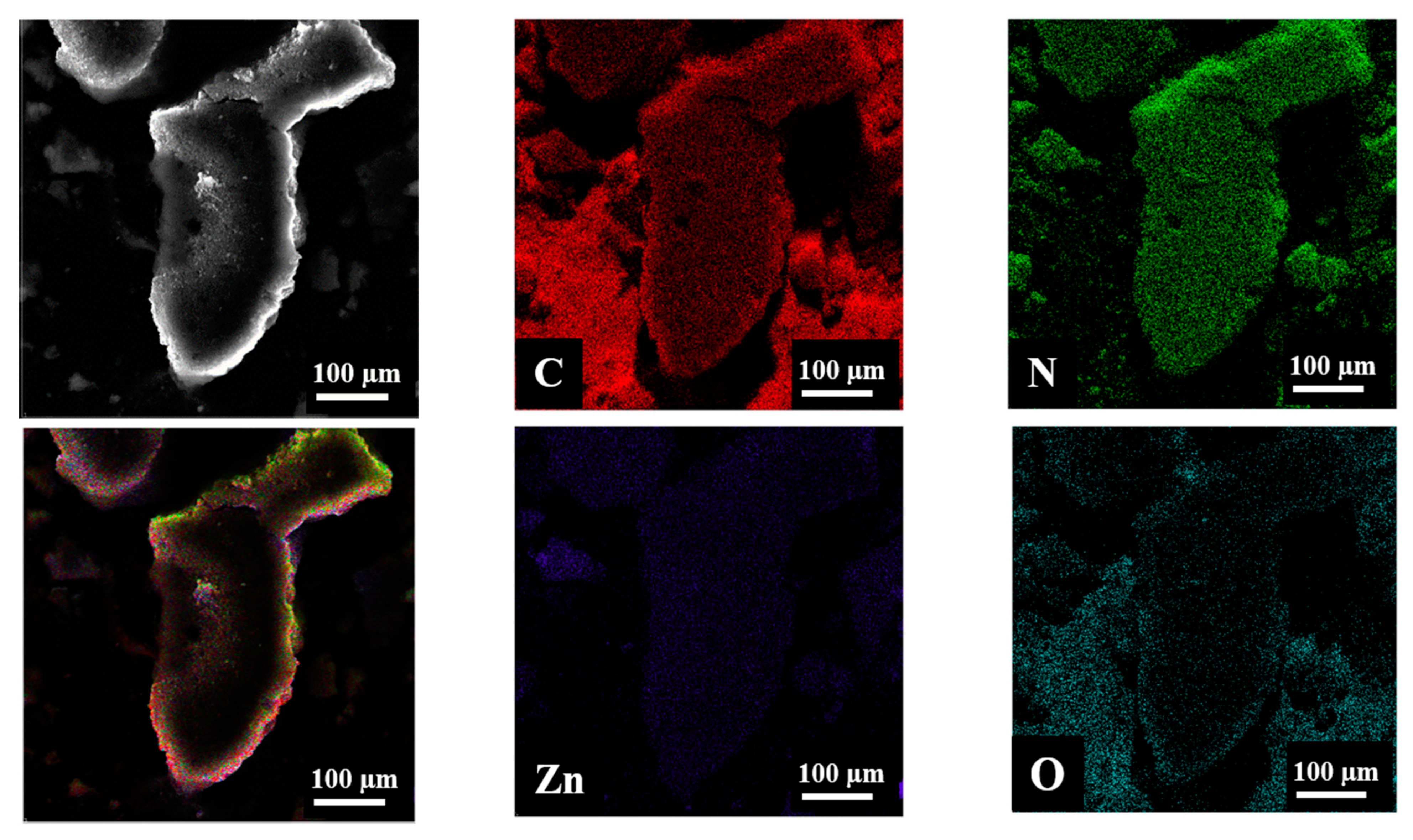
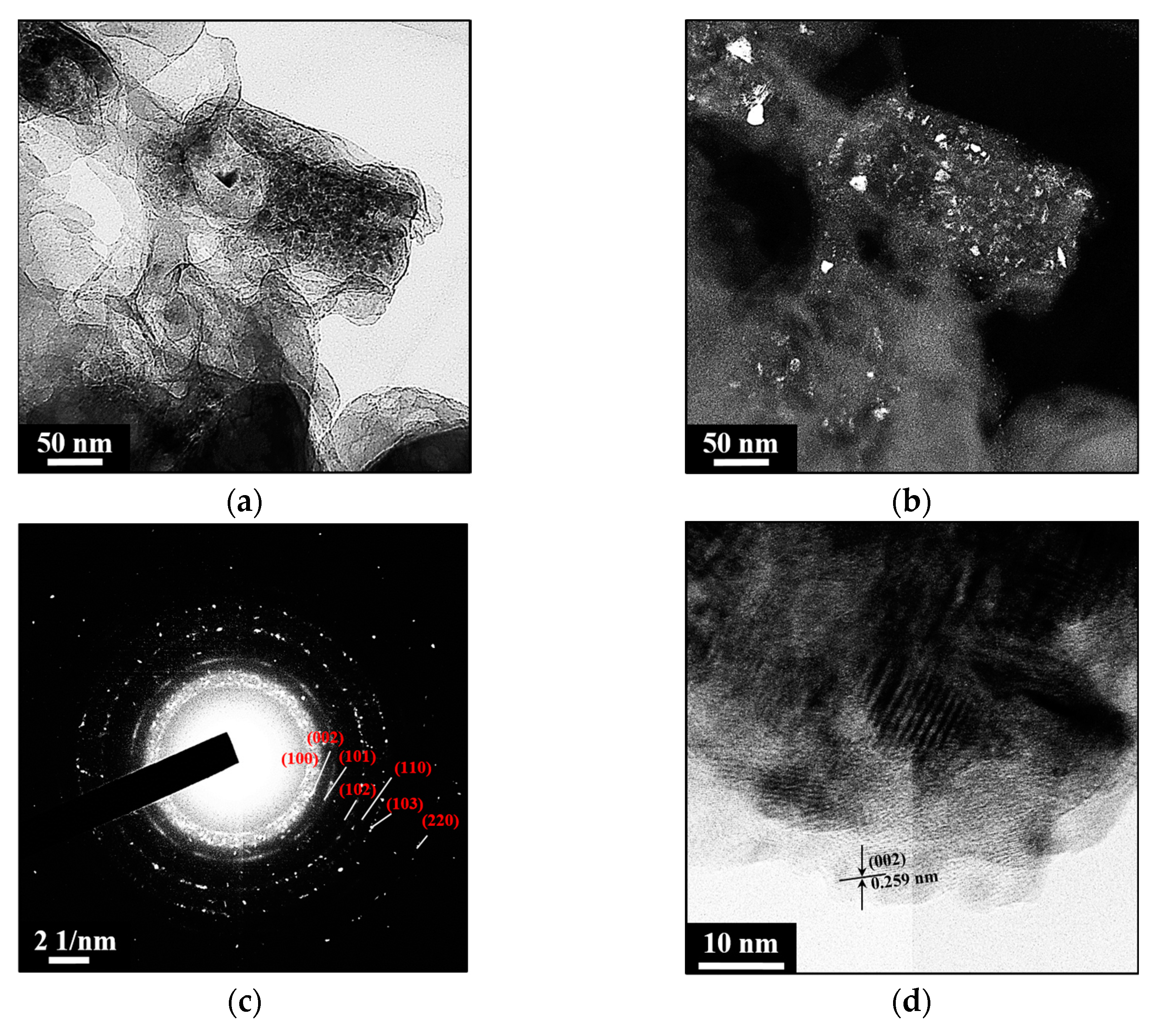
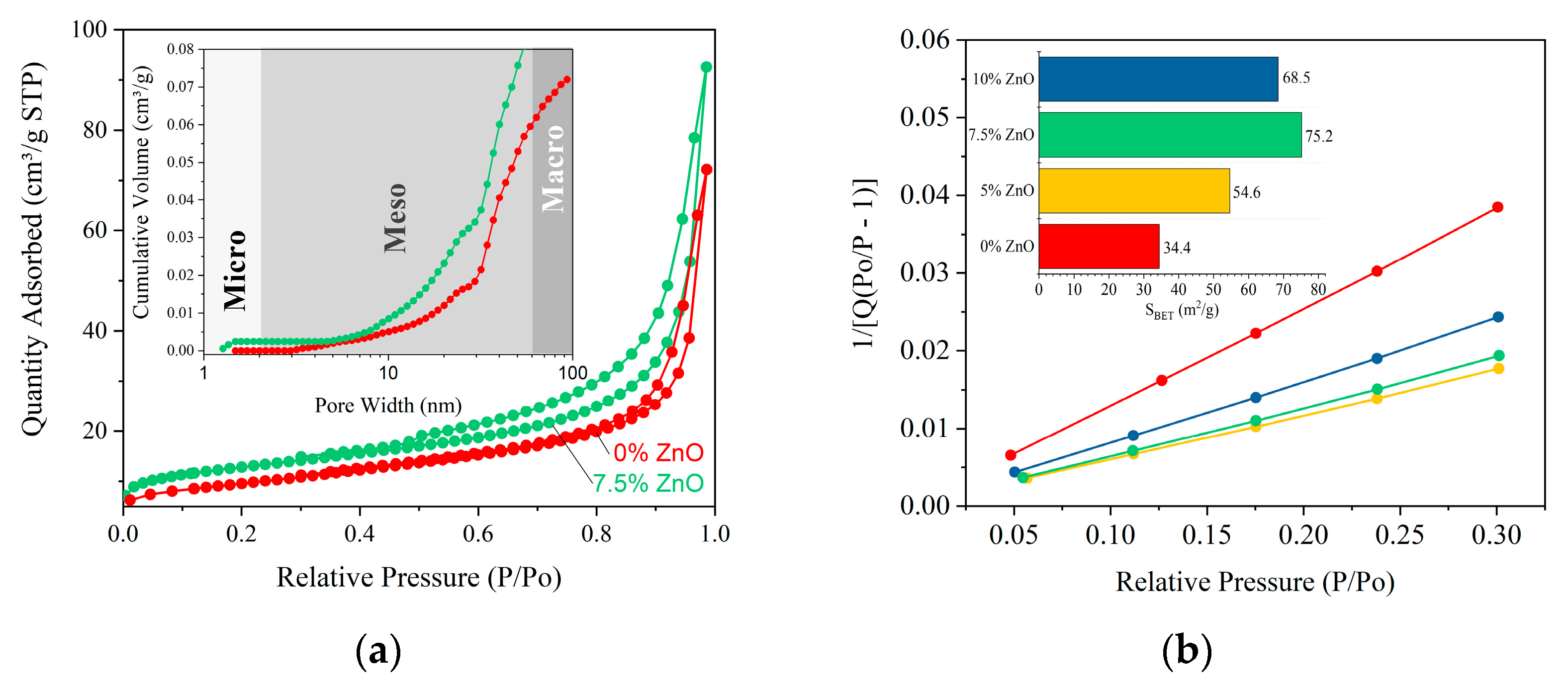
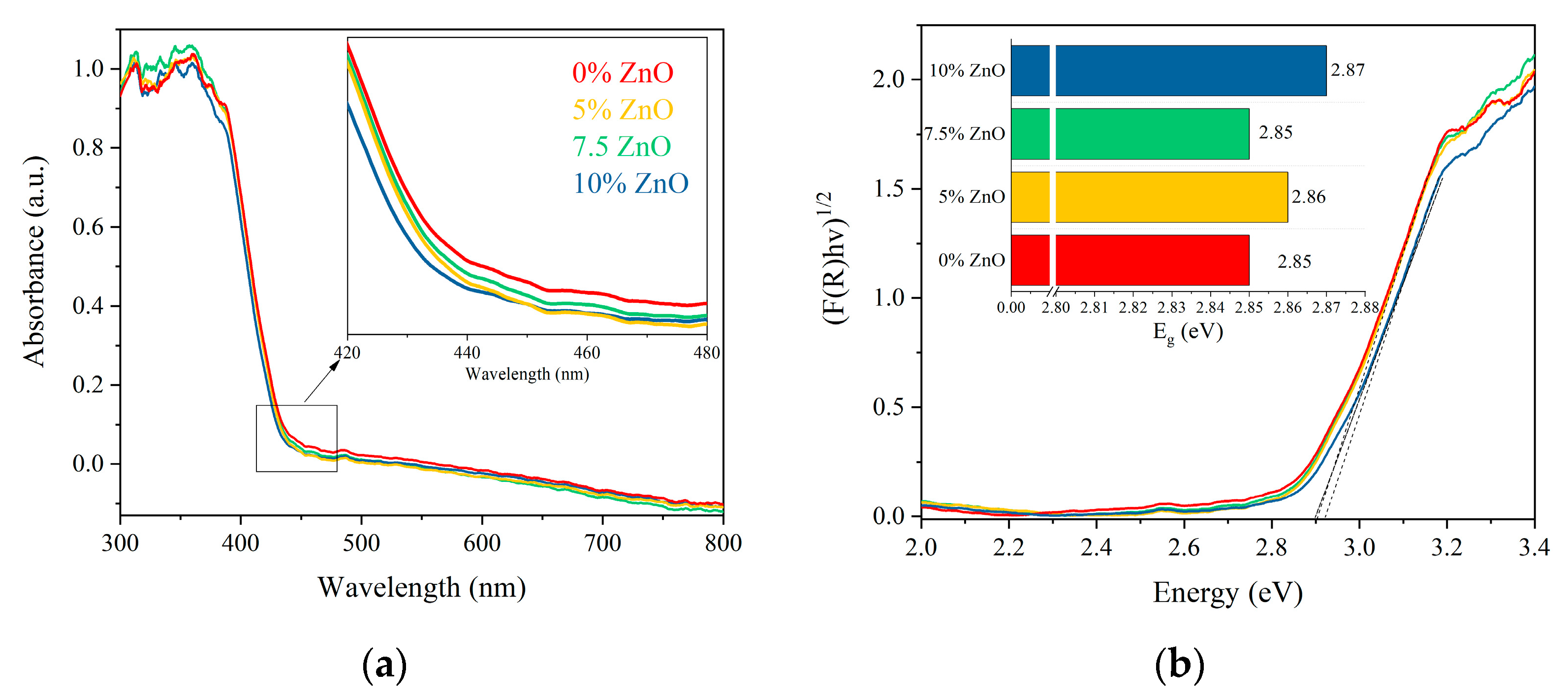
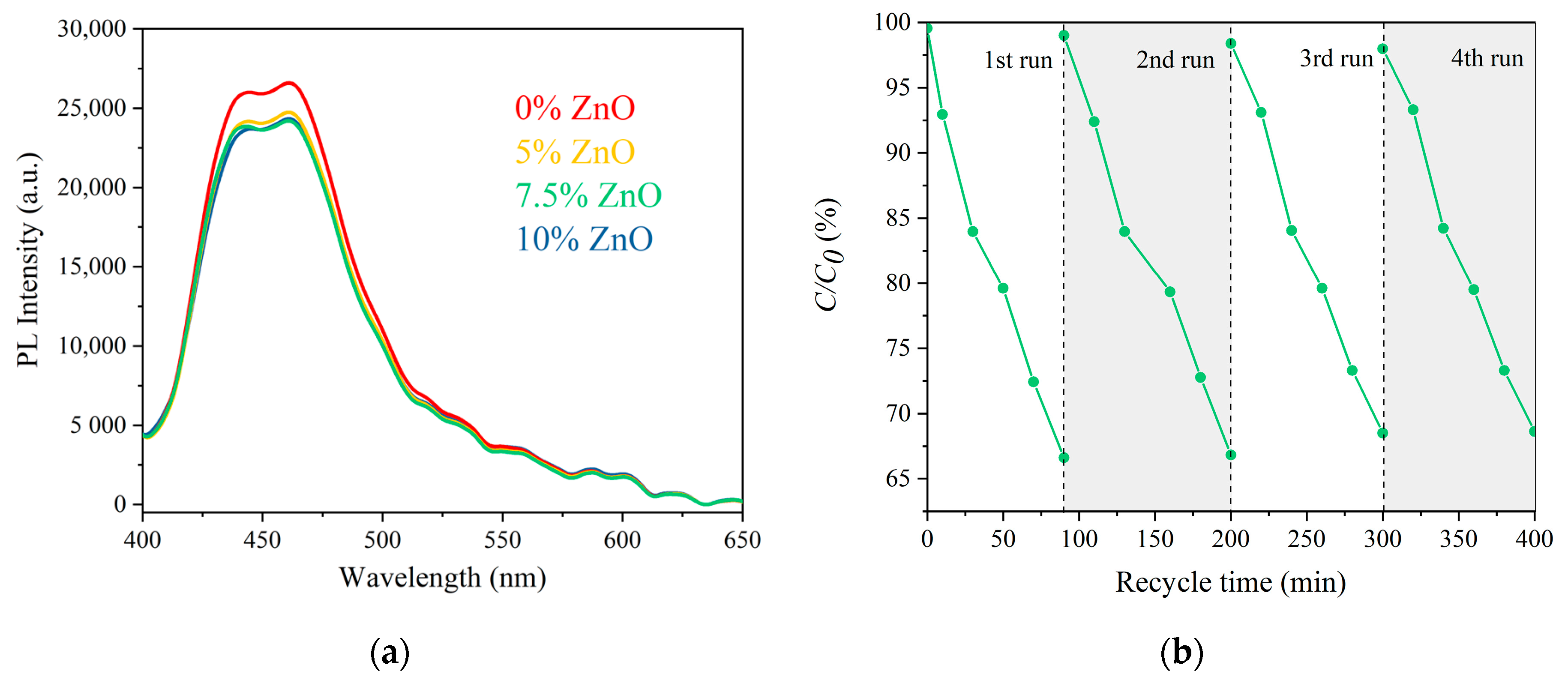
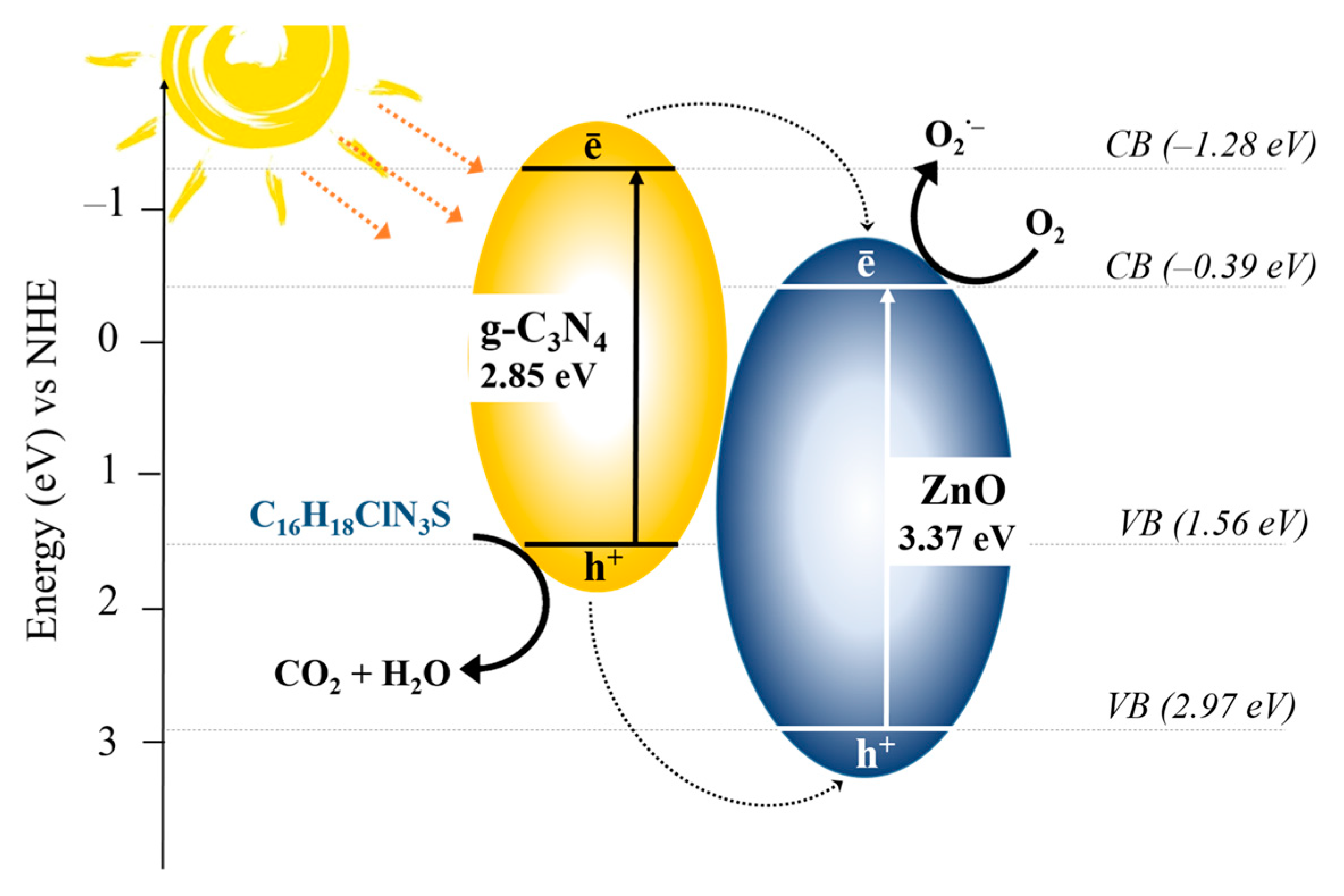
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Initial g-C3N4 Nanopowder
3.3. Synthesis of Exfoliated g-C3N4/ZnO Nanocomposites
3.4. Physico-Chemical Characterization
3.5. Photocatalytic Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marin, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Green-synthesized nanocatalysts and nanomaterials for water treatment: Current challenges and future perspectives. J. Hazard. Mater. 2020, 401, 123401. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Mohapatra, L.; Parida, K. Visible light-driven novel g-C3N4/NiFe-LDH composite photocatalyst with enhanced photocatalytic activity towards water oxidation and reduction reaction. J. Mater. Chem. A 2015, 3, 18622–18635. [Google Scholar] [CrossRef]
- Mahmood, A.; Muhmood, T.; Ahmad, F. Carbon nanotubes heterojunction with graphene like carbon nitride for the enhancement of electrochemical and photocatalytic activity. Mater. Chem. Phys. 2022, 278, 125640. [Google Scholar] [CrossRef]
- Muhmood, T.; Xia, M.; Lei, W.; Wang, F. Under vacuum synthesis of type-I heterojunction between red phosphorus and graphene like carbon nitride with enhanced catalytic, electrochemical and charge separation ability for photodegradation of an acute toxicity category-III compound. Appl. Catal. B Environ. 2018, 238, 568–575. [Google Scholar] [CrossRef]
