Abstract
Metal hydride (MH) hydrogen storage and compression systems with near-atmospheric H2 suction pressure are necessary for the utilization of the low-pressure H2 produced by solid oxide electrolyzers or released as a byproduct of chemical industries. Such systems should provide reasonably high productivity in the modes of both charge (H2 absorption at PL ≤ 1 atm) and discharge (H2 desorption at PH = 2–5 atm), which implies the provision of H2 equilibrium pressures Peq < PL at the available cooling temperature (TL = 15–20 °C) and, at the same time, Peq > PH when heated to TH = 90–150 °C. This work presents results of the development of such systems based on AB5-type intermetallics characterized by Peq of 0.1–0.3 atm and 3–8 atm for H2 absorption at TL = 15 °C and H2 desorption at TH = 100 °C, respectively. The MH powders mixed with 1 wt.% of Ni-doped graphene-like material or expanded natural graphite for the improvement of H2 charge dynamics were loaded in a cylindrical container equipped with internal and external heat exchangers. The developed units with a capacity of about 1 Nm3 H2 were shown to exhibit H2 flow rates above 10 NL/min during H2 charge at ≤1 atm when cooled to ≤20 °C with cold water and H2 release at a pressure above 2 and 5 atm when heated to 90 and 120 °C with hot water and steam, respectively.
1. Introduction
High-temperature steam electrolysis, primarily based on solid oxide cells (SOCs), is a promising method of hydrogen production [1]. However, due to technical difficulties related to cell sealing, almost all commercial SOCs operate at near-atmospheric pressures. In doing so, the generated hydrogen releases at P~1 atm (absolute), thus requiring further compression. Similar near-atmospheric pressures are typical for hydrogen byproducts released in vast amounts by several chemical industries. For example, the hydrogen production capacity of chlor-alkali plants in the U.S.A. ranges from 20 to 100 thousand tons annually [2], and the utilization of this hydrogen could bring significant benefits. At the same time, the minimum input/suction pressure of conventional mechanical hydrogen compressors does not typically exceed 4–5 atm [3]. Thus, it will be promising to collect low-pressure H2 in a metal hydride (MH) and to further deliver it at a higher pressure using thermally driven compression [4].
An obvious material solution to realize this approach is the use of hydride-forming material characterized by hydrogen equilibrium pressure (Peq) below 1 atm when absorbing the feed H2. However, the challenge will be in providing reasonably high productivities in both charge (H2 absorption) and discharge (H2 desorption) modes. Indeed, maximizing the pressure driving force for H2 absorption requires the values of Peq to be close to zero. Such MH materials (hydrogen getters) are well known [5]. However, hydrogen release from the H-saturated getter, even at low pressures requires too high temperatures, up to 650 °C [6]. At the same time, for the target application, the MH should provide sufficiently high H2 discharge pressure (at least the 2–5 atm necessary for the suction in the next compression stage) when heated up to a moderate temperature, from 70 to 90 °C (provided by solar collectors) to 150 °C (provided by low-grade industrial steam).
The most suitable hydride-forming materials which satisfy these requirements are AB5-type intermetallics based on LaNi5 where Ni is substituted with elements (Al, Mn, Co, etc.), increasing the thermal stability of the intermetallic hydride as compared to LaNi5Hx. Such intermetallics can be easily activated, and their H sorption characteristics are less sensitive to the impurities in H2 (first of all, water vapors) than the Ti-based AB- and AB2-type hydrogen storage alloys [7]. The influence of various substituting elements in LaNi5−xMx intermetallics on their structural, thermodynamic, magnetic, and hydrogen sorption properties has been analyzed by Joubert et al. in their review [8]. The substitution of Ni with Al [8,9,10] and Mn [11] has the strongest effect on the increase in the stability of the intermetallic hydrides. Multicomponent AB5-type alloys (A − La or La + Ce; B − Ni, Co, Al, and Mn) were shown to exhibit H2 plateau pressures at ambient temperature far below 1 atm and to compress H2 to 5–10 atm when heated to 150–170 °C [12,13].
Apart from thermodynamic constraints specifying the values of Peq to be below H2 charge pressure, PL ≈ 1 atm, at the cooling temperature, TL ≈ 15–20 °C, and above H2 discharge pressure, PH ≈ 2–5 atm, at the heating temperature, TH ≈ 90–150 °C, high rates of H2 uptake and release should also be provided on both the material and system level. Considering the limited pressure driving force when absorbing near-atmospheric pressure H2, the acceleration of this process is the most critical problem. On the material level, it relates to the improvement in hydrogenation kinetics, while the improvement in H2 uptake dynamics in the system mainly depends on the augmentation of heat exchange between the MH material and the cooling means of the MH reactor.
The improvement in hydrogenation kinetics, along with facilitating activation and adding poisoning tolerance, can be achieved by introducing catalysts of the dissociative H2 chemisorption, including Pd or Pd–Ni nanoparticles deposited onto the AB5 substrate [14]. The less expensive but still efficient approach is the creation of MH-based composites containing minor additives of graphene-like material with deposited Ni nanoparticles (Ni/GLM). Apart from catalyzing hydrogenation reactions, such composites are characterized by improved effective thermal conductivity (ETC), thus assisting in the acceleration of the H2 charge dynamics of the MH reactor [15]. Alternatively, ETC can be increased by adding expanded natural graphite (ENG) to the MH material [16].
Finally, the thermal augmentation methods include optimising the size and geometry of the MH reactors. The most frequently used methods combine the increase in (1) ETC (e.g., by using ENG), (2) heat transfer surface area (finned or coiled heat exchangers), and (3) the overall heat transfer coefficient (internal heat exchanger + external cooling jacket) [17].
This work presents results of the activities of the authors representing FRC PCP&MC RAS (Russia) and HySA Systems (South Africa) on the development of systems which can absorb low-pressure H2 and to further desorb it at higher pressures. The studies of the Russian team aimed toward the development of MH H2 storage and supply unit fed by H2 from a solid oxide electrolyzer were focused on the use of LaNi5−xAlx intermetallics with Ni/GLM additives. The activities of the HySA Systems team in South Africa were focused on the development of an MH hydrogen compression unit with suction pressure below 1 atm comprising a powder mixture of a multi-component AB5-type battery alloy with ENG additives loaded into a standard HySA Systems MH container for medium-pressure hydrogen compression [4].
