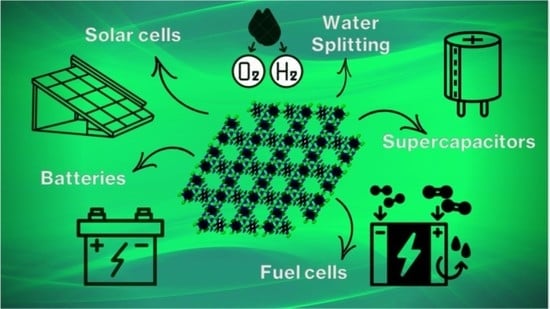
MOFs for Electrochemical Energy Conversion and Storage
Abstract
:1. Introduction
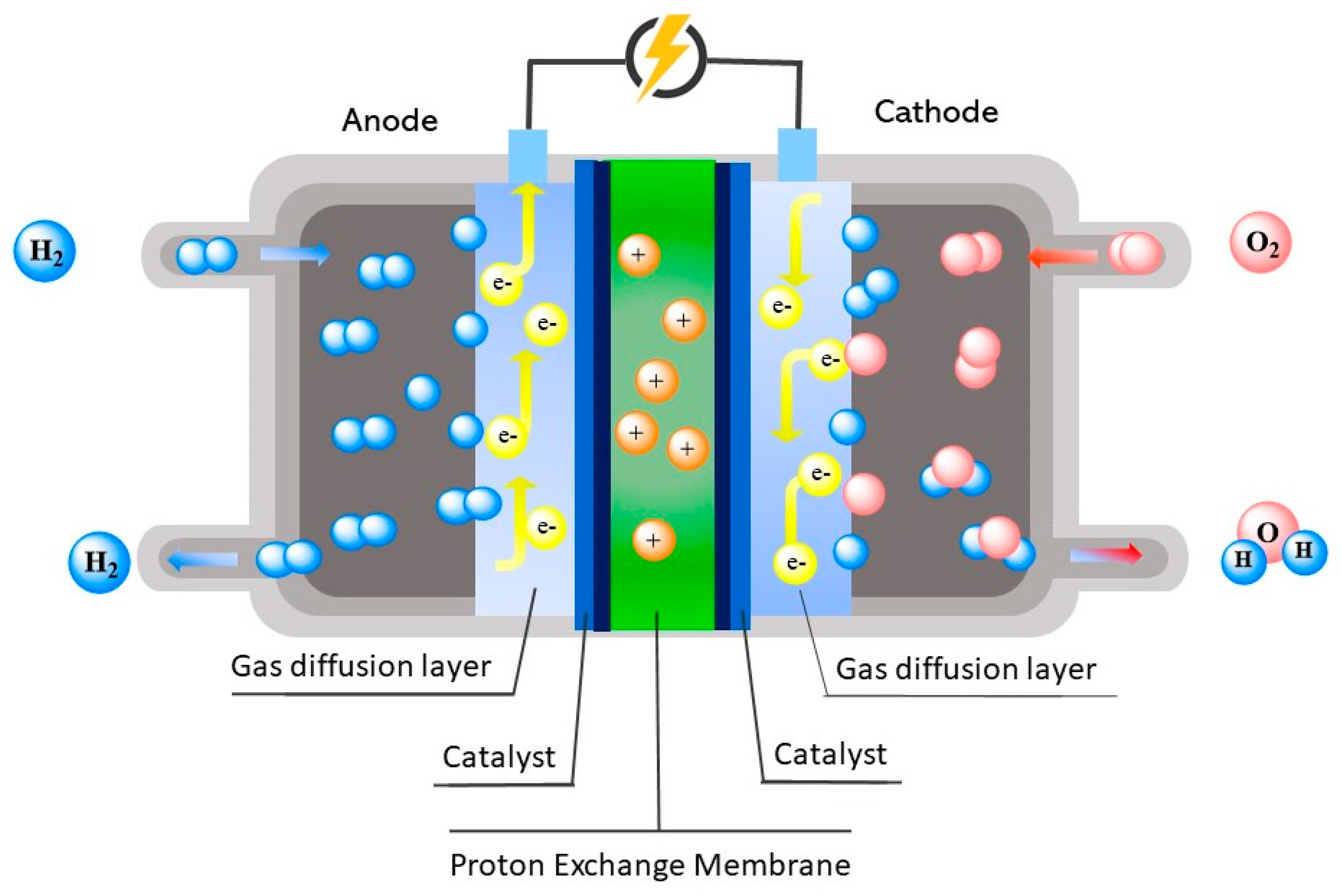
2. Fuel Cells
2.1. PEMFC
2.2. DMFC
3. Solar Cells
3.1. PSCs
3.2. Other Solar Cells
4. Batteries
4.1. Li-ion Batteries (LIBs)
4.2. Na-Ion Batteries
4.3. Other Metal-Ion, Metal Air Batteries
5. Supercapacitors
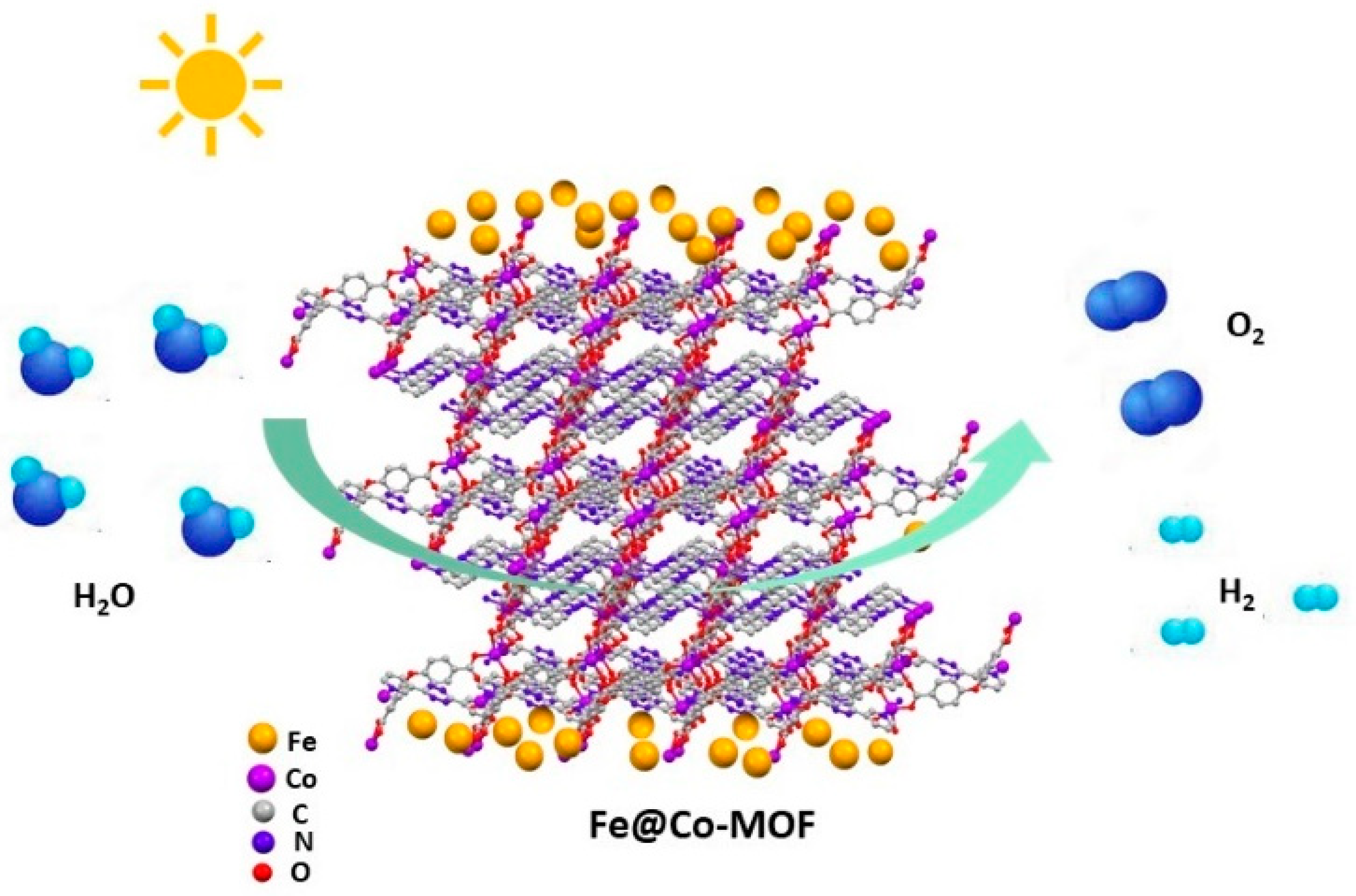
6. Water Splitting Reaction
7. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lawson, H.D.; Walton, S.P.; Chan, C. Metal-Organic Frameworks for Drug Delivery: A Design Perspective. ACS Appl. Mater. Interfaces 2021, 13, 7004–7020. [Google Scholar] [CrossRef] [PubMed]
- Rowsell, J.L.C.; Spencer, E.C.; Eckert, J.; Howard, J.A.K.; Yaghi, O.M. Gas adsorption sites in a large-pore metal-organic framework. Science 2005, 309, 1350–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.C. Recent advances in gas storage and separation using metal–organic frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Xia, W.; Mahmood, A.; Zou, R.; Xu, Q. Metal–organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ. Sci. 2015, 8, 1837–1866. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; Keeffe, M.O.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks The Chemistry and Applications of. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Chen, S.; Chen, Y.; Zhao, M.; Losic, D.; Zhang, S. Metal-organic frameworks containing solid-state electrolytes for lithium metal batteries and beyond. Mater. Chem. Front. 2021, 5, 1771–1794. [Google Scholar] [CrossRef]
- Calbo, J.; Golomb, M.J.; Walsh, A. Redox-active metal–organic frameworks for energy conversion and storage. J. Mater. Chem. A 2019, 7, 16571–16597. [Google Scholar] [CrossRef]
- Cheng, H.; Shapter, J.G.; Li, Y.; Gao, G. Recent progress of advanced anode materials of lithium-ion batteries. J. Energy Chem. 2021, 57, 451–468. [Google Scholar] [CrossRef]
- Baumann, A.E.; Burns, D.A.; Liu, B.; Thoi, V.S. Metal-organic framework functionalization and design strategies for advanced electrochemical energy storage devices. Commun. Chem. 2019, 2, 86. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.B.; Lou, X.W. Metal-organic frameworks and their derived materials for electrochemical energy storage and conversion: Promises and challenges. Sci. Adv. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Dědek, I.; Kupka, V.; Jakubec, P.; Šedajová, V.; Jayaramulu, K.; Otyepka, M. Metal-organic framework/conductive polymer hybrid materials for supercapacitors. Appl. Mater. Today 2022, 26, 101387. [Google Scholar] [CrossRef]
- Nik Zaiman, N.F.H.; Shaari, N.; Harun, N.A.M. Developing metal-organic framework-based composite for innovative fuel cell application: An overview. Int. J. Energy Res. 2022, 46, 471–504. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Q.; Liu, J.; Zhou, J.-E.; Lin, X.; Zeb, A.; Chenna, R.; Reddy, K.; Xu, X. Recent advances in Fe-based metal–organic framework derivatives for battery applications. Sustain. Energy Fuels 2022, 6, 2665–2691. [Google Scholar] [CrossRef]
- Wei, T.; Wang, Z.-M.; Zhang, Q.; Zhou, Y.; Sun, C.; Wang, M.; Liu, Y.; Qiu, X.-Y.; Xu, S.; Qin, S. Metal-Organic Frameworks-Based Solid-State Electrolytes for All Solid-State Lithium Metal Batteries: A Review. CrystEngComm 2022, 2, 5014–5030. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Q.; Xie, Q.; Ou, H.; Lin, X.; Zeb, A.; Hu, L.; Wu, Y.; Ma, G. Recent progress in Co–based metal–organic framework derivatives for advanced batteries. J. Mater. Sci. Technol. 2022, 96, 262–284. [Google Scholar] [CrossRef]
- Peng, Y.; Xu, J.; Xu, J.; Ma, J.; Bai, Y.; Cao, S.; Zhang, S.; Pang, H. Metal-organic framework (MOF) composites as promising materials for energy storage applications. Adv. Colloid Interface Sci. 2022, 307, 102732. [Google Scholar] [CrossRef]
- Zeeshan, M.; Shahid, M. State of the art developments and prospects of metal–organic frameworks for energy applications. Dalt. Trans. 2022, 51, 1675–1723. [Google Scholar] [CrossRef]
- Yan, J.; Liu, T.; Liu, X.; Yan, Y.; Huang, Y. Metal-organic framework-based materials for flexible supercapacitor application. Coord. Chem. Rev. 2022, 452, 214300. [Google Scholar] [CrossRef]
- Zhou, D.; Wu, T.; Xiao, Z. Self-supported metal-organic framework nanoarrays for alkali metal ion batteries. J. Alloys Compd. 2022, 894, 162415. [Google Scholar] [CrossRef]
- Chen, Y.; Du, W.; Dou, B.; Chen, J.; Hu, L.; Zeb, A.; Lin, X. Metal–organic frameworks and their derivatives as electrode materials for Li-ion batteries: A mini review. CrystEngComm 2022, 24, 2729–2743. [Google Scholar] [CrossRef]
- Ye, Z.; Jiang, Y.; Li, L.; Wu, F.; Chen, R. Rational Design of MOF-Based Materials for Next-Generation Rechargeable Batteries. Nano Micro Lett. 2021, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.F.; Fang, Y.; Luan, D.; Lou, X.W.D. Metal−organic frameworks derived functional materials for electrochemical energy storage and conversion: A mini review. Nano Lett. 2021, 21, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Gordeeva, L.G.; Tu, Y.D.; Pan, Q.; Palash, M.L.; Saha, B.B.; Aristov, Y.I.; Wang, R.Z. Metal-organic frameworks for energy conversion and water harvesting: A bridge between thermal engineering and material science. Nano Energy 2021, 84, 105946. [Google Scholar] [CrossRef]
- Chuhadiya, S.; Himanshu; Suthar, D.; Patel, S.L.; Dhaka, M.S. Metal organic frameworks as hybrid porous materials for energy storage and conversion devices: A review. Coord. Chem. Rev. 2021, 446, 214115. [Google Scholar] [CrossRef]
- Verma, J.; Kumar, D. Metal-ion batteries for electric vehicles: Current state of the technology, issues and future perspectives. Nanoscale Adv. 2021, 3, 3384–3394. [Google Scholar] [CrossRef]
- Qiu, T.; Liang, Z.; Guo, W.; Tabassum, H.; Gao, S.; Zou, R. Metal–Organic Framework-Based Materials for Energy Conversion and Storage. ACS Energy Lett. 2020, 5, 520–532. [Google Scholar] [CrossRef] [Green Version]
- El Boutaybi, M.; Taleb, A.; Touzani, R.; Bahari, Z. Metal-organic frameworks based on pyrazole subunit for batteries applications: A systematic review. Mater. Today Proc. 2020, 31, S96–S102. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, N.; Li, S.; Ke, Q.; Li, Z.; Zhou, M. Metal-organic framework composites for energy conversion and storage. J. Semicond. 2020, 41, 091707. [Google Scholar] [CrossRef]