- Ren, Q.; Nie, M.; Yang, L.; Wei, F.; Ding, B.; Chen, H.; Liu, Z.; Liang, Z. Synthesis of MOFs for RhB Adsorption from Wastewater. Inorganics 2022, 10, 27. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Chon, K. Green Technologies for Sustainable Water and Wastewater Treatment: Removal of Organic and Inorganic Contaminants. Separations 2022, 9, 335. [Google Scholar] [CrossRef]
- Klaczyński, E.; Ratajczak, P. Oczyszczalnie ścieków—Układy technologiczne (Waste water treatment plants—Process systems). Wodociągi Kanaliz. 2013, 4, 36–39. [Google Scholar]
- Narayanan, C.M.; Narayan, V. Biological wastewater treatment and bioreactor design: A review. Sustain. Environ. Res. 2019, 29, 33. [Google Scholar] [CrossRef] [Green Version]
- Vukšić, M.; Kocijan, M.; Ćurković, L.; Radošević, T.; Vengust, D.; Podlogar, M. Photocatalytic Properties of Immobilised Graphitic Carbon Nitride on the Alumina Substrate. Appl. Sci. 2022, 12, 9704. [Google Scholar] [CrossRef]
- Kocijan, M.; Ćurković, L.; Radošević, T.; Podlogar, M. Enhanced Photocatalytic Activity of Hybrid rGO@TiO2/CN Nanocomposite for Organic Pollutant Degradation under Solar Light Irradiation. Catalysts. 2021, 11, 1023. [Google Scholar] [CrossRef]
- Kocijan, M.; Ćurković, M.; Gonçalves, G.; Podlogar, M. The Potential of rGO@TiO2 Photocatalyst for the Degradation of Organic Pollutants in Water. Sustainability 2022, 14, 12703. [Google Scholar] [CrossRef]