2. Results
2.1. MH Materials
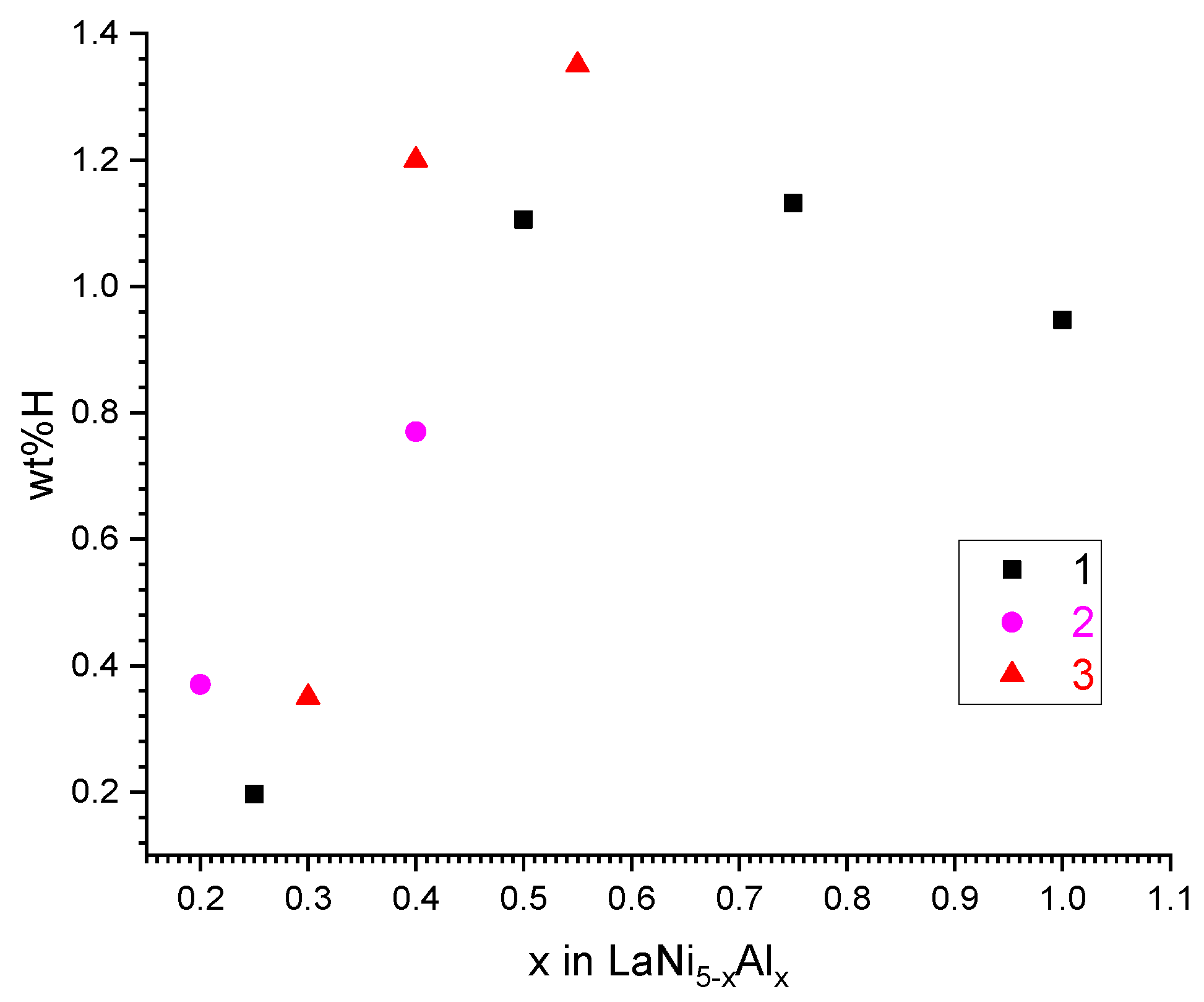
The analysis of data in the literature [8,9,10] and our own preliminary results on hydrogen absorption in aluminium-substituted LaNi5 at P(H2) = 1 atm and T = 20–40 °C (Figure 1) shows that the maximum hydrogen absorption capacity can be achieved when the content of aluminium in LaNi5−xAlx corresponds to x = 0.55. At the lower x values, when Peq in the plateau region of the pressure–composition isotherm approaches 1 atm, the capacity drop, especially pronounced when increasing the absorption temperature, is observed. A further increase in x results in reducing the H absorption capacity that is typical for the Al-substituted alloys, despite significant decreases in the plateau pressures in H–LaNi5−xAlx systems when increasing x [8]. In addition, hydrogen desorption from the hydrogenated alloys LaNi5−xAlx (x > 0.55) at moderate (<100 °C) temperatures is characterized by lower plateau pressures resulting in high residual H concentrations and, in turn, low reversible H sorption capacities at the operating conditions.
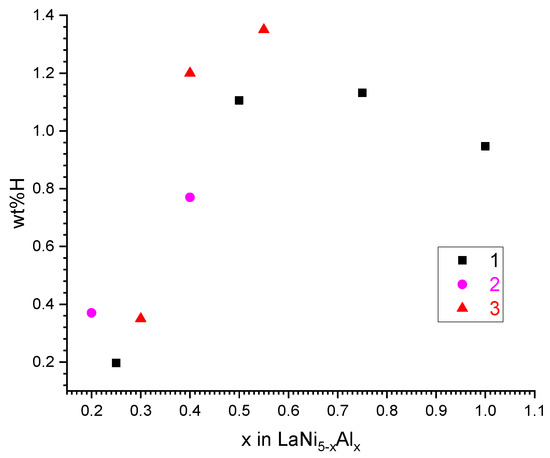
Figure 1.
Hydrogen absorption capacity of LaNi5−xAlx intermetallics at P(H2) = 1 atm. 1—[10], T = 40 °C; 2—[9], T = 20 °C; 3—this work, T = 20 °C.
The LaNi4.45Al0.55 alloy was prepared in an upscaled (×10 kg) amount and used in further studies by the Russian co-authors of this work.
In the studies carried out by HySA Systems in South Africa, a multi-component AB5-type alloy was used. According to the results of EDX analysis (see Supplementary Information; Figure S1 and Table S1), the composition of the used alloy corresponded to the formula La0.41Ce0.59Ni3.71Co0.52Mn0.36Al0.29 (B/A = 4.88).
XRD analysis of the as-delivered and hydrogenated alloys confirmed that each sample contains a single phase of CaCu5-type intermetallic (intermetallic hydride), space group P6/mmm/#191 (Figure S2 and Table S2). Rietveld refinement of the XRD patterns yielded acceptable residuals when Rp values varied between 3.1 and 5.4%, except for the starting LaNi4.45Al0.55 alloy (Rp = 13.6%), which exhibited a bigger crystallite size and noticeable preferred orientation in the (1 0 0) plane (Table S2).
The data on lattice periods and unit cell volumes calculated from the results of Rietveld refinement of the XRD patterns are summarized in Table 1. The lattice periods and unit cell volume of LaNi4.45Al0.55 are in good correspondence with the reference data for LaNi4.5Al0.5: a = 5.03 Å, c = 4.03 Å, c/a = 0.80, V = 88.3Å3 [18]. At the same time, the multi-component AB5 intermetallic studied in this work exhibits a unit cell volume 1.6–2.4% lower than the values reported in [19,20] for the battery alloys of similar compositions but containing La-rich mischmetal (~70 at.% La) as an A-component. The reason for the deviation appears to be the higher content of cerium (atomic radius 185 pm) in our case, while the intermetallics studied in [19,20] contained more lanthanum, characterized by a bigger atomic radius (195 pm).

Table 1.
Lattice periods and unit cell volumes of the studied samples calculated from Rietveld refinement of their XRD patterns.
Both alloys were found to exhibit reproducible hydrogen absorption/desorption performances after 1 h long evacuation at T = 90 °C followed by exposure in hydrogen at P = 5 atm, cooling to ambient temperature and being kept under H2 pressure for 5–6 h.
