Hybrid Gold-Based Perovskite Derivatives: Synthesis, Properties, and Prospects in Photovoltaics
Abstract
1. Introduction
2. Results and Discussions
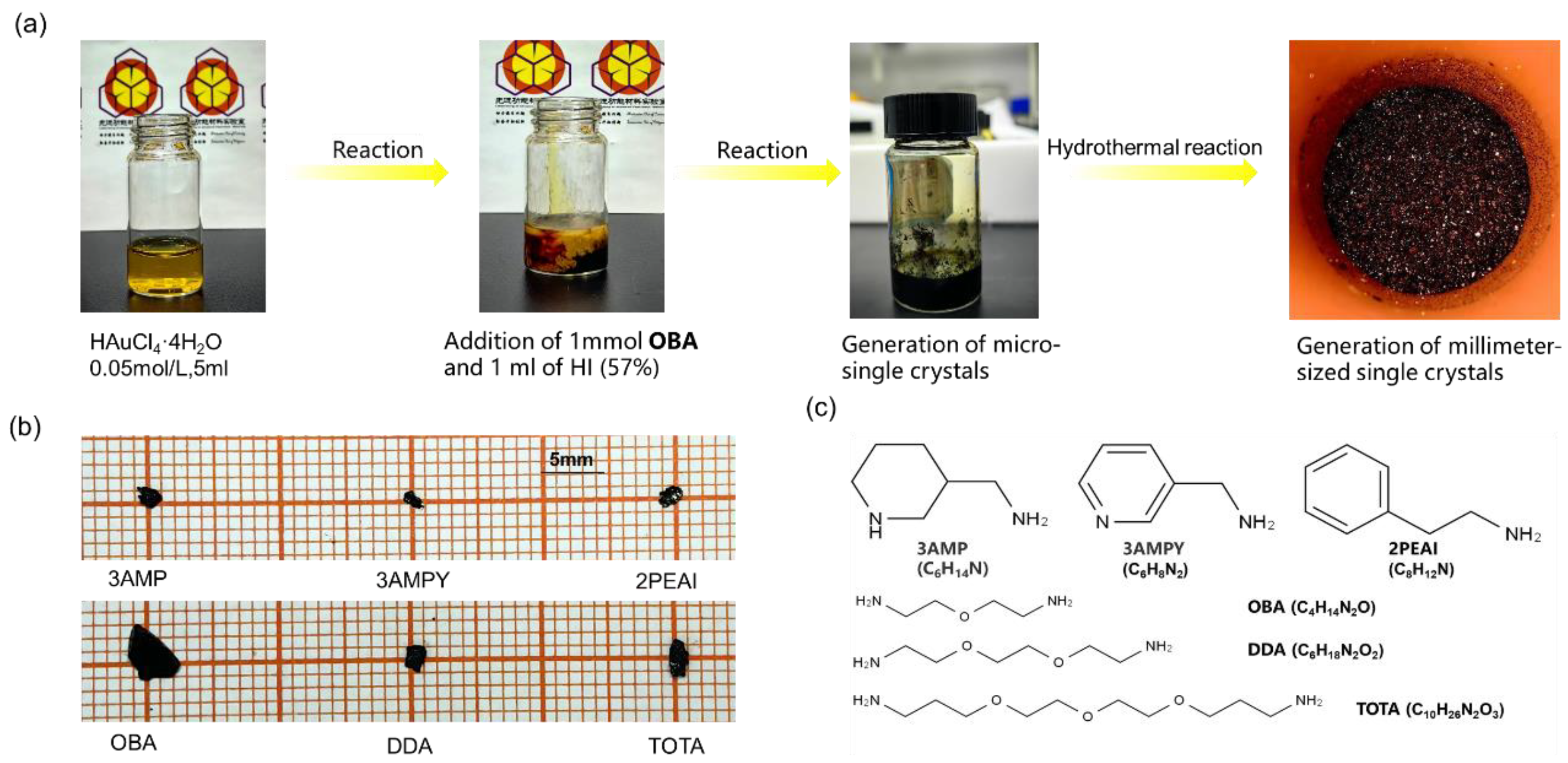
2.1. The Synthesis Process of Single Crystals
2.2. The Analysis of Single-Crystal Structures
- (1)
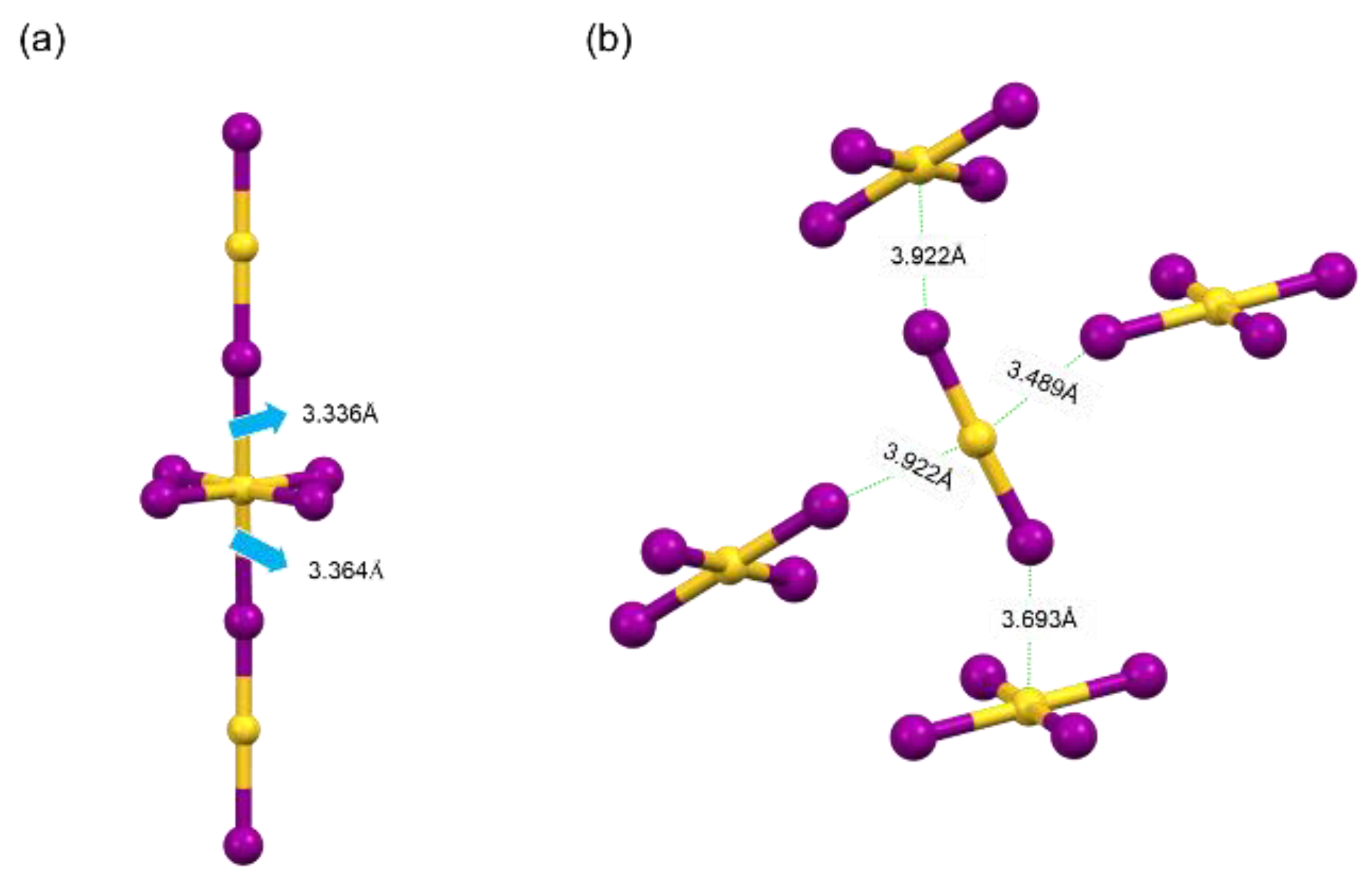
- In the crystal structures of 3AMP and 3AMPY, the anions consist of [AuII2]− and [AuIIII4]− units. Specifically, 3AMP comprises [AuII2]− and [AuIIII4]− anion units, along with one divalent organic cation to achieve charge balance. It is noteworthy that in 3AMP, there exists an [AuI6] octahedron, forming a zero-dimensional perovskite-like structure. These octahedra are connected by Au···I−-Au bonds formed by sharing I− ions in the vertical direction, with one [AuIIII4]− unit and two [AuII2]− units. Otherwise, 3AMPY comprises three [AuII2]− and one [AuIIII4]− anion units, balanced by two divalent organic cations. The [AuII2]− and [AuIIII4]− anion units exist independently, forming a zero-dimensional non-perovskite structure. This significant structural difference arises from the distinct spatial distribution of [AuII2]− and [AuIIII4]− ions between the two crystals. As shown in Figure 3a, in the crystal structure of 3AMP, the [AuII2]− is positioned directly above the center of the [AuIIII4]− unit. The Au···I bond lengths between adjacent [AuII2]− and [AuIIII4]− are 3.336 Å and 3.364 Å, respectively. Therefore, considering the shorter distance between the structural units and appropriate spatial distribution, [AuII2]− and [AuIIII4]− can form [AuI6] octahedra. In the crystal structure of 3AMPY, the Au···I bond lengths between [AuIIII4]− and the [AuII2]− unit positioned directly above it are 3.922 Å and 3.693 Å, respectively. The longer distances render the Au···I bonds unstable, preventing the formation of [AuI6] octahedra. As a result, [AuII2]− and [AuIIII4]− units exist independently without forming an octahedral structure.
