Understanding the Catalytic Deactivation upon Hydrothermal Aging at 850 °C of WO3/Fe-Cu-ZSM-5 Catalyst for Selective Catalytic Reduction of NO by NH3
Abstract
:1. Introduction
2. Results
3. Discussion
- -
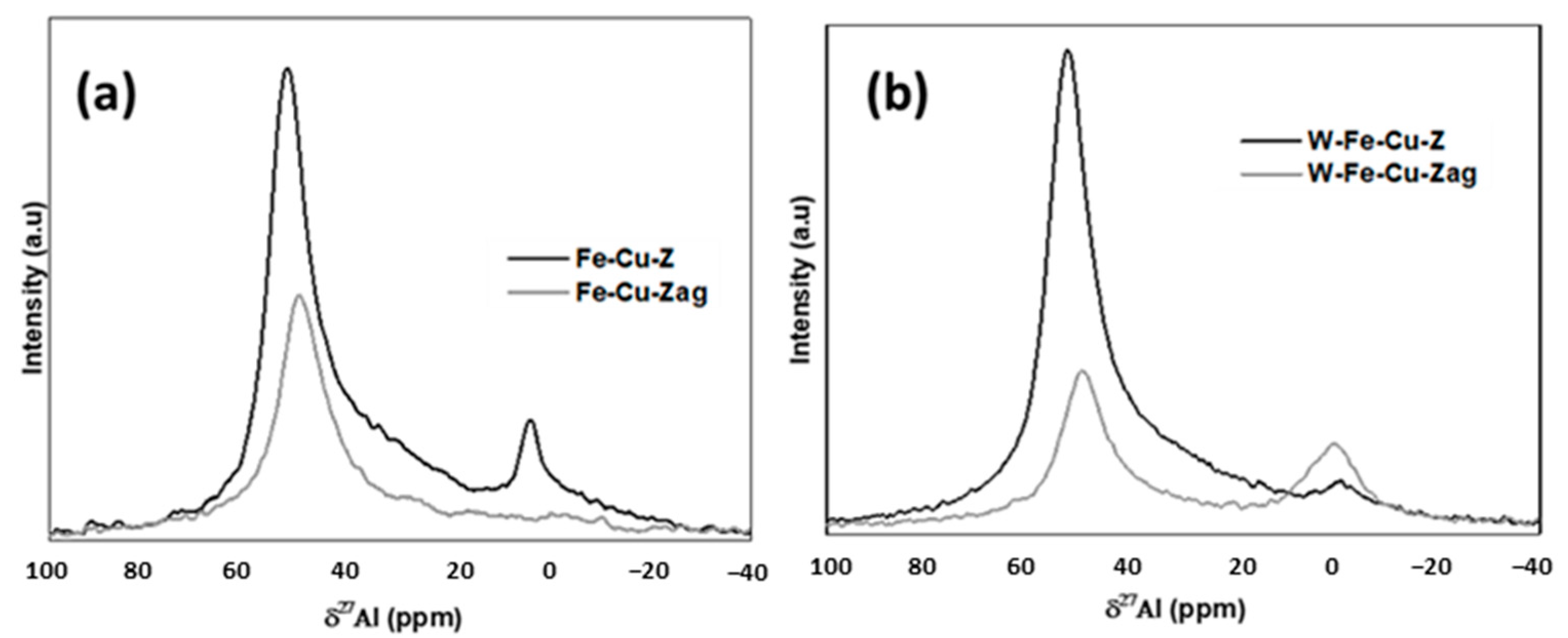
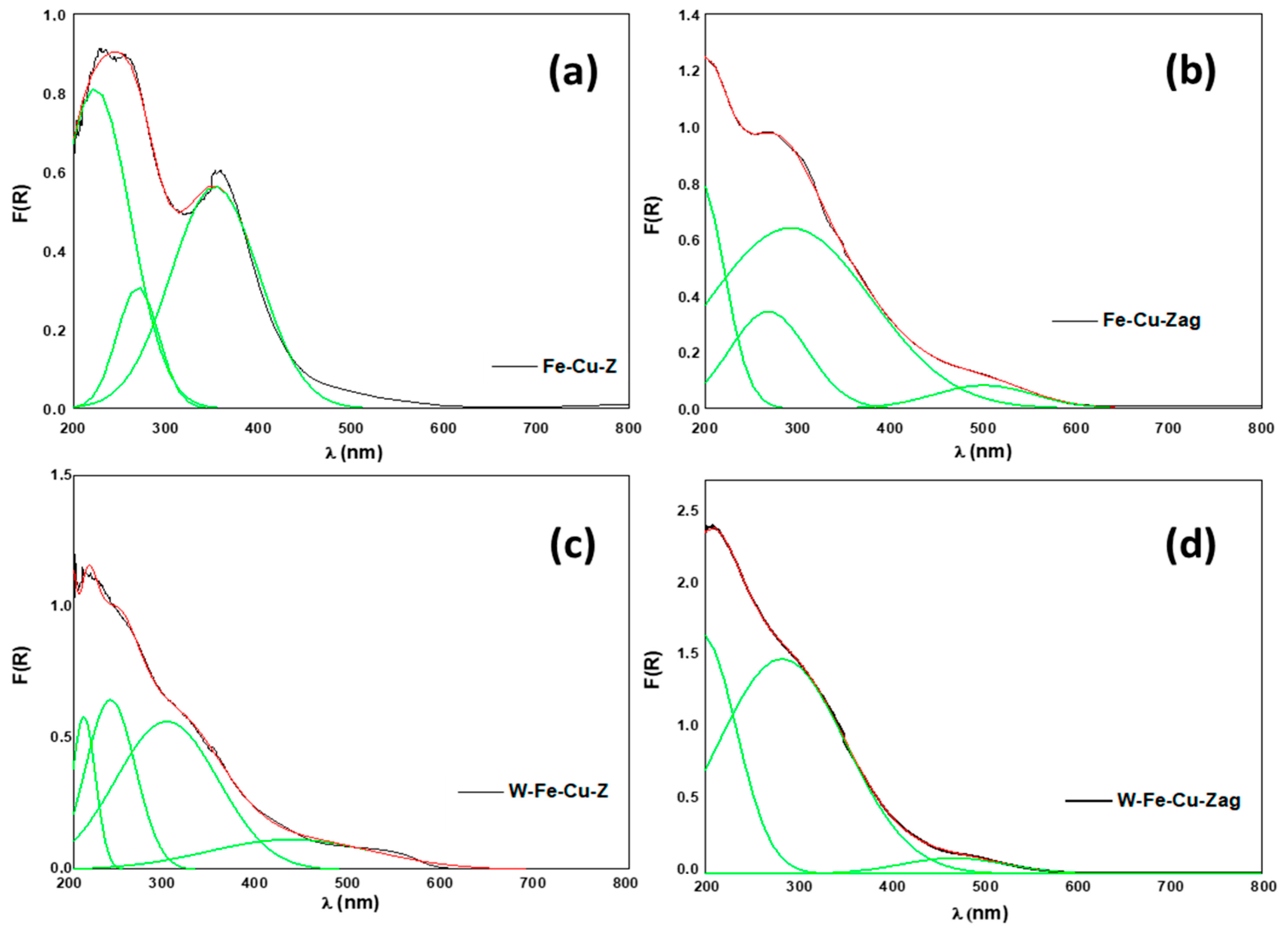
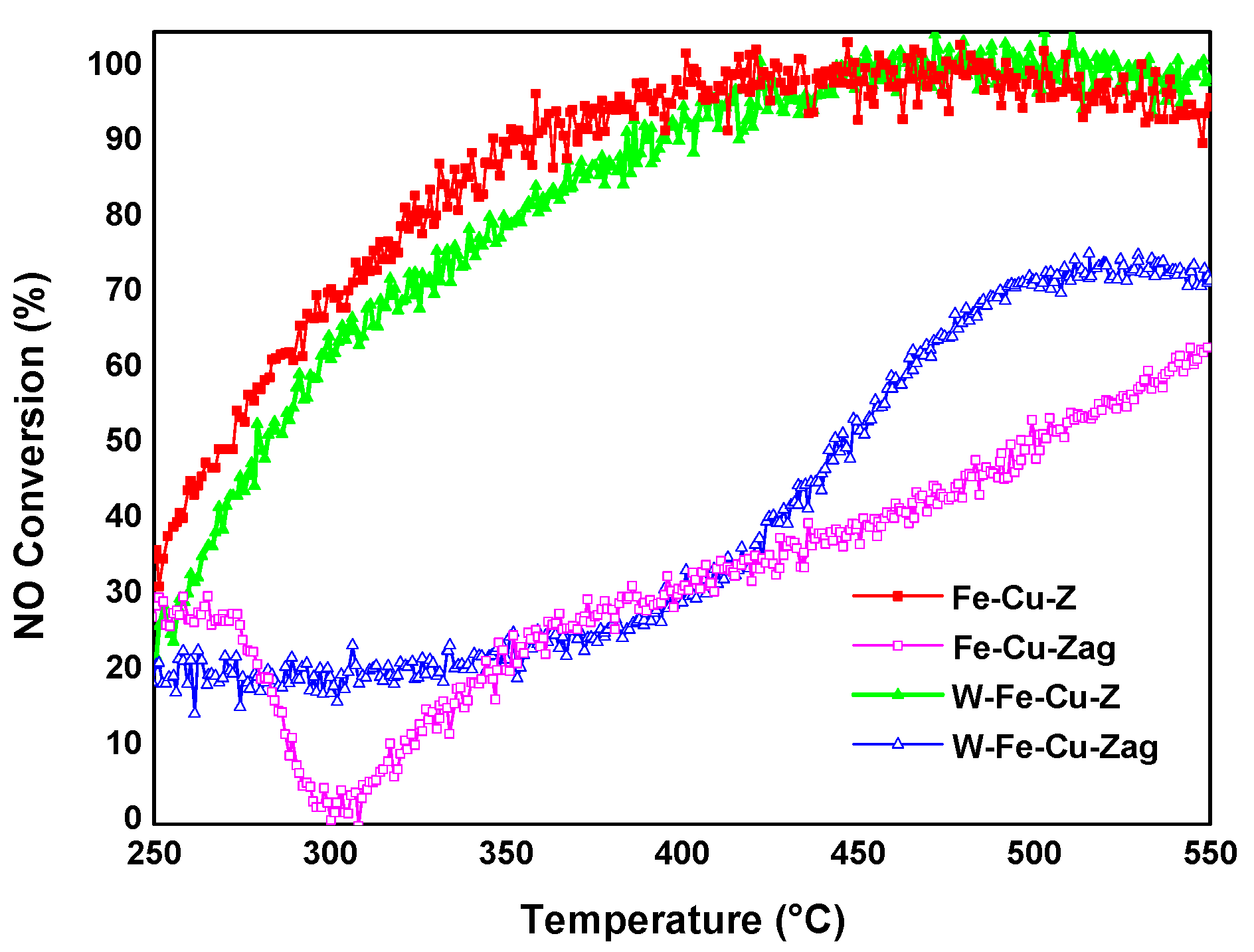
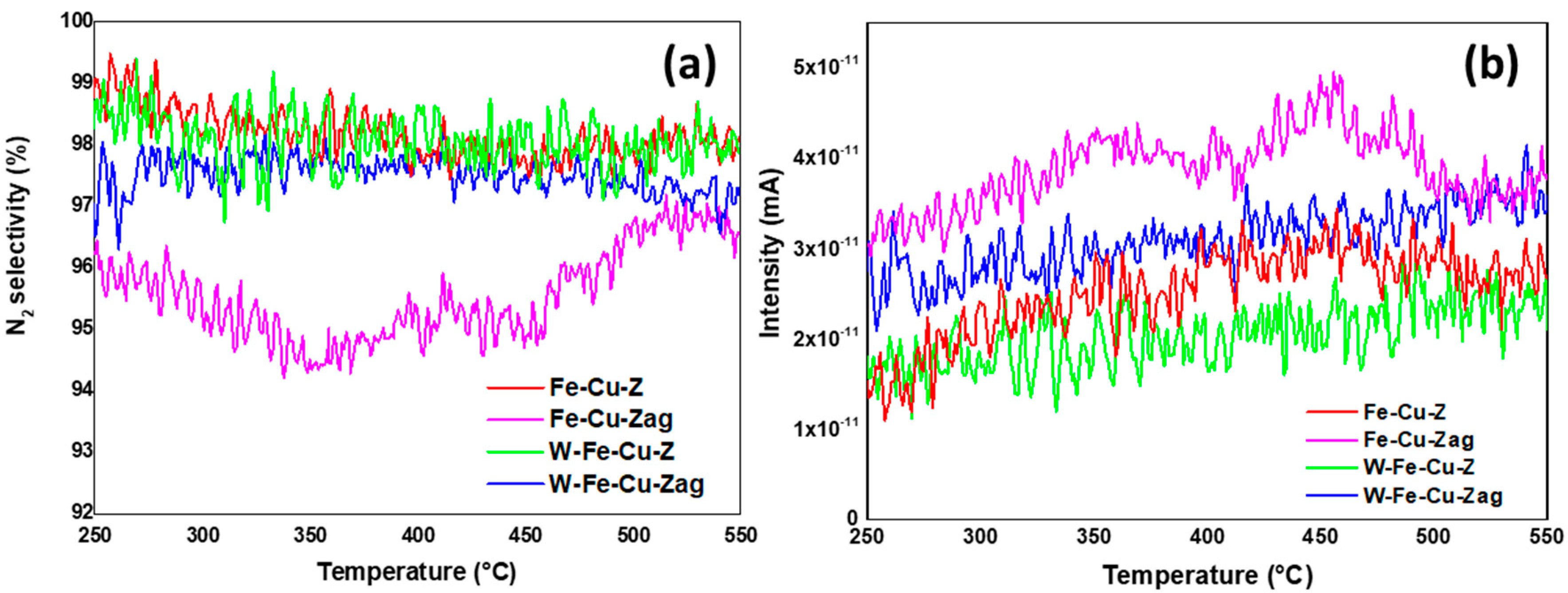
- Iron and copper are known for their good ability to oxidize NO to NO2, which is the rate-determining step of the standard NH3-SCR reaction in the presence of O2 [5,6]. The addition of tungsten into the fresh Fe-Cu-Z did not improve its low temperature catalytic activity at low temperatures probably due to the lack of acidity and/or redox active components. Furthermore, as detected using the UV-vis technique, a fresh Fe-Cu-Z catalyst encloses oligonuclear Fe3+xOy clusters, known as an active site for the NH3-SCR of NO reaction [44]. The ameliorated activity of a fresh W-based catalyst above 450 °C may be ascribed to the presence of a tetrahedrally W(VI) species known as an active site for SCR process [11]. W-O-Si and W-O-Al active sites are the two main types of W species on W-ZSM-5, according to Chen et al. [11]. The presence of the tetrahedral W-O-Al structure was proved using 27Al NMR on a W-Fe-Cu-Z sample.
