CuFe2O4 Nanofiber Incorporated with a Three-Dimensional Graphene Sheet Composite Electrode for Supercapacitor and Electrochemical Sensor Application
Abstract
:1. Introduction
2. Preparation of Three-Dimensional Graphene Sheets/Copper Ferrite Fiber Composite (3DGS/CuFe2O4)
2.1. 3DGS Preparation
2.2. Transition Metal Ferrite Fiber Preparation
2.3. The 3DGS/CuFe2O4 Fiber Composite Preparation
2.4. Supercapacitor Electrode Preparation and Studies
2.5. Glassy Carbon Electrode Entrapped with 3DGS/CuFe2O4 Nanofiber Composite (GCE/3DGS/CuFe2O4) Preparation
2.6. Capacitance and Energy Density Calculation
3. Characterization Studies
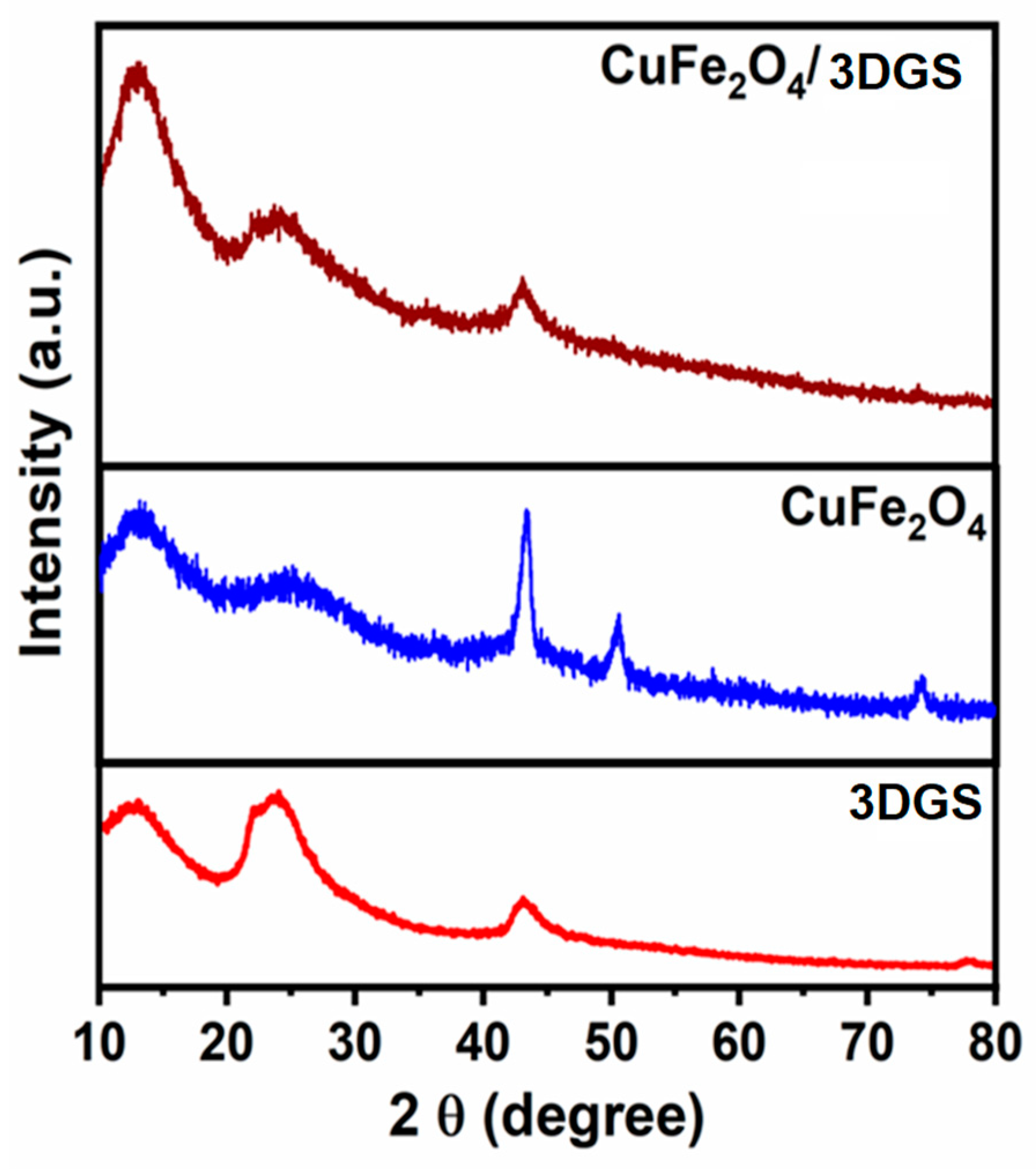
3.1. Crystalline Structure
3.2. FESEM and TEM Studies
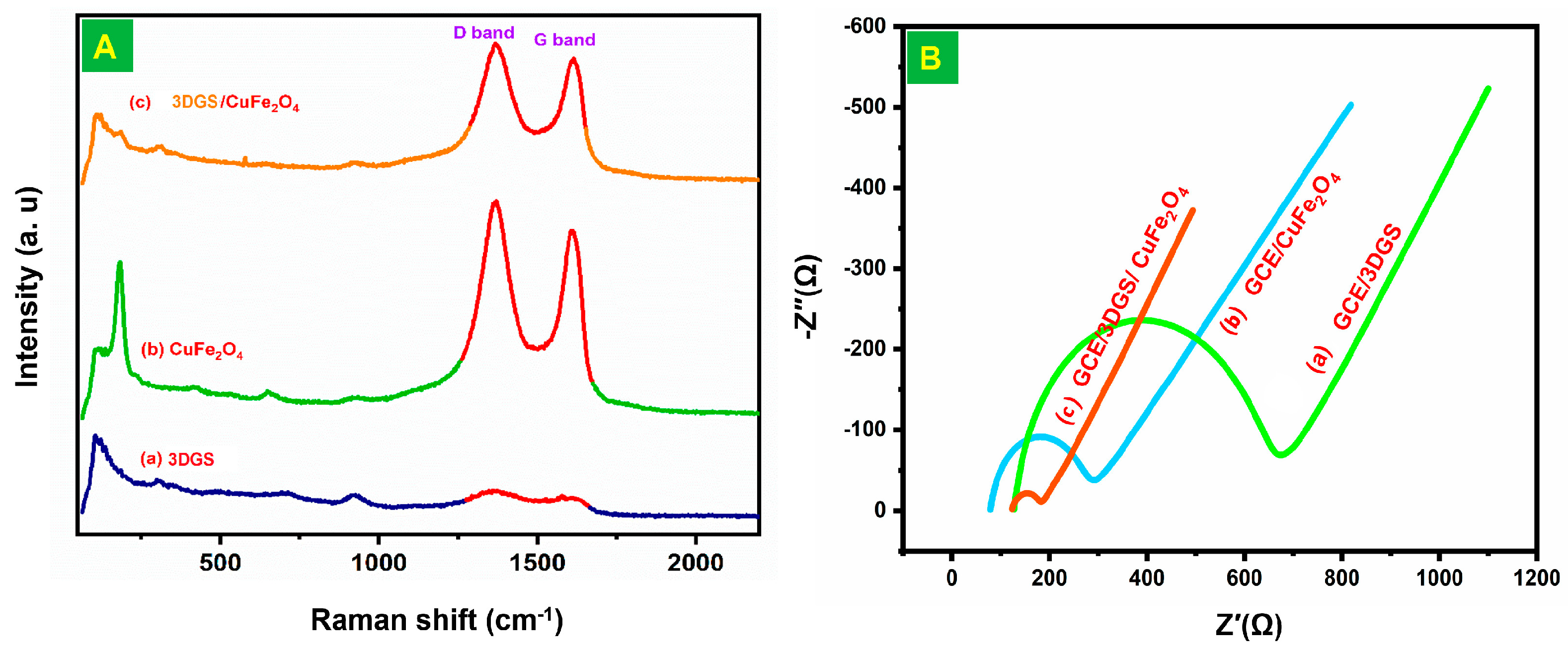
3.3. Raman and EIS Studies
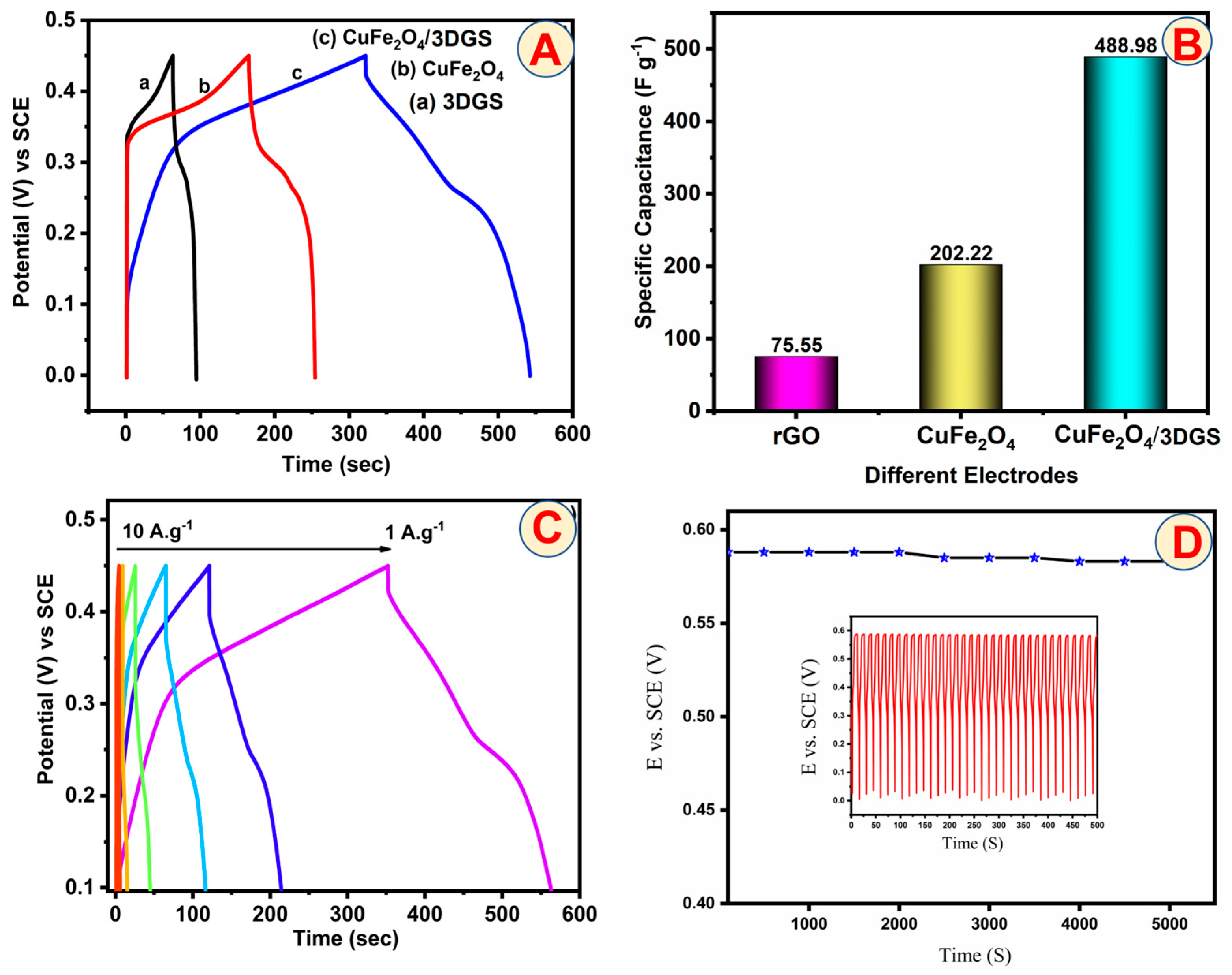
4. Supercapacitor Studies
5. Electrochemical Sensor Performance
5.1. Different Modified Electrode Studies toward the Detection of CAP
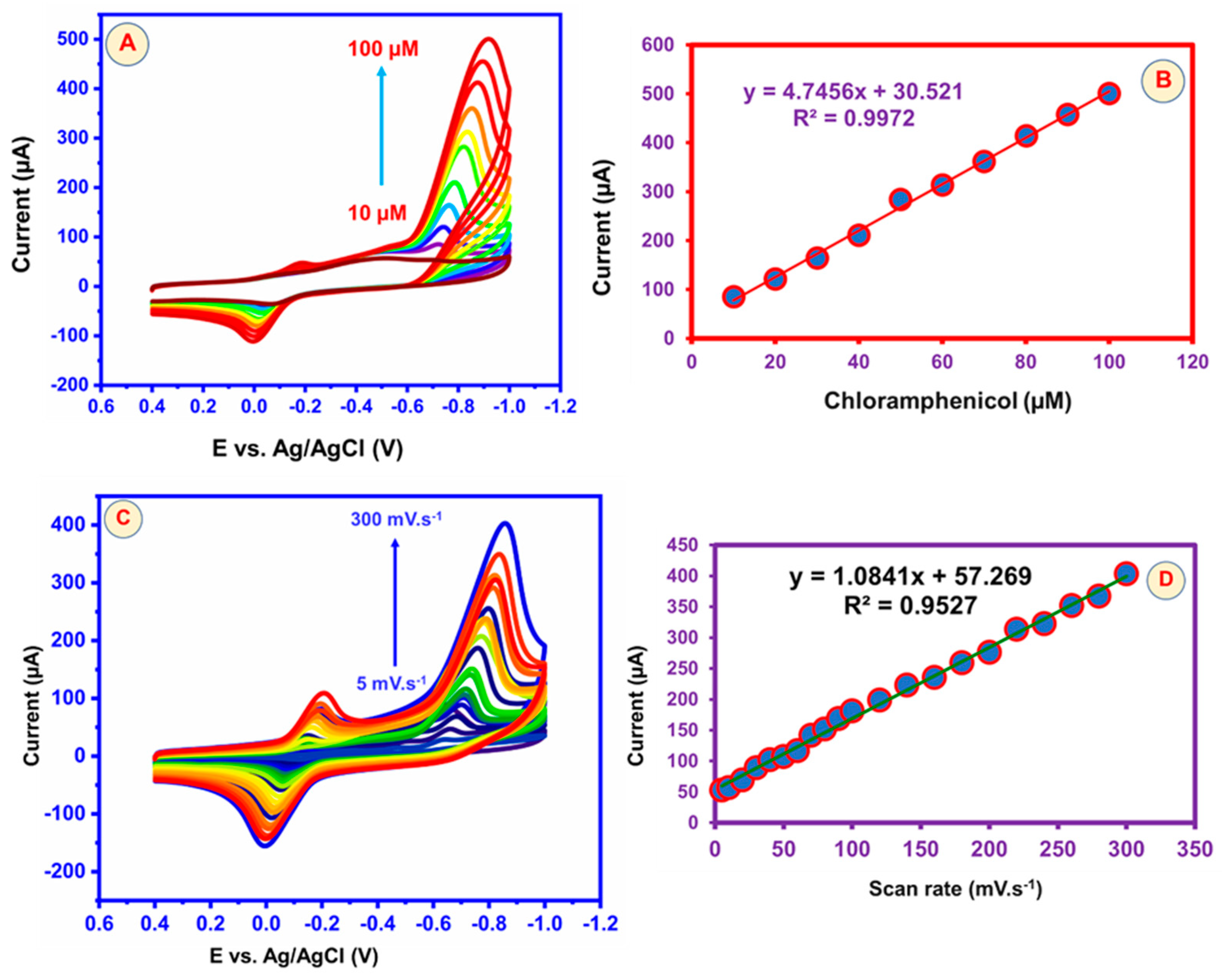
5.2. Effect of Different Concentration and Scan Rate Studies
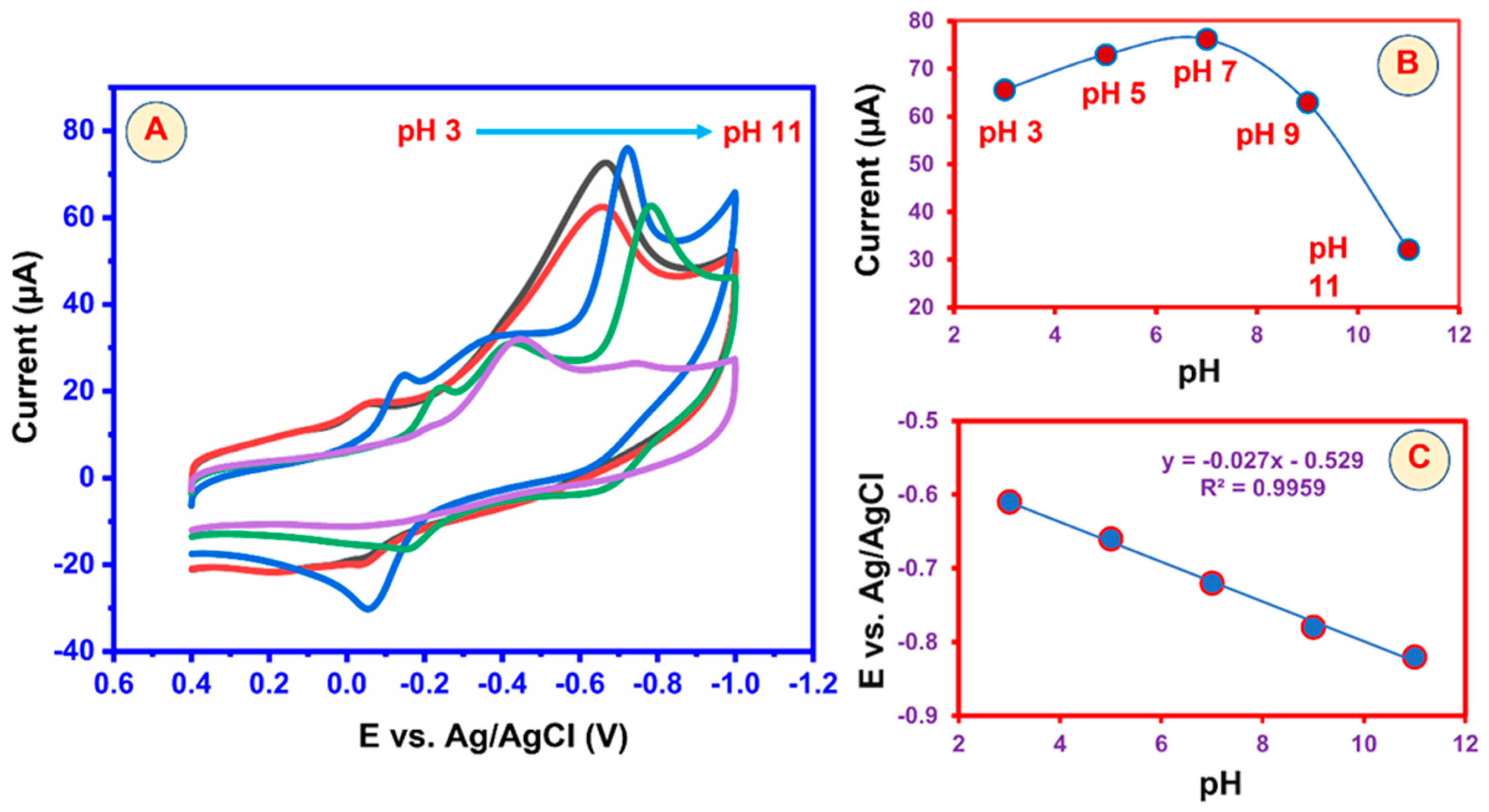
5.3. Effect of pH
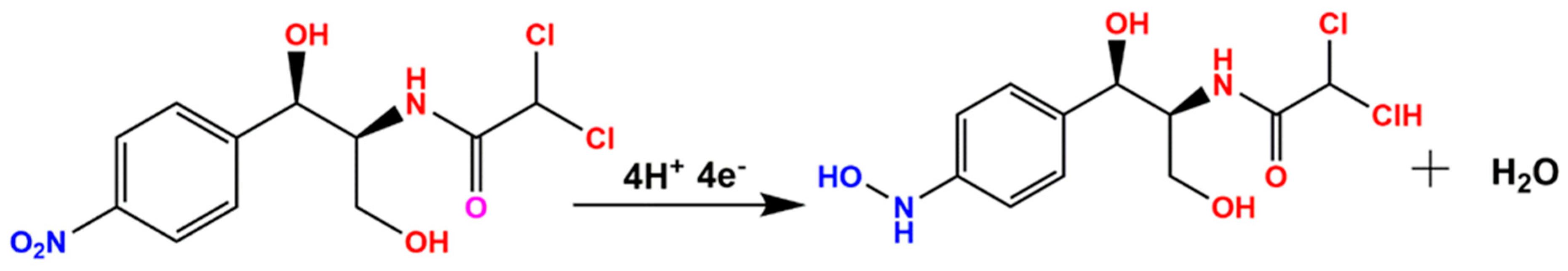
5.4. DPV Studies toward the Detection of CAP over the GCE/3DGS/CuFe2O4 Electrode
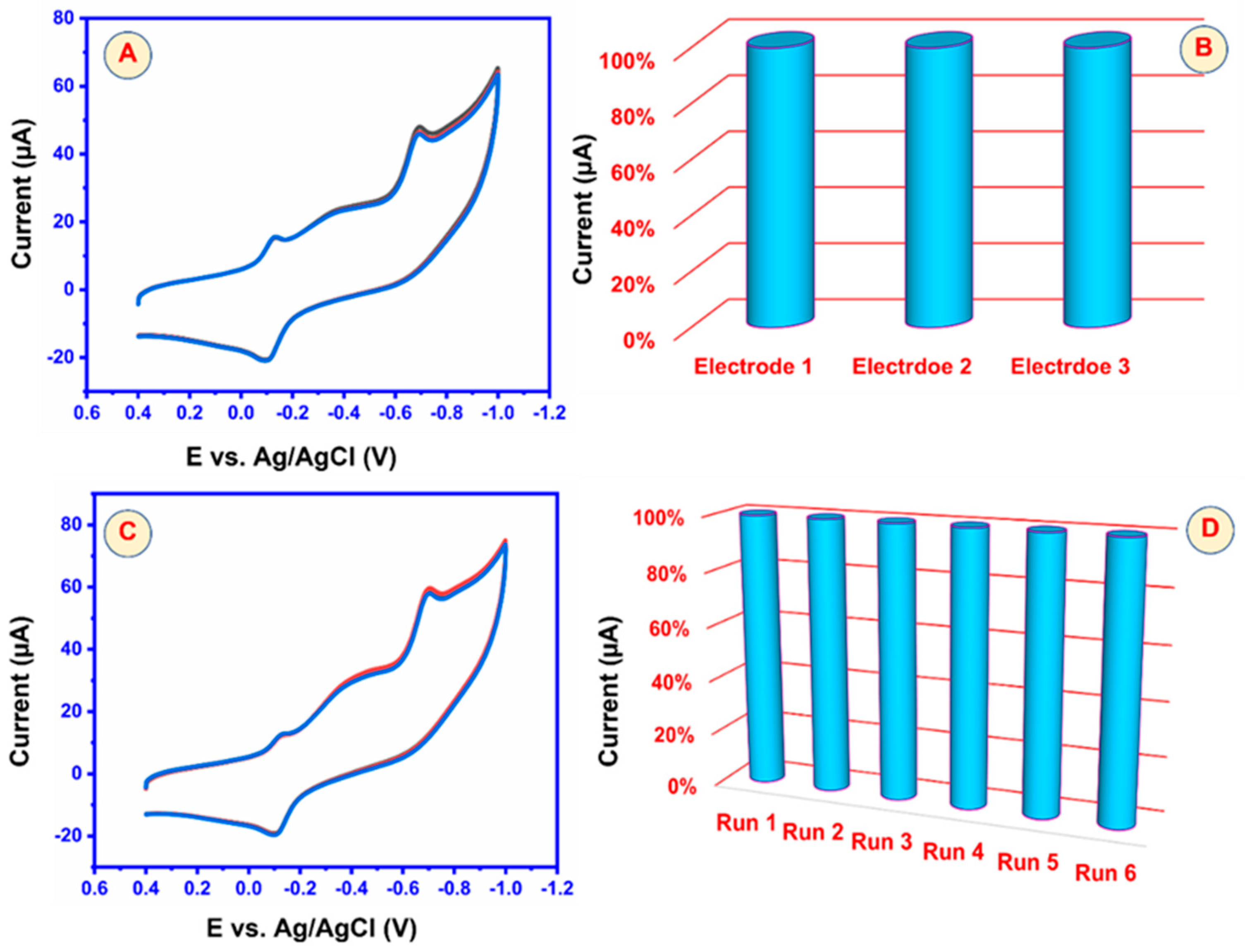
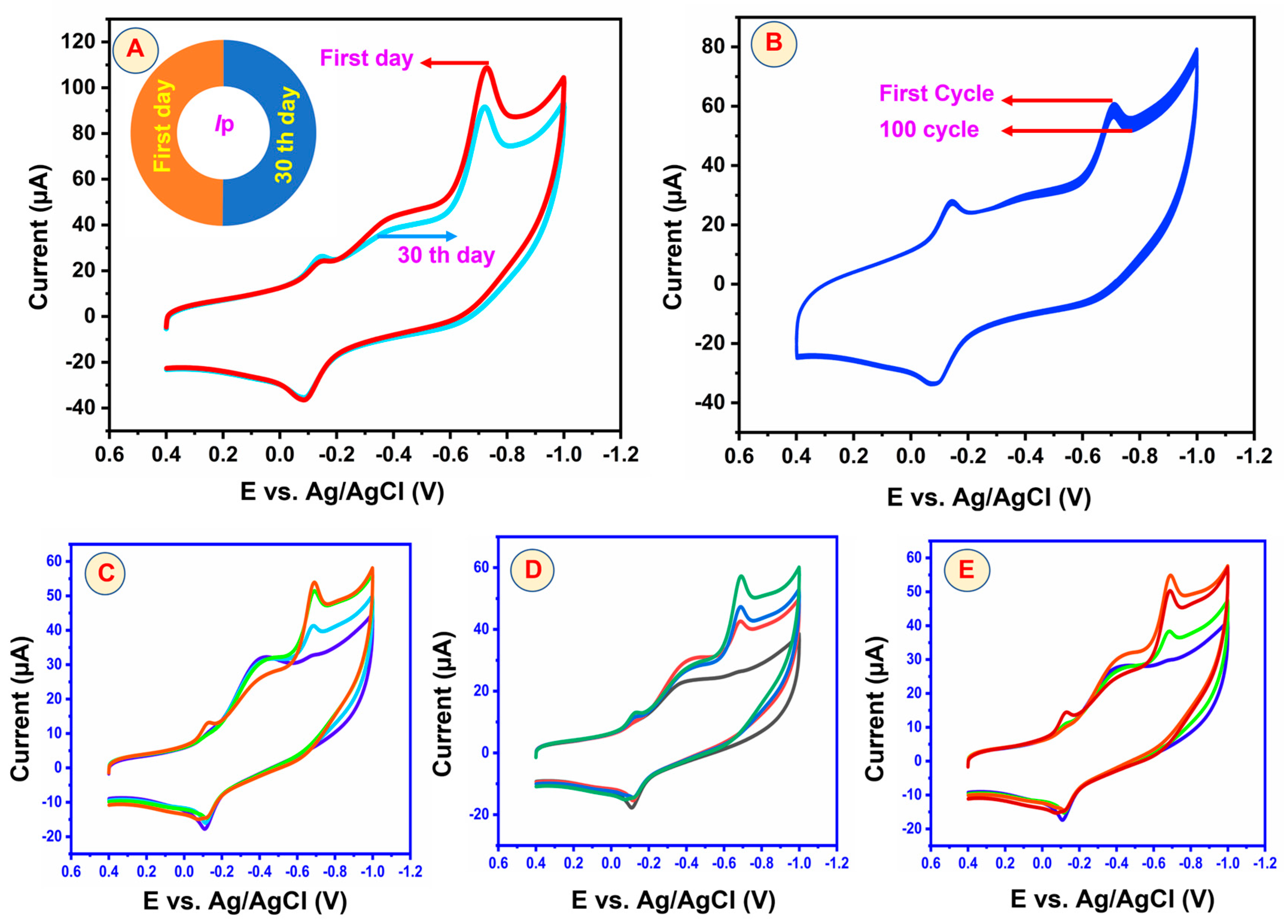
5.5. Repeatability, Reproducibility, and Stability of GCE/3DGS/CuFe2O4 Electrodes
5.6. Real Sample Analysis of the GCE/3DGS/CuFe2O4 Electrode
6. Materials and Methods
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakthinathan, S.; Rajakumaran, R.; Keyan, A.K.; Yu, C.L.; Wu, C.F.; Vinothini, S.; Chen, S.M.; Chiu, T.W. Novel construction of carbon nanofiber/CuCrO2 composite for selective determination of 4-nitrophenol in environmental samples and for supercapacitor application. RSC Adv. 2021, 11, 15856. [Google Scholar] [CrossRef] [PubMed]
- Samy, M.M.; Mohamed, M.G.; Mahdy, A.F.M.E.; Mansoure, T.H.; Wu, K.C.W.; Kuo, S.W. High-performance supercapacitor electrodes prepared from dispersions of tetrabenzonaphthalene based conjugated microporous polymers and carbon nanotubes. ACS Appl. Mater. Interfaces 2021, 13, 51906–51916. [Google Scholar] [CrossRef]
- Nasiri, F.; Fotouhi, L.; Shahrokhian, S.; Zirak, M. Cobalt sulfide flower-like derived from metal organic frameworks on nickel foam as an electrode for fabrication of asymmetric supercapacitors. Sci. Rep. 2024, 14, 6045. [Google Scholar] [CrossRef]
- Kumar, S.; Saeed, G.; Zhu, L.; Hui, K.N.; Kim, N.H.; Lee, J.H. 0D to 3D carbon-based networks combined with pseudocapacitive electrode material for high energy density supercapacitor: A review. J. Chem. Eng. 2021, 403, 126352. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hou, H.; Xu, W.; Duan, G.; He, S.; Liu, K.; Jiang, S. Recent progress in carbon based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2021, 56, 173–200. [Google Scholar] [CrossRef]