- (2)
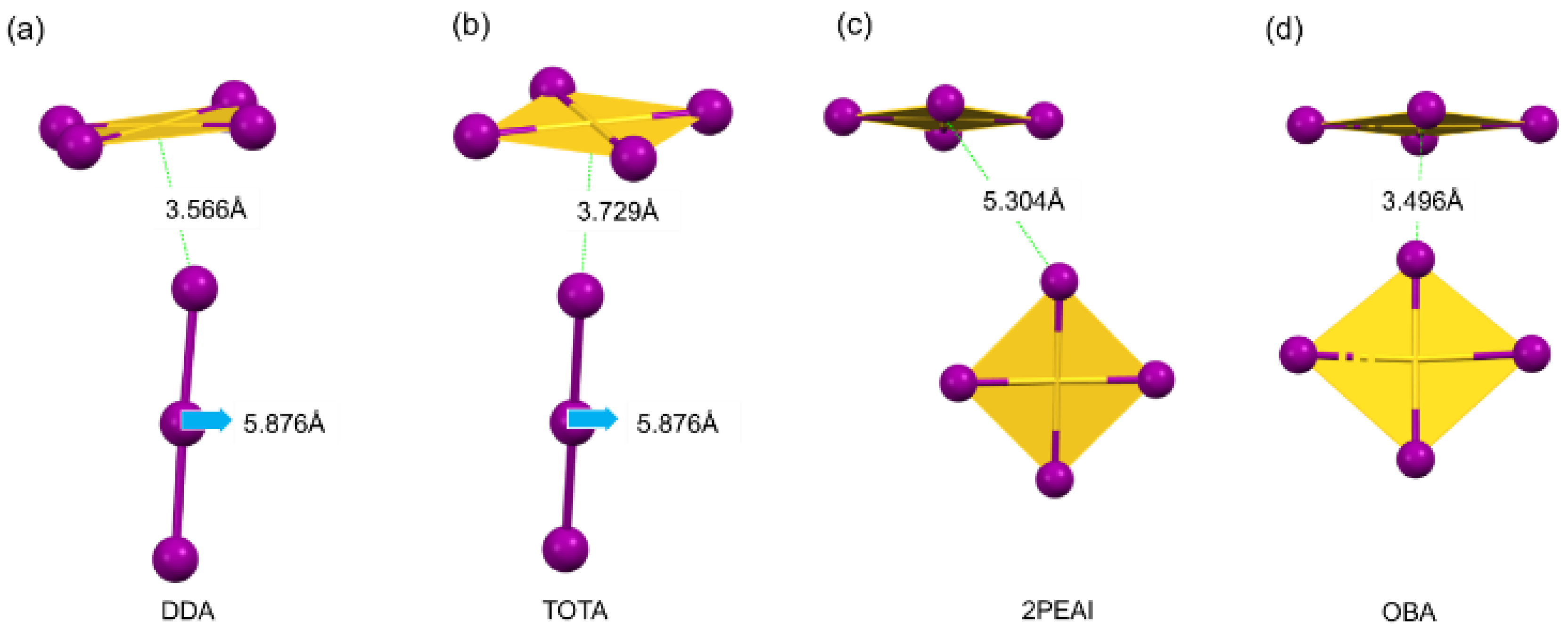
- In the DDA and TOTA crystal structures, the anions consist of [AuIIII4]− and [I3]− units. Specifically, DDA is balanced by two [AuIIII4]− and one [I3]− anion units along with two divalent organic cations, while TOTA is balanced by one [AuIIII4]− and one [I3]− anion units along with one divalent organic cation. As shown in Figure 4a,b, the length of the [I3]− structural unit is 5.876 Å. In both structures, the distance between the adjacent [AuIIII4]− units is much greater than the length of [I3]−, allowing for the incorporation of [I3]− units. Consistent with previous studies, the presence of [I3]− units in hybrid gold-based perovskite derivatives facilitates charge transport. [23] Moreover, the closer the distance between [I3]− and [AuIIII4]− units, the faster the carrier migration along [I3]−, resulting in better conductivity. This suggests that DDA and TOTA are expected to exhibit superior conductivity due to their favorable arrangement for charge transport.