- Srivastava, R.R.; Vishwakarma, P.K.; Yadav, U.; Rai, S.; Umrao, S.; Giri, R.; Saxena, P.S.; Srivastava, A. 2D SnS2 Nanostructure-Derived Photocatalytic Degradation of Organic Pollutants Under Visible Light. Front. Nanotechnol. 2021, 3, 711368. [Google Scholar] [CrossRef]
- Lebedev, L.A.; Chebanenko, M.I.; Dzhevaga, E.V.; Martinson, K.D.; Popkov, V.I. Solvothermal modification of graphitic C3N4 with Ni and Co phthalocyanines. Mendeleev Commun. 2022, 32, 317–319. [Google Scholar] [CrossRef]
- Kocijan, M.; Ćurković, L.; Ljubas, D.; Mužina, K.; Bačić, I.; Radošević, T.; Podlogar, M.; Bdikin, I.; Otero-Irurueta, G.; Hortigüela, M.J.; et al. Graphene-Based TiO2 Nanocomposite for Photocatalytic Degradation of Dyes in Aqueous Solution under Solar-Like Radiation. Appl. Sci. 2021, 11, 3966. [Google Scholar] [CrossRef]
- Kocijan, M.; Ćurković, L.; Bdikin, I.; Otero-Irurueta, G.; Hortigüela, M.J.; Gonçalves, G.; Radošević, T.; Vengust, T.; Podlogar, M. Immobilised rGO/TiO2 Nanocomposite for Multi-Cycle Removal of Methylene Blue Dye from an Aqueous Medium. Appl. Sci. 2022, 12, 385. [Google Scholar] [CrossRef]
- Nazim, M.; Parwaz Khan, A.A.; Asiri, A.M.; Kim, J.H. Exploring Rapid Photocatalytic Degradation of Organic Pollutants with Porous CuO Nanosheets: Synthesis, Dye Removal, and Kinetic Studies at Room Temperature. ACS Omega 2021, 6, 2601–2612. [Google Scholar] [CrossRef]
