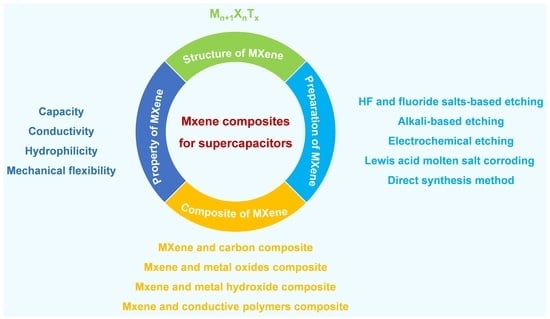
Structure, Properties, and Preparation of MXene and the Application of Its Composites in Supercapacitors
Abstract
1. Introduction
2. Structure and Properties of MXene
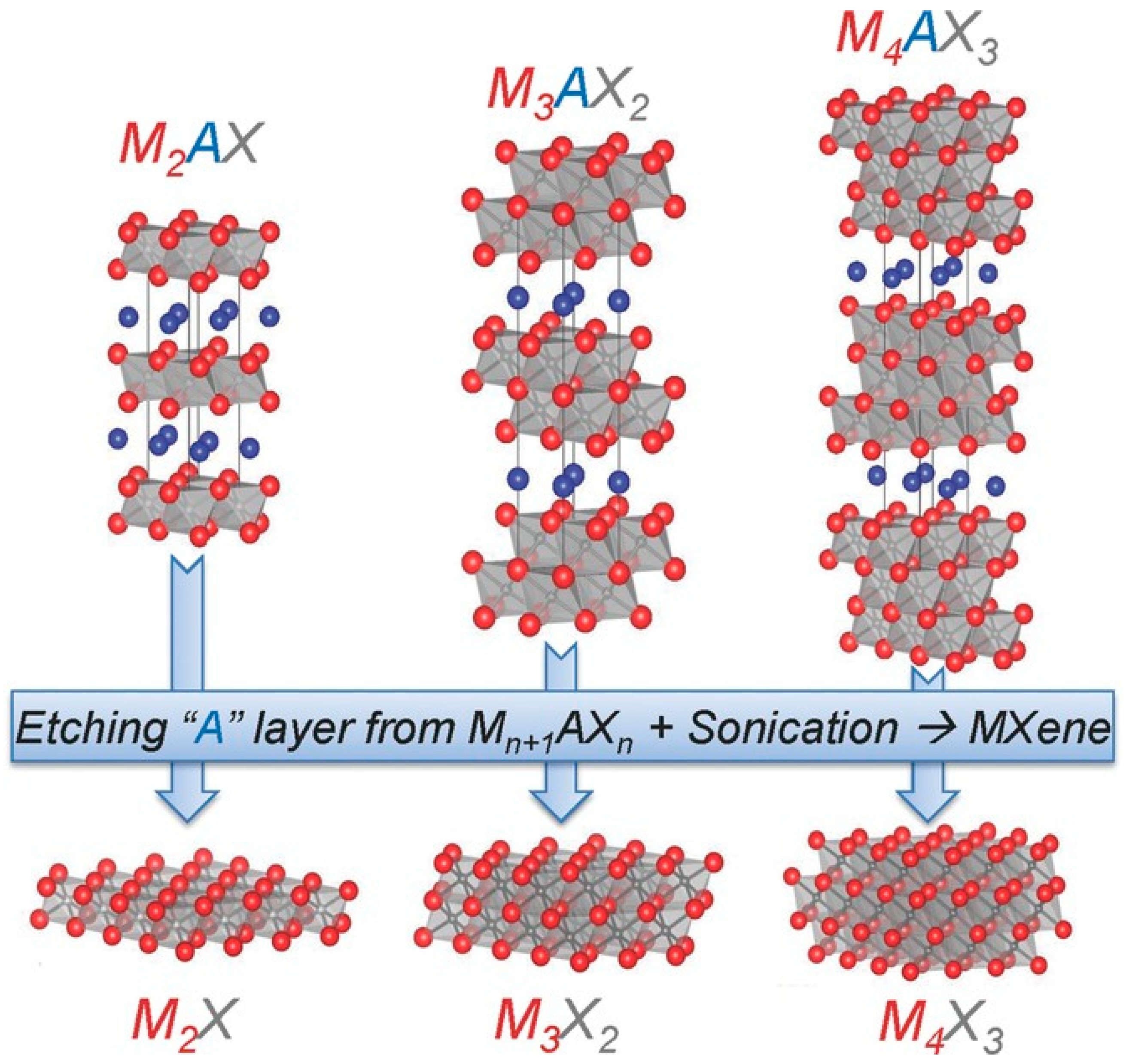
2.1. Structure of MXene
2.2. Properties of MXene
2.2.1. Capacitive Properties
2.2.2. Conductivity
2.2.3. Hydrophilicity
2.2.4. Mechanical Flexibility
3. Preparation of MXene
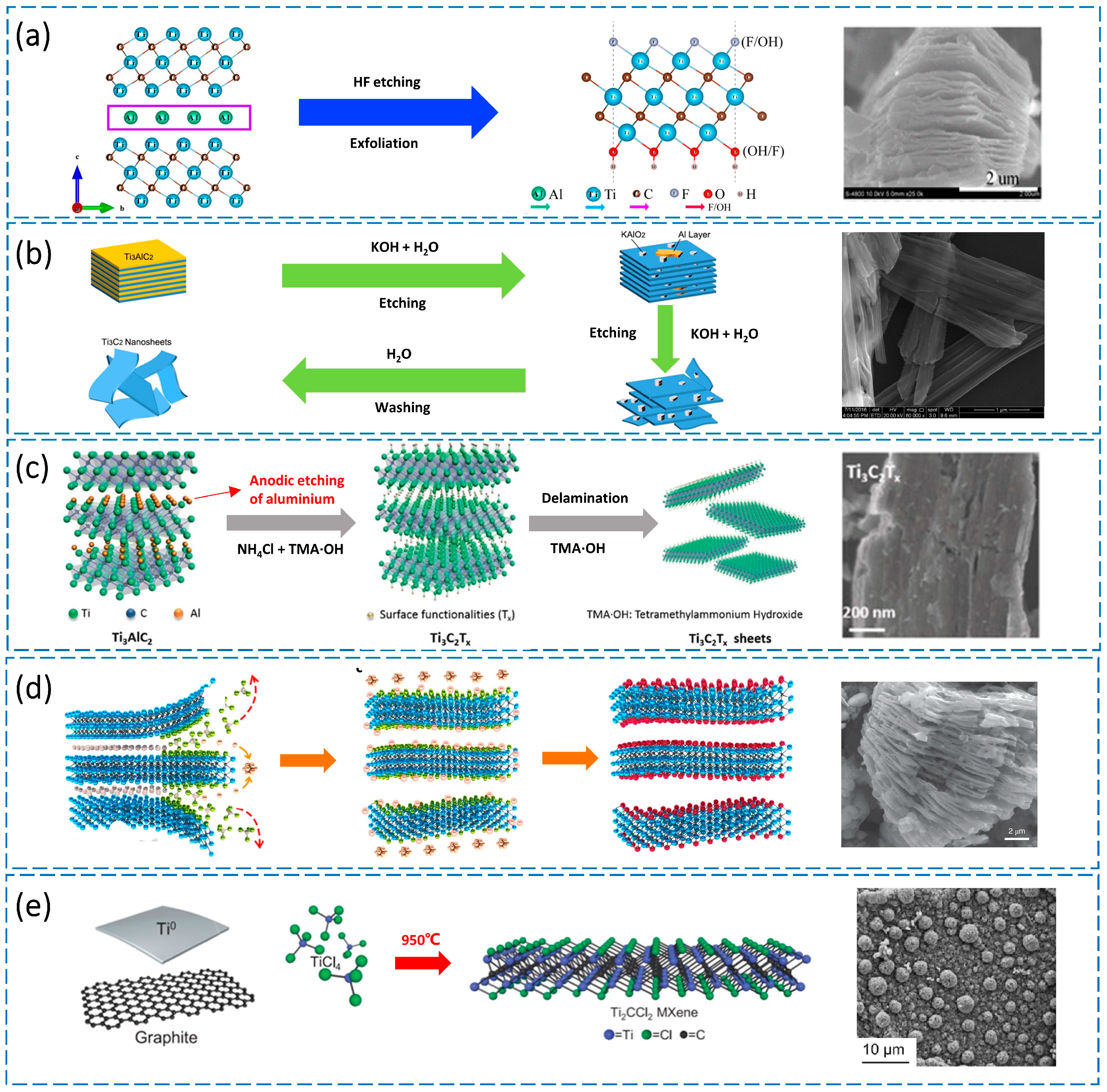
3.1. Etching Methods Based on HF and Fluoride Salts
3.2. Alkali-Based Etching Method
3.3. Electrochemical Etching Method
3.4. Lewis Acid Molten Salt Corroding Method
3.5. Direct Synthesis Method
4. Application of MXene-Based Composite Materials in Supercapacitors