- (3)
- In the crystal structures of 2PEAI and OBA, the anions consist of [AuIIII4]− units. Specifically, 2PEAI is balanced by one [AuIIII4]− anion unit and one monovalent organic cation, while OBA is balanced by two [AuIIII4]− anion units and one divalent organic cation. Due to the absence of [AuII2]− units, the [AuIIII4]− units in these two crystals cannot form octahedra, resulting in zero-dimensional non-perovskite structures. Additionally, as shown in Figure 4c,d, because of the excessively short distances between the adjacent [AuIIII4]− units, measured at 5.304 Å and 3.496 Å, respectively, [I3]− units cannot be accommodated in the structure. As a result, the anions consist solely of [AuIIII4]− units.
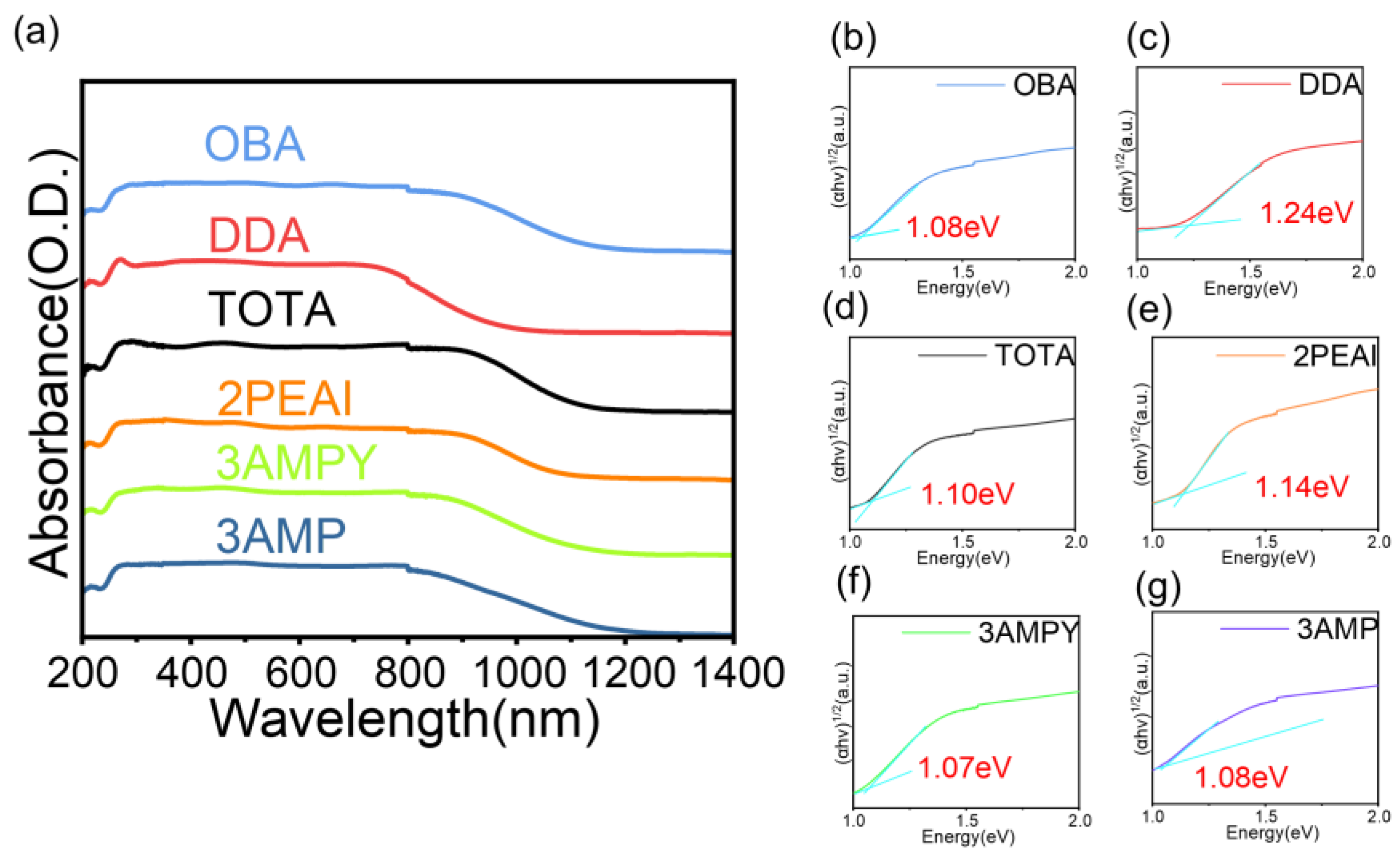
2.3. The Characterization of Optical Properties and Thermal Stability
- (1)
- For 3AMP, 2PEAI, 3AMPY, DDA, and TOTA, the thermal decomposition process can be divided into two steps:
- a.
- The first decomposition occurs around 100 °C, resulting in a weight loss of approximately 35%, corresponding to the organic cations.
- b.
- The second stage of decomposition begins at around 230 °C, corresponding to the loss of I2. The final products are AuI and Au.
- (2)
- For OBA, the thermal decomposition process can be divided into three steps:
- a.
- At around 100 °C, approximately 20% of the weight is lost, corresponding to the weight percentage of organic cations.
- b.
- At around 180 °C, the second stage of decomposition begins, with the loss of mass corresponding to I2. At this point, the products are AuI3 and Au.
- c.
- Around 240 °C, the final decomposition reaction occurs, with AuI3 decomposing into Au and AuI. After this, the thermogravimetric curve stabilizes, indicating that the final decomposition products are AuI and Au.
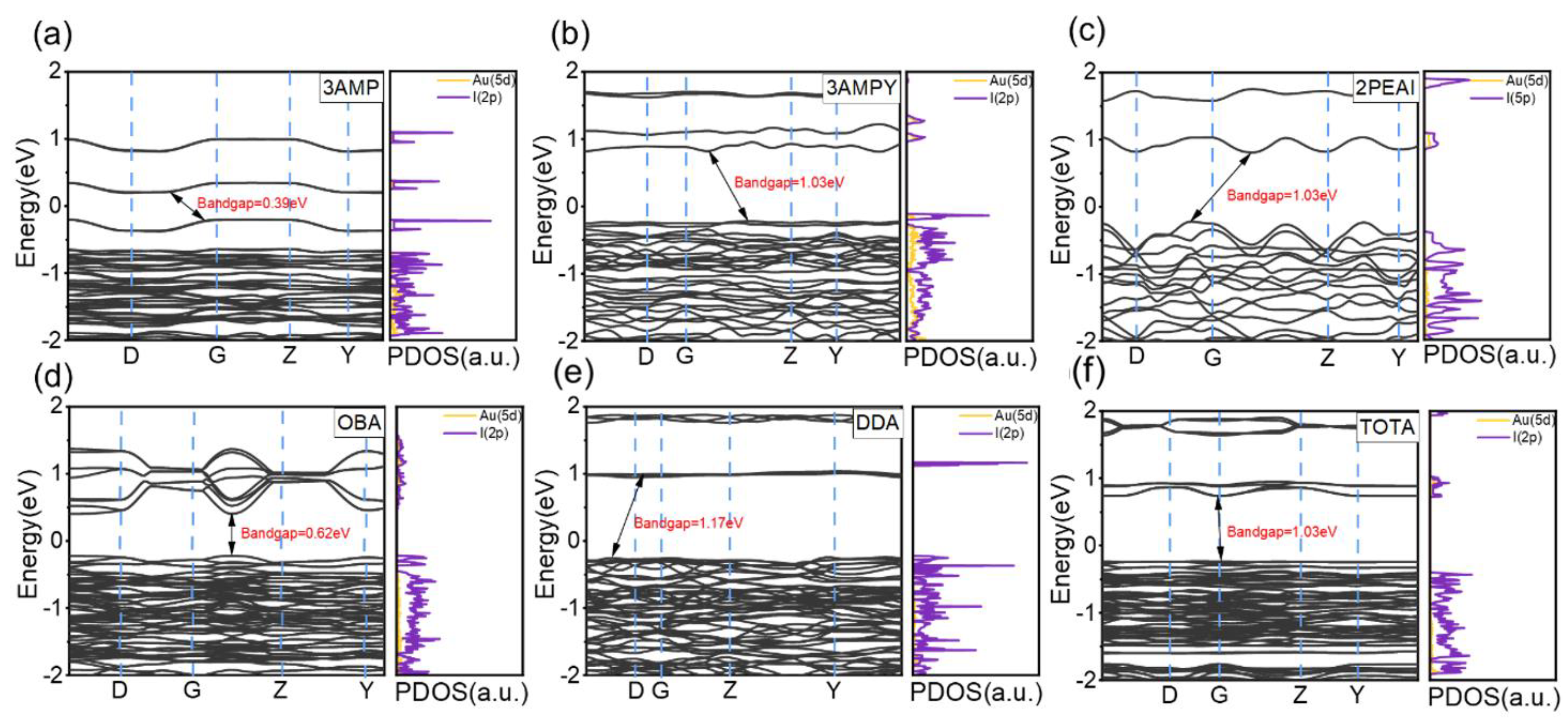
2.4. First-Principles Calculations
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Single Crystals