- Lasio, B.; Malfatti, L.; Innocenzi, P. Photodegradation of Rhodamine 6G dimers in silica sol-gel films. J. Photochem. Photobiol. 2013, 271, 93–98. [Google Scholar] [CrossRef]
- Buthiyappan, A.; Abdul Aziz, A.R.; Wan Daud, W.M.A.; Daud, W. Recent advances and prospects of catalytic advanced oxidation process in treating textile effluents. Rev. Chem. Eng. 2016, 32, 1–47. [Google Scholar] [CrossRef]
- Shi, S.; Xu, J.; Li, L. Preparation and Photocatalytic Activity of ZnO Nanorods and ZnO/Cu2O Nanocomposites. Main Group Chem. 2017, 16, 47–55. [Google Scholar] [CrossRef]
- Chebanenko, M.I.; Martinson, K.D.; Matsukevich, I.V.; Popkov, V.I. The effect of MgO additive on the g-C3N4 performance in electrochemical reforming of water-ethanol solution. Nanosyst. Phys. Chem. Math. 2020, 11, 474–479. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Hee Lee, J.; Kim, H.-Y.; Park, S.-J. Novel magnetically separable silver-iron oxide nanoparticles decorated graphitic carbon nitride nano-sheets: A multifunctional photocatalyst via one-step hydrothermal process. J. Colloid Interface Sci. 2017, 496, 343–352. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Kim, H.-Y.; Park, S.-J. Ag-ZnO photocatalyst anchored on carbon nanofibers: Synthesis, characterization, and photocatalytic activities. Synth. Met. 2016, 220, 533–537. [Google Scholar] [CrossRef]
- Elshafie, M.; Younis, S.A.; Serp, P.; Gad, E.A.M. Preparation characterization and non-isothermal decomposition kinetics of different carbon nitride sheets. Egypt. J. Pet. 2019, 29, 21–29. [Google Scholar] [CrossRef]