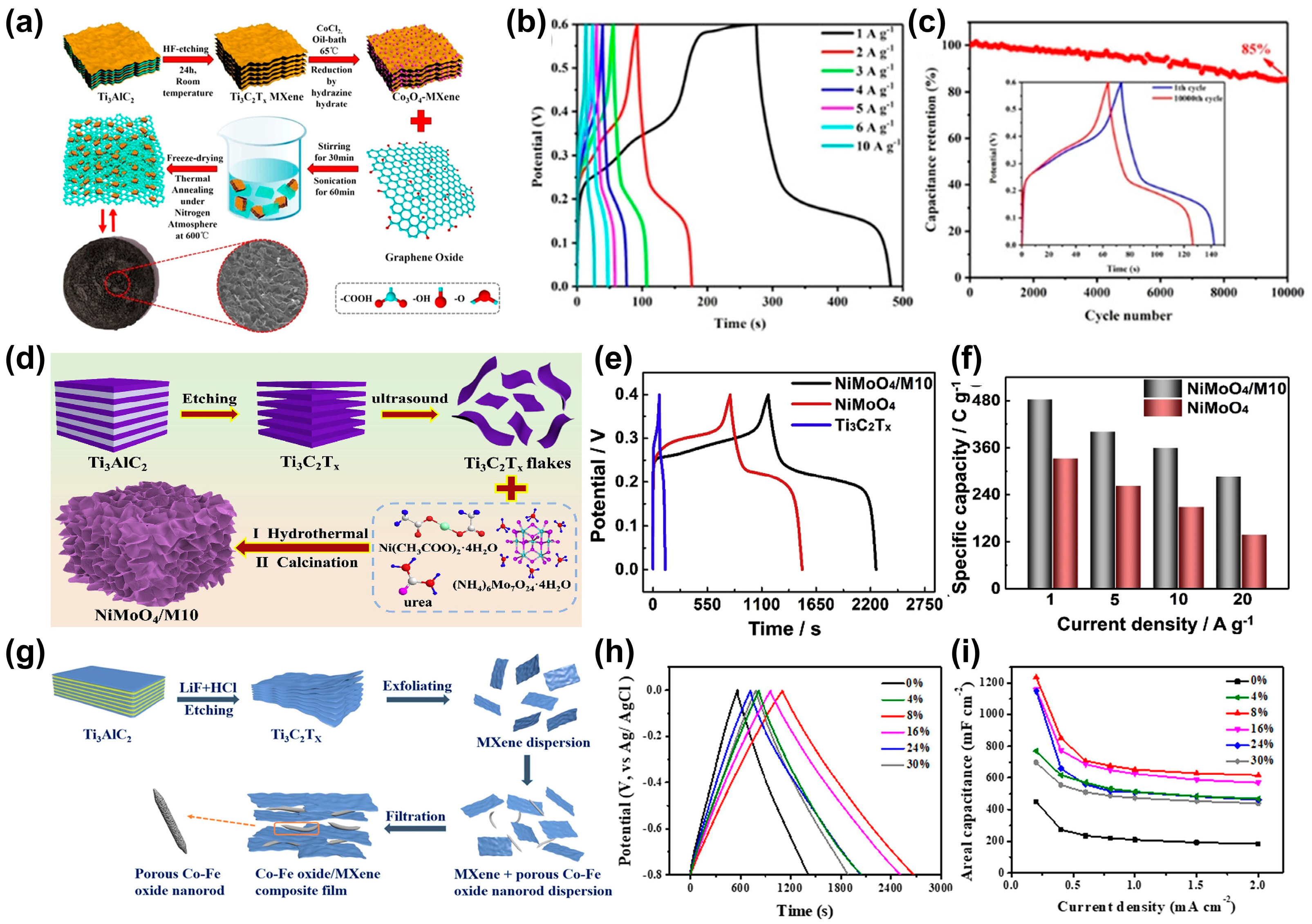
4.1. Composite of MXene and Carbon Materials
4.2. Composite of MXene and Metal Oxides Materials
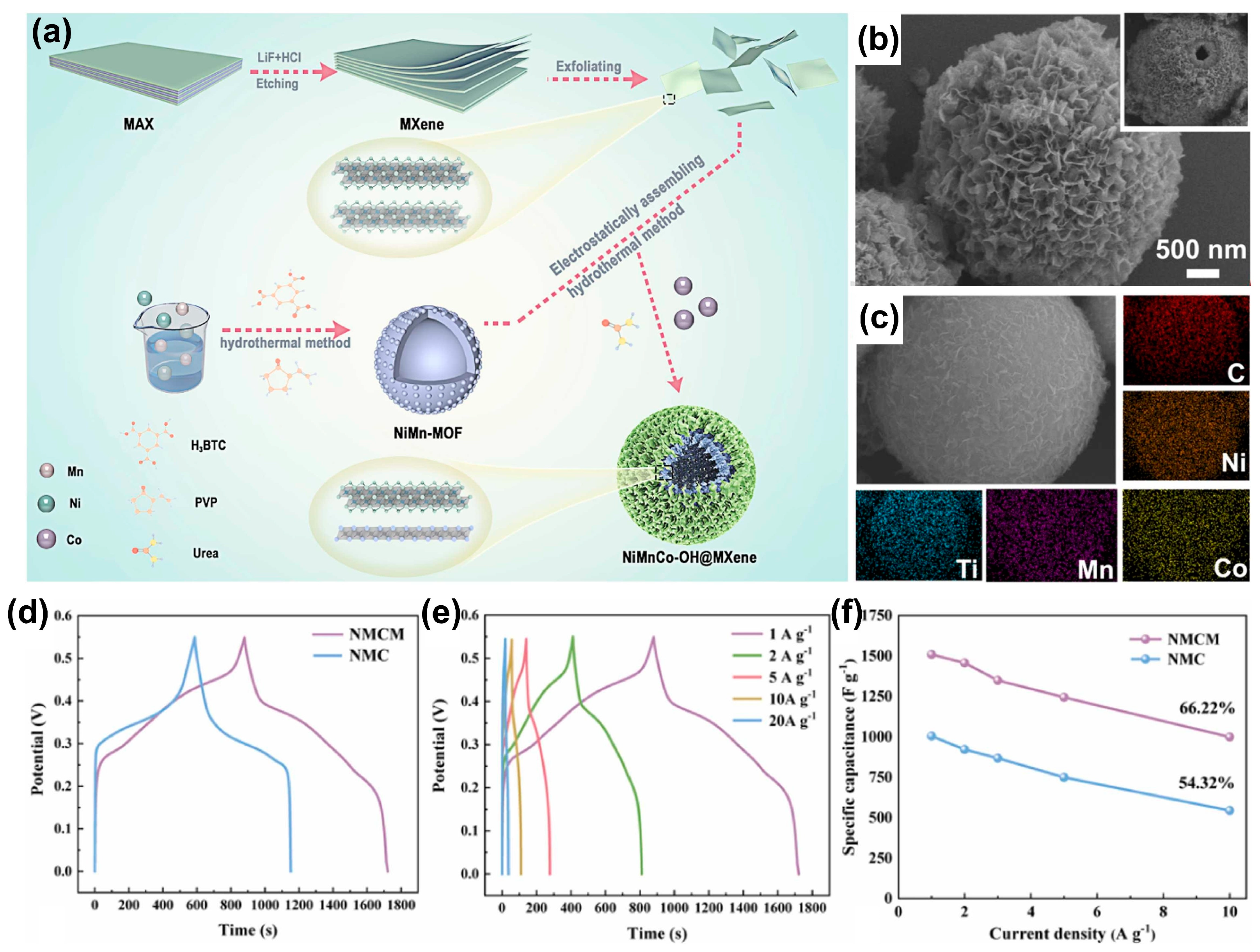
4.3. Composite of MXene and Metal Hydroxide Materials
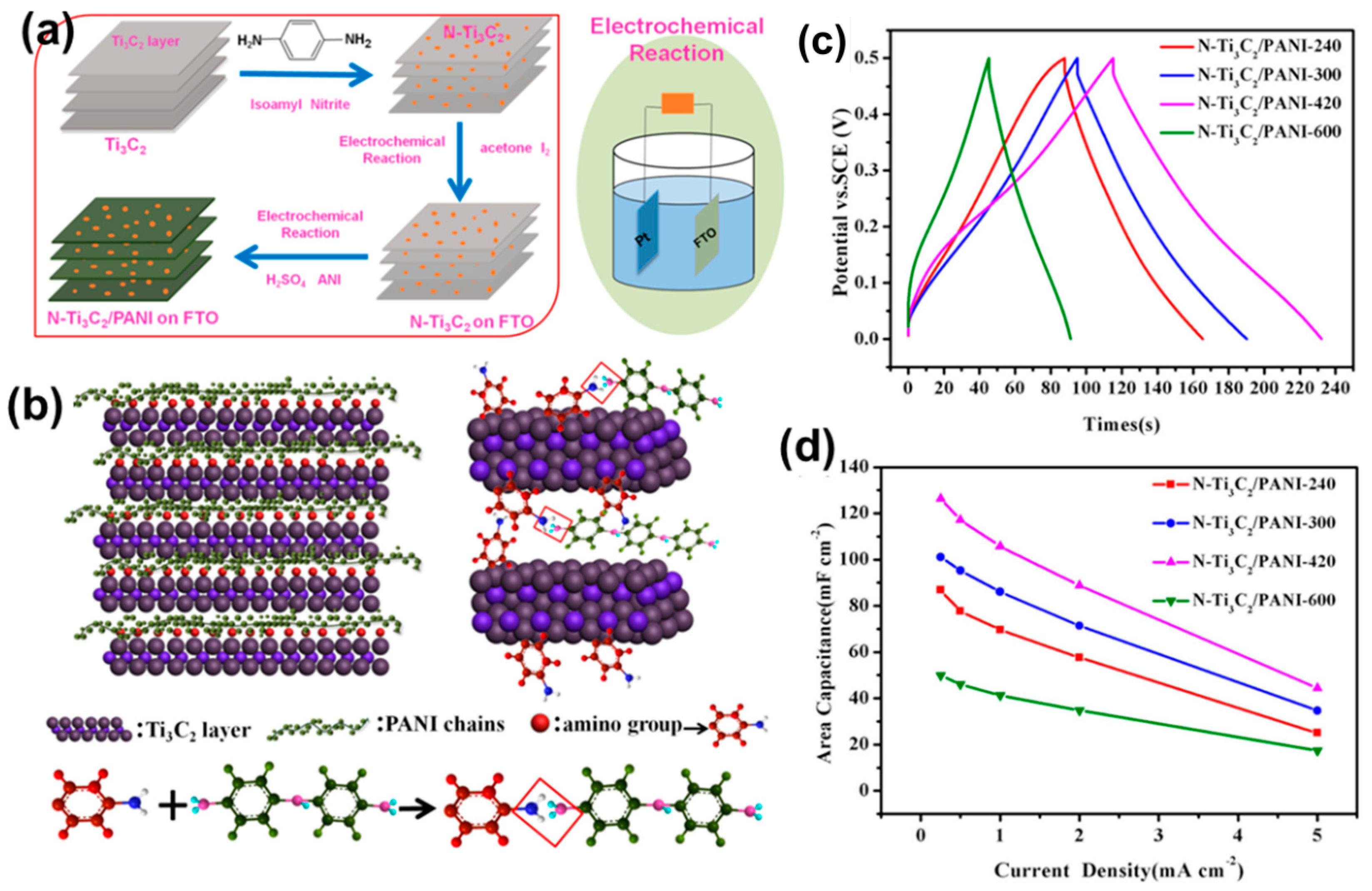
4.4. Composite of MXene and Conductive Polymer Materials
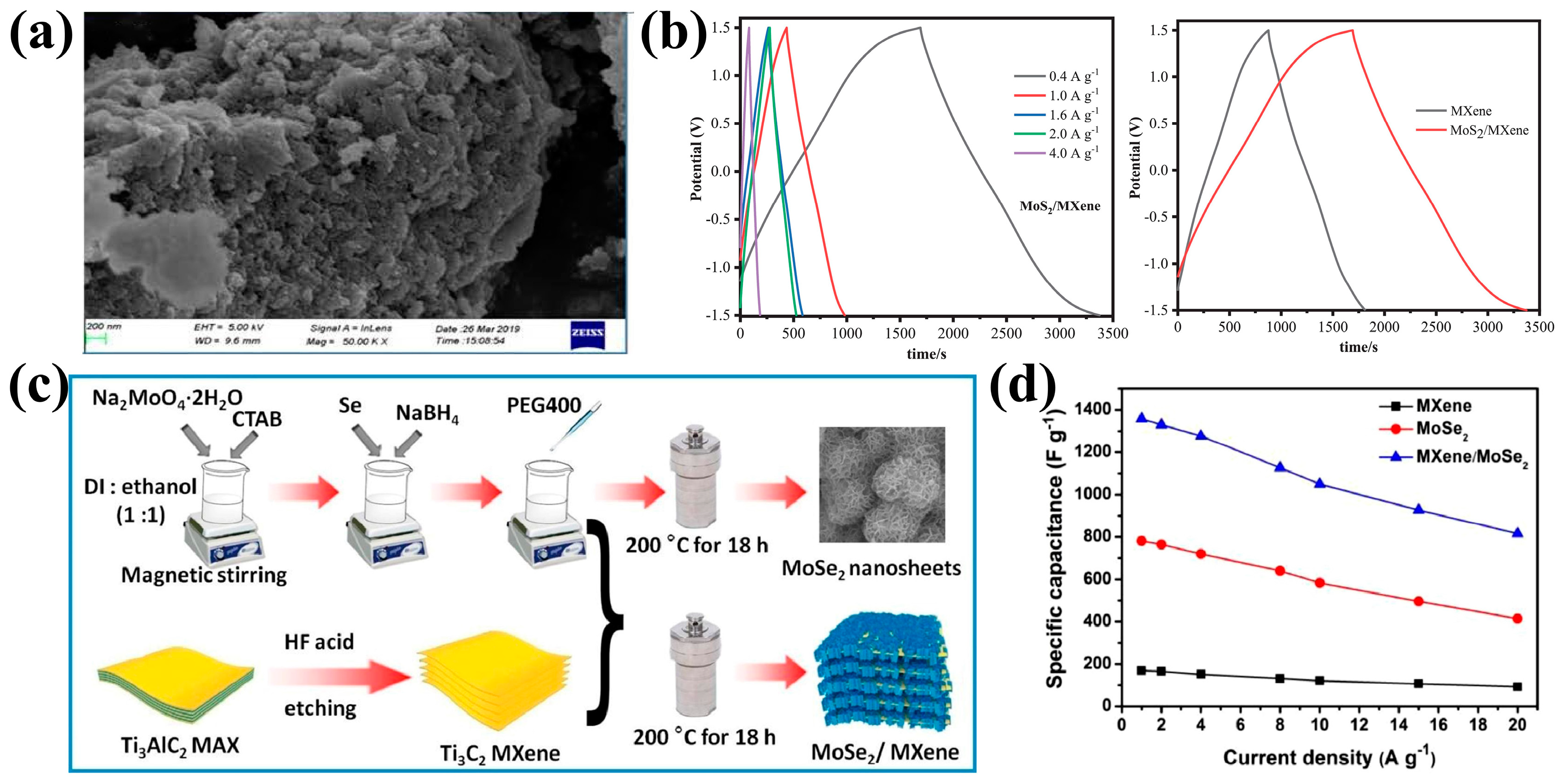
4.5. Composites of MXene and 2D Materials
- Reduce electrolyte resistance: MXene has a high conductivity and can effectively reduce the resistance of the electrolyte when it is combined with polymers. This will reduce the resistance loss inside the capacitor and improve the efficiency of its energy transfer.