- -
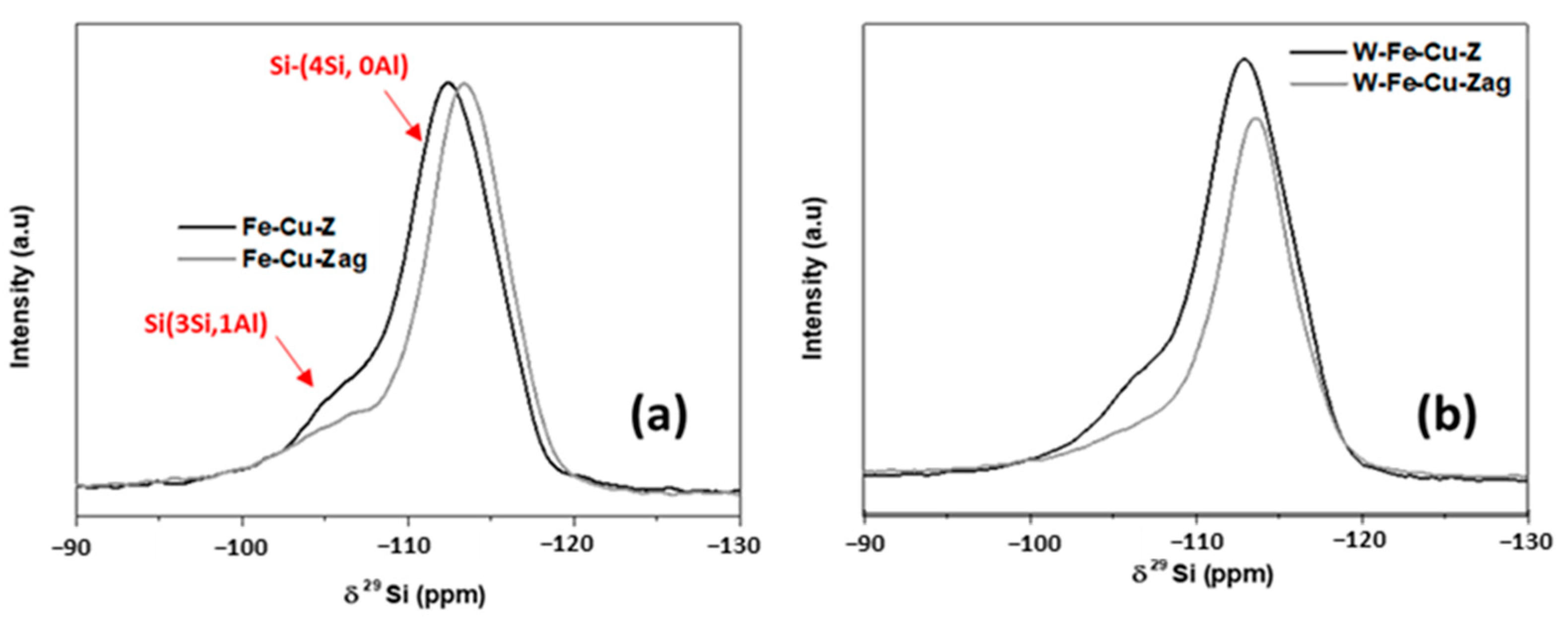
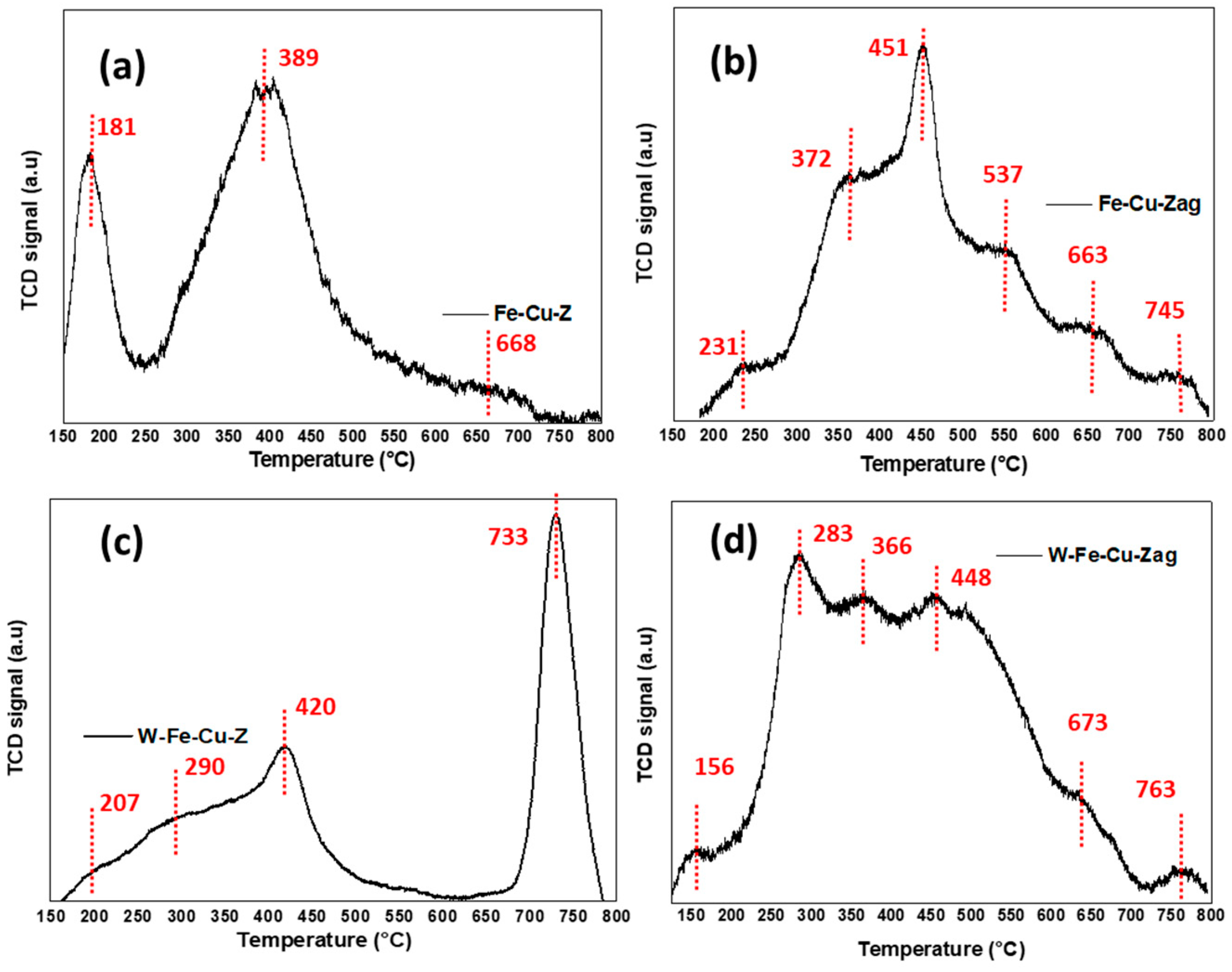
- The aging process considerably deteriorated the NO conversion over the Fe-Cu-Z catalyst; however, this sample showed a better acidity compared to the trimetallic sample above 400 °C. This explains its best starting activity, which dropped to zero as Cu atoms were displaced from their counter-cation locations in the support and formed CuAl2O4 and CuO aggregates detected mainly owing to STEM and H2-TPR techniques, which is in line with our previous study that also draws attention to copper oxide production following high-temperature aging for an Fe-Cu-ZSM-5 system [45]. These agglomerates are responsible for catalytic deactivation and are known to be very active for high temperature NO oxidation, as well NH3 oxidation, with a high selectivity in nitrogen oxide formation [46,47].