- Huang, R.; Wu, J.; Zhang, M.; Liu, B.; Zheng, Z.; Luo, D. Strategies to enhance photocatalytic activity of graphite carbon nitride-based photocatalysts. Mater. Des. 2019, 210, 110040. [Google Scholar] [CrossRef]
- Muhmood, T.; Asim Khan, M.; Xia, M.; Lei, W.; Wang, F.; Ouyang, Y. Enhanced photo-electrochemical, photo-degradation and charge separation ability of graphitic carbon nitride (g-C3N4) by self-type metal free heterojunction formation for antibiotic degradation. J. Photochem. Photobiol. A Chem. 2017, 348, 118–124. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, L.; Lin, H.; Nong, Q.; Cui, M.; Wu, Y.; He, Y. Fabrication and characterization of hollow CdMoO4 coupled g-C3N4 heterojunction with enhanced photocatalytic activity. J. Hazard. Mater. 2015, 299, 333–342. [Google Scholar] [CrossRef]
- Sun, S.; Ding, H.; Mei, L.; Chena, Y.; Hao, Q.; Chen, W.; Xu, Z.; Chen, D. Construction of SiO2-TiO2/g-C3N4 composite photocatalyst for hydrogen production and pollutant degradation: Insight into the effect of SiO2. Chin. Chem. Lett. 2020, 31, 2287–2294. [Google Scholar] [CrossRef]
- Mo, Z.; She, X.; Li, Y.; Liu, L.; Huang, L.; Chen, Z.; Zhang, Q.; Xu, H.; Li, H. Synthesis of g-C3N4 at different temperatures for superior visible/UV photocatalytic performance and photoelectrochemical sensing of MB solution. RSC Adv. 2015, 5, 101552–101562. [Google Scholar] [CrossRef]
