Abstract
Binary transition metal selenides (BTMSs) are more promising than single transition metal selenides (TMS) as anode materials of sodium-ion batteries (SIBs). However, it is still very challenging to prepare high-performance BTMSs in the pure phase, instead of a mixture of two TMSs. In this study, a binary metal center-based MOF derived selenization strategy was developed to prepare iron–cobalt selenide (Fe2CoSe4@NC) and iron–nickel selenide (Fe2NiSe4@NC) nanocomposites in the single phase and when wrapped with carbon layers. As the anode material of SIBs, Fe2CoSe4@NC exhibits higher long-term cycling performance than Fe2NiSe4@NC, maintaining a capacity of 352 mAh g−1 after 2100 cycles at 1.0 A g−1, which is ascribed to the higher percentage of the nanopores, larger lattice spacing, and faster Na+ diffusion rate in the electrode materials of the former rather than the latter.
1. Introduction
As a promising candidate for energy storage systems, sodium-ion batteries (SIBs) have garnered significant attention owing to the low cost and wide availability of sodium [1,2,3,4,5,6,7]. However, the larger radius of sodium ions (1.02 Å) than lithium ions (0.76 Å) leads to a slow kinetic of sodium ion insertion and extraction [8,9,10,11,12]. The commonly used cathode materials for LIBs are Na3V2(PO4)3 and Prussian blue (PB), etc., with large open frames [13,14], while there more options for anode materials of LIBs [15,16,17]. The pursuit of optimal cathode and anode materials towards the achievement of SIBs with prolonged cycle life and substantial specific capacity presents a significant and formidable challenge for researchers [18,19].
In recent years, transition metal selenides (TMSs) have garnered extensive attention as anode materials for sodium-ion batteries (SIBs), owing to their high theoretical capacity [20,21]. For example, our group has developed ZIF-67-derived CoSe2 nanoparticles (CoSe2@NCF/CNTs) wrapped with N-doped and CNT-entangled carbonaceous materials [8]. The overall structural morphology of CoSe2@N-CF/CNTs composites is well preserved, as anode materials of SIBs, even after 100 cycles at a current density of 1 A g−1, demonstrate the effectiveness of carbonaceous material encapsulation in maintaining structural integrity. However, the electrochemical properties of TMSs anodes are constrained by challenges such as volume expansion and dissolution of polyselenide during cycling, leading to reduced conductivity and inferior electrochemical performance [17,22,23,24].
Binary transition metal chalcogenides (BTMCs), due to their improved electrochemical performance compared to single-metal compounds, have garnered significant attention. The improved performance of binary transition metal selenides (BTMS) as the anode materials of SIBs is ascribed to their superior conductivity, endowed by the smart choice of metal elements in combination, and coupled, with the engineering of nanostructures [8,25,26]. For instance, hierarchically porous nanospheres of binary iron-cobalt selenide (Fe2CoSe4) were prepared using a hydrothermal method to obtain Fe-Co glycerate and further selenization using Se powder as a selenium precursor in hydrogen gas atmosphere, which exhibits an impressive rate capability and extended life cycle [8]. In another study, three types of binary transition metal selenides based on nickel–cobalt, nickel–iron, and cobalt–iron combinations were prepared with nanosheet structures, and nickel–iron selenide exhibits the longest cycling performance and highest charge capacity [25]. However, exploration towards the synthesis of BTMS and their application as anode materials SIBs are still very limited, since it is still very challenging to prepare a binary transition metal selenide (M1xM2ySez, M1, and M2 are two different transition metal atoms) in the single phase, instead of mixed two types of metal selenides (M1xSey/M2xSez) [27]. Additionally, the mechanism behind the difference in the electrochemical performance of different combinations of these transition metal selenides remains unexplored.
Herein, we study MOF-derived bimetallic selenides, namely, iron–cobalt selenide (Fe2CoSe4@NC) and nickel–iron selenide (Fe2NiSe4@NC). Both Fe2CoSe4@NC and Fe2NiSe4@NC exhibited outstanding long-term cycling stability and rate capability, maintaining a capacity of 352 and 282.2 mAh g−1 after 2100 cycles at 1.0 A g−1, respectively. Both the electrolyte’s penetration of the porous electrode surface and the sodium ion conductance in the anode materials might govern the electrochemical performance of sodium-ion storage.