- -
- This research points out that the stability of the tungsten-based catalyst at low reaction temperatures against hydrothermal treatment is attributed to its good redox properties as demonstrated from H2-TPR. Since the reducibility of the metal ions controls the extent of low temperature NO conversion in metal exchanged zeolite catalysts, the easier the reduction of metal species, the higher their oxidation ability in the SCR process [48].
- -
- Our results suggest that the studied samples reached the “severe” stage of aging (observed between 750 and 850 °C) as described by Luo et al. [46]. This scenario shows no structural breakdown of the zeolite and is accompanied by an agglomeration of the metal atoms and a lowering of Brönsted acid sites, leading to low temperature NH3 storage and NO conversion, which is in accordance with the findings of our study. In the case of W-Fe-Cu-Zag, the agglomeration of W and Fe as seen from EDX mapping images was beneficial to its catalytic stability. According to earlier research, adding surface tungsten oxide to SCR catalysts increases their reactivity while also having an inhibitory influence on the generation of unwanted N2O during the reaction process [49,50].
4. Materials and Methods
4.1. Catalysts Preparation
4.2. Catalysts Aging
4.3. Physical and Chemical Characterization
4.4. Catalytic Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, J.; Chang, H.; Ma, L.; Hao, J.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts-A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Brandenberger, S.; Kröcher, O.; Tissler, A.; Althoff, R. The State of the Art in Selective Catalytic Reduction of NOx by Ammonia Using Metal-Exchanged Zeolite Catalysts. Catal. Rev. Sci. Eng. 2008, 50, 492–531. [Google Scholar] [CrossRef]
- Kanazawa, T. MFI zeolite as a support for automotive catalysts with reduced Pt sintering. Appl. Catal. B 2006, 65, 185–190. [Google Scholar] [CrossRef]
- Bi, Y.; Chen, L.; Lu, G. Constructing surface active centres using Pd–Fe–O on zeolite for CO oxidation. J. Mol. Catal. A 2007, 266, 173–179. [Google Scholar] [CrossRef]
- Jouini, H.; Mejri, I.; Petitto, C.; Martinez-Ortigosa, J.; Vidal-Moya, A.; Mhamdi, M.; Blasco, T.; Delahay, G. Characterization and NH3-SCR reactivity of Cu-Fe-ZSM-5 catalysts prepared by solid state ion exchange: The metal exchange order effect. Microporous Mesoporous Mat. 2018, 260, 217–226. [Google Scholar] [CrossRef]
- Jouini, H.; Mejri, I.; Martinez-Ortigosa, J.; Cerrillo, J.L.; Mhamdi, M.; Palomares, A.E.; Delahay, G.; Blasco, T. Selective catalytic reduction of nitric oxide with ammonia over Fe-Cu modified highly silicated zeolites. Solid State Sci. 2018, 84, 75–85. [Google Scholar] [CrossRef]
- Jouini, H.; Martinez-Ortigosa, J.; Mejri, I.; Mhamdi, M.; Blasco, T.; Delahay, G. On the performance of Fe-Cu-ZSM-5 catalyst for the selective catalytic reduction of NO with NH3: The influence of preparation method. Res. Chem. Intermed. 2019, 45, 1057–1072. [Google Scholar] [CrossRef]
- Jouini, H.; Mejri, I.; Martinez-Ortigosa, J.; Cerrillo, J.L.; Petitto, C.; Mhamdi, M.; Blasco, T.; Delahay, G. Alkali poisoning of Fe-Cu-ZSM-5 catalyst for the selective catalytic reduction of NO with NH3. Res. Chem. Intermed. 2022, 48, 3415–3428. [Google Scholar] [CrossRef]