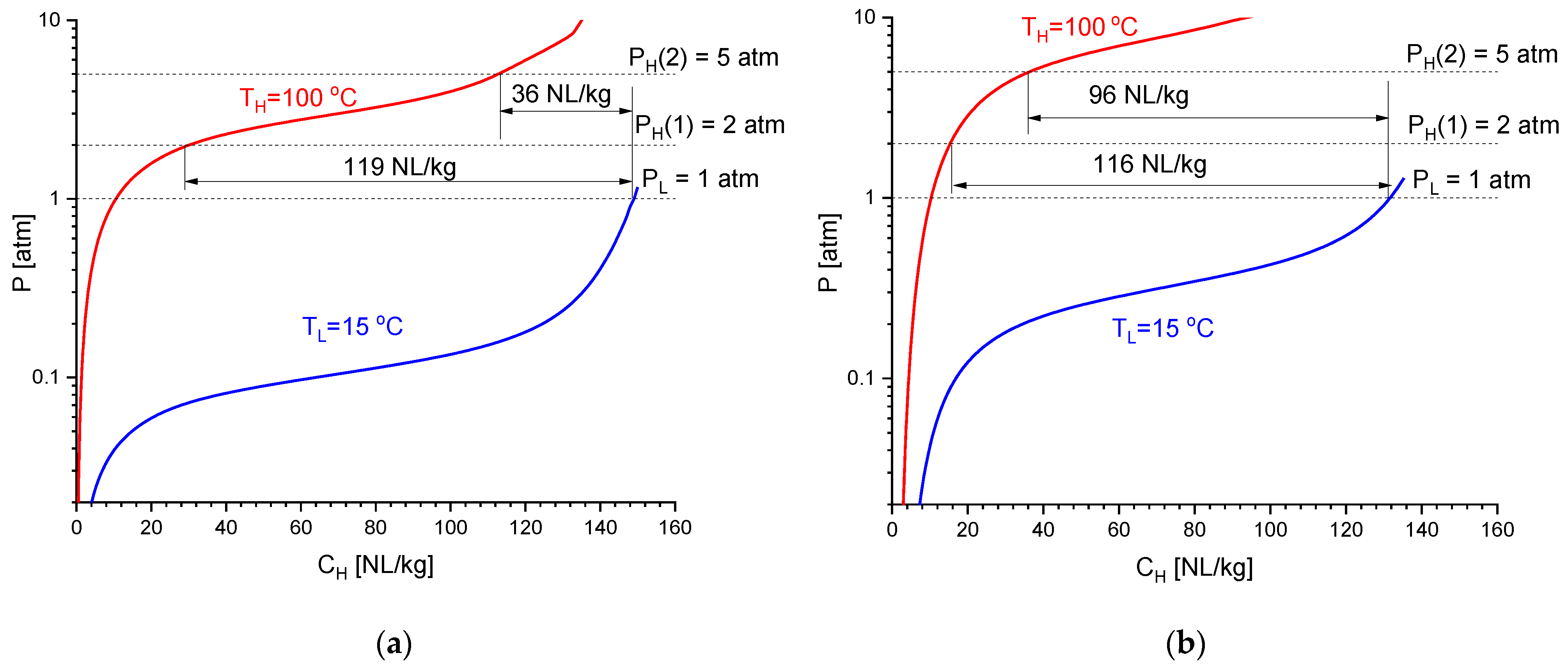
Figure 2 presents isotherms of hydrogen absorption and desorption for the studied intermetallics. For the correct comparison, the experimental PCT data taken for LaNi4.45Al0.55 (20–90 °C; 0.04–15 atm) and La0.41Ce0.59Ni3.71Co0.52Mn0.36Al0.29 (20–120 °C, 0.02–30 atm) were processed using the model of phase equilibria in hydrogen–metal systems [21], and the absorption (TL = 15 °C) and desorption (TH = 100 °C) isotherms shown in Figure 2 were calculated using the refined fitting parameters.
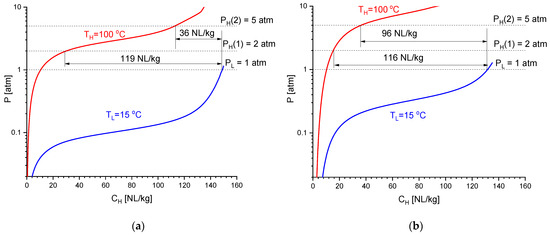
Figure 2.
Calculated isotherms of H2 absorption at TL = 15 °C and H2 desorption at TH = 100 °C for the low-pressure AB5-type intermetallics LaNi4.45Al0.55 (a) and La0.41Ce0.59Ni3.71Co0.52Mn0.36Al0.29 (b).
As it can be seen, both intermetallics exhibit similar reversible hydrogen sorption capacities: 116–119 NL/kg (1.03–1.05 wt.% H) when operating in the range of TL = 15 °C/PL = 1 atm − TH = 100 °C/PH = 2 atm. However, due to the slightly lower thermal stability of La0.41Ce0.59Ni3.71Co0.52Mn0.36Al0.29 hydride (the fitted values of hydrogenation entropy and enthalpy correspond to ΔSo = −112.20 ± 0.01 J/(mol H2 K) and ΔHo = −35.615 ± 0.004 kJ/mol H2) as compared to LaNi4.45Al0.55Hx (ΔSo = −108.22 ± 0.02 J/(mol H2 K); ΔHo = −36.90 ± 0.02 kJ/mol H2), the latter exhibits a significant drop (by 3.3 times) in the reversible capacity when the discharge pressure increases to PH = 5 bar, while for the multi-component AB5-type alloy, the capacity remains close to 100 NL/kg (0.85 wt.% H). Apparently, low errors of the refined values of ΔSo and ΔHo are related to the specifics of the modeling procedure [21], which assumes the error of the refined fitting parameter to be equal to its biggest increment (decrement), which results in the 1% increase in the sum of the squared shortest distances of the experimental points from the calculated PCI curves built in coordinates C/Cmax − ln P.
Thus, we recommend using LaNi4.45Al0.55 for buffer hydrogen storage in systems characterized by near-atmospheric pressures of the produced H2 and relatively low (up to 1 atm gage) pressures of H2 consumption typical for the renewable energy systems based on reversible solid oxide fuel cells [1]. The La0.41Ce0.59Ni3.71Co0.52Mn0.36Al0.29 alloy can be used as a material for the first stage of a thermally driven MH H2 compressor with a near-atmospheric suction pressure for the utilization of low-pressure H2.