- Indra, A.; Song, T.; Paik, U. Metal Organic Framework Derived Materials: Progress and Prospects for the Energy Conversion and Storage. Adv. Mater. 2018, 30, 1705146. [Google Scholar] [CrossRef]
- Mehtab, T.; Yasin, G.; Arif, M.; Shakeel, M.; Korai, R.M.; Nadeem, M.; Muhammad, N.; Lu, X. Metal-organic frameworks for energy storage devices: Batteries and supercapacitors. J. Energy Storage 2019, 21, 632–646. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Mekhilef, S.; Saidur, R.; Safari, A. Comparative study of different fuel cell technologies. Renew. Sustain. Energy Rev. 2012, 16, 981–989. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef] [Green Version]
- Heinzel, A.; Barragán, V.M. A review of the state-of-the-art of the methanol crossover in direct methanol fuel cells. J. Power Sources 1999, 84, 70–74. [Google Scholar] [CrossRef]
- Hjuler, H.A.; Aili, D.; Jensen, J.O. High temperature polymer electrolyte membrane fuel cells: Approaches, status, and perspectives. In High Temperature Polymer Electrolyte Membrane Fuel Cells; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–545. [Google Scholar]
- Dicks, A.L. Molten carbonate fuel cells. Curr. Opin. Solid State Mater. Sci. 2004, 8, 379–383. [Google Scholar] [CrossRef]
- Stonehart, P. Development of alloy electrocatalysts for phosphoric acid fuel cells (PAFC). J. Appl. Electrochem. 1992, 22, 995–1001. [Google Scholar] [CrossRef]
- McLean, G.F.; Niet, T.; Prince-Richard, S.; Djilali, N. An assessment of alkaline fuel cell technology. Int. J. Hydrogen Energy 2002, 27, 507–526. [Google Scholar] [CrossRef]
- Singhal, S.C. Advances in solid oxide fuel cell technology. Solid State Ion. 2000, 135, 305–313. [Google Scholar] [CrossRef]
- Walkowiak-Kulikowska, J.; Wolska, J.; Koroniak, H. Polymers application in proton exchange membranes for fuel cells (PEMFCs). Phys. Sci. Rev. 2017, 2. [Google Scholar] [CrossRef]
- Sadakiyo, M.; Yamada, T.; Kitagawa, H. Rational designs for highly proton-conductive metal-organic frameworks. J. Am. Chem. Soc. 2009, 131, 9906–9907. [Google Scholar] [CrossRef]
- Nagao, Y.; Kubo, T.; Nakasuji, K.; Ikeda, R.; Kojima, T.; Kitagawa, H. Preparation and proton transport property of N,N′- diethyldithiooxamidatocopper coordination polymer. Synth. Met. 2005, 154, 89–92. [Google Scholar] [CrossRef]
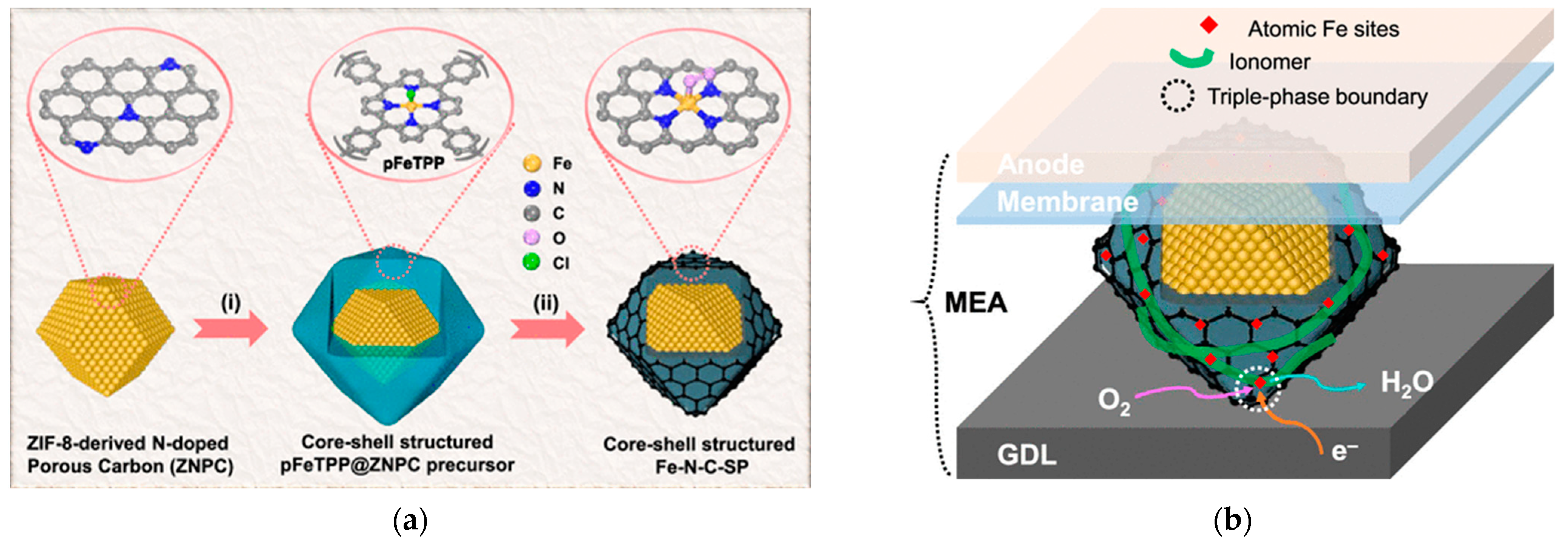
- Zhu, J.; Fang, Z.; Yang, X.; Chen, M.; Chen, Z.; Qiu, F.; Wang, M.; Liu, P.; Xu, Q.; Zhuang, X.; et al. Core–Shell Structured Fe–N–C Catalysts with Enriched Iron Sites in Surface Layers for Proton-Exchange Membrane Fuel Cells. ACS Catal. 2022, 12, 6409–6417. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Y.; Zhao, J.; Liu, J.; Wang, L.; Lei, J.; Xue, R. Enhanced proton conductivity of Nafion membrane induced by incorporation of MOF-anchored 3D microspheres: A superior and promising membrane for fuel cell applications. Chem. Commun. 2022, 58, 2906–2909. [Google Scholar] [CrossRef]
- Taylor, J.M.; Dekura, S.; Ikeda, R.; Kitagawa, H. Defect control to enhance proton conductivity in a metal-organic framework. Chem. Mater. 2015, 27, 2286–2289. [Google Scholar] [CrossRef]
- Asl, M.H.; Moosavi, F.; Akbari, S. Mixed membrane matrices (MMMs) based on Nafion® pristine/defected-UiO-66(Zr) MOFs: Assessment of the effects of dopants on cluster morphology. Mol. Syst. Des. Eng. 2022, 7, 969–985. [Google Scholar]
- Si, G.R.; Yang, F.; He, T.; Kong, X.J.; Wu, W.; Li, T.C.; Wang, K.; Li, J.R. Enhancing proton conductivity in Zr-MOFs through tuning metal cluster connectivity. J. Mater. Chem. A 2022, 10, 1236–1240. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Gao, Z.; Guo, L.; Li, M.; Su, J. Porous Hierarchical Iron/Nitrogen co-doped Carbon Etched by g-C3N4 Pyrolysis as Efficient Non-noble Metal Catalysts for PEM Fuel Cells. ChemElectroChem 2022, 9, e202101681. [Google Scholar] [CrossRef]
- Xie, X.; Shang, L.; Xiong, X.; Shi, R.; Zhang, T. Fe Single-Atom Catalysts on MOF-5 Derived Carbon for Efficient Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cells. Adv. Energy Mater. 2022, 12, 2102688. [Google Scholar] [CrossRef]
- Ponnada, S.; Kiai, M.S.; Gorle, D.B.; Nowduri, A.; Sharma, R.K. Insight into the Role and Strategies of Metal-Organic Frameworks in Direct Methanol Fuel Cells: A Review. Energy Fuels 2021, 35, 15265–15284. [Google Scholar] [CrossRef]
- Raj, V. Direct methanol fuel cells in portable applications: Materials, designs, operating parameters, and practical steps toward commercialization. Direct Methanol Fuel Cell Technol. 2020, 495–525. [Google Scholar] [CrossRef]
- Duan, Y.; Ru, C.; Li, J.; Sun, Y.N.; Pu, X.; Liu, B.; Pang, B.; Zhao, C. Enhancing proton conductivity and methanol resistance of SPAEK membrane by incorporating MOF with flexible alkyl sulfonic acid for DMFC. J. Memb. Sci. 2022, 641, 119906. [Google Scholar] [CrossRef]
- Noor, T.; Mohtashim, M.; Iqbal, N.; Naqvi, S.R.; Zaman, N.; Rasheed, L.; Yousuf, M. Graphene based FeO/NiO MOF composites for methanol oxidation reaction. J. Electroanal. Chem. 2021, 890, 115249. [Google Scholar] [CrossRef]
- Liang, X.; Zhou, X.; Ge, C.; Lin, H.; Satapathi, S.; Zhu, Q.; Hu, H. Advance and prospect of metal-organic frameworks for perovskite photovoltaic devices. Org. Electron. 2022, 106, 106546. [Google Scholar] [CrossRef]
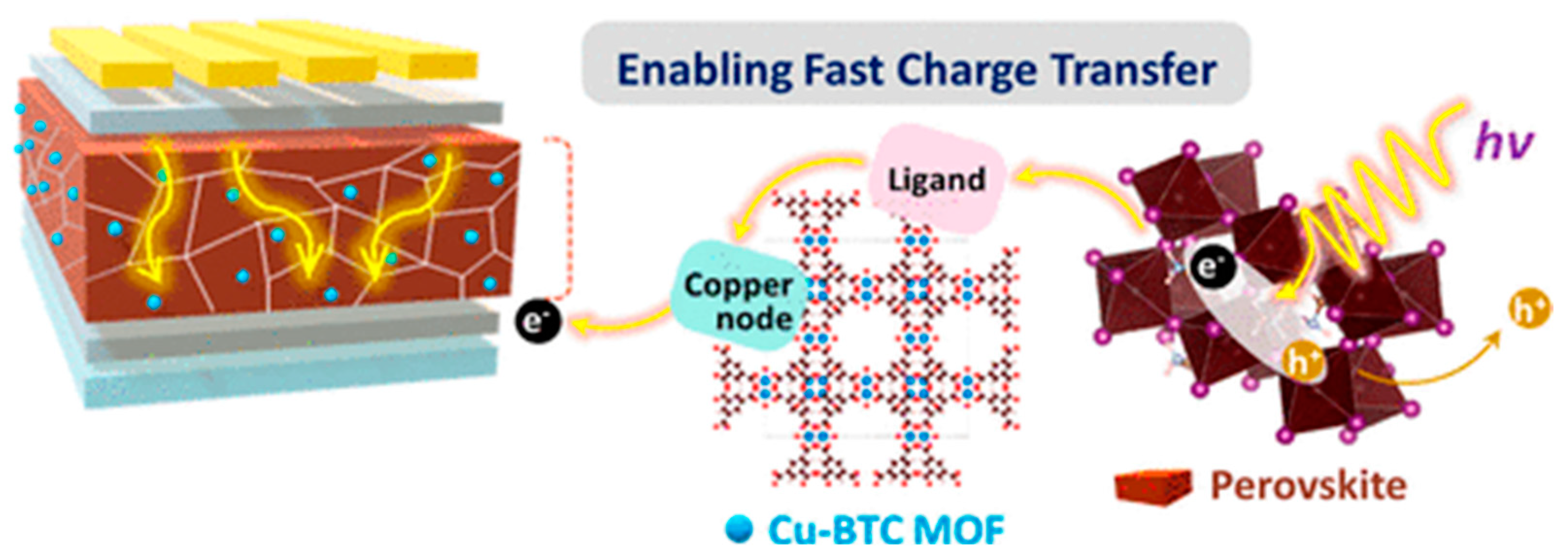
- Lee, J.; Tsvetkov, N.; Shin, S.R.; Kang, J.K. Fast Charge Transfer and High Stability via Hybridization of Hygroscopic Cu-BTC Metal-Organic Framework Nanocrystals with a Light-Absorbing Layer for Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2022, 14, 35495–35503. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Yang, Y.; Yang, C.; Dong, Y.; Lin, K.; Xia, D.; Fan, R. Functionalized Rare-Earth Metal Cluster-Based Materials as Additives for Enhancing the Efficiency of Perovskite Solar Cells. ACS Appl. Energy Mater. 2022, 2022, 13318–13326. [Google Scholar] [CrossRef]