3.3. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, P.; Grätzel, M.; Nazeeruddin, M.K. Organohalide Lead Perovskites for Photovoltaic Applications. Energy Environ. Sci. 2014, 7, 2448–2463. [Google Scholar] [CrossRef]
- Gao, P.; Bin Mohd Yusoff, A.R.; Nazeeruddin, M.K. Dimensionality Engineering of Hybrid Halide Perovskite Light Absorbers. Nat. Commun. 2018, 9, 5028. [Google Scholar] [CrossRef]
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide Perovskite Photovoltaics: Background, Status, and Future Prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Yun, H.-S.; Paik, M.J.; Noh, E.; Mun, H.J.; Kim, M.G.; Shin, T.J.; Seok, S.I. Controlled Growth of Perovskite Layers with Volatile Alkylammonium Chlorides. Nature 2023, 616, 724–730. [Google Scholar] [CrossRef]
- Dou, L.; Yang, Y.; You, J.; Hong, Z.; Chang, W.-H.; Li, G.; Yang, Y. Solution-Processed Hybrid Perovskite Photodetectors with High Detectivity. Nat. Commun. 2014, 5, 5404. [Google Scholar] [CrossRef]
- Li, N.; Jia, Y.; Guo, Y.; Zhao, N. Ion Migration in Perovskite Light-Emitting Diodes: Mechanism, Characterizations, and Material and Device Engineering. Adv. Mater. 2022, 34, 2108102. [Google Scholar] [CrossRef]
- Babayigit, A. Toxicity of Organometal Halide Perovskite Solar Cells. Nat. Mater. 2016, 15, 247–251. [Google Scholar] [CrossRef]
- Lyu, M.; Yun, J.; Chen, P.; Hao, M.; Wang, L. Addressing Toxicity of Lead: Progress and Applications of Low-Toxic Metal Halide Perovskites and Their Derivatives. Adv. Energy Mater. 2017, 7, 1602512. [Google Scholar] [CrossRef]
- Xiao, Z.; Song, Z.; Yan, Y. From Lead Halide Perovskites to Lead-Free Metal Halide Perovskites and Perovskite Derivatives. Adv. Mater. 2019, 31, e1803792. [Google Scholar] [CrossRef]
- Igbari, F.; Wang, Z.-K.; Liao, L.-S. Progress of Lead-Free Halide Double Perovskites. Adv. Energy Mater. 2019, 9, 1803150. [Google Scholar] [CrossRef]
- Ghosh, B.; Febriansyah, B.; Harikesh, P.C.; Koh, T.M.; Hadke, S.; Wong, L.H.; England, J.; Mhaisalkar, S.G.; Mathews, N. Direct Band Gap Mixed-Valence Organic–Inorganic Gold Perovskite as Visible Light Absorbers. Chem. Mater. 2020, 32, 6318–6325. [Google Scholar] [CrossRef]