- Wu, T.; Li, S.; Yuan, G.; Zhao, D.; Chen, S.; Xu, J.; Hua, T. Enhanced selectivity of propylene in butylene catalytic cracking over W-ZSM-5. Fuel Process Technol. 2018, 173, 143–152. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, G.; Guo, Y.; Guo, Y.; Zhang, Z.; Wang, Y.; Gong, X.-Q. High Performance and Stability of the Pt-W/ZSM-5 Catalyst for the Total Oxidation of Propane: The Role of Tungsten. Chem. Cat. Chem. 2013, 5, 2495–2503. [Google Scholar] [CrossRef]
- Chen, J.; Yan, H.; Gong, H.; Zhang, H.; Zhou, Y.; Gao, C.; Liu, Y.; Chen, X.; Yang, C. Tetrahedrally coordinated W(VI) species induced Lewis acid for stable catalytic cracking of 1-hexene to propene. Chem. Eng. J. 2022, 448, 137504. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Yu, Q.; Yang, H. Tungsten promoted HZSM-5 in the SCR of NO by acetylene. Microporous Mesoporous Mater. 2008, 109, 298–304. [Google Scholar] [CrossRef]
- Liu, H.; You, C.; Wang, H. Experimental and Density Functional Theory Studies on the Zeolite-Based Fe–Ni–W Trimetallic Catalyst for High-Temperature NOx Selective Catalytic Reduction: Identification of Active Sites Suppressing Ammonia Over-oxidation. ACS Catal. 2021, 11, 1189–1201. [Google Scholar] [CrossRef]
- Mohan, S.; Dinesha, P.; Kumar, S. NOx reduction behaviour in copper zeolite catalysts for ammonia SCR systems: A review. Chem. Eng. J. 2019, 123253. [Google Scholar] [CrossRef]
- Yuanqing, Z.; Weihao, Z.; Chong, X.; Qichen, H. Application and Development of Selective Catalytic Reduction Technology for Marine Low-Speed Diesel Engine: Trade-Off among High Sulfur Fuel, High Thermal Efficiency, and Low Pollution Emission. Atmosphere 2022, 13, 731. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 78, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Abid, R.; Delahay, G.; Tounsi, H. Selective catalytic reduction of NO by NH3 on cerium modified faujasite zeolite prepared from aluminum scraps and industrial metasilicate. J. Rare Earths 2019, 38, 250–256. [Google Scholar] [CrossRef]
- Wang, L.-C.; Zhang, Y.; Xu, J.; Diao, W.; Karakalos, S.; Liu, B.; Song, X.; Wu, W.; He, T.; Ding, D. Non-oxidative dehydrogenation of ethane to ethylene over ZSM-5 zeolite supported iron catalysts. Appl. Catal. 2019, 256, 117816. [Google Scholar] [CrossRef]
- Iwasaki, M.; Yamazaki, K.; Banno, K.; Shinjoh, H. Characterization of Fe/ZSM-5 DeNOx catalysts prepared by different methods: Relationships between active Fe sites and NH3-SCR performance. J. Catal. 2008, 260, 205–216. [Google Scholar] [CrossRef]
- Hongrutai, N.; Watmanee, S.; Wannakao, S.; Praserthdam, P.; Panpranot, J. Effect of small amounts of Al on the surface silanol structure and their correlation to the improved catalytic performances of WOx/SiO2–Al2O3 in the propene self-metathesis. Mater. Today Chem. 2021, 21, 100492. [Google Scholar] [CrossRef]
- Wan, C.; Hu, M.Y.; Jaegers, N.R.; Shi, D.; Wang, H.; Gao, F.; Qin, Z.; Wang, Y.; Hu, J.Z. Investigating the Surface Structure of γ-Al2O3 Supported WOX Catalysts by High Field 27Al MAS NMR and Electronic Structure Calculations. J. Phys. Chem. C 2016, 120, 23093–23103. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Yang, K.; Li, Y.; Wang, Y.; Xu, Y.; Wu, P. Effect of hydrothermal treatment on catalytic properties of PtSnNa/ZSM-5 catalyst for propane dehydrogenation. Microporous Mesoporous Mater. 2006, 96, 245–254. [Google Scholar] [CrossRef]