- Rong, B.; Wei, Y.; Chen, X.; Ding, Y.; Chen, Y.; Liu, H.; Huang, Y.; Fan, L.; Wu, J. Electron transport improvement of perovskite solar cells via intercalation of Na doped TiO2 from metal-organic framework MIL-125(Ti). Appl. Surf. Sci. 2022, 574, 151735. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Q.; Wang, C.; Li, Y. Interfacial dipole in organic and perovskite solar cells. J. Am. Chem. Soc. 2020, 142, 18281–18292. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, J.; Yang, Y.; Wang, J.; Hu, B.; Wang, W.; Cao, W.; Gai, S.; Xia, D.; Lin, K.; et al. Multifunctional nanostructured host-guest POM@MOF with lead sequestration capability induced stable and efficient perovskite solar cells. Nano Energy 2022, 97, 107184. [Google Scholar] [CrossRef]
- Uğur, A.; Gencer Imer, A.; Gülcan, M. Enhancement in the photovoltaic efficiency of dye-sensitized solar cell by doping TiO2 with MIL-101 MOF structure. Mater. Sci. Semicond. Process. 2022, 150, 106951. [Google Scholar] [CrossRef]
- Selvaraj, B.; Shanmugam, G.; Kamaraj, S.; Thirugnanasambandam, E.; Gunasekeran, A.; Sambandam, A. Effect of an aqueous copper gel electrolyte with cobalt metal organic framework based additive on performance of aqueous-dye-sensitized solar cells. Sol. Energy 2022, 236, 586–598. [Google Scholar] [CrossRef]
- Thong, C.H.; Priyanga, N.; Ng, F.L.; Pappathi, M.; Periasamy, V.; Phang, S.M. Metal organic frameworks (MOFs) as potential anode materials for improving power generation from algal biophotovoltaic (BPV) platforms. Catal. Today 2022, 397–399, 419–427. [Google Scholar] [CrossRef]
- Nevruzoglu, V.; Demir, S.; Karaca, G.; Tomakin, M.; Bilgin, N.; Yilmaz, F. Improving the stability of solar cells using metal–organic frameworks. J. Mater. Chem. A 2016, 4, 7930–7935. [Google Scholar] [CrossRef]
- Ifraemov, R.; Mukhopadhyay, S.; Rozenberg, I.; Hod, I. Metal-Organic-Framework-Based Photo-electrochemical Cells for Solar Fuel Generation. J. Phys. Chem. C 2022, 126, 5079–5091. [Google Scholar] [CrossRef]
- Brodd, R.J.; Bullock, K.R.; Leising, R.A.; Middaugh, R.L.; Miller, J.R.; Takeuchi, E. Batteries, 1977 to 2002. J. Electrochem. Soc. 2004, 151, K1. [Google Scholar] [CrossRef]
- Song, J.Y.; Wang, Y.Y.; Wan, C.C. Review of gel-type polymer electrolytes for lithium-ion batteries. J. Power Sources 1999, 77, 183–197. [Google Scholar] [CrossRef]
- Waghorne, W.E. Viscosities of electrolyte solutions. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 2001, 359, 1529–1543. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Mechili, M.; Vaitsis, C.; Argirusis, N.; Pandis, P.K.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Research Progress in Metal-Organic Framework Based Nanomaterials Applied in Battery Cathodes. Energies 2022, 15, 5460. [Google Scholar] [CrossRef]
- Chen, W.; Liu, S.; Shen, J. A review of MOFs and its derivatives for lithium ion battery anodes. IOP Conf. Ser. Earth Environ. Sci. 2021, 634, 012042. [Google Scholar]
- Cao, X.; Tan, C.; Sindoro, M.; Zhang, H. Hybrid micro-/nano-structures derived from metal–organic frameworks: Preparation and applications in energy storage and conversion. Chem. Soc. Rev. 2017, 46, 2660–2677. [Google Scholar] [CrossRef]
- Xu, G.; Nie, P.; Dou, H.; Ding, B.; Li, L.; Zhang, X. Exploring metal organic frameworks for energy storage in batteries and supercapacitors. Mater. Today 2017, 20, 191–209. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Tang, J.; Salunkhe, R.R.; Jiang, X.; Yu, A.; Wu, K.C.W.; Yamauchi, Y. Nanoarchitectured Design of Porous Materials and Nanocomposites from Metal-Organic Frameworks. Adv. Mater. 2017, 29, 1604898. [Google Scholar] [CrossRef] [PubMed]
- Nzereogu, P.U.; Omah, A.D.; Ezema, F.I.; Iwuoha, E.I.; Nwanya, A.C. Anode materials for lithium-ion batteries: A review. Appl. Surf. Sci. Adv. 2022, 9, 100233. [Google Scholar] [CrossRef]
- Bennett, T.D.; Tan, J.C.; Yue, Y.; Baxter, E.; Ducati, C.; Terrill, N.J.; Yeung, H.H.M.; Zhou, Z.; Chen, W.; Henke, S.; et al. Hybrid glasses from strong and fragile metal-organic framework liquids. Nat. Commun. 2015, 6, 8079. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, S.S.; Østergaard, M.B.; Stepniewska, M.; Johra, H.; Yue, Y.; Smedskjaer, M.M. Metal-Organic Framework Glasses Possess Higher Thermal Conductivity than Their Crystalline Counterparts. ACS Appl. Mater. Interfaces 2020, 12, 18893–18903. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, Z.; Qi, S.; Wang, P.; Rosgaard Jensen, L.; Johansen, M.; Kolle Christensen, C.; Zhang, Y.; Bomholdt Ravnsbaek, D.; Yue, Y.; et al. Metal-Organic Framework Glass Anode with an Exceptional Cycling-Induced Capacity Enhancement for Lithium-Ion Batteries. Adv. Mater. 2022, 34, 2110048. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, W.; Cao, X.; Wei, L.; Ye, C.; Meng, C.; Yuan, A.; Pang, H.; Yu, C.; Cai, Y.J.; et al. Synthesis of Tostadas-Shaped Metal-Organic Frameworks for Remitting Capacity Fading of Li-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2109927. [Google Scholar] [CrossRef]
- Li, C.; Zhang, C.; Xie, J.; Wang, K.; Li, J.; Zhang, Q. Ferrocene-based metal-organic framework as a promising cathode in lithium-ion battery. Chem. Eng. J. 2021, 404, 126463. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, M.; Wu, Y.; Su, X.; Li, X.; Xu, S.; Zhuo, S.; Ma, J.; Yuan, D.; Wang, C.; et al. A One-Dimensional π–d Conjugated Coordination Polymer for Sodium Storage with Catalytic Activity in Negishi Coupling. Angew. Chemie 2019, 131, 14873–14881. [Google Scholar] [CrossRef]
- Xie, J.; Cheng, X.-F.; Cao, X.; He, J.-H.; Guo, W.; Li, D.-S.; Xu, Z.J.; Huang, Y.; Lu, J.-M.; Zhang, Q. Nanostructured Metal–Organic Conjugated Coordination Polymers with Ligand Tailoring for Superior Rechargeable Energy Storage. Small 2019, 15, 1903188. [Google Scholar] [CrossRef]
- Li, C.; Zhang, C.; Wang, K.; Yu, F.; Xie, J.; Zhang, Q. Multi-thiol-supported dicarboxylate-based metal-organic framework with excellent performance for lithium-ion battery. Chem. Eng. J. 2022, 431, 133234. [Google Scholar] [CrossRef]
- Yang, D.X.; Wang, P.F.; Liu, H.Y.; Zhang, Y.H.; Sun, P.P.; Shi, F.N. Facile synthesis of ternary transition metal-organic framework and its stable lithium storage properties. J. Solid State Chem. 2022, 309, 122947. [Google Scholar] [CrossRef]
- Zheng, W.; Hu, T.; Fang, Y.; Li, L.; Yuan, W. High capacity of microspheric manganese and cobalt trimesic dual-metal organic framework for Li-ion battery. J. Solid State Chem. 2022, 306, 122719. [Google Scholar] [CrossRef]
- Lv, X.N.; Zhang, Y.H.; Sun, P.P.; Wang, P.F.; Tang, J.J.; Yang, G.; Shi, Q.; Shi, F.N. One pot synthesis of lanthanide-iron-sodium trimetallic metal-organic frameworks as anode materials for lithium-ion batteries. J. Solid State Chem. 2022, 306, 122786. [Google Scholar] [CrossRef]