- Improve the frequency response of the capacitor: After MXene is combined with a polymer, the surface area of the capacitor may be increased, and its effective capacitance may be improved. At the same time, in the high-frequency band, the impedance characteristic curve of the composite material may be closer to the ideal behavior of the capacitor so that the capacitor has a better performance in high-frequency applications.
- Improve the cycle stability of capacitors: MXene’s high chemical stability can improve the cycle life and stability of capacitors. A change in the impedance curve of a composite material may manifest as a smaller phase angle drift and a more stable electrochemical interface, which reduces the performance attenuation of the capacitor over a long cycle of charge and discharge.
- Adjust the charge and energy storage behavior of the capacitor: Changes in the impedance characteristic curves of MXene and polymer composites may lead to changes in the charge storage mechanism and energy storage behavior of capacitors. This may include adjustments in capacitors’ capacity, voltage response, charge/ion transfer rate, etc., that improve their performance.
5. Influence of Electrolytes on the Performance of MXene-Based Supercapacitors
5.1. Alkaline Electrolytes
5.2. Neutral Electrolytes
5.3. Acidic Electrolytes
5.4. Organic Electrolytes
5.5. Solid Electrolytes
6. Summary and Outlook
6.1. Summary
6.2. Outlook
- The preparation methods used to make MXene still primarily rely on etching, whether acid etching or alkaline etching, and especially HF etching, which is dangerous and involves handling strong corrosive substances such as waste acids and alkalis. These methods are polluting, low-yielding, and expensive. Although direct synthesis methods have been proven effective in preparing MXene and avoiding the generation of pollutants, their production yield still needs to be improved. Therefore, new MXene preparation processes need to be developed to transition from the laboratory to production on a larger scale and, eventually, commercialized operations.
- The energy density of MXene remains relatively low, so it is necessary to enhance the structural design of MXene by enlarging and utilizing the space between each of its layers, matching the radius of the ions diffused in the electrolyte as much as possible, maximize the energy storage performance of MXene, establish the influence of MXene’s structure on its energy storage mechanisms, and guide the subsequent structural design of MXene electrodes.
- Different types of MXene composite materials have unique characteristics. For example, MXene/carbon composites offer advantages in terms of their high specific capacitance and cycling stability in supercapacitors, but their energy density and conductivity are limited. MXene/metal oxide composites have a higher specific capacitance, but their cycling capability and power density are still limited due to their poor electrochemical kinetics. MXene/metal hydroxide composites effectively reduce the self-stacking effect of MXene, improving the electrochemical performance of the electrode. However, they also have some limitations to their energy density and conductivity. MXene/conductive polymer composites effectively suppress layer stacking, increase the exposure of the active surface, and facilitate electron transfer and rapid reactions while improving the mechanical properties and stability of the material. However, the energy density of conductive polymers is typically lower, limiting their use in supercapacitors intended for certain high-energy density applications. Therefore, further research and improvements are needed.
- The preparation of MXene-based composite materials is an effective means to improve the energy storage performance of MXene, but the selection of the materials used in these composites still lacks specificity. Therefore, in the creation of composite materials using MXene, selecting heteromaterials with excellent energy storage performance remains a necessary research direction for alleviating the impact of the self-stacking of MXene and synergistically improving the material’s energy storage performance.
- The easy oxidation of MXene remains a significant factor limiting the application of MXene supercapacitors. Enhancing the thermal stability and electrochemical stability of MXene by adding other substances and preparing MXene-based composites is also an important topic in the study of MXene-based capacitors.
- Wearable electronic devices are a growing trend, and developing flexible electrodes is an important part of the research in this area. Further research is needed to prepare flexible, miniaturized, and cost-effective supercapacitor devices.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, Z.; Li, L.; Xi, C.; Li, X.; Feng, S.; Wang, C.; Wang, H.; Li, T.; Ma, Y. Fabrication of the hollow dodecahedral NiCoZn layered double hydroxide for high-performance flexible asymmetric supercapacitor. J. Colloid Interface Sci. 2024, 657, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, H.; Han, Z.; Bo, Z.; Yan, J.; Cen, K.; Ostrikov, K.K. MXene-Based Electrodes for Supercapacitor Energy Storage. Energy Fuels 2022, 36, 2390–2406. [Google Scholar] [CrossRef]
- Wei, J.; Sajjad, M.; Zhang, J.; Li, D.; Mao, Z. The rise of novel 2D materials beyond graphene: A comprehensive review of their potential as supercapacitor electrodes. Surf. Interfaces 2023, 42, 103334. [Google Scholar] [CrossRef]
- Sahoo, B.B.; Pandey, V.S.; Dogonchi, A.S.; Mohapatra, P.K.; Thatoi, D.N.; Nayak, N.; Nayak, M.K. A state-of-art review on 2D material-boosted metal oxide nanoparticle electrodes: Supercapacitor applications. J. Energy Storage 2023, 65, 107335. [Google Scholar] [CrossRef]
- Rohith, R.; Prasannakumar, A.T.; Mohan, D.R.R.; Manju, V.; Varma, D.S.J. Advances in 2D Molybdenum Disulfide-Based Functional Materials for Supercapacitor Applications. ChemistrySelect 2022, 7, e202203068. [Google Scholar]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chen, C.; Zhao, X.; Chu, X.; Du, F.; Chen, G.; Gogotsi, Y.; Gao, Y.; Dall, Y. Flexible Nb4C3Tx Film with Large Interlayer Spacing for High-Performance Supercapacitors. Adv. Funct. Mater. 2020, 30, 2000815. [Google Scholar] [CrossRef]
- Xu, P.; Xiao, H.; Liang, X.; Zhang, T.; Zhang, F.; Liu, C.; Lang, B.; Gao, Q. A MXene-based EDA-Ti3C2Tx intercalation compound with expanded interlayer spacing as high performance supercapacitor electrode material. Carbon 2021, 173, 135–144. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Zhang, X.; Javed, M.S.; Ali, S.; Ahmad, A.; Shah, S.S.A.; Hussain, I.; Choi, D.; Tighezza, A.M.; Tag-Eldin, E.; Xia, C.; et al. Band engineering in Ti2N/Ti3C2Tx-MXene interface to enhance the performance of aqueous NH4+-ion hybrid supercapacitors. Nano Energy 2024, 120, 109108. [Google Scholar] [CrossRef]
- Soundiraraju, B.; George, B.K. Two-Dimensional Titanium Nitride (Ti2N) MXene: Synthesis, Characterization, and Potential Application as Surface-Enhanced Raman Scattering Substrate. ACS Nano 2017, 11, 8892–8900. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Unocic, R.R.; Armstronga, B.L.; Nanda, J. Large-scale delamination of multi-layers transition metal carbides; carbonitrides “MXenes”. Dalton Trans. 2015, 44, 9353–9358. [Google Scholar] [CrossRef] [PubMed]
- Vattikuti, S.V.P.; Shim, J.; Rosaiah, P.; Mauger, A.; Julien, C.M. Recent Advances and Strategies in MXene-Based Electrodes for Supercapacitors: Applications, Challenges and Future Prospects. Nanomaterials 2024, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Alli, Y.A.; Bamisaye, A.; Nancy, P.; Zachariah, S.M.; Oladoye, P.O.; Bankole, O.M.; Akamo, D.O.; Chkirida, S.; Anuar, H.; Thomas, S. MXene composites: Properties, synthesis and its emerging application in rechargeable batteries. J. Energy Storage 2024, 77, 109954. [Google Scholar] [CrossRef]
- Xu, H.; Dong, H.; Liu, X.; Qiao, H.; Chen, G.; Du, F.; Dall’Agnese, Y.; Gao, Y. High-Temperature Oxidized Mo2CTx MXene for a High-Performance Supercapacitor. ACS Appl. Mater. Interfaces 2023, 15, 53549–53557. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.; Zhou, J.; Ye, Y.; Qiu, H.; Zhou, P.; Luo, Z.; Guo, Q. Self-chargeable supercapacitor made with MXene-bacterial cellulose nanofiber composite for wearable devices. J. Colloid Interface Sci. 2023, 647, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Otgonbayar, Z.; Yang, S.; Kim, I.-J.; Oh, W.-C. Recent advances in 2D MXene and solid state electrolyte for energy storage applications: Comprehensive review. Chem. Eng. J. 2023, 472, 144801. [Google Scholar] [CrossRef]
- Gandla, D.; Zhuang, Z.; Jadhav, V.V.; Tan, D.Q. Lewis acid molten salt method for 2D MXene synthesis and energy storage applications: A review. Energy Storage Mater. 2023, 63, 102977. [Google Scholar] [CrossRef]