- Maeda, K.; Wang, X.; Nishihara, Y.; Lu, D.; Antonietti, M.; Domen, K. Photocatalytic Activities of Graphitic Carbon Nitride Powder for Water Reduction and Oxidation under Visible Light. J. Phys. Chem. C 2009, 113, 4940–4947. [Google Scholar] [CrossRef]
- Dong, F.; Wu, L.; Sun, Y.; Fu, M.; Wu, Z.; Lee, S.C. Efficient synthesis of polymeric g-C3N4 layered materials as novel efficient visible light driven photocatalysts. J. Mater. Chem. 2011, 21, 15171–15174. [Google Scholar] [CrossRef]
- Kharlamov, A.; Bondarenko, M.; Kharlamova, G.; Gubareni, N. Features of the synthesis of carbon nitride oxide (g-C3N4)O at urea pyrolysis. Diam. Relat. Mater. 2016, 66, 16–22. [Google Scholar] [CrossRef]
- Chidhambaram, N.; Ravichandran, K. Single step transformation of urea into metal-free g-C3N4 nanoflakes for visible light photocatalytic applications. Mater. Lett. 2017, 207, 44–48. [Google Scholar] [CrossRef]
- Chebanenko, M.I.; Zakharova, N.V.; Lobinsky, A.A.; Popkov, V.I. Ultrasonic-Assisted Exfoliation of Graphitic Carbon Nitride and its Electrocatalytic Performance in Process of Ethanol Reforming. Semiconductors 2019, 53, 28–33. [Google Scholar] [CrossRef]
- Chebanenko, M.I.; Lobinsky, A.A.; Nevedomskiy, V.N.; Popkov, V.I. NiO-decorated Graphitic Carbon Nitride toward Electrocatalytic Hydrogen Production from Ethanol. Dalton Trans. 2020, 49, 12088–12097. [Google Scholar] [CrossRef] [PubMed]
- Chebanenko, M.I.; Lebedev, L.A.; Ugolkov, V.L.; Prasolov, N.D.; Nevedomskiy, V.N.; Popkov, V.I. Chemical and structural changes of g-C3N4 through oxidative physical vapor deposition. Appl. Surf. Sci. 2022, 600, 154079. [Google Scholar] [CrossRef]