- Kuehl, G.H.; Timken, H.K.C. Acid sites in zeolite Beta: Effects of ammonium exchange and steaming. Microporous Mesoporous Mater. 2005, 21, 35–36. [Google Scholar] [CrossRef]
- Rhimi, B.; Mhamdi, M.; Kalevaru, V.N.; Martin, A. Synergy between vanadium and molybdenum in bimetallic ZSM-5 supported catalysts for ethylene ammoxidation. RSC Adv. 2016, 6, 65866–65878. [Google Scholar] [CrossRef]
- Engelhardt, G.; Lohse, U.; Samoson, A.; Mägi, M.; Tarmak, M.; Lippmaa, E. High resolution 29Si n.m.r. of dealuminated and ultrastable Y-zeolites. Zolites 1982, 2, 59–62. [Google Scholar] [CrossRef]
- Ismagilov, Z.R.; Yashnik, S.A.; Anufrienko, V.F.; Larina, T.V.; Vasenin, N.T.; Bulgakov, N.N.; Vosel, S.V.; Tsykoza, L.T. Linear nanoscale clusters of CuO in Cu-ZSM-5 catalysts. Appl. Surf. Sci. 2004, 226, 88–93. [Google Scholar] [CrossRef]
- Wilken, N.; Nedyalkova, R.; Kamasamudram, K.; Li, J.; Currier, N.W.; Vedaiyan, R.; Yezerets, A.; Olsson, L. Investigation of the Effect of Accelerated Hydrothermal Aging on the Cu Sites in a Cu-BEA Catalyst for NH3-SCR Applications. Top. Catal. 2013, 56, 317–322. [Google Scholar] [CrossRef]
- Gutiérrez-Alejandre, A.; Ramírez, J.; Busca, G. The electronic structure of oxide-supported tungsten oxide catalysts as studied by UV spectroscopy. Catal. Lett. 1998, 56, 29–33. [Google Scholar] [CrossRef]
- Ramanathan, A.; Subramaniam, B.; Badloe, D.; Hanefeld, U.; Maheswari, R. Direct incorporation of tungsten into ultra-large-pore three-dimensional mesoporous silicate framework: W-KIT-6. J. Porous Mater. 2012, 19, 961–968. [Google Scholar] [CrossRef]
- Zhao, D.; Rodriguez, A.; Dimitrijevic, N.M.; Rajh, T.; Koodali, R.T. Comparative Influence of Surface Tungstate Species and Bulk Amorphous WO3 Particles on the Acidity and Catalytic Activity of Tungsten Oxide Supported on Silica. J. Phys. Chem. C 2010, 114, 15728–15734. [Google Scholar] [CrossRef]
- Chauvin, J.; Thomas, K.; Clet, G.; Houalla, M. Comparative Influence of Surface Tungstate Species and Bulk Amorphous WO3 Particles on the Acidity and Catalytic Activity of Tungsten Oxide Supported on Silica. J. Phys. Chem. C 2015, 119, 12345–12355. [Google Scholar] [CrossRef]
- Delahay, G.; Coq, B.; Broussous, L. Selective catalytic reduction of nitrogen monoxide by decane on copper-exchanged beta zeolites. Appl. Catal. B Environ. 1997, 12, 49–59. [Google Scholar] [CrossRef]
- Delahay, G.; Guzmán-Vargas, A.; Valade, D.; Coq, B. Selective Catalytic Reduction of NO by NH3 on Fe-ZSM-5 Elaborated from Different Methods. Stud. Surf. Sci. Catal. C 2004, 154, 2501–2508. [Google Scholar]
- Kim, Y.J.; Lee, J.K.; Min, K.M.; Hong, S.B.; Nam, I.-S.; Cho, B.K. Hydrothermal stability of CuSSZ13 for reducing NOx by NH3. J. Catal. 2014, 311, 447–457. [Google Scholar] [CrossRef]
- Cavataio, G.; Jen, H.-W.; Warner, J.R.; Girard, J.W.; Kim, J.Y.; Lambert, C.K. Enhanced Durability of a Cu/Zeolite Based SCR Catalyst. SAE Int. J. Fuels Lubr. 2008, 1, 477–487. [Google Scholar] [CrossRef]
- Putluru, S.S.R.; Schill, L.; Jensen, A.D.; Fehrmann, R.S.N. Selective Catalytic Reduction of NOx with NH3 on Cu-, Fe-, and Mn-Zeolites Prepared by Impregnation: Comparison of Activity and Hydrothermal Stability. J. Chem. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [Green Version]