- Fu, X.-Y.; Shu, R.-Y.; Ma, C.-J.; Zhang, Y.-Y.; Jiang, H.-B.; Yao, M.-N. Self-assembled MXene-graphene oxide composite enhanced laser-induced graphene based electrodes towards conformal supercapacitor applications. Appl. Surf. Sci. 2023, 631, 157549. [Google Scholar] [CrossRef]
- Kim, M.C.; Saeed, G.; Alam, A.; Choi, Y.; Zhang, L.; Lee, D.; Kwon, S.H.; Mathur, S.; Kim, K.H. Ultrafine nanoparticles of tin-cobalt-sulfide decorated over 2D MXene sheets as a cathode material for high-performance asymmetric supercapacitor. J. Ind. Eng. Chem. 2023, 124, 294–303. [Google Scholar]
- Jimmy, J.; Kandasubramanian, B. Mxene functionalized polymer composites: Synthesis and applications. Eur. Polym. J. 2020, 122, 109367. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, Z.; Hou, X.; Wang, T.; Guo, X.; Liu, X.; Tian, M.; Qu, L.; Zhang, X. Flexible and ultra-thin graphene@MXene@Fe3O4 composites with excellent microwave absorption performance. Ceram. Int. 2024, 50, 6624–6633. [Google Scholar] [CrossRef]
- Amani, A.M.; Tayebi, L.; Abbasi, M.; Vaez, A.; Kamyab, H.; Chelliapan, S.; Vafa, E. The Need for Smart Materials in an Expanding Smart World: MXene-Based Wearable Electronics and Their Advantageous Applications. ACS Omega 2024, 9, 3123–3142. [Google Scholar] [CrossRef]
- Li, H.; Fan, K.; Xiong, P.; Zhou, H.; Lin, Z.; Tao, K.; Liu, T.; Guo, X.; Zhu, Y.; Zhuang, L.; et al. Selective grafting of phosphorus onto Ti3C2Tx MXene enables a two-proton process and enhanced charge storage. J. Mater. Chem. A 2024, 12, 3449–3459. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, L.; Wang, L.; Pan, L.; Zhang, P.; Sun, Z. MXene nanomesh for high-performance supercapacitor. J. Alloys Compd. 2024, 976, 173065. [Google Scholar] [CrossRef]
- Ahmad, R.; Iqbal, N.; Noor, T.; Nemani, S.K.; Zhu, L.; Anasori, B. Metal–Organic Framework/Ti3C2Tx MXene-Derived Functional Nanostructures for High-Performance Supercapacitors. ACS Appl. Nano Mater. 2024, 7, 253–266. [Google Scholar] [CrossRef]
- Paul, T.K.; Parvez, M.S.; Ahmed, C.M. Recent Progress and Prospects of MXene/Cellulose-Based Composite Electrodes: A Sustainable Pathway towards Supercapacitor Application. ChemElectroChem 2024, 11, e202300435. [Google Scholar] [CrossRef]
- Khan, U.; Gao, B.; Kong, L.B.; Chen, Z.; Que, W. Green synthesis of fluorine-free MXene via hydrothermal process: A sustainable approach for proton supercapacitor electrodes. Electrochim. Acta 2024, 475, 143651. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, B.; Dong, L.; Li, X.; Liu, D.; Hou, S.; Zhang, Y.; Niu, H.; Zhang, F.-M. MXene-derived composite catalyst with micro-holes by a solvothermal method with tiny amount of solvent for high-efficiency catalytic hydrogen production. Int. J. Hydrogen Energy 2024, 51, 1161–1169. [Google Scholar] [CrossRef]
- Kalidasan, B.; Pandey, A.K.; Saidur, R.; Han, T.K.; Mishra, Y.N. MXene-based eutectic salt hydrate phase change material for efficient thermal features, corrosion resistance & photo-thermal energy conversion. Mater. Today Sustain. 2024, 25, 100634. [Google Scholar]
- Tang, Q.; Zhou, Z.; Shen, P. Are MXenes Promising Anode Materials for Li Ion Batteries Computational Studies on Electronic Properties and Li Storage Capability of Ti3C2 and Ti3C2X2 (X = F, OH) Monolayer. J. Am. Chem. Soc. 2012, 134, 16909–16916. [Google Scholar] [CrossRef]
- Liang, C.; Meng, Y.; Zhang, Y.; Zhang, H.; Wang, W.; Lu, M.; Wang, G. Insights into the impact of interlayer spacing on MXene-based electrodes for supercapacitors: A review. J. Energy Storage 2023, 65, 107341. [Google Scholar] [CrossRef]
- Levitt, A.S.; Alhabeb, M.; Hatter, C.B.; Sarycheva, A.; Dionb, G.; Gogotsi, Y. Electrospun MXene/carbon nanofibers as supercapacitor electrodes. J. Mater. Chem. A 2019, 7, 269–277. [Google Scholar] [CrossRef]
- Tian, Y.; Ju, M.; Luo, Y.; Bin, X.; Lou, X.; Que, W. In situ oxygen doped Ti3C2Tx MXene flexible film as supercapacitor electrode. Chem. Eng. J. 2022, 446, 137451. [Google Scholar] [CrossRef]
- Wang, X.; Bak, S.-M.; Han, M.; Shuck, C.E.; McHugh, C.; Li, K.; Li, J.; Tang, J.; Gogotsi, Y. Surface Redox Pseudocapacitance of Partially Oxidized Titanium Carbide MXene in Water-in-Salt Electrolyte. ACS Energy Lett. 2022, 7, 30–35. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, X.; Zhuo, J.; Cao, L.; Song, Y.; Yin, Y.; Wang, X.; Yang, G.; Yi, F. Self-Supporting, Binder-Free, and Flexible Ti3C2Tx MXene-Based Supercapacitor Electrode with Improved Electrochemical Performance. ACS Nano 2022, 16, 9713–9727. [Google Scholar] [CrossRef]
- Cai, M.; Wei, X.; Huang, H.; Yuan, F.; Li, C.; Xu, S.; Liang, X.; Zhou, W.; Guo, J. Nitrogen-doped Ti3C2Tx MXene prepared by thermal decomposition of ammonium salts and its application in flexible quasi-solid-state supercapacitor. Chem. Eng. J. 2023, 458, 141338. [Google Scholar] [CrossRef]
- Kawai, K.; Fujita, M.; Iizuka, R.; Yamada, A.; Okubo, M. Influence of surface termination groups on electrochemical charge storage of MXene electrodes. 2D Mater. 2023, 10, 014012. [Google Scholar] [CrossRef]
- Ma, R.; Chen, Z.; Zhao, D.; Zhang, X.; Zhuo, J.; Yin, Y.; Wang, X.; Yang, G.; Yi, F. Ti3C2Tx MXene for electrode materials of supercapacitors. J. Mater. Chem. A 2021, 9, 11501–11529. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, N.; Hegh, D.; Usman, K.A.S.; Guan, G.; Qin, S.; Jurewicz, I.; Yang, W.; Razal, J.M. Freezing Titanium Carbide Aqueous Dispersions for Ultra-long-term Storage. ACS Appl. Mater. Interfaces 2020, 12, 34032–34040. [Google Scholar] [CrossRef]
- De, S.; Maity, C.K.; Sahoo, S.; Nayak, G.C. Polyindole Booster for Ti3C2Tx MXene Based Symmetric and Asymmetric Supercapacitor Devices. ACS Appl. Energy Mater. 2021, 4, 3712–3723. [Google Scholar] [CrossRef]
- Sun, S.; Zhu, X.; Wu, X.; Xu, M.; Hu, Y.; Bao, N.; Wu, G. Covalent-architected molybdenum disulfide arrays on Ti3C2Tx MXene fiber towards robust capacitive energy storage. J. Mater. Sci. Technol. 2023, 139, 23–30. [Google Scholar] [CrossRef]
- Kasprzak, D.; Mayorga-Martinez, C.C.; Alduhaish, O.; Pumera, M. Wearable and Flexible All-Solid-State Supercapacitor Based on MXene and Chitin. Energy Technol. 2023, 11, 2201103. [Google Scholar] [CrossRef]
- De, S.; Maity, C.K.; Kim, M.J.; Nayak, G.C. Tin(IV) selenide anchored-biowaste derived porous carbon-Ti3C2Tx (MXene) nanohybrid: An ionic electrolyte enhanced high performing flexible supercapacitor electrode. Electrochim. Acta 2023, 463, 142811. [Google Scholar] [CrossRef]
- Karim, G.M.; Dutta, P.; Majumdar, A.; Patra, A.; Deb, S.K.; Das, S.; Dambhare, N.V.; Rath, A.K.; Maiti, U.N. Ultra-fast electro-reduction and activation of graphene for high energy density wearable supercapacitor asymmetrically designed with MXene. Carbon 2023, 203, 191–201. [Google Scholar] [CrossRef]
- Lorencova, L.; Kasak, P.; Kosutova, N.; Jerigova, M.; Noskovicova, E.; Vikartovska, A.; Barath, M.; Farkas, P.; Tkac, J. MXene-based electrochemical devices applied for healthcare applications. Microchim. Acta 2024, 191, 88. [Google Scholar] [CrossRef]
- Zeraati, A.S.; Mirkhani, S.A.; Sun, P.; Naguib, M.; Braun, P.V.; Sundararaj, U. Improved synthesis of Ti3C2Tx MXenes resulting in exceptional electrical conductivity, high synthesis yield, and enhanced capacitance. Nanoscale 2021, 13, 3572–3580. [Google Scholar] [CrossRef]
- Mathis, T.S.; Maleski, K.; Goad, A.; Sarycheva, A.; Anayee, M.; Foucher, A.C.; Hantanasirisakul, K.; Shuck, C.E.; Stach, E.A.; Gogotsi, Y. Modified MAX Phase Synthesis for Environmentally Stable and Highly Conductive Ti3C2 MXene. ACS Nano 2021, 15, 6420–6429. [Google Scholar] [CrossRef]
- Jia, L.; Zhou, S.; Ahmed, A.; Yang, Z.; Liu, S.; Wang, H.; Li, F.; Zhang, M.; Zhang, Y.; Sun, L. Tuning MXene electrical conductivity towards multifunctionality. Chem. Eng. J. 2023, 475, 146361. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, N.; Uzun, S.; Levitt, A.; Seyedin, S.; Lynch, P.A.; Qin, S.; Han, M.; Yang, W.; Liu, J.; et al. Scalable Manufacturing of Free-Standing, Strong Ti3C2Tx MXene Films with Outstanding Conductivity. Adv. Mater. 2020, 32, 2001093. [Google Scholar] [CrossRef]
- Aravind, A.M.; Tomy, M.; Kuttapan, A. Ann Mary Kakkassery Aippunny, Xavier Thankappan Suryabai, Progress of 2D MXene as an Electrode Architecture for Advanced Supercapacitors: A Comprehensive Review. ACS Omega 2023, 8, 44375–44394. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, C.; Chen, T.; Li, W.; Zheng, S.; Pi, Y.; Luo, Y.; Pang, H. MXene-Copper/Cobalt Hybrids via Lewis Acidic Molten Salts Etching for High Performance Symmetric Supercapacitors. Angew. Chem. Int. Ed. 2021, 60, 25318–25322. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, C.; Zhao, Y.; Pan, W.; Xie, K.; Yue, P.; Zhang, G.; Omar, A.; Liu, L.; Yu, M.; et al. Freestanding MXene-based macroforms for electrochemical energy storage applications. Susmat 2023, 3, 471–497. [Google Scholar] [CrossRef]