- Gupta, S.D. The double auric iodides of substituted ammonium bases. J. Am. Chem. Soc. 1914, 36, 747–751. [Google Scholar] [CrossRef]
- Strähle, J.; Gelinek, J.; Kölmel, M. Über den thermischen Abbau einiger Alkalimetall- und Ammoniumhalogenoaurate(III) und die Kristallstruktur der Zersetzungsprodukte Rb2Au2Br6, Rb3Au3Cl8 und Au(NH3)Cl3. Z. Anorg. Allg. Chem. 1979, 456, 241–260. [Google Scholar] [CrossRef]
- Strähle, J.; Bärnighausen, H. Notizen: Die Kristallstruktur von Rubidium-tetrachloro-aurat(III) RbAuCl4. Z. Naturforschung B 1970, 25, 1186–1187. [Google Scholar] [CrossRef]
- Kojima, N.; Kitagawa, H.; Ban, T.; Amita, F.; Nakahara, M. Semiconductor-to-Metal and Metal-to-Metal Transitions in the Three-Dimensional Mixed-Valence Compound Cs2Au2I6 under High Pressures. Solid State Commun. 1990, 73, 743–745. [Google Scholar] [CrossRef]
- Debbichi, L.; Lee, S.; Cho, H.; Rappe, A.M.; Hong, K.; Jang, M.S.; Kim, H. Mixed Valence Perovskite Cs2Au2I6: A Potential Material for Thin-Film Pb-Free Photovoltaic Cells with Ultrahigh Efficiency. Adv. Mater. 2018, 30, 1707001. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Zhang, Z.; Gao, P. Alternative Lead-Free Mixed-Valence Double Perovskites for High-Efficiency Photovoltaic Applications. J. Energy Chem. 2023, 84, 347–353. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Q.; Zhang, Z.; Lien, S.-Y.; Xie, Y.; Liang, W.; Gao, P. Gold-Based Double Perovskite-Related Polymorphs: Low Dimensional with an Ultranarrow Bandgap. Chem. Mater. 2022, 34, 1544–1553. [Google Scholar] [CrossRef]
- Walusiak, B.W.; Raghavan, A.; Cahill, C.L. I−/I3−Redox-Assisted Synthesis and Properties of Low Dimensional, Mixed-Valent Gold Iodide Perovskite Derivatives. Cryst. Growth Des. 2024, 24, 678–687. [Google Scholar] [CrossRef]
- Evans, H.A.; Schueller, E.C.; Smock, S.R.; Wu, G.; Seshadri, R.; Wudl, F. Perovskite-Related Hybrid Noble Metal Iodides: Formamidinium Platinum Iodide [(FA)2PtIVI6] and Mixed-Valence Methylammonium Gold Iodide [(MA)2AuIAuIIII6]. Inorg. Chim. Acta 2017, 468, 280–284. [Google Scholar] [CrossRef]