- Zhu, Y.; Peng, L.; Fang, Z.; Yan, C.; Zhang, X.; Yu, G. Structural Engineering of 2D Nanomaterials for Energy Storage and Catalysis. Adv. Mater. 2018, 30, 1706347. [Google Scholar] [CrossRef]
- Xiong, P.; Sun, B.; Sakai, N.; Ma, R.; Sasaki, T.; Wang, S.; Zhang, J.; Wang, G. 2D Superlattices for Efficient Energy Storage and Conversion. Adv. Mater. 2020, 32, 1902654. [Google Scholar] [CrossRef] [PubMed]
- Pomerantseva, E.; Gogotsi, Y. Two-dimensional heterostructures for energy storage. Nat. Energy 2017, 27, 1–6. [Google Scholar] [CrossRef]
- Zhang, H.; Hussain, I.; Brust, M.; Butler, M.F.; Rannard, S.P.; Cooper, A.I. Aligned two- and three-dimensional structures by directional freezing of polymers and nanoparticles. Nat. Mater. 2005, 4, 787–793. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Li, X.; Jin, H.; Ali, W.; Song, Z.; Ding, S. Metal–organic-framework derived Co@CN modified horizontally aligned graphene oxide array as free-standing anode for lithium-ion batteries. J. Mater. Chem. A 2022, 10, 699–706. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, W.; Gao, M.; Xu, H.; Guo, Y.; Sun, L.; Zheng, D.; Lin, J. A truncated octahedron metal-organic framework derived TiO2@C@MoS2 composite with superior lithium-ion storage properties. J. Power Sources 2022, 518, 230746. [Google Scholar] [CrossRef]
- Tang, Y.; Kang, H.; Zheng, J.; Li, H.; Wang, R.; Zhang, L.; Ma, Q.; Xiong, X.; Zhou, T.; Zhang, C. Metal-Organic Framework derived Bi2S3 hybrid nanofibers for enhanced lithium-ion storage. J. Power Sources 2022, 520, 230895. [Google Scholar] [CrossRef]
- Moustafa, M.G.; Aboraia, A.M.; Butova, V.V.; Elmasry, F.; Guda, A.A. Facile synthesis of ZnNC derived from a ZIF-8 metal-organic framework by the microwave-assisted solvothermal technique as an anode material for lithium-ion batteries. New J. Chem. 2022, 46, 9138–9145. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, C.; Liu, H.; Liu, T.; Wang, L.; Zhu, J. Hierarchical architecture of two-dimensional Ti3C2 nanosheets@Metal-Organic framework derivatives as anode for hybrid li-ion capacitors. J. Colloid Interface Sci. 2022, 623, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.B.; Shao, M.Y.; Li, K.; Ding, L.; Zeng, M. Facile synthesis and lithium storage mechanism study of directly usable tin-based metal organic framework. J. Electroanal. Chem. 2022, 912, 116268. [Google Scholar] [CrossRef]
- Liu, J.; Xie, D.; Xu, X.; Jiang, L.; Si, R.; Shi, W.; Cheng, P. Reversible formation of coordination bonds in Sn-based metal-organic frameworks for high-performance lithium storage. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Yao, H.; Yan, L.; Shen, J.; Wang, T.; Chen, P.; Cong, X.; Zhang, S.; Jiang, H.; Zhao, X. Controllably regulating ion transport in lithium metal batteries via pore effect of metal–organic framework-based separators. Appl. Surf. Sci. 2022, 589, 152885. [Google Scholar] [CrossRef]
- Tu, Z.; Choudhury, S.; Zachman, M.J.; Wei, S.; Zhang, K.; Kourkoutis, L.F.; Archer, L.A. Designing Artificial Solid-Electrolyte Interphases for Single-Ion and High-Efficiency Transport in Batteries. Joule 2017, 1, 394–406. [Google Scholar] [CrossRef] [Green Version]
- Tu, Z.; Zachman, M.J.; Choudhury, S.; Wei, S.; Ma, L.; Yang, Y.; Kourkoutis, L.F.; Archer, L.A.; Tu, Z.; Ma, L.; et al. Nanoporous Hybrid Electrolytes for High-Energy Batteries Based on Reactive Metal Anodes. Adv. Energy Mater. 2017, 7, 1602367. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Shen, L.; Wu, H.B.; Liu, F.; Zhang, C.; Ma, S.; Le, Z.; Lu, Y. Anchoring anions with metal–organic framework-functionalized separators for advanced lithium batteries. Nanoscale Horiz. 2019, 4, 705–711. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Shi, J.; Liu, Y.; Zheng, S.; Zou, H.; Chen, Y.; Kuang, W.; Ding, K.; Chen, L.; et al. A 3D interconnected metal-organic framework-derived solid-state electrolyte for dendrite-free lithium metal battery. Energy Storage Mater. 2022, 47, 262–270. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Yuan, S.; Wang, Y.; Xia, Y. To mitigate self-discharge of lithium–sulfur batteries by optimizing ionic liquid electrolytes. Energy Environ. Sci. 2016, 9, 224–231. [Google Scholar] [CrossRef]
- Bhauriyal, P.; Heine, T. Catalysing the performance of Li–sulfur batteries with two-dimensional conductive metal organic frameworks. J. Mater. Chem. A 2022, 10, 12400–12408. [Google Scholar] [CrossRef]
- Geng, P.; Du, M.; Guo, X.; Pang, H.; Tian, Z.; Braunstein, P.; Xu, Q. Bimetallic Metal-Organic Framework with High-Adsorption Capacity toward Lithium Polysulfides for Lithium–sulfur Batteries. Energy Environ. Mater. 2022, 5, 599–607. [Google Scholar] [CrossRef]
- Hu, X.; Huang, Q.; Zhang, Y.; Zhong, H.; Lin, Z.; Lin, X.; Zeb, A.; Xu, C.; Xu, X. A metal–organic framework approach to engineer mesoporous ZnMnO 3/C towards enhanced lithium storage. Sustain. Energy Fuels 2022, 6, 1175–1185. [Google Scholar] [CrossRef]
- Shi, X.; Lei, D.; Qiao, S.; Zhang, Q.; Wang, Q.; Deng, X.; Liu, J.; He, G.; Zhang, F. Metal-Organic Framework-Derived NiSe2Nanoparticles on Graphene for Polysulfide Conversion in Lithium-Sulfur Batteries. ACS Appl. Nano Mater. 2022, 16, 6. [Google Scholar]
- Chen, X.; Li, L.; Shan, Y.; Zhou, D.; Cui, W.; Zhao, Y. Synergistic effect of cerium oxide with core-shell structure embedded in porous carbon for high-performance lithium-sulfur batteries. Mater. Today Commun. 2021, 27, 102381. [Google Scholar] [CrossRef]
- Feng, W.; Chen, J.; Niu, Y.; Zhao, W.; Zhang, L. CeO2 composite metal organic framework is used to construct high-performance lithium-sulfur batteries. J. Alloys Compd. 2022, 906, 164341. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, X.; Gong, W.; Guo, S.; Ouyang, Y.; Li, D.; Xiao, Y.; Tan, C.; Xie, L.; Lu, H.; et al. Copolymerization of Sulfur Chains with Vinyl Functionalized Metal−Organic Framework for Accelerating Redox Kinetics in Lithium−Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2104074. [Google Scholar] [CrossRef]
- Capková, D.; Kazda, T.; Čech, O.; Király, N.; Zelenka, T.; Čudek, P.; Sharma, A.; Hornebecq, V.; Fedorková, A.S.; Almáši, M. Influence of metal-organic framework MOF-76(Gd) activation/carbonization on the cycle performance stability in Li-S battery. J. Energy Storage 2022, 51, 104419. [Google Scholar] [CrossRef]