- Garg, R.; Agarwal, A.; Agarwal, M. Effect of vanadium doping on MXene-based supercapacitor. J. Mater. Sci. Mater. Electron. 2021, 32, 22046–22059. [Google Scholar] [CrossRef]
- Jiang, Q.; Lei, Y.; Liang, H.; Xi, K.; Xia, C.; Alshareef, H.N. Review of MXene electrochemical microsupercapacitors. Energy Storage Mater. 2020, 27, 78–95. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; El-Demellawi, J.K.; Jiang, Q.; Ge, G.; Liang, H.; Lee, K.; Dong, X.; Alshareef, H.N. MXene hydrogels: Fundamentals and applications. Chem. Soc. Rev. 2020, 49, 7229–7251. [Google Scholar] [CrossRef]
- Idumah, C.I. Influence of surfaces and interfaces on MXene and MXene hybrid polymeric nanoarchitectures, properties, and applications. J. Mater. Sci. 2022, 57, 14579–14619. [Google Scholar] [CrossRef]
- Xiong, D.; Li, X.; Bai, Z.; Lu, S. Recent Advances in Layered Ti3C2Tx MXene for Electrochemical Energy Storage. Small 2018, 14, 1703419. [Google Scholar] [CrossRef]
- Song, F.; Li, G.; Zhu, Y.; Wu, Z.; Xie, X.; Zhang, N. Rising from the horizon: Three-dimensional functional architectures assembled with MXene nanosheets. J. Mater. Chem. A 2020, 8, 18538–18559. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Li, X.; Li, H.; Wang, Z.; Tang, Z.; Ma, L.; Mo, F.; Zhi, C. Recent Progress of MXene-Based Nanomaterials in Flexible Energy Storage and Electronic Devices. Energy Environ. Mater. 2018, 1, 183–195. [Google Scholar] [CrossRef]
- Luo, Y.; Que, W.; Bin, X.; Xia, C.; Kong, B.; Gao, B.; Kong, L.B. Flexible MXene-Based Composite Films: Synthesis, Modification, and Applications as Electrodes of Supercapacitors. Small 2022, 18, 2201290. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Deshmukh, K.; Pasha, S.K.K.; Theerthagiri, J.; Manickam, S.; Choi, M.Y. MXene based emerging materials for supercapacitor applications: Recent advances, challenges, and future perspectives. Coord. Chem. Rev. 2022, 462, 214518. [Google Scholar] [CrossRef]
- Mazhar, S.; Qarni, A.A.; Haq, Y.U.; Haq, Z.U.; Murtaza, I. Promising PVC/MXene based flexible thin film nanocomposites with excellent dielectric, thermal and mechanical properties. Ceram. Int. 2020, 46, 12593–12605. [Google Scholar] [CrossRef]
- Tang, B.; Yang, Y.; Shi, Y.; Nie, H.; Xia, H.; Shen, X. Improved mechanical performances of short aramid fiber-reinforced polypropylene composites by Ti3C2 Mxene nanosheets. Polym. Compos. 2021, 42, 2010–2018. [Google Scholar] [CrossRef]
- Chu, N.; Luo, C.; Chen, X.; Li, L.; Liang, C.; Chao, M.; Yan, L. Ti3C2Tx MXene/polyimide composites film with excellent mechanical properties and electromagnetic interference shielding properties. J. Alloys Compd. 2023, 955, 170241. [Google Scholar] [CrossRef]
- Shekhirev, M.; Shuck, C.E.; Sarycheva, A.; Gogotsi, Y. Characterization of MXenes at every step, from their precursors to single flakes and assembled films. Prog. Mater. Sci. 2021, 120, 100757. [Google Scholar] [CrossRef]
- Guo, Z.; Zhou, J.; Si, C.; Sun, Z. Flexible two-dimensional Tin+1Cn (n = 1, 2 and 3) and their functionalized MXenes predicted by density functional theories. Phys. Chem. Chem. Phys. 2015, 17, 15348–15354. [Google Scholar] [CrossRef]
- Iravani, S.; Rabiee, N.; Makvandi, P. Advancements in MXene-based composites for electronic skins. J. Mater. Chem. B 2024, 12, 895–915. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Y.; Huang, J.; Li, J.; Chen, M.; Hu, X.; Liu, Y.; Wang, R.; Shen, Y.; Li, J.; et al. Size-refinement enhanced flexibility and electrochemical performance of MXene electrodes for flexible waterproof supercapacitors. J. Energy Chem. 2021, 63, 594–603. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, K.; Zhang, T.; Luo, M.; Li, M.; Li, Z.; Liu, C.; Ling, Y.; Chen, W.; Zhou, X. A facile “thick to thin” strategy for integrating high volumetric energy density and excellent flexibility into MXene/wood free-standing electrode for supercapacitors. Chem. Eng. J. 2023, 460, 141733. [Google Scholar] [CrossRef]
- Mashtalir, O.; Naguib, M.; Dyatkin, B.; Gogotsi, Y.; Barsoum, M.W. Kinetics of aluminum extraction from Ti3AlC2 in hydrofluoric acid. Mater. Chem. Phys. 2013, 139, 147–152. [Google Scholar] [CrossRef]
- Wang, X.; Shen, X.; Gao, Y.; Wang, Z.; Yu, R.; Chen, L. Atomic-Scale Recognition of Surface Structure and Intercalation Mechanism of Ti3C2X. J. Am. Chem. Soc. 2015, 137, 2715–2721. [Google Scholar] [CrossRef]
- Halim, J.; Lukatskaya, M.R.; Cook, K.M.; Lu, J.; Smith, C.R.; Näslund, L.-Å.; May, S.J.; Hultman, L.; Gogotsi, Y.; Eklund, P.; et al. Transparent Conductive Two-Dimensional Titanium Carbide Epitaxial Thin Films. Chem. Mater. 2014, 26, 2374–2381. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Xue, Y.; Li, L.; Chen, S.; Nie, Y.; Ding, W.; Wei, Z. Surface Al leached Ti3AlC2 as a substitute for carbon for use as a catalyst support in a harsh corrosive electrochemical system. Nanoscale 2014, 6, 11035–11040. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tan, L.; Zhang, Y.; Wu, B.; Li, L. Highly Efficiently Delaminated Single-Layered MXene Nanosheets with Large Lateral Size. Langmuir 2017, 33, 9000–9006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhu, J.; Shi, P.; Wu, W.; Wang, F. Fluoride-free synthesis and microstructure evolution of novel two-dimensional Ti3C2(OH)2 nanoribbons as high-performance anode materials for lithium-ion batteries. Ceram. Int. 2019, 45, 8395–8405. [Google Scholar] [CrossRef]
- Li, T.; Yao, L.; Liu, Q.; Gu, J.; Luo, R.; Li, J.; Yan, X.; Wang, W.; Liu, P.; Chen, B.; et al. Fluorine-Free Synthesis of High-Purity Ti3C2Tx (T=OH, O) via Alkali Treatment. Angew. Chem. Int. Ed. 2018, 57, 6115–6119. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, T.; Liu, Q.; Gu, J. Fluorine-free Ti3C2Tx as anode materials for Li-ion batteries. Electrochem. Commun. 2019, 104, 106472. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, P.; Wang, F.; Ricciardulli, A.G.; Lohe, M.R.; Blom, P.W.M.; Feng, X. Fluoride-Free Synthesis of Two-Dimensional Titanium Carbide (MXene) Using A Binary Aqueous System. Angew. Chem. Int. Ed. 2018, 57, 15491–15495. [Google Scholar] [CrossRef]
- Pang, S.-Y.; Wong, Y.-T.; Yuan, S.; Liu, Y.; Tsang, M.-K.; Yang, Z.; Huang, H.; Wong, W.-T.; Hao, J. Universal Strategy for HF-Free Facile and Rapid Synthesis of Two-dimensional MXenes as Multifunctional Energy Materials. J. Am. Chem. Soc. 2019, 141, 9610–9616. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, J.; Luo, K.; Li, Y.; Chang, K.; Chen, K.; Zhou, J.; Rosen, J.; Hultman, L.; Eklund, P.; et al. Element Replacement Approach by Reaction with Lewis Acidic Molten Salts to Synthesize Nanolaminated MAX Phases and MXene. J. Am. Chem. Soc. 2019, 141, 4730–4737. [Google Scholar] [CrossRef]
- Li, Y.; Shao, H.; Lin, Z.; Lu, J.; Liu, L.; Duployer, B.; Persson, P.O.Å.; Eklund, P.; Hultman, L.; Li, M.; et al. A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte. Nat. Mater. 2020, 19, 894–899. [Google Scholar] [CrossRef]
- Kamysbayev, V.; Filatov, A.S.; Hu, H.; Rui, X.; Lagunas, F.; Wang, D.; Klie, R.F.; Talapin, D.V. Covalent surface modifications and superconductivity of two-dimensional metal carbide MXenes. Science 2020, 369, 979–983. [Google Scholar] [CrossRef]
- Gogotsi, Y. Transition metal carbides go 2D. Nat. Mater. 2015, 14, 1079–1080. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Filatov, A.S.; Cho, W.; Lagunas, F.; Wang, M.; Vaikuntanathan, S.; Liu, C.; Klie, R.F.; Talapin, D.V. Direct synthesis and chemical vapor deposition of 2D carbide and nitride MXenes. Science 2023, 379, 1242–1247. [Google Scholar] [CrossRef]
- Zhan, C.; Naguib, M.; Lukatskaya, M.; Kent, P.R.C.; Gogotsi, Y.; Jiang, D.-E. Understanding the MXene Pseudocapacitance. J. Phys. Chem. Lett. 2018, 9, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Y.; Sun, H.; Zhou, J.; Yang, F.; Li, H.; Chen, H.; Chen, Y.; Liu, Z.; Qiu, Z.; et al. Progress and Perspective: MXene and MXene-Based Nanomaterials for High-Performance Energy Storage Devices. Adv. Electron. Mater. 2021, 7, 2000967. [Google Scholar] [CrossRef]
- Xu, K.; Ji, X.; Zhang, B.; Chen, C.; Ruan, Y.; Miao, L.; Jiang, J. Charging/discharging dynamics in two-dimensional titanium carbide (MXene) slit nanopore: Insights from molecular dynamic study. Electrochim. Acta 2016, 196, 75–83. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef]
- Guan, Y.; Zhao, R.; Cong, Y.; Chen, K.; Wu, J.; Zhu, H.; Dong, Z.; Zhang, Q.; Yuan, G.; Li, Y.; et al. Flexible Ti2C MXene film: Synthesis, electrochemical performance and capacitance behavior. Chem. Eng. J. 2022, 433, 133582. [Google Scholar] [CrossRef]
- Guan, Y.; Jiang, S.; Cong, Y.; Wang, J.; Dong, Z.; Zhang, Q.; Yuan, G.; Li, Y.; Li, X. A hydrofluoric acid-free synthesis of 2D vanadium carbide (V2C) MXene for supercapacitor electrodes. 2D Mater. 2020, 7, 025010. [Google Scholar] [CrossRef]