2.2. System Development
2.2.1. MH Hydrogen Storage Unit Charged from Solid Oxide Electrolyzer
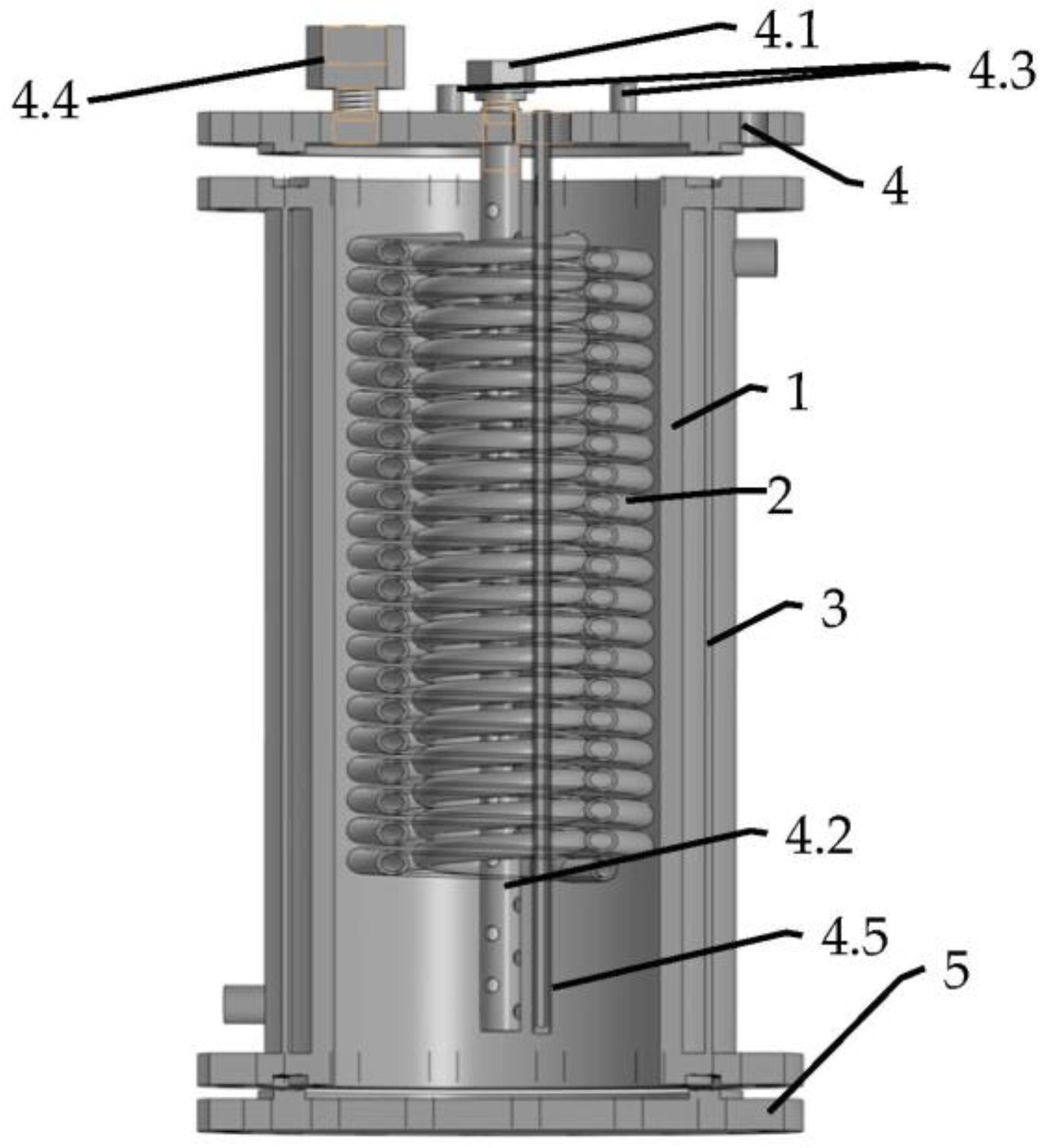
The low-pressure hydrogen storage unit developed by FRC PCP&MC RAS (Figure 3) comprises a stainless-steel cylindrical container (1) equipped with an inner heat exchanger (2) made as a double-circuit coiled copper tubing, and an outer heating/cooling jacket (3). The containment ends are closed by two flanges (4,5). The top flange (4) carries a H2 inlet/outlet port (4.1) connected to tubular filter (4.2), as well as ports (4.3) for the input and output of cooling/heating fluid (cold and hot water), a connector for the pressure sensor (4.4), and a thermocouple probe (4.5) for measuring the temperature of the MH bed. The inner space of the containment (1), 20 mm in the diameter and 290 mm in the height, was filled with 8.5 kg of LaNi4.45Al0.55 powder mixed with 100 g (1.16 wt.%) of Ni/GLM powder for the improvement in hydrogen absorption kinetics and the increase in the effective thermal conductivity of the MH bed [15].
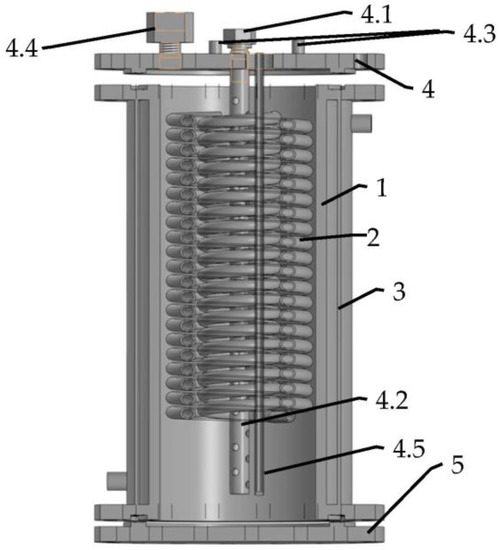
Figure 3.
Layout of hydrogen storage unit developed at FRC PCP&MC RAS. 1—cylindrical containment; 2—heat exchanger; 3—heating/cooling jacket; 4—top flange: 4.1—H2 inlet/outlet port, 4.2—tubular filter, 4.3—heating/cooling ports, 4.4—connector for pressure sensor, 4.5—thermocouple probe; 5—bottom flange.
The developed unit exhibited the maximum hydrogen absorption capacity of 1200 NL, of which, about 1000 NL was absorbed at P(H2) ≤ 1 atm when cooled to 15–20 °C with running water. The amount of hydrogen further desorbed at P(H2) ≥ 2 atm during water heating to 70–90 °C exceeded 900 NL, which corresponds to 100 NL/kg, or ~84% of the theoretical (equilibrium) reversible H storage capacity of LaNi4.45Al0.55 in the range of operating temperatures and pressures 15–100 °C and 1–2 atm, respectively (see Figure 2a). The unit was successfully tested in integration with a solid oxide electrolyzer.
2.2.2. MH Unit for Low-Pressure Hydrogen Compression
As mentioned above, the low-pressure H2 compression unit developed by HySA Systems used a standard MH container for medium-pressure hydrogen compression, described in ref. [4]. The cylindrical stainless-steel container (625 mm long and 2.68 dm3 in the inner volume) with an inner heat exchanger (finned tube) and an outer heating/cooling jacket was filled with 8.3 kg of the powdered mixture of La0.41Ce0.59Ni3.71Co0.52Mn0.36Al0.29 with 1wt.% of ENG.