- de Lucas, A.; Valverde, J.L.; Rodriguez, L.; Sanchez, P.; Garcia, M.T. Modified W/HZSM-5 catalysts: Structure and catalytic properties. J. Mol. Catal. A 2001, 171, 195–203. [Google Scholar] [CrossRef]
- Wang, C.; Yang, S.; Chang, H.; Peng, Y.; Li, J. Dispersion of tungsten oxide on SCR performance of V2O5 single bond WO3/TiO2: Acidity, surface species and catalytic activity. Chem. Eng. J. 2013, 225, 520–527. [Google Scholar] [CrossRef]
- Bahmanpour, A.M.; Le Monnier, B.P.; Du, Y.-P.; Héroguel, F.; Luterbacher, J.S.; Kröcher, O. Increasing the activity of the Cu/CuAl2O4/Al2O3 catalyst for the RWGS through preserving the Cu2+ ions. Chem. Comm. 2021, 57, 1153–1156. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of Integrated Molar Extinction Coefficients for Infrared Absorption Bands of Pyridine Adsorbed on Solid Acid Catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Bhuiyan, T.I.; Arudra, P.; Akhtar, M.N.; Aitani, A.M.; Abudawoud, R.H.; Al-Yami, M.A.; Al-Khattaf, S.S. Metathesis of 2-butene to propylene over W-mesoporous molecular sieves: A comparative study between tungsten containing MCM-41 and SBA-15. Appl. Catal. A Gen. 2013, 467, 224–234. [Google Scholar] [CrossRef]
- Väliheikki, A.; Kolli, T.; Huuhtanen, M.; Maunula, T.; Kinnunen, T.; Keiski, R.L. The Effect of Biofuel Originated Potassium and Sodium on the NH3-SCR Activity of Fe–ZSM-5 and W–ZSM-5 Catalysts. Top. Catal. 2013, 56, 602–610. [Google Scholar] [CrossRef]
- Madia, G.; Koebel, M.; Elsener, M.; Wokaun, A. Side Reactions in the Selective Catalytic Reduction of NOx with Various NO2 Fractions. Ind. Eng. Chem. Res. 2002, 41, 4008–4015. [Google Scholar] [CrossRef]
- Heinrich, F.; Schmidt, C.; Löffler, E.; Menzel, M.; Grünert, W. Fe–ZSM-5 Catalysts for the Selective Reduction of NO by Isobutane—The Problem of the Active Sites. J. Catal. 2002, 212, 157–172. [Google Scholar] [CrossRef]
- Jouini, H.; Mejri, I.; Rhimi, B.; Mhamdi, M.; Blasco, T.; Delahay, G. Ce-promoted Fe–Cu–ZSM-5 catalyst: SCR-NO activity and hydrothermal stability. Res. Chem. Intermed. 2021, 47, 2901–2915. [Google Scholar] [CrossRef]
- Luo, J.; An, H.; Kamasamudram, K.; Currier, N.; Yezerets, A.; Watkins, T.; Allard, L. Impact of Accelerated Hydrothermal Aging on Structure and Performance of Cu-SSZ-13 SCR Catalysts. SAE Int. J. Eng. 2015, 8, 1181–1186. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, J.; Guo, L.; Zhang, W.; Zhao, H.; Wu, X. Research progress of hydrothermal stability of metal-based zeolite catalysts in NH3-SCR reaction. J. Fuel Chem. Technol. 2020, 48, 1193–1207. [Google Scholar] [CrossRef]
- Arakawa, K.; Matsuda, S.; Kinoshita, H. SOx poisoning mechanism of NOx selective reduction catalysts. Appl. Surf. Sci. 1997, 121–122, 382–386. [Google Scholar] [CrossRef]
- Jaegers, N.R.; Lai, J.-K.; He, Y.; Walter, E.; Dixon, D.A.; Vasiliu, M.; Chen, Y.; Wang, C.; Hu, M.Y.; Mueller, K.T.; et al. Mechanism by which Tungsten Oxide Promotes the Activity of Supported V2O5/TiO2 Catalysts for NOX Abatement: Structural Effects Revealed by 51V MAS NMR Spectroscopy. Angew. Chem. 2019, 131, 12739–12746. [Google Scholar] [CrossRef]
- Ye, D.; Qu, R.; Song, H.; Zheng, C.; Gao, X.; Luo, Z.; Ni, M.; Cen, K. Investigation of the promotion effect of WO3 on the decomposition and reactivity of NH4HSO4 with NO on V2O5–WO3/TiO2 SCR catalysts. RSC Adv. 2016, 6, 55584–55592. [Google Scholar] [CrossRef]