- Chen, D.; Mukherjee, S.; Zhang, C.; Li, Y.; Xiao, B.; Singh, C.V. Two-dimensional square metal organic framework as promising cathode material for lithium-sulfur battery with high theoretical energy density. J. Colloid Interface Sci. 2022, 613, 435–446. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Zhao, C.; Hu, Y.S.; Titirici, M.M.; Li, H.; Huang, X.; Chen, L. Recent advances of electrode materials for low-cost sodium-ion batteries towards practical application for grid energy storage. Energy Storage Mater. 2017, 7, 130–151. [Google Scholar] [CrossRef]
- He, H.; Sun, D.; Tang, Y.; Wang, H.; Shao, M. Understanding and improving the initial Coulombic efficiency of high-capacity anode materials for practical sodium ion batteries. Energy Storage Mater. 2019, 23, 233–251. [Google Scholar] [CrossRef]
- Kim, S.W.; Seo, D.H.; Ma, X.; Ceder, G.; Kang, K. Electrode Materials for Rechargeable Sodium-Ion Batteries: Potential Alternatives to Current Lithium-Ion Batteries. Adv. Energy Mater. 2012, 2, 710–721. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Wu, F.; Bai, Y.; Wu, C. Boost sodium-ion batteries to commercialization: Strategies to enhance initial Coulombic efficiency of hard carbon anode. Nano Energy 2021, 82, 105738. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, X.; Mi, L.; Liu, C.; Zhang, J.; Cui, S.; Feng, X.; Cao, Y.; Shen Chen, C.W.; Zhang, X.; et al. High-Performance Flexible Freestanding Anode with Hierarchical 3D Carbon-Networks/Fe7S8/Graphene for Applicable Sodium-Ion Batteries. Adv. Mater. 2019, 31, 1806664. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Y.; Giuli, G.; Diemant, T.; Behm, R.J.; Geiger, D.; Kaiser, U.; Ulissi, U.; Passerini, S.; Bresser, D. Conversion/alloying lithium-ion anodes—Enhancing the energy density by transition metal doping. Sustain. Energy Fuels 2018, 2, 2601–2608. [Google Scholar] [CrossRef] [Green Version]
- Sanati, S.; Morsali, A.; Garcia, H. First-row transition metal-based materials derived from bimetallic metal–organic frameworks as highly efficient electrocatalysts for electrochemical water splitting. Energy Environ. Sci. 2022, 15, 3119–3151. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Xue, H.; Pang, H.; Xu, Q. Metal–organic frameworks as a platform for clean energy applications. EnergyChem 2020, 2, 100027. [Google Scholar] [CrossRef]
- Yu, M.; Dong, R.; Feng, X. Two-Dimensional Carbon-Rich Conjugated Frameworks for Electrochemical Energy Applications. J. Am. Chem. Soc. 2020, 142, 12903–12915. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, C.; Chen, Y.; Wu, Y.; Wang, C. The chemical states of conjugated coordination polymers. Chem 2021, 7, 1224–1243. [Google Scholar] [CrossRef]
- Wang, B.; Li, J.; Ye, M.; Zhang, Y.; Tang, Y.; Hu, X.; He, J.; Chao Li, C.; Wang, B.; Li, J.; et al. Dual-Redox Sites Guarantee High-Capacity Sodium Storage in Two-Dimension Conjugated Metal–Organic Frameworks. Adv. Funct. Mater. 2022, 32, 2112072. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Guo, H.; Huang, Y.; Li, W.; Wang, C.; Wang, Y. Phase transformation induced benzene rings activation in a metal–organic framework to boost sodium storage performance. Chem. Eng. J. 2022, 433, 133508. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gao, Y.J.; Wang, X.; Ye, Q.; Zhang, Y.; Wu, Y.; Chen, S.H.; Ruan, B.; Shi, D.; Jiang, T.; et al. MoTe2 on metal-organic framework derived MoO2/N-doped carbon rods for enhanced sodium-ion storage properties. Energy 2022, 243, 123043. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Ding, Y.; Li, Y.; Ren, S.B.; Shen, M.; Chen, Y.X.; Li, W.; Han, D.M. Metal–organic framework-derived nitrogen-doped carbon-confined CoSe2 anchored on multiwalled carbon nanotube networks as an anode for high-rate sodium-ion batteries. Dalt. Trans. 2022, 51, 5184–5194. [Google Scholar] [CrossRef]
- Feng, J.; Luo, S.H.; Lin, Y.; Zhan, Y.; Yan, S.; Hou, P.; Wang, Q.; Zhang, Y. hui. Metal-organic framework derived CoSe2/N-doped carbon core-shell nanoparticles encapsulated in porous N-doped carbon nanotubes as high-performance anodes for sodium-ion batteries. J. Power Sources 2022, 535, 231444. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Z.; Xu, F.; Qin, Z.; Sun, R.; Wang, C.; Lu, S.; Zhang, Y.; Fan, H. Ni3Se4@CoSe2 hetero-nanocrystals encapsulated into CNT-porous carbon interpenetrating frameworks for high-performance sodium ion battery. J. Colloid Interface Sci. 2022, 611, 718–725. [Google Scholar] [CrossRef]
- Qian, Z.; Wang, X.; Liu, T.; Zhang, L.; Yu, J. Nickel-cobalt selenide@N-doped carbon towards high-performance anode materials for sodium-ion batteries. J. Energy Storage 2022, 51, 104522. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, M.; Huang, M.; Xiong, Y.; Yang, X.; Miao, Z.; Yang, Z.; Yu, J. Co0.85Se@carbon nanotubes surface-seeding grown on carbon microplates as superior anode material for sodium ion batteries. Electrochim. Acta 2022, 414, 140167. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, X.; Cao, P.; Wang, Y.; Li, F.; Li, L.; Zhang, W. Employing ZIF-67 architectures into 1D binder-free Co3O4-based carbon fiber composites for advanced sodium-ion storage application. J. Alloys Compd. 2022, 890, 161907. [Google Scholar] [CrossRef]
- Liang, H.; Li, X.; Liu, X.; Sun, R.; Qin, Z.; Zhang, Y.; Fan, H. Epitaxial growth induced multilayer yolk-shell structured CoSe2 with promoting transport kinetics of sodium ion half/full batteries. J. Power Sources 2022, 517, 230729. [Google Scholar] [CrossRef]
- Wang, L.; Liu, B.; Zhu, Y.; Yang, M.; Du, C.; Han, Z.; Yao, X.; Ma, X.; Cao, C. General metal–organic framework-derived strategy to synthesize yolk-shell carbon-encapsulated nickelic spheres for sodium-ion batteries. J. Colloid Interface Sci. 2022, 613, 23–34. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Zarrabeitia, M.; Liang, H.-P.; Geiger, D.; Kaiser, U.; Varzi, A.; Passerini, S.; Li, H.; Zhang, H.; et al. Metal–Organic Framework Derived Copper Chalcogenides-Carbon Composites as High-Rate and Stable Storage Materials for Na Ions. Adv. Sustain. Syst. 2022, 6, 2200109. [Google Scholar] [CrossRef]