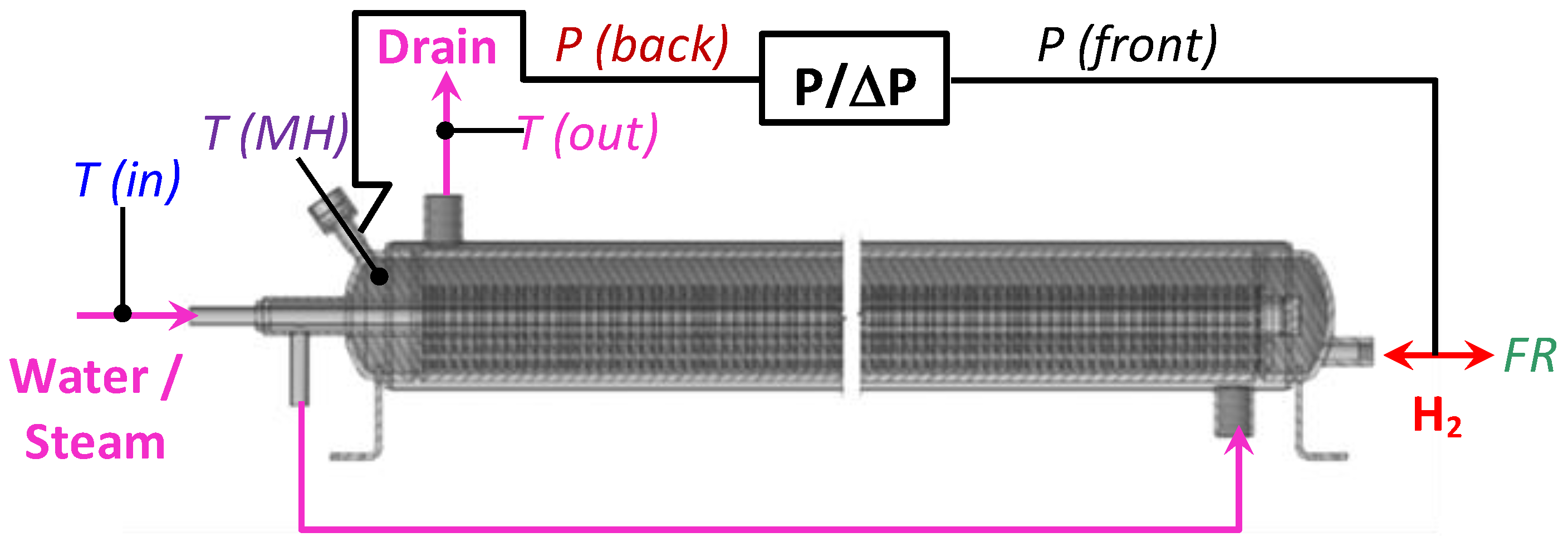
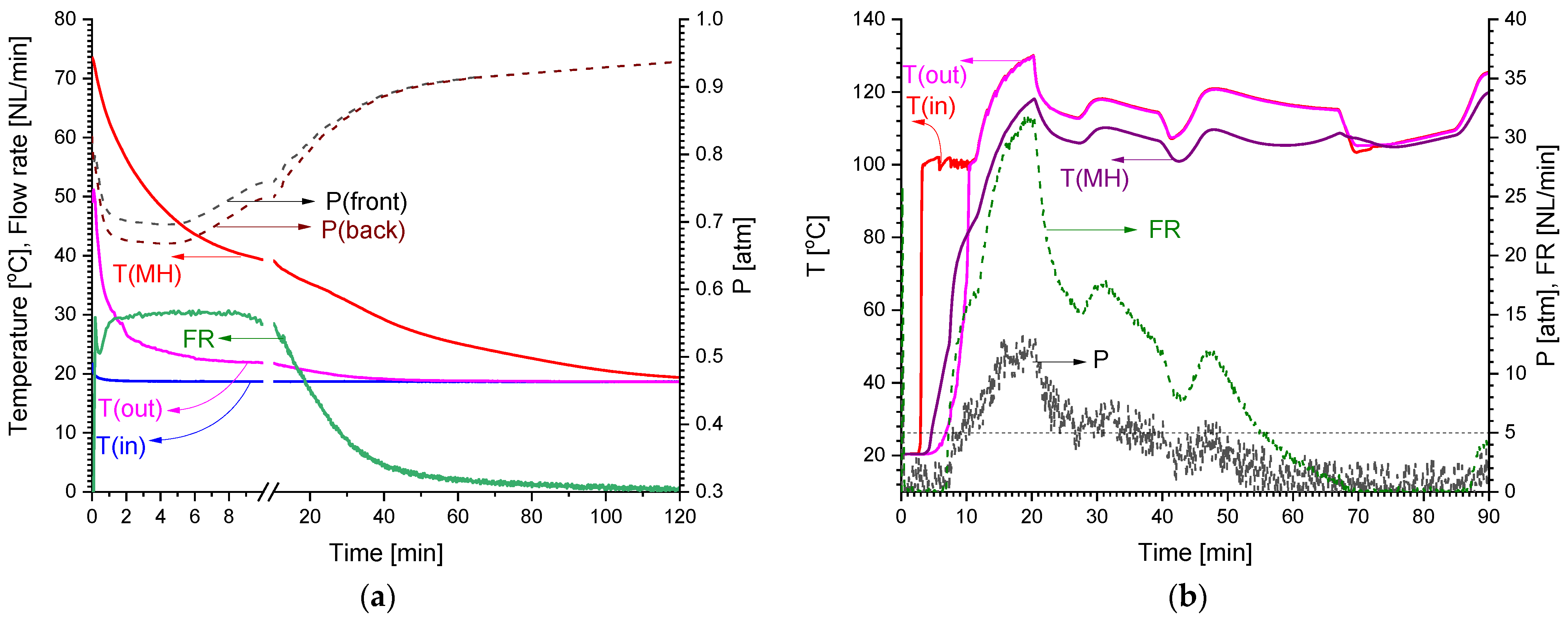
Figure 4 schematically shows the connection of the MH container to the testrig. During the tests, hydrogen pressure was measured both at the front (H2 supply/removal pipeline; P(front)) and back (P(back)) sides of the container using a two-channel absolute/differential PD39 pressure sensor (0–10 atm absolute). For the connection of the rear side of the container to the pressure sensor, as well as for the input of a thermocouple measuring the temperature in the MH bed, the MH loading port of the container was used. Additionally, the mass flow rate (FR) of hydrogen supplied to or released from the MH container was measured. The temperatures of the supplied (T(in)) and drained (T(out)) cooling/heating fluid (water/steam), along with the MH bed temperature (T(MH)) in the container, close to its back side, were measured as well.
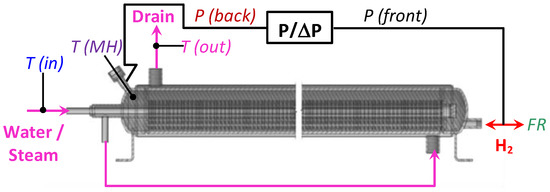
Figure 4.
Schematics of the tests of MH container for the low-pressure H2 compression. T(in)—steam (water) inlet temperature, T(out)—steam (water) outlet temperature, T(MH)—temperature in the MH bed, P/ΔP—absolute/differential pressure sensor, P(front)—H2 pressure at the input/output of the MH container, P(back)—H2 pressure from the opposite side, FR—mass flow rate of the absorbed/desorbed H2.