- Starkholm, A.; Kloo, L.; Svensson, P.H. Gold Polyiodide Hybrid Perovskite Solar Cells. ACS Mater. Lett. 2023, 5, 406–412. [Google Scholar] [CrossRef]
- Braathen, G.; Chou, P.-T.; Frei, H. Time-resolved reaction of oxygen (1.DELTA.) with iodide in aqueous solution. J. Phys. Chem. 1988, 92, 6610–6615. [Google Scholar]
- Murasugi, H.; Kumagai, S.; Iguchi, H.; Yamashita, M.; Takaishi, S. Organic–Inorganic Hybrid Gold Halide Perovskites: Structural Diversity through Cation Size. Chem. A Eur. J. 2019, 25, 9885–9891. [Google Scholar] [CrossRef]
- Kojima, Norimichi, Gold Valence Transition and Phase Diagram in the Mixed-Valence Complexes, M2[AuIX2] [AuIIIX4] (M = Rb, Cs; X = Cl, Br, and I). Bull. Chem. Soc. Jpn. 2000, 73, 1445–1460. [CrossRef]
- Wu, Y.; Xie, F.; Chen, H.; Yang, X.; Su, H.; Cai, M.; Zhou, Z.; Noda, T.; Han, L. Thermally Stable MAPbI3 Perovskite Solar Cells with Efficiency of 19.19% and Area over 1 Cm 2 Achieved by Additive Engineering. Adv. Mater. 2017, 29, 1701073. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Monika; Pachori, S.; Agrawal, R.; Choudhary, B.L.; Verma, A.S. An Efficient and Stable Lead-Free Organic–Inorganic Tin Iodide Perovskite for Photovoltaic Device: Progress and Challenges. Energy Rep. 2022, 8, 5753–5763. [Google Scholar] [CrossRef]
- Machín, A.; Márquez, F. Advancements in Photovoltaic Cell Materials: Silicon, Organic, and Perovskite Solar Cells. Materials 2024, 17, 1165. [Google Scholar] [CrossRef]
- Lu, X.; Fan, X.; Zhang, H.; Xu, Q.; Ijaz, M. Review on Preparation of Perovskite Solar Cells by Pulsed Laser Deposition. Inorganics 2024, 12, 128. [Google Scholar] [CrossRef]



| 2PEAI | 3AMP | 3AMPY | OBA | DDA | TOTA | |
|---|---|---|---|---|---|---|
| Formula | C8H12N (AuI4) | C6H14N2(AuI4) (AuI2) | C6H8N2 (AuI4)(AuI2) | C4H14N2O (AuI4)2 | (C6H18N2O2)3 (AuI4)4(I3)2 | C10H26N2O3 (AuI4)(I3) |
| Crystal system | monoclinic | monoclinic | triclinic | monoclinic | triclinic | monoclinic |