- Young, C.; Park, T.; Yi, J.W.; Kim, J.; Hossain, M.S.A.; Kaneti, Y.V.; Yamauchi, Y. Advanced functional carbons and their hybrid nanoarchitectures towards supercapacitor applications. ChemSusChem 2018, 11, 3546–3558. [Google Scholar] [CrossRef]
- Lim, J.M.; Jang, Y.S.; Nguyen, H.V.T.; Kim, J.S.; Yoon, Y.; Park, B.J.; Seo, D.H.; Lee, K.K.; Han, Z.; Ostrikov, K.; et al. Advances in high-voltage supercapacitors for energy storage systems: Materials and electrolyte tailoring to implementation. Nanoscale Adv. 2023, 5, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Fang, R.; Chu, D.; Wanga, D.W.; Ostrikovd, K. Introduction to supercapacitors. Nanoscale Adv. 2023, 5, 4015–4017. [Google Scholar] [CrossRef] [PubMed]
- Veeramani, V.; Madhu, R.; Chen, S.M.; Sivakumar, M. Flower like nickel cobalt oxide decorated dopamine-derived carbon nanocomposite for high-performance supercapacitor applications. ACS Sustain. Chem. Eng. 2016, 4, 5013–5020. [Google Scholar] [CrossRef]
- Veeramani, V.; Madhu, R.; Chen, S.M.; Sivakumar, M.; Hung, C.T.; Miyamoto, N.; Liu, S.B. NiCo2O4 decorated porous carbon nanosheets for high-performance supercapacitors. Electrochim. Acta 2017, 247, 288–295. [Google Scholar] [CrossRef]
- Veeramani, V.; Sivakumar, M.; Chen, S.M.; Madhu, R.; Alamri, H.R.; Alothman, Z.A.; Hossain, M.S.A.; Chen, C.K.; Yamauchi, Y.; Miyamoto, N.; et al. Lignocellulosic biomass-derived, graphene sheetlike porous activated carbon for electrochemical supercapacitor and catechin sensing. RSC Adv. 2017, 7, 45668–45675. [Google Scholar] [CrossRef]
- Xu, G.; Han, J.; Ding, B.; Nie, P.; Pan, J.; Dou, H.; Li, H.; Zhang, X. Biomass-derived porous carbon materials with sulfur and nitrogen dual-doping for energy storage. Green Chem. 2015, 17, 1668–1674. [Google Scholar] [CrossRef]
- Madhu, R.; Veeramani, V.; Chen, S.M.; Veerakumar, P.; Liu, S.B. Functional porous carbon/nickel oxide nanocomposites as binder-free electrodes for supercapacitors. Chem. Eur. J. 2015, 21, 8200–8206. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Ye, L.; Huang, Z.H.; Xu, Q.; Bai, Y.; Kang, F.; Yang, Q.H. A honeycomb-like porous carbon derived from pomelo peel for use in high-performance supercapacitors. Nanoscale 2014, 6, 13831–13837. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cai, X.; Cai, S.; Shao, Y.; Hu, C.; Lu, S.; Ding, S. High energy lithium-ion batteries: Recent progress and a promising future in applications. Energy Environ. Mater. 2023, 6, e12450. [Google Scholar] [CrossRef]
- Liu, W.; Placke, T.; Chau, K.T. Overview of batteries and battery management for electric vehicles. Energy Rep. 2022, 8, 4058–4084. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, M.; Chen, X. Supercapacitors for renewable energy applications: A review. Micro Nano Eng. 2023, 21, 100229. [Google Scholar] [CrossRef]