The results of the tests (see Figure 5) showed that the total hydrogen capacity of the MH container exceeded 1000 NL, of which, about 850 NL was absorbed at the pressure below 1 atm during the cooling of the container with running water at T = 16–18 °C. About 80% of the low-pressure hydrogen (670 NL) was absorbed in 30 min with a maximum flow rate of 30 NL/min. The MH temperature increased to ~75 °C almost immediately after the start of the low-pressure H2 supply, and the pressure drop between the entrance to the container, P(front), and its rear side, P(back), did not exceed 0.05 atm. Further heating of the container by steam (T~120 °C) resulted in the desorption of hydrogen at higher pressures; in doing so, about 650 NL H2 was desorbed from the container at a pressure above 5 atm and a flow rate of 10–30 NL/min.
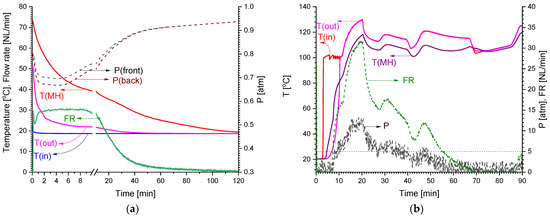
Figure 5.
Results of tests of MH container for low-pressure H2 compression: H2 absorption (a) and H2 desorption (b).
3. Discussion
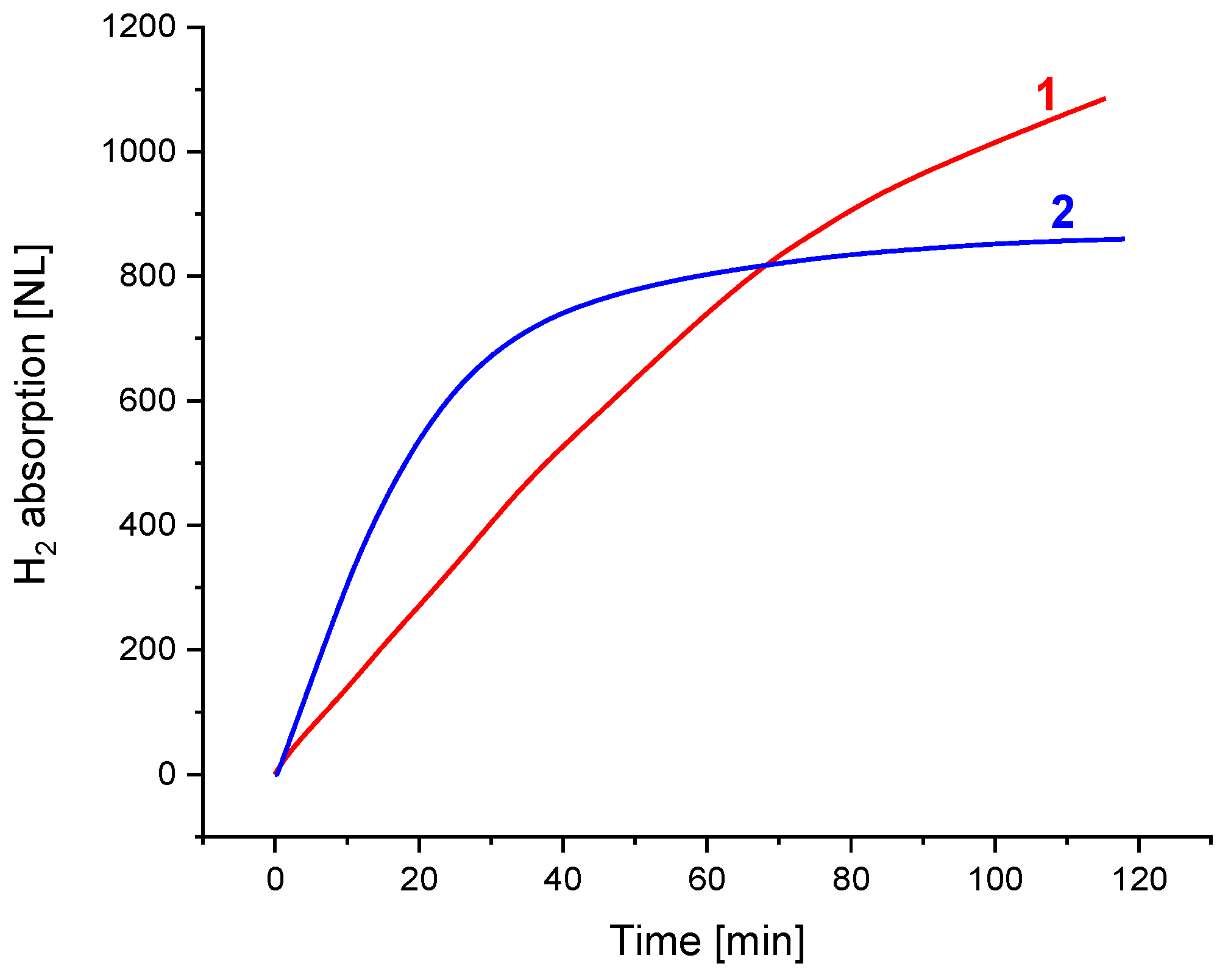
Figure 6 compares the H2 charge dynamic performance of the low-pressure hydrogen storage unit developed at FRC PCP&MC RAS (1) and the unit for the low-pressure H2 compression developed by HySA Systems (2). Both units can absorb hydrogen supplied below atmospheric pressure; the hydrogen absorption capacity achieved after 2 h long operation was about 90% of the maximum one observed after the charge at P(H2) = 5 atm and ambient temperature. In doing so, the average H absorption rate exceeded 10 NL/min for the MH load between 8.3 and 8.6 kg, which corresponded to the specific rate of more than 1.15–1.20 NL/(min kg). This shows the technical feasibility of charging MH units with near-atmospheric-pressure hydrogen with reasonable productivity when the absorption plateau pressure of the used AB5-type MH material is about 0.1–0.3 atm (Figure 2).
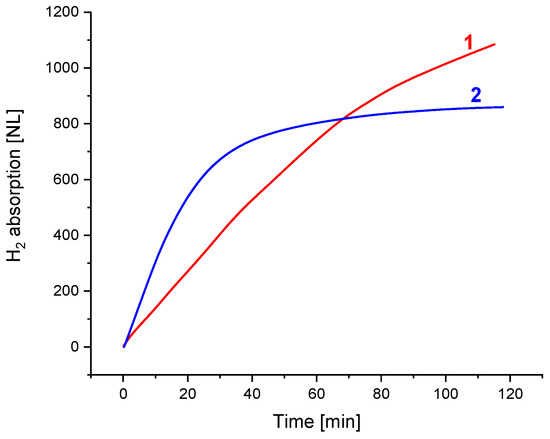
Figure 6.
Dynamics of H2 charge of the MH hydrogen storage (1: FRC PCP&MC RAS) and compression (2: HySA Systems) units at the pressure of the feed H2 below 1 atm (absolute).