| Space group | (14) | (8) | (2) | (14) | (2) | (14) |
| a, Å | 6.025 (4) | 19.084 (2) | 8.3376 (3) | 15.7263 (8) | 8.1710 (5) | 9.1421 (5) |
| b, Å | 25.425 (13) | 8.3142 (9) | 9.3799 (3) | 8.8418 (4) | 10.8475 (8) | 24.0782 (12) |
| c, Å | 9.2683 (6) | 13.762 (2) | 14.0131 (5) | 17.2007 (8) | 20.3167 (14) | 12.9102 (6) |
| α, ° | 90 | 90 | 90.1030(10) | 90 | 80.611(3) | 90 |
| β, ° | 92.634 (2) | 111.702 (5) | 103.457 (2) | 98.458 (2) | 85.264 (3) | 102.001 (2) |
| γ−γ | 90 | 90 | 115.317(10) | 90 | 73.480(3) | 90 |
| Volume, Å3 | 1418.5 (15) | 2028.8 (4) | 957.03 (6) | 2365.7 (2) | 1702.1 (2) | 2779.7 (2) |
| Z | 4 | 2 | 2 | 4 | 2 | 4 |
| Radiation, Å | MoKα (λ = 0.71073 Å) | MoKα (λ = 0.71073 Å) | MoKα (λ = 0.71073 Å) | MoKα (λ = 0.71073 Å) | MoKα (λ = 0.71073 Å) | MoKα (λ = 0.71073 Å) |
| Temp., K | 200.00 | 200.00 | 200.00 | 200.00 | 200.00 | 200.00 |
| Compound | 3AMP | 3AMPY | 2PEAI | OBA | DDA | TOTA |
|---|---|---|---|---|---|---|
| Temperature (°C) | 82 | 98 | 104 | 102 | 94 | 110 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Fu, X.; Nan, Z.-A.; Zhang, Z.; Meng, L.; Gao, P. Hybrid Gold-Based Perovskite Derivatives: Synthesis, Properties, and Prospects in Photovoltaics. Inorganics 2024, 12, 157. https://doi.org/10.3390/inorganics12060157
Liu C, Fu X, Nan Z-A, Zhang Z, Meng L, Gao P. Hybrid Gold-Based Perovskite Derivatives: Synthesis, Properties, and Prospects in Photovoltaics. Inorganics. 2024; 12(6):157. https://doi.org/10.3390/inorganics12060157
Chicago/Turabian StyleLiu, Chang, Xifeng Fu, Zi-Ang Nan, Zilong Zhang, Lingyi Meng, and Peng Gao. 2024. "Hybrid Gold-Based Perovskite Derivatives: Synthesis, Properties, and Prospects in Photovoltaics" Inorganics 12, no. 6: 157. https://doi.org/10.3390/inorganics12060157
APA StyleLiu, C., Fu, X., Nan, Z.-A., Zhang, Z., Meng, L., & Gao, P. (2024). Hybrid Gold-Based Perovskite Derivatives: Synthesis, Properties, and Prospects in Photovoltaics. Inorganics, 12(6), 157. https://doi.org/10.3390/inorganics12060157