2. Results
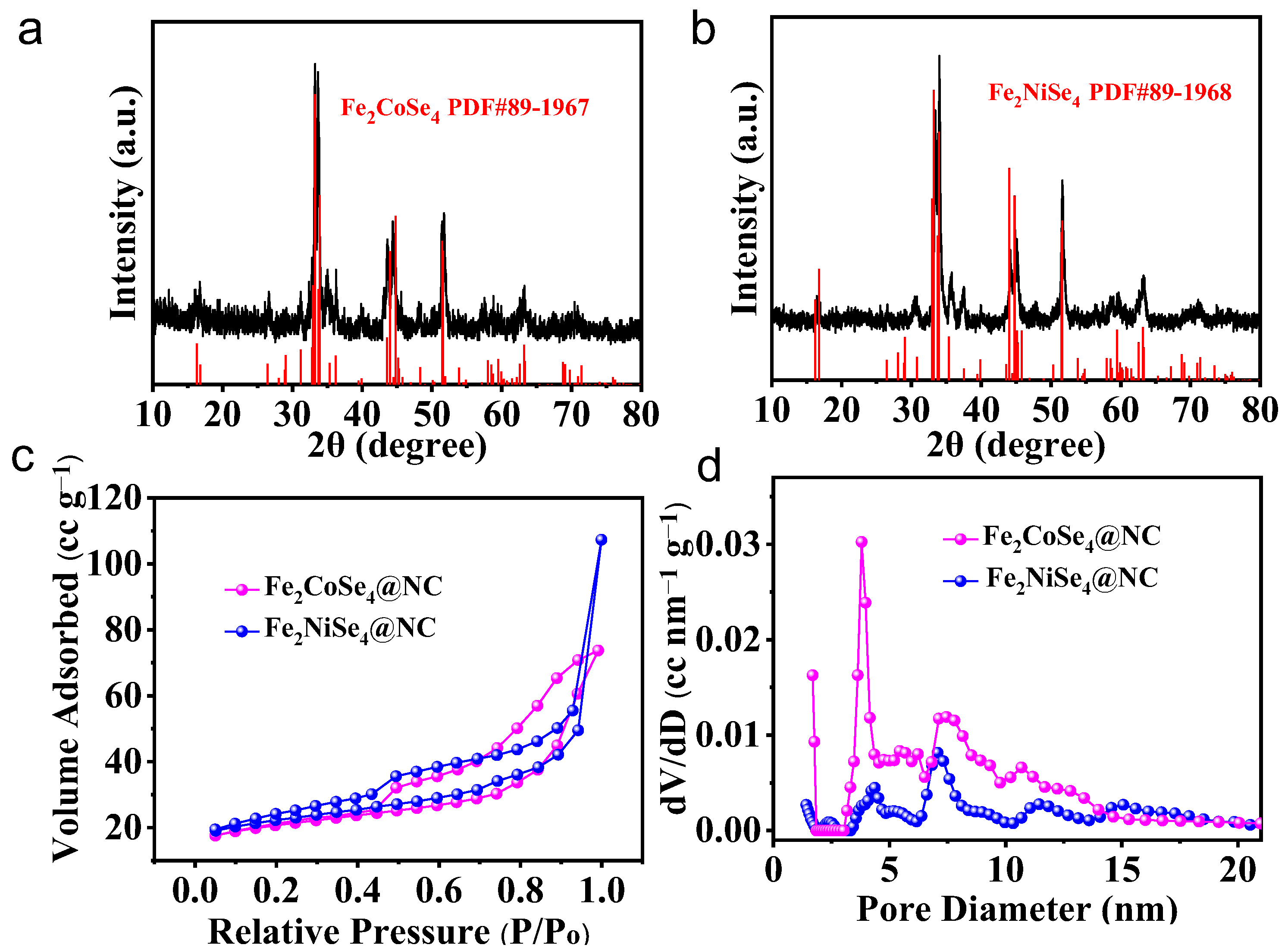
Fe2CoSe4@NC and Fe2NiSe4@NC were synthesized with the selenization of a binary transition metal organic framework (MOF). Initially, a mixture of FeCo-MOF or FeNi-MOF and selenium powder was maintained at 300 °C for 4 h to ensure the complete selenization of the metal ligands. Subsequently, the sample was further annealed at 700 °C to fully carbonize the MOF structure to obtain carbon-wrapped BTMS. The crystal structure of the Fe2CoSe4@NC and Fe2NiSe4@NC samples was initially examined using XRD, as depicted in Figure 1a,b. The diffraction pattern in Figure 1a,b exhibits several peaks that can be accurately indexed to Fe2CoSe4@NC (JCPDS 89-1967) and Fe2NiSe4@NC (JCPDS 89-1968) without any detectable impurities [8]. This confirms the efficacy of our proposed strategy for the facile synthesis of BTMS in the pure phase. Fe2CoSe4@NC and Fe2NiSe4@NC show very similar diffraction patterns. The four diffraction peaks, with a high intensity identified at 32.9° to 33.9°, were ascribed to the reflection of (–2 0 2), (–1 1 2), (1 1 2), and (2 0 2), and the two prominent peaks at 44° and 44.7° were indexed to (–1 1 4) and (1 1 4). Another two evident peaks were identified