- Gupta, H.; Kumar, M.; Sarkar, D.; Menezes, P.W. Recent technological advances in designing electrodes and electrolytes for efficient zinc ion hybrid supercapacitors. Energy Adv. 2023, 2, 1263–1293. [Google Scholar] [CrossRef]
- Islam, M.S.; Hoque, S.M.; Rahaman, M.; Islam, M.R.; Irfan, A.; Sharif, A. Superior cyclic stability and capacitive performance of cation and water molecule pre intercalated δ-MnO2/h-WO3 nanostructures as supercapacitor electrodes. ACS Omega 2024, 9, 10680–10693. [Google Scholar] [CrossRef]
- Reddy, A.K.J.S.; Acharya, S.; Lakshmi, B.; Deepak, K.; Naveen, C.S.; Harishd, K.N.; Ramakrishna, S. A review on nanomaterial-based electrodes for the electrochemical detection of chloramphenicol and furazolidone antibiotics. Anal. Methods 2022, 14, 3228–3249. [Google Scholar]
- Xia, Y.M.; Zhang, W.; Li, M.Y.; Xia, M.; Zou, L.J.; Gao, W.W. Effective electrochemical determination of chloramphenicol and florfenicol based on graphene/copper phthalocyanine nanocomposites modified glassy carbon electrode. J. Electrochem. Soc. 2019, 166, B654–B663. [Google Scholar] [CrossRef]
- Peng, Y.; Li, M.; Jia, X.; Su, J.; Zhao, X.; Zhang, S.; Zhang, H.; Zhou, X.; Chen, J.; Huang, Y.; et al. Cu nanoparticle-decorated boron carbon nitrogen nanosheets for electrochemical determination of chloramphenicol. ACS Appl. Mater. Interfaces 2022, 14, 28956–28964. [Google Scholar] [CrossRef] [PubMed]
- Kaewnu, K.; Promsuwan, K.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. A simple and sensitive electrochemical sensor for chloramphenicol detection in pharmaceutical samples. J. Electrochem. Soc. 2020, 167, 087506. [Google Scholar] [CrossRef]
- Chuanuwatanakul, S.; Chailapakul, O.; Motomizu, S. Electrochemical analysis of chloramphenicol using boron-doped diamond electrode applied to a flow-injection system. Anal. Sci. 2008, 24, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Sakthinathan, S.; Kubendhiran, S.; Chen, S.M.; Karuppiah, C.; Chiu, T.W. Novel bifunctional electrocatalyst for ORR activity and methyl parathion detection based on reduced graphene oxide/palladium tetraphenyl porphyrin nanocomposite. Phys. Chem. C 2017, 121, 14096–14107. [Google Scholar] [CrossRef]
- Sakthinathan, S.; Keyan, A.K.; Vasu, D.; Vinothini, S.; Nagaraj, K.; Mangesh, V.L.; Chiu, T.W. Graphitic carbon nitride incorporated europium molybdate composite as an enhanced sensing platform for electrochemical detection of carbendazim in agricultural products. J. Electrochem. Soc. 2022, 169, 127504. [Google Scholar] [CrossRef]
- Chand, P.; Vaish, S.; Kumar, P. Structural, optical and dielectric properties of transition metal (MFe2O4; M = Co, Ni and Zn) nanoferrites. Phys. B Condens. Matter 2017, 524, 53–63. [Google Scholar] [CrossRef]