- Muhmood, T.; Xia, M.; Lei, W.; Wang, F.; Mahmood, A. Fe-ZrO2 imbedded graphene like carbon nitride for acarbose (ACB) photo-degradation intermediate study. Adv. Powder Technol. 2018, 29, 3233–3240. [Google Scholar] [CrossRef]
- Muhmood, T.; Xia, M.; Lei, W.; Wang, F.; Khan, M.A. Design of Graphene Nanoplatelet/Graphitic Carbon Nitride Heterojunctions by Vacuum Tube with Enhanced Photocatalytic and Electrochemical Response. ur. J. Inorg. Chem. 2018, 2018, 1726–1732. [Google Scholar] [CrossRef]
- Xiuling Guo, X.; Duan, J.; Li, C.; Zhang, Z.; Wang, W. Highly efficient Z-scheme g-C3N4/ZnO photocatalysts constructed by co-melting-recrystallizing mixed precursors for wastewater treatment. J. Mater. Sci. 2020, 55, 2018–2031. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, L.; Kim, E.J.; Hahn, S.H. Electronic structure and optical properties of Zn(OH)2: LDA+U calculations and intense yellow luminescence. RSC Adv. 2015, 5, 87496. [Google Scholar] [CrossRef]
- Guan, R.; Li, J.; Zhang, J.; Zhao, Z.; Wang, D.; Zhai, H.; Sun, D. Photocatalytic Performance and Mechanistic Research of ZnO/g-C3N4 on Degradation of Methyl Orange. ACS Omega 2019, 4, 20742–20747. [Google Scholar] [CrossRef]
- Gayathri, M.; Sakar, M.; Satheeshkumar, E.; Sundaravadivel, E. Insights into the mechanism of ZnO/g-C3N4 nanocomposites toward photocatalytic degradation of multiple organic dyes. J. Mater. Sci. Mater. Electron. 2022, 33, 9347–9357. [Google Scholar] [CrossRef]