at 51.49° and 51.59°, which were from the (310) and (0 2 0) planes. Although the diffraction positions of these prominent peaks are quite similar to each other, for Fe2CoSe4@NC and Fe2NiSe4@NC, the relative peak intensities were different from one another. For instance, the peak intensity of (–1 1 2) is slightly higher than that of (1 1 2) for Fe2CoSe4@NC, but it is the opposite case for Fe2NiSe4@NC, indicating the orientation difference of the lattice reflections of Fe2CoSe4@NC and Fe2NiSe4@NC. Additionally, the diffraction peak position of (–1 1 4) and (1 1 4) of Fe2CoSe4@NC negatively shifted compared to the standard peak, due to the lattice extension of (–1 1 4) and (1 1 4) planes, while no apparent shift was observed for Fe2NiSe4@NC. It should also be noted that the peaks of (310) and (0 2 0) planes are well resolved for Fe2CoSe4@NC, but emerged as one peak for Fe2NiSe4@NC, indicating the higher crystallinity of Fe2CoSe4@NC compared to Fe2NiSe4@NC.
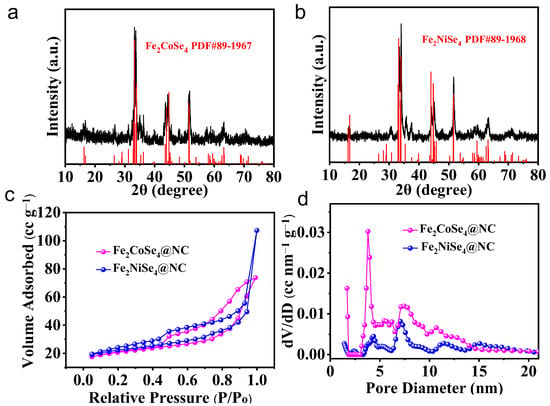
Figure 1.
XRD patterns of (a) Fe2CoSe4@NC and (b) Fe2NiSe4@NC. (c) N2 adsorption/desorption isotherms and (d) the corresponding Barret–Joyner–Halenda (BJH) pore size distribution of Fe2CoSe4@NC and Fe2NiSe4@NC.