- Bhujun, B.; Tan, M.T.T.; Shanmugam, A.S. Study of mixed ternary transition metal ferrites as potential electrodes for supercapacitor applications. Results Phys. 2017, 7, 345–353. [Google Scholar] [CrossRef]
- Sharif, S.; Rahimi, K.; Yazdani, A. Highly improved supercapacitance properties of MnFe2O4 nanoparticles by MoS2 nanosheets. Sci. Rep. 2021, 11, 8378. [Google Scholar] [CrossRef]
- Kiey, S.A.A.; Ramadan, R.; Masry, M.M.E. Synthesis and characterization of mixed ternary transition metal ferrite nanoparticles comprising cobalt, copper and binary cobalt-copper for high-performance supercapacitor applications. Appl. Phys. A 2022, 128, 473. [Google Scholar] [CrossRef]
- Wang, L.; Bock, D.C.; Li, J.; Stach, E.A.; Marschilok, A.C.; Takeuchi, K.J.; Takeuchi, E.S. Synthesis and characterization of CuFe2O4 nano/submicron wire carbon nanotube composites as binder-free anodes for Li-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 8770–8785. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Lan, W.; Gong, H.; Bai, J.; Ramachandran, R.; Liu, S.; Wang, F. Highly sensitive acetone-sensing properties of Pt-decorated CuFe2O4 nanotubes prepared by electrospinning. Ceram. Int. 2018, 44, 2856–2863. [Google Scholar] [CrossRef]
- Peng, S.; Li, L.; Srinivasan, M. Electrospun CuFe2O4 nanotubes as anodes for high-performance lithium-ion batteries. J. Energy Chem. 2014, 23, 301–307. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, Z.; Chen, B.; Liao, L.; Wang, X. Cobalt oxide porous nanofibers directly grown on conductive substrate as a binder/additive-free lithium-ion battery anode with high capacity. Nanoscale Res. Lett. 2017, 12, 302. [Google Scholar] [CrossRef] [PubMed]
- Kanwade, A.; Gupta, S.; Kankane, A.; Tiwari, M.K.; Srivastava, A.; Kumar, J.A.; Subhash, S.; Yadav, C.; Shirage, P.M. Transition metal oxides as a cathode for indispensable Na-ion batteries. RSC Adv. 2022, 12, 23284–23310. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Guan, X.; Xu, B.; Gong, J.; Gao, Y.; Li, M. Surface porous microstructured fibers with customized functionalities for 1D functional materials. Compos. Part. B. Eng. 2021, 223, 109112. [Google Scholar] [CrossRef]
- Israr, M.; Iqbal, J.; Arshad, A.; Sadaf, A.; Rani, M.; Rani, M.; Jabeen, S. CuFe2O4/GNPs nanocomposites for symmetric supercapacitors and photocatalytic applications. J. Phys. D Appl. Phys. 2021, 54, 395501. [Google Scholar] [CrossRef]
- Atacan, K. CuFe2O4/reduced graphene oxide nanocomposite decorated with gold nanoparticles as a new electrochemical sensor material for ʟ-cysteine detection. J. Alloys Compd. 2019, 791, 391–401. [Google Scholar] [CrossRef]
- Yang, H.; Shi, X.; Pan, X.; Lin, S.; Zhang, X.; Du, Y.; Liu, J.; Fan, D.; Wang, Y.; Lei, M. Defect locating: One-step to monodispersed CoFe2O4/rGO nanoparticles for oxygen reduction and oxygen evolution. Int. J. Hydrogen Energy 2017, 42, 17075–17083. [Google Scholar] [CrossRef]