Slower H2 charge dynamics of the FRC PCP&MC RAS unit (1), first of all, are caused by the lower efficiency of the used inner heat exchanger (coiled tube) as compared to the HySA Systems unit (2), in which the heat exchanger was made as a finned tube with fin pitch of 5 mm. However, the advantage of the former unit is the almost constant hydrogen absorption flow rate throughout the operation period, which improves the overall system performance when operating in the integration with the solid oxide electrolyzer. The further heating of the unit to a moderate temperature, 70–90 °C, would allow hydrogen to be released at P ≥ 2 atm, which is sufficient for H2 supply to a solid oxide fuel cell stack, for example, when the SOC unit is used in the reversible “electrolyzer–fuel cell” mode.
The analysis of the low-pressure hydrogen compression performance of the HySA Systems unit (2; see also Figure 5) allows us to conclude that the productivity of hydrogen compression from PL = 1 atm (TL = 15 °C) to PH = 5 atm (TH = 120 °C) at the typical duration of an absorption–desorption cycle of 50–60 min [22] can achieve ~0.1 Nm3/h per 1 kg of the used multi-component AB5-type alloy. A further increase in hydrogen compression productivity can be achieved by optimising the MH material, first of all, towards the acceleration of hydrogen absorption kinetics at a low pressure driving force. The replacement of the ENG additive with Ni/GLM, along with the optimization of its content in the composite [15], can be promising in solving this problem.
4. Materials and Methods
The laboratory samples of LaNi5–xAlx intermetallics (x = 0.2–0.8) used in preliminary studies were prepared via arc melting of the components (La–98%; Ni, Al–99.5+%) under purified argon; the ingots were turned over and re-melted 3 times. The LaNi4.45Al0.55 alloy for further studies and use in the low-pressure MH hydrogen storage unit was prepared via induction melting in an alumina crucible under an argon atmosphere. All the alloys were studied in as-cast forms; no heat treatments were applied.
The Ni/GLM material was prepared via the reduction of a freeze-dried suspension of graphite oxide in Ni(CH3COO)2 aqueous solution in hydrogen flow at T = 300–500 °C; further details are presented in Ref. [23]. The LaNi4.45Al0.55–Ni/GLM composite was prepared by mixing the powders of the components (8.5 kg and 100 g, respectively).
The multi-component AB5-type alloy used in HySA Systems studies was supplied in x10 kg amount by Guangdong Provincial Key Laboratory of Rare Earth Development and Application (Guangzhou, China). The composition of a standard battery alloy was modified by the supplier, in consultation with HySA Systems, to provide the desired H2 absorption/desorption performance: minimum H2 absorption plateau pressure (below 1 atm) at near-ambient temperature and maximum H2 desorption plateau pressure (above 2 atm) at the temperatures above 70–90 °C.
The expanded natural graphite (ENG) was prepared via the heating of graphite oxide powder (KP50 expandable graphite, Bolun Industrial Limited/Huaian, China; 500 μ, 99.9+%) to 900 °C in an open tube furnace for 1 h. The powders of 8.3 kg of La0.41Ce0.59Ni3.71Co0.52Mn0.36Al0.29 alloy and 83 g of ENG were mixed and loaded into the MH container for low-pressure H2 compression.
XRD studies were carried out using Thermo Scientific (Waltham, MA, USA) ARL XʹTRA (FRC PCP&MC RAS) and Rigaku Miniflex 600 (HySA Systems), Tokyo, Japan, instruments, using Cu-Kα radiation, in the range of Bragg angles 2θ = 20–90°. The hydrogenated samples taken for the XRD measurements were stabilized via exposure to carbon monoxide (FRC PCP&MC RAS) or ambient air at liquid nitrogen temperature (HySA Systems). A standard α-Al2O3 sample was used for the determination of the instrumental contribution into peak profile parameters. The XRD patterns were processed via Rietveld analysis using GSAS software (2001 release, last update in 2009). During the refinements, the Gaussian profile parameters (GU, GV, and GW) were fixed (kept the same as for the Al2O3 standard), and only two Lorentzian profile parameters were refined: LX (size broadening) and LY (strain broadening).
EDX analysis of the multi-component AB5-type alloy was carried out using a Zeiss Auriga scanning electron microscope (Zeiss, Oberkochen, Germany); imaging in secondary electrons was carried out at the acceleration voltage of 15–20 kV.
Hydrogen absorption/desorption PCT studies were carried out using Sieverts setups in the range of temperatures of 20–120 °C and pressures of 0.01–40 atm, with a sample weight of 1–2 g, a sample holder volume of 12 cm3, and the reference volume, depending on the instrument used, being from 12 to 35 cm3. The as-collected PCT data were processed using the model of phase equilibria in hydrogen–metal systems [21]; the average fitting error corresponded to ΔC/Cmax below 0.06%.
Testing of the developed units was carried out using in-house built testrigs, allowing us to monitor the H2 charge/discharge flow rates, hydrogen pressures, and temperatures.
5. Conclusions
This work has shown the technical feasibility of the storage and thermally driven compression of low-pressure (1 atm absolute) hydrogen with a compression ratio from 2 to 5 in the temperature range from 15 to 20 to 70 to 120 °C by using AB5-type hydride-forming intermetallics characterized by plateau pressures of 0.1–0.3 atm at the ambient temperature and 3–8 atm at T = 100 °C. The use of powdered mixtures of these alloys with minor additives of the reduced graphite oxide (expanded natural graphite) or graphene-like material with deposited nickel nanoparticles allowed us to achieve the rate of hydrogen uptake and release above 10 NL/min for the 1 Nm3 H2 capacity unit, or above 1.2 NL/min per 1 kg of the used material.
6. Patents
The following patented solutions contributed to the results reported in this work:
- Metal hydride bed, metal hydride container, and method for the making thereof, by M. Lototskyy, M.W. Davids, B.G. Pollet, V.M. Linkov and Y. Klochko. WO 2015/189758 A1 (2015).
- Nickel-graphene hydrogenation catalyst and method of its production, by A.A. Arbuzov, S.A. Mozhzhukhin, A.A. Volodin, P.V. Fursikov and B.P. Tarasov. RU 2660232 C1 (2018).
- Low-pressure hydrogen accumulator of multiple action, by B.P. Tarasov, A.A. Arbuzov, S.A. Mozhzhukhin, A.A. Volodin, P.V. Fursikov and M.V. Lototskyy. Application RU2023108442 (2023).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/inorganics11070290/s1, Figure S1: Energy dispersion spectrum and low-magnification SEM image of the multi-component AB5-type alloy; Table S1: Summary of EDX analysis of the multi-component AB5-type alloy; Figure S2: Refined XRD patterns of the studied samples; Table S2: Summary of Rietveld refinement of XRD patterns of the studied samples.