- Chen, J.-J.; Ye, J.-C.; Zhang, X.-G.; Symes, M.D.; Fan, S.-C.; Long, D.-L.; Zheng, M.-S.; Wu, D.-Y.; Cronin, L.; Dong, Q.-F.; et al. Design and Performance of Rechargeable Sodium Ion Batteries, and Symmetrical Li-Ion Batteries with Supercapacitor-Like Power Density Based upon Polyoxovanadates. Adv. Energy Mater 2018, 8, 1701021. [Google Scholar] [CrossRef]
- Cao, D.; Sha, Q.; Wang, J.; Li, J.; Ren, J.; Shen, T.; Bai, S.; He, L.; Song, Y.F. Advanced Anode Materials for Sodium-Ion Batteries: Confining Polyoxometalates in Flexible Metal-Organic Frameworks by the ‘breathing Effect’. ACS Appl. Mater. Interfaces 2022, 14, 22186–22196. [Google Scholar] [CrossRef]
- Wang, J.; Yue, X.; Liu, Z.; Xie, Z.; Zhao, Q.; Abudula, A.; Guan, G. Trimetallic sulfides derived from tri-metal-organic frameworks as anode materials for advanced sodium ion batteries. J. Colloid Interface Sci. 2022, 625, 248–256. [Google Scholar] [CrossRef]
- Li, C.; Li, A.; Li, M.; Xiong, P.; Liu, Y.; Cheng, M.; Geng, D.; Xu, Y. Ultrafast Synthesis of Layered Transition-Metal Oxide Cathodes from Metal–Organic Frameworks for High-Capacity Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 24462–24468. [Google Scholar] [CrossRef]
- Yang, C.; Shang, S.; Gu, Q.; Shang, J.; Li, X. Metal-organic framework-derived carbon nanotubes with multi-active Fe-N/Fe sites as a bifunctional electrocatalyst for zinc-air battery. J. Energy Chem. 2022, 66, 306–313. [Google Scholar] [CrossRef]
- Shin, S.; Yoon, Y.; Shin, M.W. Co/Zn-based bimetallic MOF-derived hierarchical porous Co/C composite as cathode material for high-performance lithium-air batteries. Int. J. Energy Res. 2022, 46, 9900–9910. [Google Scholar] [CrossRef]
- Dong, X.; Sun, J.; Mu, Y.; Yu, Y.; Hu, T.; Miao, C.; Huang, C.; Meng, C.; Zhang, Y. RGO/Manganese Silicate/MOF-derived carbon Double-Sandwich-Like structure as the cathode material for aqueous rechargeable Zn-ion batteries. J. Colloid Interface Sci. 2022, 610, 805–817. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Liu, Y.; Liu, X.; Gao, S. Self-catalyzed growth of Zn/Co-N-C carbon nanotubes derived from metal-organic frameworks as efficient oxygen reduction catalysts for Zn-air battery. Sci. China Mater. 2021, 65, 653–662. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal–air batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Deng, Y.; Wang, H.; Wang, R.; Jin, J.; Gong, Y.; Zhao, L. Metal organic framework derived perovskite/spinel heterojunction as efficient bifunctional oxygen electrocatalyst for rechargeable and flexible Zn-air batteries. J. Colloid Interface Sci. 2022, 625, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Díaz, R.; Orcajo, M.G.; Botas, J.A.; Calleja, G.; Palma, J. Co8-MOF-5 as electrode for supercapacitors. Mater. Lett. 2012, 68, 126–128. [Google Scholar] [CrossRef]
- Lee, D.Y.; Yoon, S.J.; Shrestha, N.K.; Lee, S.H.; Ahn, H.; Han, S.H. Unusual energy storage and charge retention in Co-based metal–organic-frameworks. Microporous Mesoporous Mater. 2012, 153, 163–165. [Google Scholar] [CrossRef]
- Arafat, Y.; Azhar, M.R.; Zhong, Y.; Xu, X.; Tadé, M.O.; Shao, Z. A Porous Nano-Micro-Composite as a High-Performance Bi-Functional Air Electrode with Remarkable Stability for Rechargeable Zinc–Air Batteries. Nano-Micro Lett. 2020, 12, 1–16. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, S.; Xu, Y.; Xue, H.; Liu, C.; Pang, H. Ultrathin two-dimensional cobalt-organic frameworks nanosheets for electrochemical energy storage. Chem. Eng. J. 2019, 373, 1319–1328. [Google Scholar] [CrossRef]
- Hong, Y.; Wang, Y.; Guo, Y.; Wang, K.; Wu, H.; Zhang, C.; Zhang, Q. Recent advances in pillar-layered metal-organic frameworks with interpenetrated and non-interpenetrated topologies as supercapacitor electrodes. Zeitschrift für Anorg. und Allg. Chemie 2022, 648, e202200115. [Google Scholar] [CrossRef]
- Pakulski, D.; Montes-García, V.; Gorczyński, A.; Czepa, W.; Chudziak, T.; Samorì, P.; Ciesielski, A. Thiol-decorated covalent organic frameworks as multifunctional materials for high-performance supercapacitors and heterogeneous catalysis. J. Mater. Chem. A 2022, 10, 16685–16696. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Y.Z.; Wang, F.X.; Zhou, S.H.; Zeng, T.Y.; Dong, Y. Research progress on metal and covalent organic framework-based materials for high-performance supercapacitors. New Carbon Mater. 2022, 37, 109–135. [Google Scholar] [CrossRef]
- Umezawa, S.; Douura, T.; Yoshikawa, K.; Tanaka, D.; Stolojan, V.; Silva, S.R.P.; Yoneda, M.; Gotoh, K.; Hayashi, Y. Zinc-Based Metal–Organic Frameworks for High-Performance Supercapacitor Electrodes: Mechanism Underlying Pore Generation. Energy Environ. Mater. 2022, 1–13. [Google Scholar] [CrossRef]
- Yao, S.; Jiao, Y.; Sun, S.; Wang, L.; Li, P.; Chen, G. Vertically Co-oriented Mn-Metal-Organic Framework Grown on 2D Cation-Intercalated Manganese Oxide via a Self-sacrificing Template Process for a High-Performance Asymmetric Supercapacitor. ACS Sustain. Chem. Eng. 2020, 8, 3191–3199. [Google Scholar] [CrossRef]
- Shi, C.; Kang, N.; Wang, C.; Yu, K.; Lv, J.; Wang, C.; Zhou, B. An inorganic–organic hybrid nanomaterial with a core–shell structure constructed by using Mn–BTC and Ag5[BW12O40] for supercapacitors and photocatalytic dye degradation. Nanoscale Adv. 2022, 4, 4358–4365. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, X.; Yu, K.; Wang, C.; Lv, J.; Wang, C.; Zhou, B. Simple preparation of Ag-BTC-modified Co3Mo7O24 mesoporous material for capacitance and H 2 O 2 -sensing performances. CrystEngComm 2022, 24, 5614–5621. [Google Scholar] [CrossRef]
- Zheng, S.; Sun, Y.; Xue, H.; Braunstein, P.; Huang, W.; Pang, H. Dual-ligand and hard-soft-acid-base strategies to optimize metal-organic framework nanocrystals for stable electrochemical cycling performance. Natl. Sci. Rev. 2022, 9, nwab197. [Google Scholar] [CrossRef]
- Sahoo, R.; Ghosh, S.; Chand, S.; Chand Pal, S.; Kuila, T.; Das, M.C. Highly scalable and pH sTable 2D Ni-MOF-based composites for high performance supercapacitor. Compos. Part B Eng. 2022, 245, 110174. [Google Scholar] [CrossRef]
- Lu, J.; Duan, H.; Zhang, Y.; Zhang, G.; Chen, Z.; Song, Y.; Zhu, R.; Pang, H. Directional Growth of Conductive Metal-Organic Framework Nanoarrays along [001] on Metal Hydroxides for Aqueous Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2022, 14, 25878–25885. [Google Scholar] [CrossRef]