- Battad, G.A.T.; Estacio, J.G.; Indiongco, J.L.C.; Mopon, M.L., Jr. Development of a CuFe2O4 Reduced Graphene Oxide-Based Electrochemical Sensor for Malathion. Key Eng. Mater. 2020, 841, 41–47. [Google Scholar] [CrossRef]
- Baghayeri, M.; Amiri, A.; Fayazi, M.; Nodehi, M.; Esmaeelnia, A. Electrochemical detection of bisphenol a on a MWCNTs/CuFe2O4 nanocomposite modified glassy carbon electrode. Mater. Chem. Phys. 2021, 261, 124247. [Google Scholar] [CrossRef]
- Tajik, S.; Askari, M.B.; Ahmadi, S.A.; Nejad, F.G.; Dourandish, Z.; Razavi, R.; Beitollahi, H.; Bartolomeo, A.D. Electrochemical Sensor Based on ZnFe2O4/RGO Nanocomposite for Ultrasensitive Detection of Hydrazine in Real Samples. Nanomaterials 2022, 12, 491. [Google Scholar] [CrossRef] [PubMed]
- Bharath, G.; Hai, A.; Kiruthiga, T.; Rambabu, K.; Sabri, M.A.; Park, J.; Choi, M.Y.; Banat, F.; Haija, M.A. Fabrication of Ru–CoFe2O4/RGO hierarchical nanostructures for high-performance photoelectrodes to reduce hazards Cr(VI) into Cr(III) coupled with anodic oxidation of phenols. Chemosphere 2022, 299, 134439. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Habib, M.; Gondal, M.A.; Khalil, A.; Rehman, Z.U.; Muhammad, Z.; Haleem, Y.A.; Wang, C.; Wu, C.Q.; Song, L. Facile synthesis of CuFe2O4–Fe2O3 composite for high-performance supercapacitor electrode applications. Mater. Res. Express 2017, 4, 105501. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H. Hydrothermal synthesis of CuFe2O4 nanoparticles for highly sensitive electrochemical detection of sunset yellow. Food Chem. Toxicol. 2022, 165, 113048. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Yang, W.; Sakib, S.; Zhitomirsky, I. Magnetic CuFe2O4 Nanoparticles with Pseudocapacitive Properties for Electrical Energy Storage. Molecules 2022, 27, 5313. [Google Scholar] [CrossRef] [PubMed]
- Bhangoji, J.C.; Kahandal, S.S.; Patil, R.S.; Lambat, T.L.; Khan, S.I.; Wadhava, G.; Mahmood, S.H.; Shendage, S.S. One Pot Synthesis of CuO-CuFe2O4@rGO Nanostructure with Synergistic Effect for Efficient Electrochemical Sensing Application of Paracetamol. ECS Adv. 2023, 2, 026503. [Google Scholar] [CrossRef]
- Mahdikhah, V.; Saadatkia, S.; Sheibani, S.; Ataie, A. Outstanding photocatalytic activity of CoFe2O4/rGO nanocomposite in degradation of organic dyes. Opt. Mater. 2020, 108, 110193. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Y.; Qi, H. Synergistic degradation of organic pollutants on CoFe2O4/rGO nanocomposites by peroxymonosulfate activation under LED irradiation. Appl. Surf. Sci. 2022, 579, 1521510. [Google Scholar] [CrossRef]
- Shi, F.; Shan, H.; Li, D.; Yin, X.; Yu, J.; Ding, B. A general strategy to fabricate soft magnetic CuFe2O4@SiO2 nanofibrous membranes as efficient and recyclable fenton-like catalysts. J. Colloid Interface Sci. 2019, 538, 620–629. [Google Scholar]