Author Contributions
Conceptualization, B.T. and M.L.; methodology, A.A. and M.L.; validation, S.M., M.W.D. and J.A.; formal analysis, A.V. and P.F.; investigation, B.T., A.A. and M.L.; resources, A.A. and M.W.D.; writing and visualization, M.L. and B.T.; supervision, B.T. and A.A.; project administration and funding acquisition, B.T. and M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Ministry of Science and Higher Education (Agreement No. 075-15-2022-1126) and the Department of Science and Innovation of South Africa (HySA Program, Key Project KP6-S02).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This work was performed using the equipment of FRC PCP&MC RAS, https://www.icp.ac.ru/en/, and HySA Systems hosted by the University of the Western Cape, http://hysasystems.com/.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brisse, A.; Schefold, J.; Léon, A. High-Temperature Steam Electrolysis. In Electrochemical Power Sources: Fundamentals, Systems, and Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 229–280. [Google Scholar]
- Lee, D.-Y.; Elgowainy, A.; Dai, Q. Life Cycle Greenhouse Gas Emissions of Hydrogen Fuel Production from Chlor-Alkali Processes in the United States. Appl. Energy 2018, 217, 467–479. [Google Scholar] [CrossRef]
- Sdanghi, G.; Maranzana, G.; Celzard, A.; Fierro, V. Review of the Current Technologies and Performances of Hydrogen Compression for Stationary and Automotive Applications. Renew. Sustain. Energy Rev. 2019, 102, 150–170. [Google Scholar] [CrossRef]
- Lototskyy, M.; Linkov, V. Thermally Driven Hydrogen Compression Using Metal Hydrides. Int. J. Energy Res. 2022, 46, 22049–22069. [Google Scholar] [CrossRef]
- Cuevas, F.; Latroche, M. Intermetallic Alloys as Hydrogen Getters. J. Alloys Compd. 2022, 905, 164173. [Google Scholar] [CrossRef]
- Lototsky, M.V.; Yartys, V.A.; Klochko, Y.V.; Borisko, V.N.; Starovoitov, R.I.; Azhazha, V.M.; V’yugov, P.N. Applications of Zr–V Hydrogen Getters in Vacuum-Plasma Devices: Phase-Structural and Hydrogen Sorption Characteristics. J. Alloys Compd. 2005, 404, 724–727. [Google Scholar] [CrossRef]
- Sandrock, G. A Panoramic Overview of Hydrogen Storage Alloys from a Gas Reaction Point of View. J. Alloys Compd. 1999, 293, 877–888. [Google Scholar] [CrossRef]
- Joubert, J.-M.; Paul-Boncour, V.; Cuevas, F.; Zhang, J.; Latroche, M. LaNi5 Related AB5 Compounds: Structure, Properties and Applications. J. Alloys Compd. 2021, 862, 158163. [Google Scholar] [CrossRef]
- Dashbabu, D.; Kumar, E.A.; Jain, I.P. Thermodynamic Analysis of a Metal Hydride Hydrogen Compressor with Aluminium Substituted LaNi5 Hydrides. Int. J. Hydrogen Energy 2022. [Google Scholar] [CrossRef]
- Diaz, H.; Percheron Guegan, A.; Achard, J.; Chatillon, C.; Mathieu, J. Thermodynamic and Structural Properties of LaNi5∓yAly Compounds and Their Related Hydrides. Int. J. Hydrogen Energy 1979, 4, 445–454. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Zhang, W.; Zhu, S.; Zhang, N.; Ke, D.; Liu, J.; Yan, K.; Cheng, H. Effect of Mn on the Long-Term Cycling Performance of AB5-Type Hydrogen Storage Alloy. Int. J. Hydrogen Energy 2021, 46, 21973–21983. [Google Scholar] [CrossRef]
- Lv, L.; Lin, J.; Yang, G.; Ma, Z.; Xu, L.; He, X.; Han, X.; Liu, W. Hydrogen Storage Performance of LaNi3.95Al0.75Co0.3 Alloy with Different Preparation Methods. Prog. Nat. Sci. Mater. Int. 2022, 32, 206–214. [Google Scholar] [CrossRef]
- Lototskyy, M.V.; Yartys, V.A.; Tarasov, B.P.; Davids, M.W.; Denys, R.V.; Tai, S. Modelling of Metal Hydride Hydrogen Compressors from Thermodynamics of Hydrogen—Metal Interactions Viewpoint: Part I. Assessment of the Performance of Metal Hydride Materials. Int. J. Hydrogen Energy 2021, 46, 2330–2338. [Google Scholar] [CrossRef]
- Lototsky, M.V.; Williams, M.; Yartys, V.A.; Klochko, Y.V.; Linkov, V.M. Surface-Modified Advanced Hydrogen Storage Alloys for Hydrogen Separation and Purification. J. Alloys Compd. 2011, 509, S555–S561. [Google Scholar] [CrossRef]
- Tarasov, B.P.; Arbuzov, A.A.; Volodin, A.A.; Fursikov, P.V.; Mozhzhuhin, S.A.; Lototskyy, M.V.; Yartys, V.A. Metal Hydride—Graphene Composites for Hydrogen Based Energy Storage. J. Alloys Compd. 2022, 896, 162881. [Google Scholar] [CrossRef]
- Atalmis, G.; Sattarkhanov, K.; Demiralp, M.; Kaplan, Y. The Effect of Expanded Natural Graphite Added at Different Ratios of Metal Hydride on Hydrogen Storage Amount and Reaction Kinetics. Int. J. Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Sreeraj, R.; Aadhithiyan, A.K.; Anbarasu, S. Integration of Thermal Augmentation Methods in Hydride Beds for Metal Hydride Based Hydrogen Storage Systems: Review and Recommendation. J. Energy Storage 2022, 52, 105039. [Google Scholar] [CrossRef]
- Kodama, T. The Thermodynamic Parameters for the LaNi5−xAlx–H2 and MmNi5−xAlx–H2 Systems. J. Alloys Compd. 1999, 289, 207–212. [Google Scholar] [CrossRef]
- Vivet, S.; Joubert, J.-M.; Knosp, B.; Percheron-Guégan, A. Effects of Cobalt Replacement by Nickel, Manganese, Aluminium and Iron on the Crystallographic and Electrochemical Properties of AB5-Type Alloys. J. Alloys Compd. 2003, 356, 779–783. [Google Scholar] [CrossRef]
- Ma, J. Effect of Heat Treatment on the Microstructure and Electrochemical Properties of AB5-Type MlNi3.60Co0.85Mn0.40Al0.15 Hydride Alloy: 1. The Microstructure and P-C Isotherms. Int. J. Hydrogen Energy 2002, 27, 57–62. [Google Scholar] [CrossRef]
- Lototskyy, M.V. New Model of Phase Equilibria in Metal—Hydrogen Systems: Features and Software. Int. J. Hydrogen Energy 2016, 41, 2739–2761. [Google Scholar] [CrossRef]
- Lototskyy, M.V.; Yartys, V.A.; Tarasov, B.P.; Denys, R.V.; Eriksen, J.; Bocharnikov, M.S.; Tai, S.; Linkov, V. Modelling of Metal Hydride Hydrogen Compressors from Thermodynamics of Hydrogen—Metal Interactions Viewpoint: Part II. Assessment of the Performance of Metal Hydride Compressors. Int. J. Hydrogen Energy 2021, 46, 2339–2350. [Google Scholar] [CrossRef]
- Tarasov, B.P.; Arbuzov, A.A.; Mozhzhuhin, S.A.; Volodin, A.A.; Fursikov, P.V. Composite Materials with 2D Graphene Structures: Applications for Hydrogen Energetics and Catalysis with Hydrogen Participation. J. Struct. Chem. 2018, 59, 830–838. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).