- Kumari, V.; Pal Singh, P.; Kaushal, S. Synthesis and applications of metal-organic frameworks and graphene-based composites: A review. Polyhedron 2022, 214, 115645. [Google Scholar] [CrossRef]
- Rahmanifar, M.S.; Hesari, H.; Noori, A.; Masoomi, M.Y.; Morsali, A.; Mousavi, M.F. A dual Ni/Co-MOF-reduced graphene oxide nanocomposite as a high performance supercapacitor electrode material. Electrochim. Acta 2018, 275, 76–86. [Google Scholar] [CrossRef]
- Gupta, A.K.; Saraf, M.; Bharadwaj, P.K.; Mobin, S.M. Dual Functionalized CuMOF-Based Composite for High-Performance Supercapacitors. Inorg. Chem. 2019, 58, 9844–9854. [Google Scholar] [CrossRef]
- Yan, Y.; Luo, Y.; Ma, J.; Li, B.; Xue, H.; Pang, H.; Yan, Y.; Luo, Y.; Ma, J.; Li, B.; et al. Facile Synthesis of Vanadium Metal-Organic Frameworks for High-Performance Supercapacitors. Small 2018, 14, 1801815. [Google Scholar] [CrossRef]
- Zaman, N.; Noor, T.; Iqbal, N. Recent advances in the metal–organic framework-based electrocatalysts for the hydrogen evolution reaction in water splitting: A review. RSC Adv. 2021, 11, 21904–21925. [Google Scholar] [CrossRef]
- Khan, S.; Noor, T.; Iqbal, N.; Pervaiz, E. Recent Advancement in Metal-Organic Framework for Water Electrolysis: A Review. ChemNanoMat 2022, 8, e202200115. [Google Scholar] [CrossRef]
- Budnikova, Y.H. Recent advances in metal–organic frameworks for electrocatalytic hydrogen evolution and overall water splitting reactions. Dalt. Trans. 2020, 49, 12483–12502. [Google Scholar] [CrossRef]
- Tan, J.B.; Li, G.R. Recent progress on metal-organic frameworks and their derived materials for electrocatalytic water splitting. J. Mater. Chem. A 2022, 8, 14326–14355. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Wang, L. Metal–Organic Framework-Based Materials for Solar Water Splitting. Small Sci. 2021, 1, 2000074. [Google Scholar] [CrossRef]
- Jaryal, R.; Kumar, R.; Khullar, S. Mixed metal-metal organic frameworks (MM-MOFs) and their use as efficient photocatalysts for hydrogen evolution from water splitting reactions. Coord. Chem. Rev. 2022, 464, 214542. [Google Scholar] [CrossRef]
- Cao, L.M.; Zhang, J.; Ding, L.W.; Du, Z.Y.; He, C.T. Metal-organic frameworks derived transition metal phosphides for electrocatalytic water splitting. J. Energy Chem. 2022, 68, 494–520. [Google Scholar] [CrossRef]
- Zhang, P.F.; Wu, D.; Yang, G.P.; Wang, Y.Y. Metal-Organic Frameworks as Heterogeneous Electrocatalysts for Water Splitting and CO2Fixation. Cryst. Growth Des. 2021, 21, 3123–3142. [Google Scholar] [CrossRef]
- Guan, J.; Pal, T.; Kamiya, K.; Fukui, N.; Maeda, H.; Sato, T.; Suzuki, H.; Tomita, O.; Nishihara, H.; Abe, R.; et al. Two-Dimensional Metal-Organic Framework Acts as a Hydrogen Evolution Cocatalyst for Overall Photocatalytic Water Splitting. ACS Catal. 2022, 12, 3881–3889. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, C.; Wu, X.; Liu, P.F.; Yang, H.G. Reverse Replacement in NH2-MIL-125 with 1,4-Dicarboxybenzene for Enhanced Photocatalytic Hydrogen Generation. Chem.—A Eur. J. 2022, 28, e202200938. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, D.; Li, B.; Li, W.; Zhang, H.; Pang, J.; Fan, L. Compositional Engineering of Co(II)MOF/Carbon-Based Overall Water Splitting Electrocatalysts: From Synergistic Effects to Structure-Activity Relationships. Cryst. Growth Des. 2022, 22, 2775–2792. [Google Scholar] [CrossRef]
- Gao, J.; Yu, X.; Li, Y.; Ma, Y. Structural Fine-Tuning and In-situ Generation of P, O Vacancies in Hollow Co-Ferrocene-MOFs Derived Phosphides for Efficient Water Oxidation. ChemCatChem 2022, 14, e202200558. [Google Scholar] [CrossRef]
- Dai, S.; Liu, Y.; Mei, Y.; Hu, J.; Wang, K.; Li, Y.; Jin, N.; Wang, X.; Luo, H.; Li, W. Iron-doped novel Co-based metal–organic frameworks for preparation of bifunctional catalysts with an amorphous structure for OER/HER in alkaline solution. Dalt. Trans. 2022, 51, 15446–15457. [Google Scholar] [CrossRef]
- Huang, P.; Miao, X.; Wu, J.; Zhang, P.; Zhang, H.; Bai, S.; Liu, W. Facile synthesis of an ultrathin ZIF-67 layer on the surface of Sn/Ti co-doped hematite for efficient photoelectrochemical water oxidation. Dalt. Trans. 2022, 51, 8848–8854. [Google Scholar] [CrossRef]
- Liu, L.; Du, S.; Guo, X.; Xiao, Y.; Yin, Z.; Yang, N.; Bao, Y.; Zhu, X.; Jin, S.; Feng, Z.; et al. Water-Stable Nickel Metal-Organic Framework Nanobelts for Cocatalyst-Free Photocatalytic Water Splitting to Produce Hydrogen. J. Am. Chem. Soc. 2022, 144, 2747–2754. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Hu, X.; Meng, Y.; She, H.; Wang, L.; Huang, J.; Zhu, G. Constructing NiFe-metal-organic frameworks from NiFe-layered double hydroxide as a highly efficient cocatalyst for BiVO4 photoanode PEC water splitting. Chem. Eng. J. 2022, 433, 133592. [Google Scholar] [CrossRef]
- Chhetri, K.; Muthurasu, A.; Dahal, B.; Kim, T.; Mukhiya, T.; Chae, S.H.; Ko, T.H.; Choi, Y.C.; Kim, H.Y. Engineering the abundant heterointerfaces of integrated bimetallic sulfide-coupled 2D MOF-derived mesoporous CoS2 nanoarray hybrids for electrocatalytic water splitting. Mater. Today Nano 2022, 17, 100146. [Google Scholar] [CrossRef]
- Ali, M.; Pervaiz, E. Effect of synthesis route on electrocatalytic water-splitting activity of MoS2/UiO-66 hybrid. Mol. Catal. 2022, 519, 112136. [Google Scholar] [CrossRef]
- Li, T.M.; Hu, B.Q.; Han, J.H.; Lu, W.; Yu, F.; Li, B. Highly Effective OER Electrocatalysts Generated from a Two-Dimensional Metal-Organic Framework Including a Sulfur-Containing Linker without Doping. Inorg. Chem. 2022, 61, 7051–7059. [Google Scholar] [CrossRef]
- Yin, Z.; Liang, J.; Zhang, Z.Y.; Luo, H.; Zhou, J. Construction of superhydrophilic metal-organic frameworks with hierarchical microstructure for efficient overall water splitting. J. Colloid Interface Sci. 2022, 623, 405–416. [Google Scholar] [CrossRef]
- Yu, H.; Wang, L.; Li, H.; Luo, Z.; Isimjan, T.T.; Yang, X. Improving the Electrocatalytic Activity of a Nickel-Organic Framework toward the Oxygen Evolution Reaction through Vanadium Doping. Chem. A Eur. J. 2022, 28, e202201784. [Google Scholar] [CrossRef]
- Ali, M.; Pervaiz, E.; Rabi, O. Enhancing the Overall Electrocatalytic Water-Splitting Efficiency of Mo2C Nanoparticles by Forming Hybrids with UiO-66 MOF. ACS Omega 2021, 6, 34219–34228. [Google Scholar] [CrossRef]