- Lin, L.; Cui, H.; Zeng, G.; Chen, M.; Zhang, H.; Xu, M.; Shen, X.; Bortolini, C.; Dong, M. Ag–CuFe2O4 magnetic hollow fibers for recyclable antibacterial materials. J. Mater. Chem. B 2013, 1, 2719–2723. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cheng, Y.; Yan, X.; Sun, D.; Zhu, F.; Xue, Q. Magnetic and electrochemical properties of CuFe2O4 hollow fibers fabricated by simple electrospinning and direct annealing. Cryst. Eng. Comm. 2012, 14, 5879–5885. [Google Scholar] [CrossRef]
- Qiao, H.; Luo, L.; Chen, K.; Fei, Y.; Cui, R.; Wei, Q. Electrospun synthesis and lithium storage properties of magnesium ferrite nanofibers. Electrochim. Acta 2015, 160, 43–49. [Google Scholar] [CrossRef]
- Han, D.; Steckl, A.J. Coaxial electrospinning formation of complex polymer fibers and their applications. Chem. Plus Chem. 2019, 84, 1453–1497. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, J.; Zeng, L.; Qiao, Z.; Liu, X.; Liu, H.; Zhang, J.; Ding, J. Fabrication of electrospun polymer nanofibers with diverse morphologies. Molecules 2019, 24, 834. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.C.; Keyan, A.K.; Hung, C.W.; Sakthinathan, S.; Yu, C.L.; Chiu, T.W.; Nagaraj, K.; Fan, F.Y.; Shan, Y.K.; Chen, P.C. Fabrication of CuYO2 nanofibers by electrospinning and applied to hydrogen harvest. Materials 2022, 15, 8957. [Google Scholar] [CrossRef] [PubMed]
- Su, S.Y.; Wang, S.S.; Sakthinathan, S.; Chiu, T.W.; Park, J.H. Preparation of CuAl2O4 submicron tubes from electrospun Al2O3 fibers. Ceram. Int. 2019, 45, 1439–1442. [Google Scholar] [CrossRef]
- Dastgeer, G.; Nisar, S.; Rasheed, A.; Akbar, K.; Chavan, V.D.; Kim, D.K.; Wabaidur, S.M.; Zulfiqar, M.W.; Eom, J. Atomically engineered, high-speed non-volatile flash memory device exhibiting multibit data storage operations. Nano Energy 2024, 119, 109106. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Z.; Huang, X.; Liu, Y.; Huang, Y.; Duan, X. Flexible solid-state supercapacitors based on three-dimensional graphene hydrogel films. ACS Nano 2013, 7, 4042–4049. [Google Scholar] [CrossRef]
- Cao, X.; Yin, Z.; Zhang, H. Three-dimensional graphene materials: Preparation, structures, and application in supercapacitors. Energy Environ. Sci. 2014, 7, 1850–1865. [Google Scholar] [CrossRef]
- Yang, M.; Lee, K.G.; Lee, S.J.; Lee, S.B.; Han, Y.K.; Choi, B.G. Three-dimensional expanded graphene-metal oxide film via solid state microwave irradiation for aqueous asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 22364–22371. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Kang, Z.; Li, F.; Huang, X. Graphene with three-dimensional architecture for high-performance supercapacitor. Carbon 2014, 67, 221–229. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Wang, K.; Zhang, H.T.; Sun, X.Z.; Ma, Y.W. Three-dimensional graphene networks for supercapacitor electrode materials. New Carbon Mater. 2015, 30, 193–206. [Google Scholar] [CrossRef]