The N2 adsorption/desorption isotherms of Fe2CoSe4@NC and Fe2NiSe4@NC (Figure 1c) exhibit a type IV isotherm with a hysteresis loop at a relatively high pressure, indicating their mesoporous characteristics [11]. Both Fe2CoSe4@NC (75.02 m2 g−1) and Fe2NiSe4@NC (80.66 m2 g−1) display similar specific surface areas. The pore-size distribution of the Fe2CoSe4@NC sample, calculated using the Barrett−Joyner−Halenda (BJH) method, ranges from 3.8 to14.4 nm (Figure 1d), including a narrow distribution at 4.8 nm and a broad distribution at around 7.5 nm, with a much higher percentage for the smaller pores than the larger ones. On the other hand, the pore size distribution of the Fe2NiSe4@NC sample was predominantly centered around 4.3~10 nm, with the maximum peak intensity being 7.3 nm. This indicates that the pore size in Fe2CoSe4@NC is relatively smaller than that of Fe2NiSe4@NC. It has been reported that the mesoporous nature and large surface area of electrode materials can enhance the electrolyte penetration, surface contact with electrolyte, and the interaction with sodium-ions, as well as increasing electronic conductivity by reducing the ion diffusion length [8].
The morphology of the nanocomposites was examined using scanning electron microscopy (SEM), as depicted in Figure 2a,b. In Figure 2a, SEM images of Fe2CoSe4@NC reveal random nanoparticles and nano-blocks, along with small pores on the surface. On the other hand, the SEM image of Fe2NiSe4@NC displays a noticeable agglomeration of nanoparticles (Figure 2b). The high-resolution transition electron microscopy (HRTEM) image of Fe2CoSe4@NC (Figure 2c) shows the calculated lattice spacing of 2.66 Å for (202) plane, which is slightly larger than that of Fe2NiSe4@NC(2.62 Å) (Figure 2d), consistent with the results obtained from the XRD patterns [25].
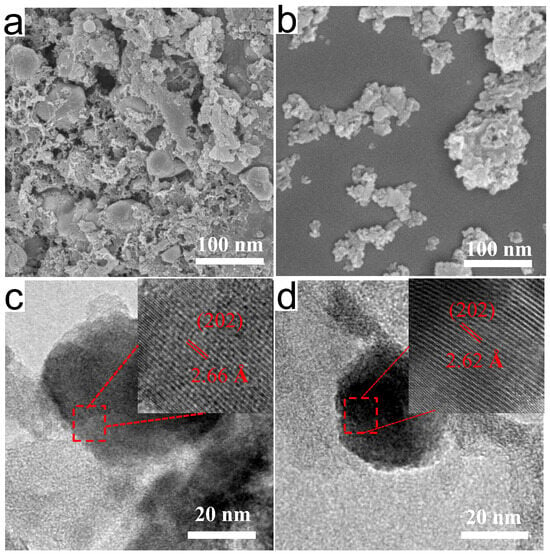
Figure 2.
The SEM images of (a) Fe2CoSe4@NC and (b) Fe2NiSe4@NC. TEM images of the nanoparticles of (c) Fe2CoSe4@NC and (d) Fe2NiSe4@NC, respectively.
The carbon content in the Fe2CoSe4@NC and Fe2NiSe4@NC composites was evaluated using TGA, as shown in Figure S1. The percentages of TGA products for Fe2CoSe4@NC and Fe2NiSe4@NC were measured as 44.68% and 41.44%, respectively. According to the XRD (Figure S2), the TGA products of Fe2CoSe4@NC and Fe2NiSe4@NC were identified to be (CoFe2)O4 and (NiFe2)O4, respectively. Based on the stoichiometry of the chemical reaction Fe2CoSe4@NC (s) + 3 O2 (g) = (CoFe2)O4 (s) + 4 SeO2 (g), the content of Fe2CoSe4 is calculated to be approximately 92.6 wt%, suggesting a carbon content of about 7.3 wt%. Similarly, the carbon content of Fe2NiSe4@NC is calculated to be about 14.0 wt%.