- Wang, X.; Lin, S.; Tong, H.; Huang, Y.; Tong, P.; Zhao, B.; Dai, J.; Liang, C.; Wang, H.; Zhu, X.; et al. Two-dimensional V4C3 MXene as high performance electrode materials for supercapacitors. Electrochim. Acta 2019, 307, 414–421. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Y.; Liu, K.; Zhao, Q.; Liang, Q.; Zhang, M.; Si, C. Ultralight MXene/carbon nanotube composite aerogel for high-performance flexible supercapacitor. Adv. Compos. Hybrid Mater. 2023, 6, 108. [Google Scholar] [CrossRef]
- Althubiti, N.A.; Aman, S. Taha Abdel Mohaymen Taha, Synthesis of MnFe2O4/MXene/NF nanosized composite for supercapacitor application. Ceram. Int. 2023, 49, 27496–27505. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, N.; Liu, Y.; Zhou, X.; Pu, B.; Qing, Y.; Zhang, M.; Jiang, X.; Huang, J.; Tang, Q.; et al. MXene/Graphdiyne nanotube composite films for Free-Standing and flexible Solid-State supercapacitor. Chem. Eng. J. 2022, 450, 138398. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, Y.; Fan, L.; Ye, D.; Xu, W.; Xu, J. Ultralight PPy@PVA/BC/MXene composite aerogels for high-performance supercapacitor eltrodes and pressure sensors. Appl. Surf. Sci. 2023, 624, 157138. [Google Scholar] [CrossRef]
- Mo, T.; Wang, Z.; Zeng, L.; Chen, M.; Kornyshev, A.A.; Zhang, M.; Zhao, Y.; Feng, G. Energy Storage Mechanism in Supercapacitors with Porous Graphdiynes: Effects of Pore Topology and Electrode Metallicity. Adv. Mater. 2023, 35, 2301118. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Wang, Y.; Xie, Z.; Wang, D.; Yuan, Y.; Kang, H.; Su, B.; Cheng, Z.; Liu, Y. Modified MXene/Holey Graphene Films for Advanced Supercapacitor Electrodes with Superior Energy Storage. Adv. Sci. 2018, 5, 1800750. [Google Scholar] [CrossRef]
- Li, H.; Chen, R.; Ali, M.; Lee, H.; Ko, M.J. In Situ Grown MWCNTs/MXenes Nanocomposites on Carbon Cloth for High-Performance Flexible Supercapacitors. Adv. Funct. Mater. 2020, 30, 2002739. [Google Scholar] [CrossRef]
- Zhou, Y.; Maleski, K.; Anasori, B.; Thostenson, J.O.; Pang, Y.; Feng, Y.; Zeng, K.; Parker, C.B.; Zauscher, S.; Gogotsi, Y.; et al. Ti3C2Tx MXene-Reduced Graphene Oxide Composite Electrodes for Stretchable Supercapacitors. ACS Nano 2020, 14, 3576–3586. [Google Scholar] [CrossRef] [PubMed]
- Ayman, I.; Rasheed, A.; Ajmal, S.; Rehman, A.; Ali, A.; Shakir, I.; Warsi, M.F. CoFe2O4 Nanoparticle-Decorated 2D MXene: A Novel Hybrid Material for Supercapacitor Applications. Energy Fuels 2020, 34, 7622–7630. [Google Scholar] [CrossRef]
- Zhu, Y.; Rajouâ, K.; Le Vot, S.; Fontaine, O.; Simon, P.; Favier, F. MnO2-MXene Composite as Electrode for Supercapacitor. J. Electrochem. Soc. 2022, 169, 030524. [Google Scholar] [CrossRef]
- Zhang, X.; Shao, B.; Guo, A.; Sun, Z.; Zhao, J.; Cui, F.; Yang, X. MnO2 nanoshells/Ti3C2Tx MXene hybrid film as supercapacitor electrode. Appl. Surf. Sci. 2021, 560, 150040. [Google Scholar] [CrossRef]
- Luo, L.; Meng, W.; Wang, G.; Qin, J.; He, H.; Huang, H. MnO2 nanoflowers-decorated MXene nanosheets with enhanced supercapacitor performance. J. Alloys Compd. 2023, 957, 170411. [Google Scholar] [CrossRef]
- Prabhakar, N.; Vasanth, S.; Ponpandian, N.; Viswanathan, C. Synthesis effect on surface functionalized Ti3C2Tx MXene supported nickel oxide nanocomposites with enhanced specific capacity for supercapacitor application. J. Energy Storage 2023, 72, 108414. [Google Scholar]
- Liu, R.; Zhang, A.; Tang, J.; Tian, J.; Huang, W.; Cai, J.; Barrow, C.; Yang, W.; Liu, J. Fabrication of Cobaltosic Oxide Nanoparticle-Doped 3D MXene/Graphene Hybrid Porous Aerogels for All-Solid-State Supercapacitors. Chem. A Eur. J. 2019, 25, 5547–5554. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, J.; Qian, X.; Zhang, Y.; Yu, L.; Niu, R.; Zhao, H.; Zhu, J. 2D/2D heterostructures of nickel molybdate and MXene with strong coupled synergistic effect towards enhanced supercapacitor performance. J. Power Sources 2019, 414, 540–546. [Google Scholar] [CrossRef]
- Xie, W.; Wang, Y.; Zhou, J.; Zhang, M.; Yu, J.; Zhu, C.; Xu, J. MOF-derived CoFe2O4 nanorods anchored in MXene nanosheets for all pseudocapacitive flexible supercapacitors with superior energy storage. Appl. Surf. Sci. 2020, 534, 147584. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, F.; Fang, L.; Hu, J.; Luo, H.; Guan, T.; Hu, B.; Zhou, M. Layered NiFe-LDH/MXene nanocomposite electrode for high-performance supercapacitor. Int. J. Hydrogen Energy 2020, 45, 13080–13089. [Google Scholar] [CrossRef]
- Liang, C.; Feng, Z.; Chen, M.; Xv, X.; Lu, M.; Wang, W. Nanoflower-like hollow NiMnCo-OH decorated with self-assembled 2D Ti3C2Tx for high-efficiency hybrid supercapacitors. J. Alloys Compd. 2024, 970, 172537. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Liu, D.; Li, X.; Xiao, H.; Ma, Y.; Xu, M.; Yuan, G.; Chen, G. Opening MXene Ion Transport Channels by Intercalating PANI Nanoparticles from the Self-Assembly Approach for High Volumetric and Areal Energy Density Supercapacitors. ACS Appl. Mater. Interfaces 2021, 13, 30633–30642. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Niu, D.; Zhu, J.; Gao, Y.; Wei, D.; Liu, X.; Wang, F.; Wang, L.; Yang, L. Organ-like Ti3C2 MXenes/polyaniline composites by chemical grafting as high-performance supercapacitors. J. Electroanal. Chem. 2019, 847, 113203. [Google Scholar] [CrossRef]
- Pathak, I.; Acharya, D.; Chhetri, K.; Lohani, P.C.; Ko, T.H.; Muthurasu, A.; Subedi, S.; Kim, T.; Saidin, S.; Dahal, B.; et al. Ti3C2Tx MXene integrated hollow carbon nanofibers with polypyrrole layers for MOF-derived freestanding electrodes of flexible asymmetric supercapacitors. Chem. Eng. J. 2023, 469, 143388. [Google Scholar] [CrossRef]
- Kosnan, M.A.; Asyadi, A.M.; Ezyanie, S.N.; Farahiyan, M.R.; Akito, T. Recent Progress of Electrode Architecture for MXene/MoS2 Supercapacitor: Preparation Methods and Characterizations. Micromachines 2022, 13, 1837. [Google Scholar] [CrossRef]
- Hussain, S.; Iqra, R.; Dhanasekaran, V.; Taqi, M.; Faisal, S.; Soo, S.Y.; Seok, K.H.; Jongwan, J. Designing the MXene/molybdenum diselenide hybrid nanostructures for high-performance symmetric supercapacitor and hydrogen evolution applications. Int. J. Energy Res. 2021, 45, 18770. [Google Scholar] [CrossRef]
- Arulkumar, C.; Gandhi, R.; Vadivel, S. Ultra-thin nanosheets of Ti3C2Tx MXene/MoSe2 nanocomposite electrode for asymmetric supercapacitor and electrocatalytic water splitting. Electrochim. Acta 2023, 462, 142742. [Google Scholar] [CrossRef]
- Chandran, M.; Anitta, T.; Asha, R.; Mari, V.; Bhagiyalakshmi, M. MoS2 Confined MXene Heterostructures as Electrode Material for Energy Storage Application. J. Energy Storage 2020, 30, 101446. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, J.; Cai, J.; Zhang, Y.; Wang, X. Nanosheets assembled layered MXene/MoSe2 nanohybrid positive electrode materials for high-performance asymmetric supercapacitors. J. Energy Storage 2021, 40, 102721. [Google Scholar] [CrossRef]
- Sharma, A.; Rout, C.S. 1T metallic vanadium disulfide hybridized with MXene and functionalized-MWCNT as a remarkable electrode for high power density asymmetric supercapacitor applications. Int. J. Energy Res. 2022, 46, 24537–24553. [Google Scholar] [CrossRef]
- Mao, K.; Shi, J.; Zhang, Q.; Hou, Y.; Wen, L.; Liu, Z.; Long, F.; Niu, K.; Liu, N.; Long, F.; et al. High-capacitance MXene anode based on Zn-ion pre-intercalation strategy for degradable micro Zn-ion hybrid supercapacitors. Nano Energy 2022, 103, 107791. [Google Scholar] [CrossRef]
- Javed, M.S.; Zhang, X.; Ali, S.; Mateen, A.; Idrees, M.; Sajjad, M.; Batool, S.; Ahmad, A.; Imran, M.; Najam, T.; et al. Heterostructured bimetallic–sulfide@layered Ti3C2T–MXene as a synergistic electrode to realize high-energy-density aqueous hybrid-supercapacitor. Nano Energy 2022, 101, 107624. [Google Scholar] [CrossRef]
- Zheng, Z.; Wu, W.; Yang, T.; Wang, E.; Du, Z.; Hou, X.; Liang, T. In situ reduced MXene/AuNPs composite toward enhanced charging/discharging and specific capacitance. J. Adv. Ceram. 2021, 10, 1061–1071. [Google Scholar] [CrossRef]
- Li, L.; Niu, H.; Robertson, J.; Jiang, Z.; Guo, Y.; Kuai, C. Cyclocrosslinked polyphosphazene modified MXene as aqueous supercapacitor. Electrochim. Acta 2023, 439, 141574. [Google Scholar] [CrossRef]
- Jia, B.; Yang, H.; Wang, L.; Zhao, Z.; Wu, X. Synergistic interface-pillared Fe-MOF on 2D Ti3C2TX MXene electrode coupling toward high energy density. Appl. Surf. Sci. 2022, 602, 154386. [Google Scholar] [CrossRef]
- Xu, H.; Fan, J.; Su, H.; Liu, C.; Chen, G.; Dall’Agnese, Y.; Gao, Y. Metal Ion-Induced Porous MXene for All-Solid-State Flexible Supercapacitors. Nano Lett. 2023, 23, 283–290. [Google Scholar] [CrossRef]