- Zaka, A.; Hayat, K.; Hayat, K.; Hayat, K.; Mittal, V. Recent trends in the use of three-dimensional graphene structures for supercapacitors. ACS Appl. Electron. Mater. 2021, 3, 574–596. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, G.; Duan, X. Self-assembled three-dimensional graphene macrostructures: Synthesis and applications in supercapacitors. Acc. Chem. Res. 2015, 48, 1666–1675. [Google Scholar] [CrossRef]
- Niu, Z.; Zhang, Y.; Zhang, Y.; Lu, X.; Liu, J. Enhanced electrochemical performance of three-dimensional graphene/carbon nanotube composite for supercapacitor application. J. Alloys Compd. 2020, 820, 153114. [Google Scholar] [CrossRef]
- Singh, K.; Kumar, S.; Agarwal, K.; Soni, K.; Gedela, V.R.; Ghosh, K. Three-dimensional graphene with MoS2 nanohybrid as potential energy storage/transfer device. Sci. Rep. 2017, 7, 9458. [Google Scholar] [CrossRef]
- Rania, B.; Sahua, N.K. Electrochemical properties of CoFe2O4 nanoparticles and its rGO composite for supercapacitor. Diam. Relat. Mater. 2020, 108, 107978. [Google Scholar] [CrossRef]
- Yin, J.; Ouyang, H.; Li, W.; Long, Y. An Effective Electrochemical platform for chloramphenicol detection based on carbon doped boron nitride nanosheets. Biosensors 2023, 13, 116. [Google Scholar] [CrossRef]
- Sebastian, N.; Yu, W.C.; Balram, D. Electrochemical detection of an antibiotic drug chloramphenicol based on a graphene oxide/hierarchical zinc oxide nanocomposite. Inorg. Chem. Front. 2019, 6, 82–93. [Google Scholar] [CrossRef]
- Yi, W.; Li, Z.; Dong, C.; Li, H.W.; Li, J. Electrochemical detection of chloramphenicol using palladium nanoparticles decorated reduced graphene oxide. Microchem. J. 2019, 148, 774–783. [Google Scholar] [CrossRef]
- Wang, W.; Sun, D. Electrochemical determination of chloramphenicol based on ZnO-NPs/SWCNTs composite modified glassy carbon electrode. Int. J. Electrochem. Sci. 2021, 16, 210216. [Google Scholar] [CrossRef]
- Hailu, T.; Li, Y.K.; Tessema, M. Electrochemical determination of chloramphenicol in milk and eye-drop using easily activated screen-printed carbon electrodes. Iran. J. Anal. Chem. 2021, 8, 93–101. [Google Scholar]
- Peng, R.; Chen, W.; Zhou, Q. Electrochemical sensor for chloramphenicol based on copper nano dendrites and carbon nanotubes. Ionics 2022, 28, 451–462. [Google Scholar] [CrossRef]
- Zhang, W.; Quan, B.; Lee, C.; Park, S.K.; Li, X.; Choi, E.; Diao, G.; Piao, Y. One-step facile solvothermal synthesis of copper ferrite–graphene composite as a high-performance supercapacitor material. ACS Appl. Mater. Interfaces 2015, 7, 2404–2414. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, Y.; Hu, X.; Yao, Y.; Li, Z. Tween modified CuFe2O4 nanoparticles with enhanced supercapacitor performance. Colloids Surf. Physicochem. Eng. Asp. 2021, 631, 127676. [Google Scholar] [CrossRef]