- Shi, L.; Wu, C.; Wang, Y.; Dou, Y.; Yuan, D.; Li, H.; Huang, H.; Zhang, Y.; Gates, I.D.; Sun, X.; et al. Rational Design of Coordination Bond Connected Metal Organic Frameworks/MXene Hybrids for Efficient Solar Water Splitting. Adv. Funct. Mater. 2022, 32, 2202571. [Google Scholar] [CrossRef]
- Lu, C.; Xiong, D.; Chen, C.; Wang, J.; Kong, Y.; Liu, T.; Ying, S.; Yi, F.Y. Indium-Based Metal-Organic Framework for Efficient Photocatalytic Hydrogen Evolution. Inorg. Chem. 2022, 61, 2587–2594. [Google Scholar] [CrossRef]
- Ibomcha Singh, T.; Rajeshkhanna, G.; Narayan Pan, U.; Kshetri, T.; Lin, H.; Hoon Kim, N.; Hee Lee, J.; Singh, T.I.; Pan, U.N.; Kshetri, T.; et al. Alkaline Water Splitting Enhancement by MOF-Derived Fe–Co–Oxide/Co@NC-mNS Heterostructure: Boosting OER and HER through Defect Engineering and In Situ Oxidation. Small 2021, 17, 2101312. [Google Scholar] [CrossRef]
- Liu, S.; Xiao, W.; Jin, C.; Xia, S.; Wang, W.; Jiang, X.; Li, L.; Wang, S.; Chen, C. MOFs derived CdS/CdO heterojunction photoanode for high-efficient water splitting. Appl. Surf. Sci. 2022, 605, 154697. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, M.; Li, X.; Shao, F.; Li, S.; Li, S.; Xu, Y.; Chen, J.; Zeng, F.; Jiao, Y. Regulating the electronic structure of Fe-based metal organic frameworks by electrodeposition of Au nanoparticles for electrochemical overall water splitting. J. Colloid Interface Sci. 2022, 626, 426–434. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Su, H.; Hong, A.N.; Wang, Y.; Yang, H.; Ge, L.; Song, W.; Liu, J.; Ma, T.; et al. Electron Redistributed S-Doped Nickel Iron Phosphides Derived from One-Step Phosphatization of MOFs for Significantly Boosting Electrochemical Water Splitting. Adv. Funct. Mater. 2022, 32, 2200733. [Google Scholar] [CrossRef]
- Kong, Y.; Xiong, D.; Lu, C.; Wang, J.; Liu, T.; Ying, S.; Ma, X.; Yi, F.Y. Vanadium-Based Trimetallic Metal-Organic-Framework Family as Extremely High-Performing and Ultrastable Electrocatalysts for Water Splitting. ACS Appl. Mater. Interfaces 2022, 14, 37804–37813. [Google Scholar] [CrossRef]
- Van Phuc, T.; Jana, J.; Ravi, N.; Kang, S.G.; Chung, J.S.; Choi, W.M.; Hur, S.H. Highly active Ni/Co-metal organic framework bifunctional electrocatalyst for water splitting reaction. Int. J. Hydrogen Energy 2022, 47, 22787–22795. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Bera, S.; Das, R.; Chakraborty, D.; Basu, A.; Banerjee, P.; Ghosh, S.; Bhaumik, A. A Ni(II) Metal-Organic Framework with Mixed Carboxylate and Bipyridine Ligands for Ultrafast and Selective Sensing of Explosives and Photoelectrochemical Hydrogen Evolution. ACS Appl. Mater. Interfaces 2022, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shi, X.; Sun, S.; Zheng, X.; Tian, D.; Jiang, D. Metal-Organic Framework-Derived Three-Dimensional Macropore Nitrogen-Doped Carbon Frameworks Decorated with Ultrafine Ru-Based Nanoparticles for Overall Water Splitting. Inorg. Chem. 2022, 61, 9685–9692. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, C.; Zhou, Z.; Ye, C.; Yu, R.; Wang, C.; Du, Y. Ultra-low Ru doped MOF-derived hollow nanorods for efficient oxygen evolution reaction. Inorg. Chem. Front. 2022, 9, 6158–6166. [Google Scholar] [CrossRef]
- Wang, R.; Sun, P.; Yuan, Q.; Nie, R.; Wang, X. MOF-derived cobalt-embedded nitrogen-doped mesoporous carbon leaf for efficient hydrogen evolution reaction in both acidic and alkaline media. Int. J. Hydrogen Energy 2019, 44, 11838–11847. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, Z.; Zou, R.; Xu, Q. Metal-Organic Frameworks for Batteries. Joule 2018, 2, 2235–2259. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Zhao, H.; Yue, L.; Liang, J.; Li, T.; Liu, Q.; Luo, Y.; Kong, X.; Lu, S.; Shi, X.; et al. Recent advances in lithium-based batteries using metal organic frameworks as electrode materials. Electrochem. Comm. 2021, 122, 106881. [Google Scholar] [CrossRef]
- Cui, X.; Dong, H.; Chen, S.; Wu, M.; Wang, Y. Progress and Perspective of Metal- and Covalent-Organic Frameworks and their Derivatives for Lithium-Ion Batteries. Batter. Supercaps. 2021, 4, 72–97. [Google Scholar] [CrossRef]

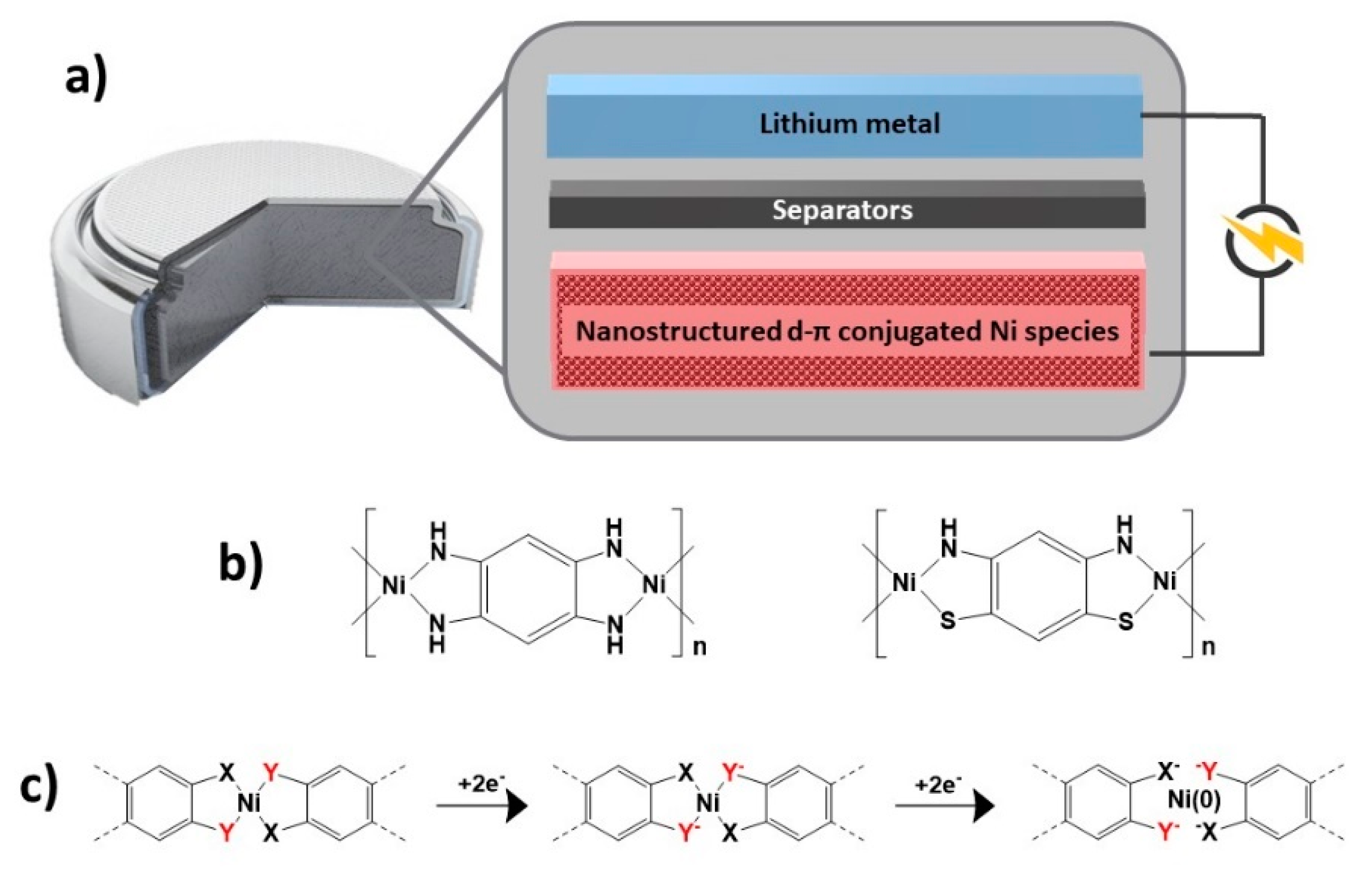
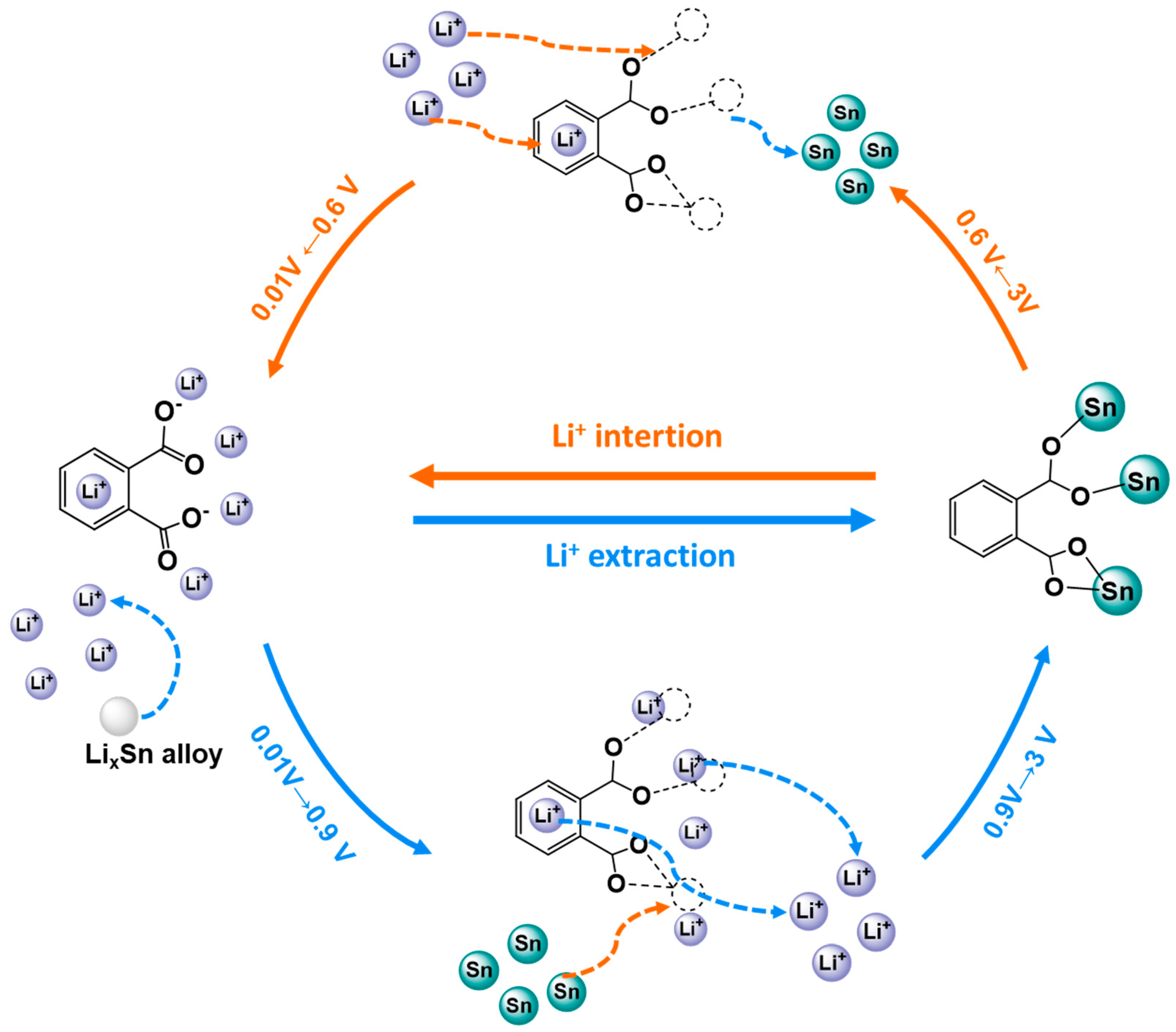
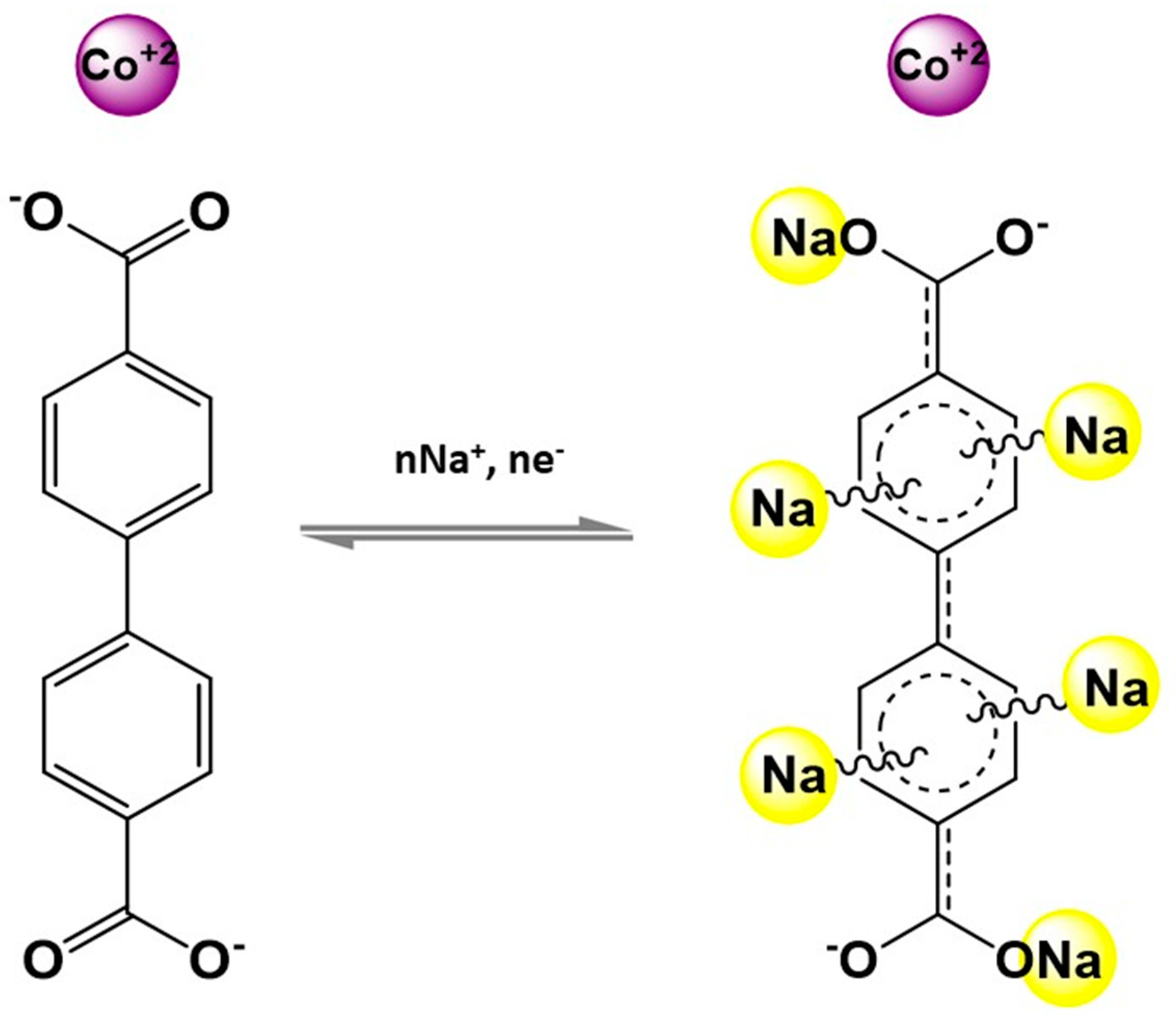
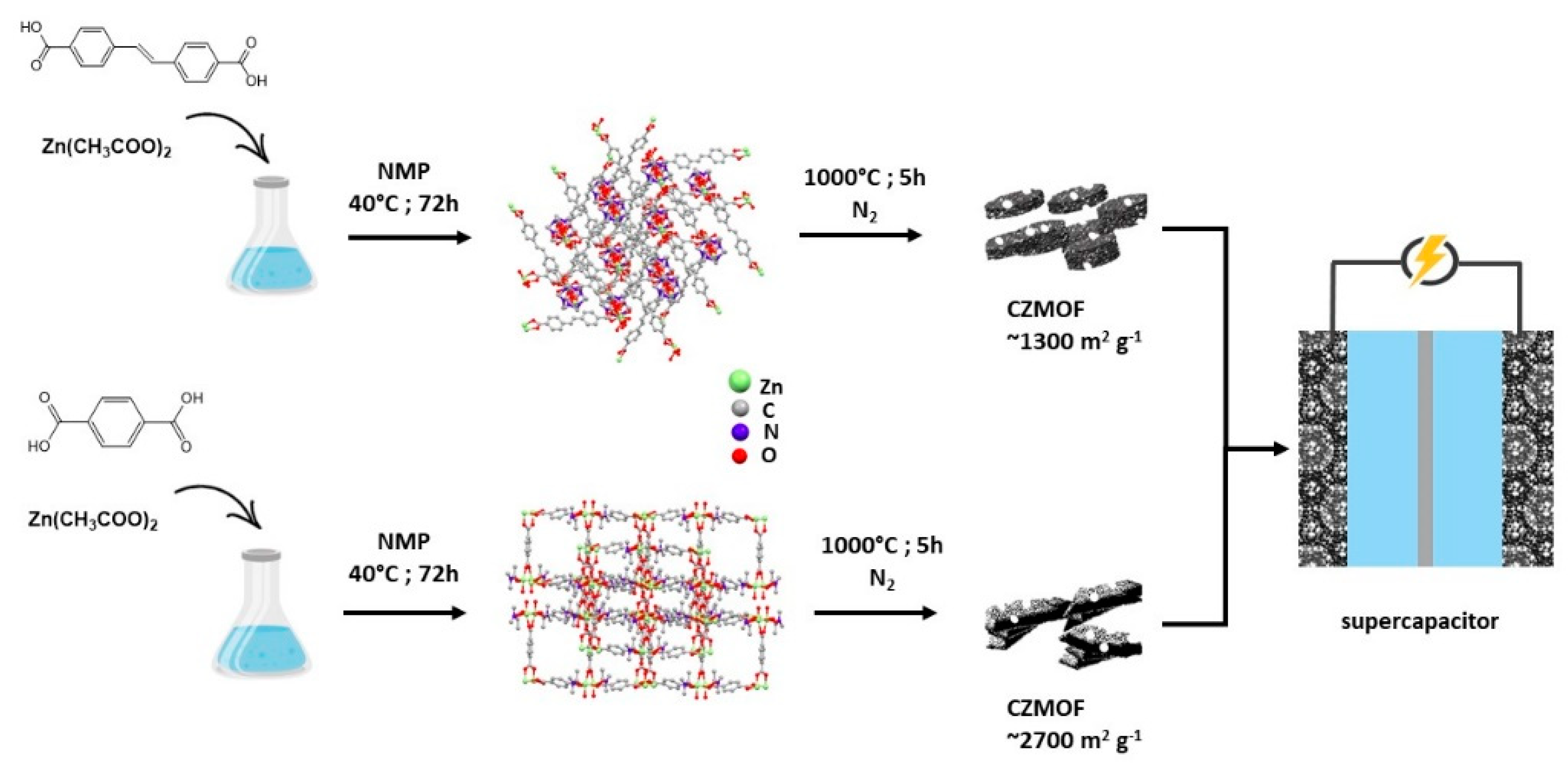
| Application | MOF | Typology | Ref. | |
|---|---|---|---|---|
| Fuel Cells | ||||
| PEMFC | ZIF-8 | Precursor | [43] | |
| ZIF-8 | Composite | [44] | ||
| UiO-66 | Composite | [46] | ||
| BUT-77 | Pristine | [47] | ||
| MOF-5 | Derivative | [49] | ||
| DMFC | MIL-101-NH2 | Composite | [52] | |
| Solar Cells | ||||
| PSC | MIL-125(Ti) | Precursor | [57] | |
| Zr-MOF | Composite | [59] | ||
| DSSC | MIL-101 | Composite | [60] | |
| Batteries | ||||
| Li-ion Batteries | ZIF-62(Co) | Composite | [77] | |
| d-p conjugated Ni CPs | Composite | [81] | ||
| ZiF-67 | Composite | [89] | ||
| MIL-125(Ti) | Precursor | [91] | ||
| CAU-17 | Precursor | [92] | ||
| ZIF-8 | Composite | [93] | ||
| Sn-MOF | Pristine | [96] | ||
| UiO-66-NH2 | Composite | [98,99,100] | ||
| UiO-66 | Composite | [111] | ||
| Na-Ion Batteries | HATN-OCu | Pristine | [124] | |
| Cobpdc-400 | Precursor | [125] | ||
| Ni-ZIF-67 | Precursor | [129] | ||
| MIL-88B | Composite | [137] | ||
| Supercapacitors | ||||
| AgBTC | Composite | [156] | ||
| Ni-MOF | Composite | [159] | ||
| MIL-47 | Pristine | [163] | ||
| Water splitting | ||||
| Co-Ferrocene | Composite | [179] | ||
| UiO-66 | Composite | [180] | ||
| In-MOF | Pristine | [187] | ||
| Cd-MOF | Precursor | [189] | ||
| Ni-MOF | Pristine | [194] | ||
| ZiF-8 | Pristine | [195] | ||
| Ru-MOF | Composite | [196] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pettinari, C.; Tombesi, A. MOFs for Electrochemical Energy Conversion and Storage. Inorganics 2023, 11, 65. https://doi.org/10.3390/inorganics11020065
Pettinari C, Tombesi A. MOFs for Electrochemical Energy Conversion and Storage. Inorganics. 2023; 11(2):65. https://doi.org/10.3390/inorganics11020065
Chicago/Turabian StylePettinari, Claudio, and Alessia Tombesi. 2023. "MOFs for Electrochemical Energy Conversion and Storage" Inorganics 11, no. 2: 65. https://doi.org/10.3390/inorganics11020065
APA StylePettinari, C., & Tombesi, A. (2023). MOFs for Electrochemical Energy Conversion and Storage. Inorganics, 11(2), 65. https://doi.org/10.3390/inorganics11020065