- Li, K.; Wang, X.; Li, S.; Urbankowski, P.; Li, J.; Xu, Y.; Gogotsi, Y. An Ultrafast Conducting Polymer@MXene Positive Electrode with High Volumetric Capacitance for Advanced Asymmetric Supercapacitors. Small 2020, 16, 1906851. [Google Scholar] [CrossRef]
- Fu, J.; Yun, J.; Wu, S.; Li, L.; Yu, L.; Kim, K.H. Architecturally Robust Graphene-Encapsulated MXene Ti2CTx@Polyaniline Composite for High-Performance Pouch-Type Asymmetric Supercapacitor. ACS Appl. Mater. Interfaces 2018, 10, 34212–34221. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Wang, H.; Zhu, C.; Lin, S.; Xu, Z.; Zhang, X. Free-standing MXene film modified by amorphous FeOOH quantum dots for high-performance asymmetric supercapacitor. Electrochim. Acta 2019, 308, 1–8. [Google Scholar] [CrossRef]
- Xia, Q.X.; Fu, J.; Yun, J.M.; Mane, R.S.; Kim, K.H. High volumetric energy density annealed-MXene-nickel oxide/MXene asymmetric supercapacitor. RSC Adv. 2017, 7, 11000–11011. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, X.; Bai, Y.; Xiao, H.; Liu, Y.; Yuan, G. Scalable fabrication of polyaniline nanodots decorated MXene film electrodes enabled by viscous functional inks for high-energy-density asymmetric supercapacitors. Chem. Eng. J. 2021, 405, 126664. [Google Scholar] [CrossRef]
- Li, L.; Zhang, N.; Zhang, M.; Zhang, X.; Zhang, Z. Flexible Ti3C2Tx/PEDOT:PSS films with outstanding volumetric capacitance for asymmetric supercapacitors. Dalton Trans. 2019, 48, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Jian, X.; He, M.; Chen, L.; Zhang, M.M.; Li, R.; Gao, L.J.; Fu, F.; Liang, Z.-H. Three-dimensional carambola-like MXene/polypyrrole composite produced by one-step co-electrodeposition method for electrochemical energy storage. Electrochim. Acta 2019, 318, 820–827. [Google Scholar] [CrossRef]
- Tong, L.; Jiang, C.; Cai, K.; Wei, P. High-performance and freestanding PPy/Ti3C2Tx composite film for flexible all-solid-state supercapacitors. J. Power Sources 2020, 465, 228267. [Google Scholar] [CrossRef]
- Li, Y.; Kamdem, P.; Jin, X.J. Hierarchical architecture of MXene/PANI hybrid electrode for advanced asymmetric supercapacitors. J. Alloys Compd. 2021, 850, 156608. [Google Scholar] [CrossRef]
- Qin, L.; Tao, Q.; El Ghazaly, A.; Fernandez-Rodriguez, J.; Persson, P.O.; Rosen, J.; Zhang, F. High-Performance Ultrathin Flexible Solid-State Supercapacitors Based on Solution Processable Mo1.33C MXene and PEDOT:PSS. Adv. Funct. Mater. 2018, 28, 1703808. [Google Scholar] [CrossRef]
- Xia, Q.X.; Shinde, N.M.; Zhang, T.; Yun, J.M.; Zhou, A.; Mane, R.S.; Mathur, S.; Kim, K.H. Seawater electrolyte-mediated high volumetric MXene-based electrochemical symmetric supercapacitors. Dalton Trans. 2018, 47, 8676–8682. [Google Scholar] [CrossRef]
- Zhu, K.; Jin, Y.; Du, F.; Gao, S.; Gao, Z.; Meng, X.; Chen, G.; Wei, Y.; Gao, Y. Synthesis of Ti2CTx MXene as electrode materials for symmetric supercapacitor with capable volumetric capacitance. J. Energy Chem. 2019, 31, 11–18. [Google Scholar] [CrossRef]
- Wang, J.; Gong, J.; Zhang, H.; Lv, L.; Liu, Y.; Dai, Y. Construction of hexagonal nickel-cobalt oxide nanosheets on metal-organic frameworks based on MXene interlayer ion effect for hybrid supercapacitors. J. Alloys Compd. 2021, 870, 159466. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Y.; Lu, Z.; Chao, Y.; Liu, W. High-performance MnO2@MXene/carbon nanotube fiber electrodes with internal and external construction for supercapacitors. J. Mater. Sci. 2022, 57, 3613–3628. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Z.; Yang, Q.; Hanif, M.; Wang, Z.; Dong, L.; Dong, M. A novel MnO2/Ti3C2Tx MXene nanocomposite as high performance electrode materials for flexible supercapacitors. Electrochim. Acta 2018, 290, 695–703. [Google Scholar] [CrossRef]
- Shen, B.; Liao, X.; Zhang, X.; Ren, H.T.; Lin, J.H.; Lou, C.W.; Li, T.T. Synthesis of Nb2C MXene-based 2D layered structure electrode material for high-performance battery-type supercapacitors. Electrochim. Acta 2022, 413, 140144. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, J.; Yuan, Z.; Zhao, L.; Wang, L.; Han, W. Assembling Co3O4 Nanoparticles into MXene with Enhanced electrochemical performance for advanced asymmetric supercapacitors. J. Colloid Interface Sci. 2021, 599, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, M.; Chaudhary, K.; Shahid, M.; Shakir, I.; Agboola, P.O.; Aadil, M. Fabrication of MoO3 Nanowires/MXene@CC hybrid as highly conductive and flexible electrode for next-generation supercapacitors applications. Ceram. Int. 2022, 48, 19314–19323. [Google Scholar] [CrossRef]
- Liu, H.; Hu, R.; Qi, J.; Sui, Y.; He, Y.; Meng, Q.; Wei, F.; Ren, Y.; Ren, Y.; Wei, W. One-Step Synthesis of Nanostructured CoS2 Grown on Titanium Carbide MXene for High-Performance Asymmetrical Supercapacitors. Adv. Mater. Interfaces 2020, 7, 1901659. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, J.; Wang, J.; Hu, C.; Xie, M.; Jin, X.; Wang, S.; Dai, Y. Facile fabrication of MXene supported nickel-cobalt selenide ternary composite via one-step hydrothermal for high-performance asymmetric supercapacitors. J. Alloys Compd. 2022, 899, 163354. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.; Lu, W.; Lei, D.; Tian, Y.; Guo, M.; Mi, P.; Qu, N.; Zhao, Y. MXenes induced formation of Ni-MOF microbelts for high-performance supercapacitors. J. Colloid Interface Sci. 2021, 592, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, S.; Cui, Y.; Wang, X.; Yong, Z.; Liang, D.; Chi, Y.; Wang, Z. Sandwich-like high-load MXene/polyaniline film electrodes with ultrahigh volumetric capacitance for flexible supercapacitors. J. Colloid Interface Sci. 2022, 620, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cui, L.; Lei, Z.; Xu, M.; Jin, X. MXene/carboxymethylcellulose-polyaniline (Ti3C2Tx/CMC-PANI) film as flexible electrode for high-performance asymmetric supercapacitors. Electrochim. Acta 2022, 436, 141408. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Han, J.; Wang, T.; Leng, Y.; Wang, Y.; Li, T.; Han, Y. Preparation of Polyaniline onto dl-Tartaric Acid Assembled MXene Surface as an Electrode Material for Supercapacitors. ACS Appl. Energy Mater. 2020, 3, 9326–9336. [Google Scholar] [CrossRef]
- Murali, S.; Quarles, N.; Zhang, L.L.; Potts, J.R.; Tan, Z.; Lu, Y.; Zhu, Y.; Ruoff, R.S. Volumetric capacitance of compressed activated microwave-expanded graphite oxide (a-MEGO) electrodes. Nano Energy 2013, 2, 764–768. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, L.; Li, Z.; Zhang, Y.; Xing, B.; Zhang, C.; Zhou, A. Electrochemical performance of Ti3C2 supercapacitors in KOH electrolyte. J. Adv. Ceram. 2015, 4, 130–134. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Kota, S.; Lin, Z.; Zhao, M.-Q.; Shpigel, N.; Levi, M.D.; Halim, J.; Taberna, P.-L.; Barsoum, M.W.; Simon, P. Ultra-high-rate pseudocapacitive energy storage in two-dimensional transition metal carbides. Nat. Energy 2017, 2, 17105. [Google Scholar] [CrossRef]
- Wang, X.; Mathis, T.S.; Li, K.; Lin, Z.; Vlcek, L.; Torita, T.; Osti, N.C.; Hatter, C.; Urbankowski, P.; Sarycheva, A. Influences from solvents on charge storage in titanium carbide MXenes. Nat. Energy 2019, 4, 241–248. [Google Scholar] [CrossRef]
- Liu, C.; Wu, H.; Wang, X.; Fan, J.; Su, H.; Yang, D.; Wei, Y.; Du, F.; Dall, Y.; Gao, Y. Flexible solid-state supercapacitor integrated by methanesulfonic acid/polyvinyl acetate hydrogel and Ti3C2Tx. Energy Storage Mater. 2023, 54, 164–171. [Google Scholar] [CrossRef]
- Hui, X.; Ge, X.; Zhao, R.; Li, Z.; Yin, L. Interface chemistry on MXene-based materials for enhanced energy storage and conversion performance. Adv. Funct. Mater. 2020, 30, 2005190. [Google Scholar] [CrossRef]
- Hu, M.; Hu, T.; Cheng, R.; Yang, J.; Cui, C.; Zhang, C.; Wang, X. MXene-coated silk-derived carbon cloth toward flexible electrode for supercapacitor application. J. Energy Chem. 2018, 27, 161–166. [Google Scholar] [CrossRef]
- Gao, Z.W.; Zheng, W.; Lee, L.Y.S. Highly enhanced pseudocapacitive performance of vanadium-doped MXenes in neutral electrolytes. Small 2019, 15, 1902649. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Yang, C.; Luo, Y.; Zhao, H.; Du, Y.; Kong, L.B.; Que, W. Understanding MXene-based “symmetric” supercapacitors and redox electrolyte energy storage. ACS Appl. Energy Mater. 2020, 3, 5006–5014. [Google Scholar] [CrossRef]
- Mu, X.; Wang, D.; Du, F.; Chen, G.; Wang, C.; Wei, Y.; Gogotsi, Y.; Gao, Y.; Dall, Y. Revealing the pseudo-intercalation charge storage mechanism of MXenes in acidic electrolyte. Adv. Funct. Mater. 2019, 29, 1902953. [Google Scholar] [CrossRef]
- Luo, J.; Fang, C.; Jin, C.; Yuan, H.; Sheng, O.; Fang, R.; Zhang, W.; Huang, H.; Gan, Y.; Xia, Y. Tunable pseudocapacitance storage of MXene by cation pillaring for high performance sodium-ion capacitors. J. Mater. Chem. A 2018, 6, 7794–7806. [Google Scholar] [CrossRef]