- Saravanakumar, B.; Ramachandran, S.P.; Ravi, G.; Ganesh, V.; Guduru, R.K.; Yuvakkumar, R. Electrochemical Performances of Monodispersed Spherical CuFe2O4 Nanoparticles for Pseudocapacitive Applications. Vacuum 2019, 168, 108798. [Google Scholar] [CrossRef]
- Polat, S.; Faris, D. Fabrication of CuFe2O4@g-C3N4@GNPs Nanocomposites as Anode Material for Supercapacitor Applications. Ceram. Int. 2022, 48, 24609–24618. [Google Scholar] [CrossRef]
- Zhu, M.; Meng, D.; Wang, C.; Diao, G. Facile fabrication of hierarchically porous CuFe2O4 nanospheres with enhanced capacitance property. ACS Appl. Mater. Interfaces 2013, 5, 6030–6037. [Google Scholar] [CrossRef]






| Modified Electrode | Linear Range (µM) | Detection Limit (µM) | References |
|---|---|---|---|
| P(EBT)/GCE) | 0.01–4.0 | 3 | [23] |
| C-BN | 0.1–700 | 0.035 | [69] |
| Cu−BCN/GCE | 0.01−1.12 | 2.41 | [22] |
| Gr/CuPc/GCE | 1–30 | 0.75 | [21] |
| GO/ZnO/GCE | 0.2–124 | 0.01 | [70] |
| rGO/PdNPs/GCE | 0.05 | 0.05–1 | [71] |
| ZnO NPs/SWCNTs | 10–140 | 0.03 | [72] |
| Activated SPCE | 0.05–100 | 0.02 | [73] |
| CuNDs/MWCNTs/GCE | 0.15–12.0 | 9.84 | [74] |
| g-C3N4/EuMoO4 | 50–400 | 0.4 | [24] |
| GCE/3DGS/CuFe2O4 | 5–400 µM | 0.5 | This work |
| Sample | Added (µM) | Found (µM) | Recovery (µM) | RSD (%) |
|---|---|---|---|---|
| Milk | 20 | 19.2 | 96 | 2.52 |
| 40 | 35.6 | 89 | 2.15 | |
| 60 | 58.7 | 97.8 | 2.19 | |
| Honey | 20 | 19.5 | 97.5 | 2.25 |
| 40 | 39.5 | 98.7 | 1.95 | |
| 60 | 59.8 | 99.6 | 2.36 | |
| Eye drop | 20 | 19.8 | 99 | 2.42 |
| 40 | 39.3 | 98.2 | 1.93 | |
| 60 | 58.6 | 97.6 | 2.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinothini, S.; Keyan, A.K.; Sakthinathan, S.; Chiu, T.-W.; Vittayakorn, N. CuFe2O4 Nanofiber Incorporated with a Three-Dimensional Graphene Sheet Composite Electrode for Supercapacitor and Electrochemical Sensor Application. Inorganics 2024, 12, 164. https://doi.org/10.3390/inorganics12060164
Vinothini S, Keyan AK, Sakthinathan S, Chiu T-W, Vittayakorn N. CuFe2O4 Nanofiber Incorporated with a Three-Dimensional Graphene Sheet Composite Electrode for Supercapacitor and Electrochemical Sensor Application. Inorganics. 2024; 12(6):164. https://doi.org/10.3390/inorganics12060164
Chicago/Turabian StyleVinothini, Sivaramakrishnan, Arjunan Karthi Keyan, Subramanian Sakthinathan, Te-Wei Chiu, and Naratip Vittayakorn. 2024. "CuFe2O4 Nanofiber Incorporated with a Three-Dimensional Graphene Sheet Composite Electrode for Supercapacitor and Electrochemical Sensor Application" Inorganics 12, no. 6: 164. https://doi.org/10.3390/inorganics12060164
APA StyleVinothini, S., Keyan, A. K., Sakthinathan, S., Chiu, T.-W., & Vittayakorn, N. (2024). CuFe2O4 Nanofiber Incorporated with a Three-Dimensional Graphene Sheet Composite Electrode for Supercapacitor and Electrochemical Sensor Application. Inorganics, 12(6), 164. https://doi.org/10.3390/inorganics12060164