| Sample Label | Theoretical Composition | Fe (wt.%) | Cu (wt.%) | W (wt.%) |
|---|---|---|---|---|
| Fe-Cu-Z | Fe (2 wt.%)-Cu (1.5 wt.%) | 1.83 | 1.40 | - |
| W-Fe-Cu-Z | W (2 wt.%)-Fe (2 wt.%)-Cu (1.5 wt.%) | 1.72 | 1.48 | 2.02 |
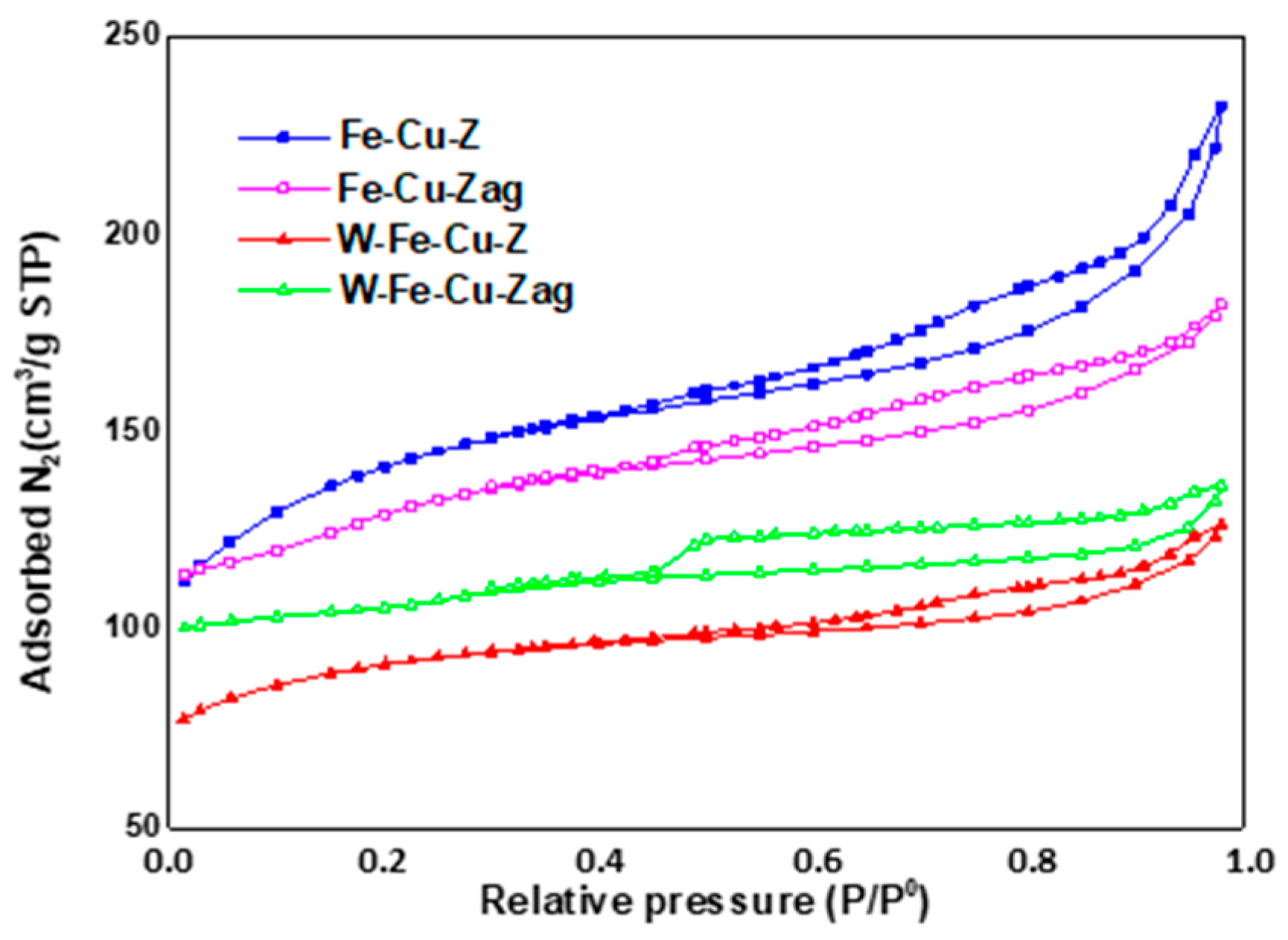
| Sample | SBET a (m2/g) | Micropore Volume b (cm3/g) | Pore Size c (Å) |
|---|---|---|---|
| Fe-Cu-Z | 327 | 0.110 | 287.90 |
| Fe-Cu-Zag | 291 | 0.046 | 56.92 |
| W-Fe-Cu-Z | 304 | 0.063 | 69.94 |
| W-Fe-Cu-Zag | 306 | 0.122 | 29.45 |
| Catalyst | λ (nm) | Attribution | Reference |
|---|---|---|---|
| 223 | isolated mononuclear Fe3+ (Td) | [25] | |
| Fe-Cu-Z | 271 | isolated mononuclear Fe3+ (Oh) | [25] |
| (Figure 7a) | 353 | oligomeric Fe3+xOy clusters | [25] |
| 201 | zeolite matrix | [26] | |
| Fe-Cu-Zag | 265 | isolated mononuclear Fe3+ (Oh) | [25] |
| (Figure 7b) | 292 | isolated mononuclear Fe3+ (Oh) | [25] |
| 501 | Bulk CuO | [27] | |
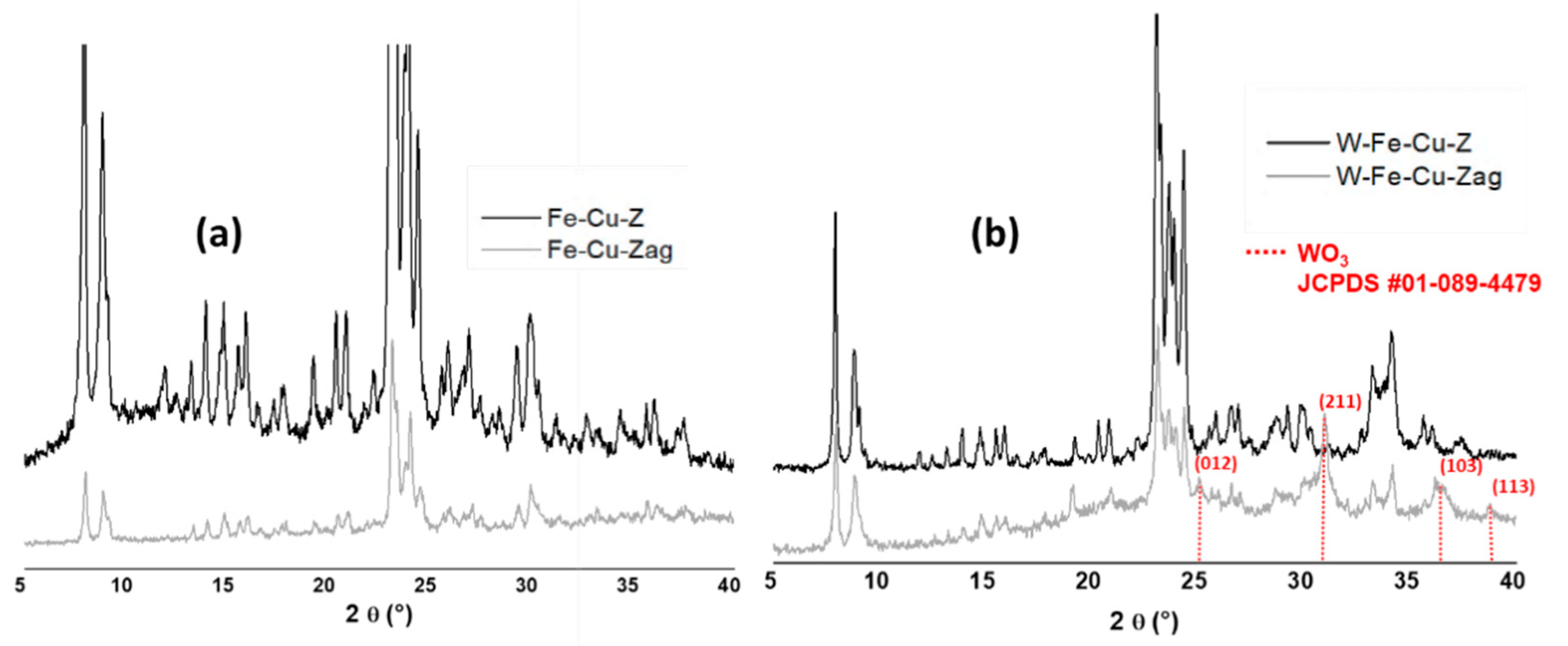
| 211 | partially polymerized W(VI)/Surface WO3 | [28,29] | |
| W-Fe-Cu-Z | 240 | W species (low nuclearity)/tetrahedrally coordinated W (VI) | [11,29] |
| (Figure 7c) | 301 | oligomeric Fe3+xOy clusters | [25] |
| 435 | Bulk WO3 | [30] | |
| W-Fe-Cu-Zag | 198 | MFI matrix | [26] |
| (Figure 7d) | 284 | isolated mononuclear Fe3+ (Oh) | [25] |
| 478 | Bulk WO3 | [30] |
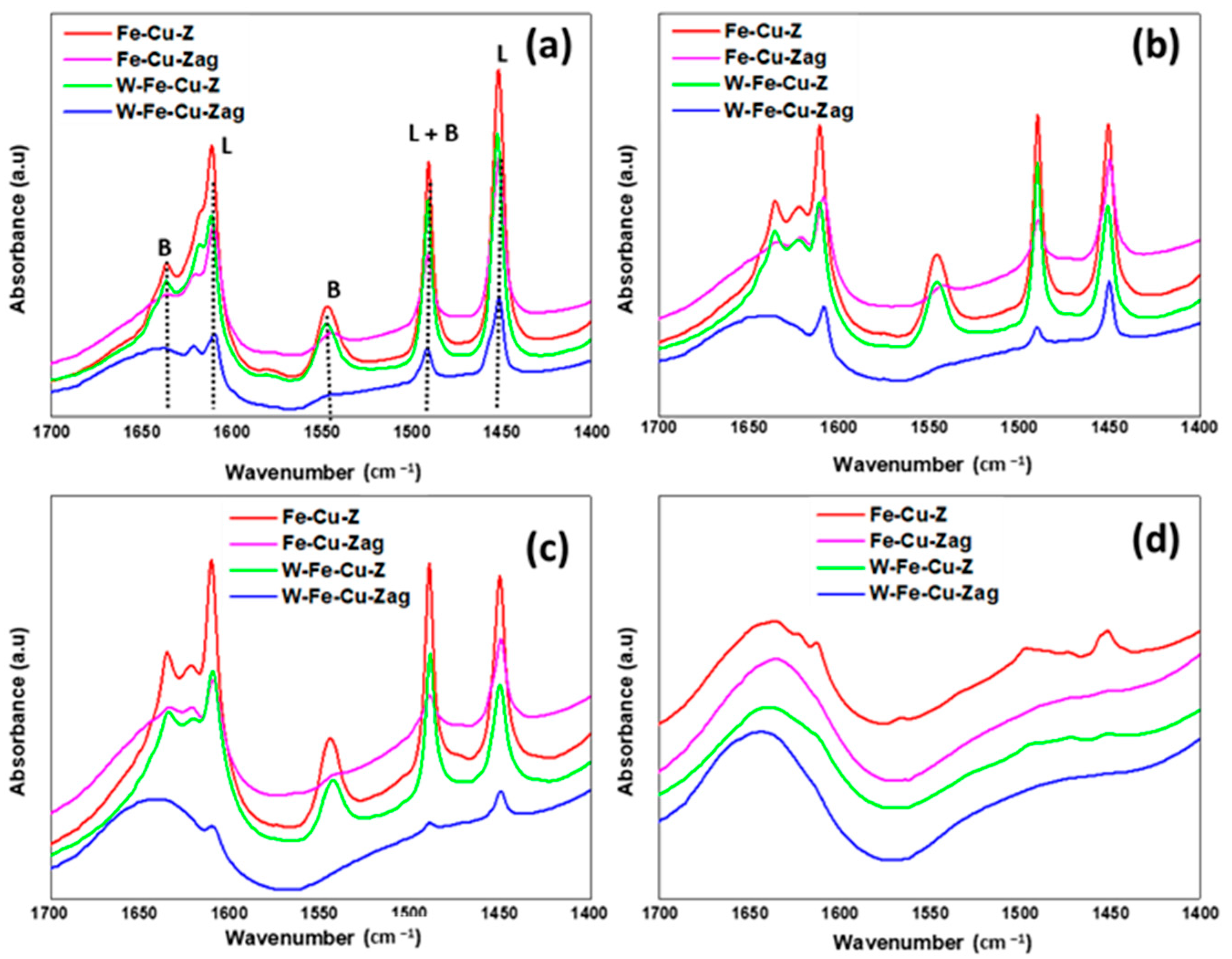
| Sample | Temperature (°C) | Acid Centers (µmol Py/gr) | |||
|---|---|---|---|---|---|
| Brönsted | Lewis | B350/B150 | L350/L150 | ||
| Fe-Cu-Z | 150 | 526 | 999 | 0.67 | 0.35 |
| 250 | 495 | 499 | |||
| 350 | 356 | 353 | |||
| Fe-Cu-Zag | 150 | 86 | 341 | 0.24 | 0.27 |
| 250 | 55 | 164 | |||
| 350 | 21 | 92 | |||
| W-Fe-Cu-Z | 150 | 380 | 715 | 0.70 | 0.31 |
| 250 | 378 | 307 | |||
| 350 | 265 | 224 | |||
| W-Fe-Cu-Zag | 150 | 34 | 185 | 0.051 | 0.20 |
| 250 | 16 | 120 | |||
| 350 | 2 | 38 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jouini, H.; de Marcos-Galán, A.; Mejri, I.; Bensouilah, R.; Mhamdi, M.; Blasco, T.; Delahay, G. Understanding the Catalytic Deactivation upon Hydrothermal Aging at 850 °C of WO3/Fe-Cu-ZSM-5 Catalyst for Selective Catalytic Reduction of NO by NH3. Inorganics 2022, 10, 180. https://doi.org/10.3390/inorganics10110180
Jouini H, de Marcos-Galán A, Mejri I, Bensouilah R, Mhamdi M, Blasco T, Delahay G. Understanding the Catalytic Deactivation upon Hydrothermal Aging at 850 °C of WO3/Fe-Cu-ZSM-5 Catalyst for Selective Catalytic Reduction of NO by NH3. Inorganics. 2022; 10(11):180. https://doi.org/10.3390/inorganics10110180
Chicago/Turabian StyleJouini, Houda, Alessandra de Marcos-Galán, Imène Mejri, Rahma Bensouilah, Mourad Mhamdi, Teresa Blasco, and Gérard Delahay. 2022. "Understanding the Catalytic Deactivation upon Hydrothermal Aging at 850 °C of WO3/Fe-Cu-ZSM-5 Catalyst for Selective Catalytic Reduction of NO by NH3" Inorganics 10, no. 11: 180. https://doi.org/10.3390/inorganics10110180
APA StyleJouini, H., de Marcos-Galán, A., Mejri, I., Bensouilah, R., Mhamdi, M., Blasco, T., & Delahay, G. (2022). Understanding the Catalytic Deactivation upon Hydrothermal Aging at 850 °C of WO3/Fe-Cu-ZSM-5 Catalyst for Selective Catalytic Reduction of NO by NH3. Inorganics, 10(11), 180. https://doi.org/10.3390/inorganics10110180