- Alharthi, F.A.; Alghamdi, A.A.; Alanazi, H.S.; Alsyahi, A.A.; Ahmad, N. Photocatalytic Degradation of the Light Sensitive Organic Dyes: Methylene Blue and Rose Bengal by Using Urea Derived g-C3N4/ZnO Nanocomposites. Catalysts. 2020, 10, 1457. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chebanenko, M.I.; Tikhanova, S.M.; Nevedomskiy, V.N.; Popkov, V.I. Synthesis and Structure of ZnO-Decorated Graphitic Carbon Nitride (g-C3N4) with Improved Photocatalytic Activity under Visible Light. Inorganics 2022, 10, 249. https://doi.org/10.3390/inorganics10120249
Chebanenko MI, Tikhanova SM, Nevedomskiy VN, Popkov VI. Synthesis and Structure of ZnO-Decorated Graphitic Carbon Nitride (g-C3N4) with Improved Photocatalytic Activity under Visible Light. Inorganics. 2022; 10(12):249. https://doi.org/10.3390/inorganics10120249
Chicago/Turabian StyleChebanenko, Maria I., Sofia M. Tikhanova, Vladimir N. Nevedomskiy, and Vadim I. Popkov. 2022. "Synthesis and Structure of ZnO-Decorated Graphitic Carbon Nitride (g-C3N4) with Improved Photocatalytic Activity under Visible Light" Inorganics 10, no. 12: 249. https://doi.org/10.3390/inorganics10120249
APA StyleChebanenko, M. I., Tikhanova, S. M., Nevedomskiy, V. N., & Popkov, V. I. (2022). Synthesis and Structure of ZnO-Decorated Graphitic Carbon Nitride (g-C3N4) with Improved Photocatalytic Activity under Visible Light. Inorganics, 10(12), 249. https://doi.org/10.3390/inorganics10120249