- Shao, H.; Lin, Z.; Xu, K.; Taberna, P.-L.; Simon, P. Electrochemical study of pseudocapacitive behavior of Ti3C2Tx MXene material in aqueous electrolytes. Energy Storage Mater. 2019, 18, 456–461. [Google Scholar] [CrossRef]
- Allah, A.E.; Wang, J.; Kaneti, Y.V.; Li, T.; Farghali, A.A.; Khedr, M.H.; Nanjundan, A.K.; Ding, B.; Dou, H.; Zhang, X.; et al. Auto-programmed heteroarchitecturing: Self-assembling ordered mesoporous carbon between two-dimensional Ti3C2Tx MXene layers. Nano Energy 2019, 65, 103991. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Zalnezhad, E.; Hui, K.N.; Hui, K.S.; Ko, M.J. 3D hierarchical transition-metal sulfides deposited on MXene as binder-free electrode for high-performance supercapacitors. J. Ind. 2020, 82, 309–316. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Ren, C.E.; Ling, Z.; Lukatskaya, M.R.; Zhang, C.; Van Aken, K.L.; Barsoum, M.W.; Barsoum, Y. Flexible MXene/carbon nanotube composite paper with high volumetric capacitance. Adv. Mater. 2014, 27, 0935–9648. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Ren, C.E.; Maleski, K.; Hatter, C.B.; Anasori, B.; Urbankowski, P.; Sarycheva, A.; Gogotsi, Y. Flexible MXene/graphene films for ultrafast supercapacitors with outstanding volumetric capacitance. Adv. Funct. Mater. 2017, 27, 1701264. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, Y.; Deng, Q.; Zhou, J.; Pei, Z.; Xue, Q.; Huang, Y.; Wang, Z.; Li, H.; Huang, Q. Highly flexible freestanding supercapacitor electrode with enhanced performance obtained by hybridizing polypyrrole chains with MXene. Adv. Energy Mater. 2016, 6, 1600969. [Google Scholar] [CrossRef]





| Property | MXene | 2D Carbon Nitride Material | 2D Transition Metal Dichalcogenides | Clay-Type 2D Material |
|---|---|---|---|---|
| Flexibility | Very high | Low | Moderate | Moderate |
| Excellent flexibility, maintains stability during bending | Relatively prone to deformation during bending | Tends to lose stability during bending | Prone to fracture during bending | |
| Elastic Recovery | Excellent | Normal | Poor | Poor |
| Demonstrates excellent elastic recovery ability | Normal elastic recovery under stress | Poor elastic recovery under stress | Significant deformation under stress | |
| Tensile Strength | Outstanding | Normal | Normal | Poor |
| Demonstrates excellent resistance to fracture | Displays ordinary performance during stretching | Susceptible to structural damage during stretching | Susceptible to rupture during stretching | |
| Post-Bending Recovery Performance | Excellent | Normal | Normal | Poor |
| Rapidly recovers to original performance after bending | Slow recovery of performance after bending | Limited recovery of performance after bending | Incomplete recovery of performance after bending | |
| Fatigue Resistance | Outstanding | Normal | Normal | Poor |
| Demonstrates outstanding fatigue resistance, maintaining high performance over extended periods of stress | Normal fatigue resistance, prolonged stress affects performance | Moderate fatigue resistance, performance diminishes over time under stress | Poor fatigue resistance, prone to fatigue failure |
| Mxene | Capacitance Value | Energy Density | Power Density | Stability | Ref. |
|---|---|---|---|---|---|
| Mxene/ZnCl2//MnO2-MWCNTs | 529.1 F g−1, 1.00 mV s−1 | 90.1 Wh kg−1 | 185.0 W kg−1 | capacity retention rate of 86.9% after 12,000 cycles | [121] |
| HS−NCS@MXene//AC-AHSC | 2637 F g−1, 2.5 A g−1 | 80 Wh kg−1 | 1196 W kg−1 | stable cycling life (96%) over 10,000 cycles | [122] |
| MXene/AuNPs | 278 F·g−1, 5 mV·s−1 | 8.82 Wh·L−1 | 264.6 W·L−1 | reaches 95.0% after 10,000 cycles. | [123] |
| MXene/PZS//AC | 380 F g−1, 2 mV s−1 | 12.26 Wh kg−1 | 125.00 W kg−1 | remarkable cycling stability without obvious deterioration after 5000 cycles | [124] |
| MIL-100(Fe)/Ti3C2 | 962.17 F g−1, 0.5 A g−1 | 85.53 Wh kg−1 | 200 W kg−1 | The capacity retention rate was 93% up to 10,000 cycles at 5 A/g | [125] |
| Ti3C2Tx-Mn | 248 F g−1, 100 A g−1 | 38.40 Wh kg−1 | 55.30 Wh kg−1 | an excellent cycle life with 85.53% retention after 100,000 cycles at a current density of 100 A g−1 | [126] |
| Devices | Electrolyte | Capacitance | Energy Density at Power Density | Ref. |
|---|---|---|---|---|
| MXene//PANI@MXene | 3 M H2SO4 | 87 Fg−1, 10 mVs−1 | 50.6 WhL−1 at 1.7 kWL−1 24.4 WhL−1 at 127 kWL−1 | [127] |
| GMP//graphene | 1 M H2SO4 | 68 Fg−1, 10Ag−1 | 42.3Whkg−1 at 950 Wkg−1 25 Whkg−1 at 18,000 Wkg−1 | [128] |
| Ti3C2Tx/Fe-15%//MnO2/CC | 1 M Li2SO4 | 115 mF cm−2, 2 mA cm−2 | 40mWh cm−2 at 8.2 mW cm−2 | [129] |
| Ni-dMXNC//Ti3C3Tx | 1 M KOH | - | 1.04×10−3 Wh cm−3 at 0.22 W cm−3 | [130] |
| MXene//PANI/MXene | 1 M H2SO4 | 231 F cm−3, 10 mV s−1 | 65.6 Wh L−1 at 1687.3 W L1 | [131] |
| Ti3C2Tx/P-100-H//rGO | 1 M H2SO4 | 117 F cm−3, 1.5 mA cm−2 | 23 mWh cm−3 at 7659 mW cm−3 | [132] |
| MXene/PPy//MXene/PPy | 1 M H2SO4 | 184 F g−1, 10 mV s−1 | - | [133] |
| 400PPy175/Ti3C2Tx// 400PPy175/Ti3C2Tx | 1 M H2SO4 | 258 F g−1, 0.5 A g−1 | 10.82 μWh mg−1 at 0.11 mW mg−1 | [134] |
| AC//MXene/PANI | 7 M KOH | 262 F g−1, 0.5 A g−1 | 22.67 Wh kg−1 at 217 W kg−1 | [135] |
| Mo1.33C MXene/PEDOT: PSS//Mo1.33C MXene/PEDOT: PSS | 1 M H2SO4 | 568 F g−1, 0.5 A g−1 | 33.3 mWh cm−3 at 19,470 mW cm−3 | [136] |
| Ti3C2Tx//Ti3C2Tx | Sea water | 27 F cm−3, 0.25 A g−1 | 1.74 × 10−3 Wh cm−3 at 0.15 W cm−3 | [137] |
| Ti2CTx//Ti2CTx | 1 M KOH | 452 F cm−3, 2 mV s−1 | 35 mWh cm−3 at 0.49 W cm−3 | [138] |
| M-NC@NCM/NF//AC | -- | 118.5 F·g−1 | 32.6 Wh·L−1/699.6 W kg−1 | [139] |
| MnO2@MXene/CNT | 1mol·L−1 H2SO4 | 371.1F·cm−3 @1 A·cm−3 | 8.22 mWh·cm−3/276.28 mW·cm−3 | [140] |
| MnO2/Ti3C2Tx | 1mol·L−1 Na2SO4 | 130.5F·g−1 @0.2 A·g−1 | - | [141] |
| Co3O4-Nb2C | 6mol·L−1 KOH | 1061 F·g−1 @2 A·g−1 | 60.3 Wh·kg−1/670 W·kg−1 | [142] |
| Co-MXene | 6mol·L−1 KOH | 1081F·g−1 @0.5 A·g−1 | 26.06 Wh·kg−1/700 W·kg−1 | [143] |
| MoO3 NWs/MXene@CC | 2mol·L−1 KOH | 775 F·g−1 @1 A·g−1 | - | [144] |
| Ti3C2Tx/CoS2 | 2mol·L−1 KOH | 1320 F·g−1 @1 A·g−1 | - | [145] |
| NiCo2Se4/MXene | 3mol·L−1 KOH | 953.8 F·g−1 @1 A·g−1 | 22.4 Wh·kg−1/800 W·kg−1 | [146] |
| Ti3C2Tx/Ni-MOFs | 6mol·L−1 KOH | 1124 F·g−1 @1 A·g−1 | 24 Wh·kg−1/8 kW·kg−1 | [147] |
| MP/FM/MP-20% | 1 M H2SO4 | 388 F·g−1 | 17.45 Wh kg−1 | [148] |
| MX/PANI NPs | -- | 377 F g−1 | 90.3 μWh cm−2 | [112] |
| Ti3C2Tx/CMC-PANI (TCP) film | 1 M H2SO4 | 1161.4 mF cm−2 @1 mA cm−2 | 158.7 μW h cm−2 at 700.1 μW cm−2 | [149] |
| TDP | 1 M H2SO4 | 452 F g−1 @1 A g−1 | - | [150] |
| Materials | Electrolyte | Volumetric Capacitance (F cm−3) | Gravimetric Capacitance (F g−1) | Areal Capacitance (mF cm−2) | Cycling Stability | Flexibility | Ref. |
|---|---|---|---|---|---|---|---|
| Ti3C2Tx/OMC | 3 M KOH | 823 (1 A g−1) | 329 (1 A g−1) | No mention | 117% retention (100 mV s−1), 10,000 cycles | No mention | [163] |
| Ti3C2Tx/NiCo2S4 | 3 M KOH | No mention | 1147.47 (1 A g−1) | No mention | 91.1% retention (10 A g−1), 3000 cycles | No mention | [164] |
| Ti3C2Tx | 2M KCl | No mention | 365.9 (2 mV s−1) | No mention | 95% retention (10 A g−1), 5000 cycles | No mention | [158] |
| Ti3C2Tx/CNT | MgSO4 | 390 (20 mV s−1) | ~125 (2 mV s−1) | No mention | ~100% retention (5 A g−1), 10,000 cycles | Flexible film | [165] |
| Ti3C2Tx/rGO | 3 M H2SO4 | 1040 (2 mV s−1) | 335 (2 mV s−1) | No mention | ~100% retention (100 mV s−1), 20,000 cycles | Flexible film | [166] |
| Ti3C2Tx/PPy | 0.5 M H2SO4 | 406 (30 mV s−1) | No mention | 203 (30 mV s−1) | ~100% retention, 20,000 cycles | Bending angles: 60°, 90°, 150° | [167] |
| Ti3C2Tx | 3 M H2SO4 | 1500 (2 mV s−1) | 380 (2 mV s−1) | 4000 (2 mV s−1) | Over 90% retention (10 A g−1), 10,000 cycles | Flexible film | [157] |
| Ti3C2Tx | MSA/PVA hydrogel | No mention | No mention | 1719 (2 mV s−1) | 92% retention (10 A g−1), 80,000 cycles | Bending angles: 0°–180° | [155] |
| Ti3C2Tx | LiTFSI-PC | 410 (2 mV s−1) | 195 (2 mV s−1) | No mention | 94% retention (100 mV s−1), 10,000 cycles | No mention | [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Ye, W.; Zhang, J.; Zheng, K. Structure, Properties, and Preparation of MXene and the Application of Its Composites in Supercapacitors. Inorganics 2024, 12, 112. https://doi.org/10.3390/inorganics12040112
Sun M, Ye W, Zhang J, Zheng K. Structure, Properties, and Preparation of MXene and the Application of Its Composites in Supercapacitors. Inorganics. 2024; 12(4):112. https://doi.org/10.3390/inorganics12040112
Chicago/Turabian StyleSun, Mingming, Wen Ye, Jingyao Zhang, and Kaining Zheng. 2024. "Structure, Properties, and Preparation of MXene and the Application of Its Composites in Supercapacitors" Inorganics 12, no. 4: 112. https://doi.org/10.3390/inorganics12040112
APA StyleSun, M., Ye, W., Zhang, J., & Zheng, K. (2024). Structure, Properties, and Preparation of MXene and the Application of Its Composites in Supercapacitors. Inorganics, 12(4), 112. https://doi.org/10.3390/inorganics12040112