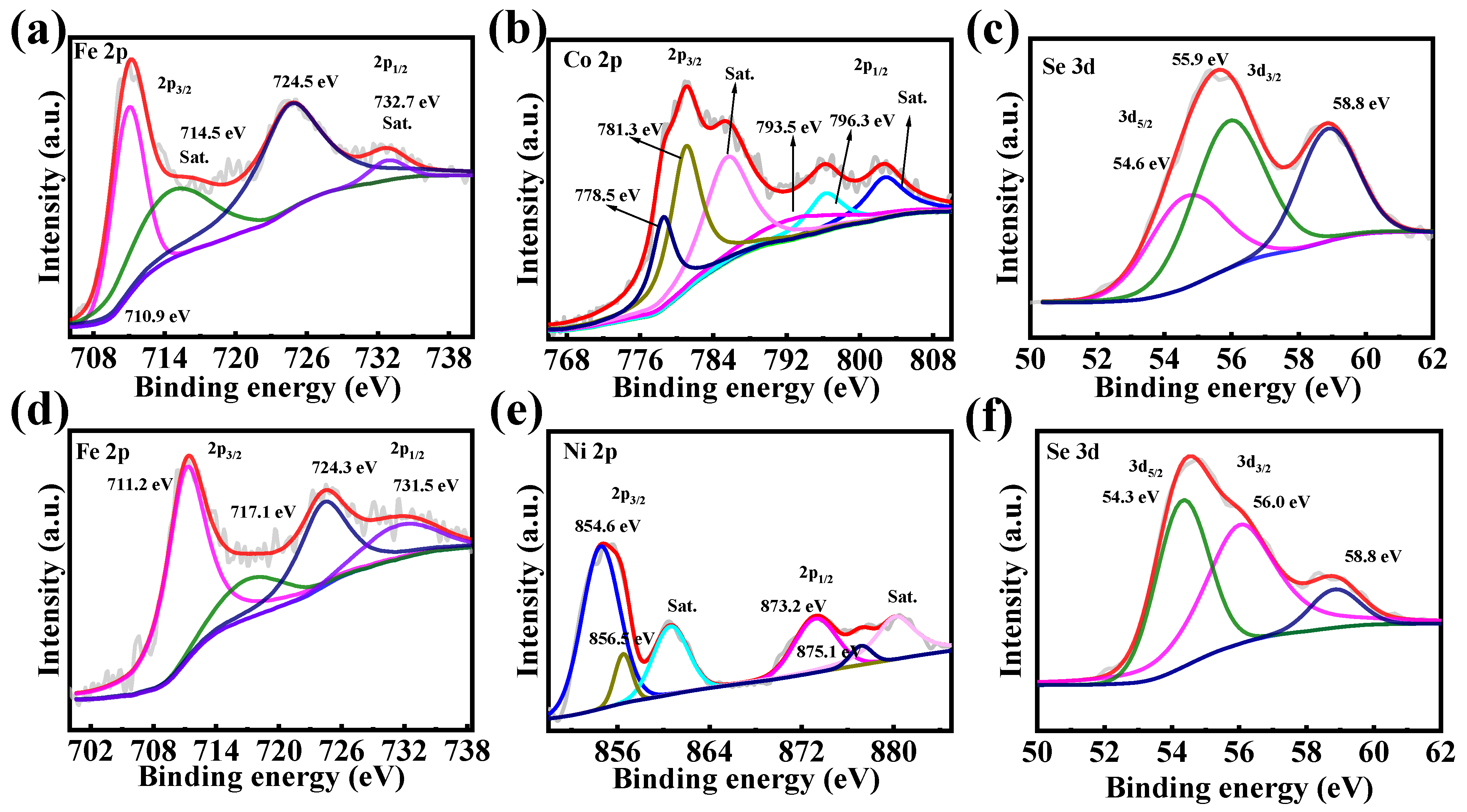
The chemical composition and valence states of elements in Fe2CoSe4@NC and Fe2NiSe4@NC composites were analyzed using X-ray Photoelectron Spectroscopy (XPS). Figure S3 presents the full survey spectra of Fe2CoSe4@NC and Fe2NiSe4@NC, revealing the presence of Fe, Co, Se, C, O, and N elements in Fe2CoSe4@NC, and Fe, Ni, Se, C, O, and N elements in Fe2NiSe4@NC, respectively. The high-resolution Fe 2p spectra of Fe2CoSe4@NC (Figure 3a) and Fe2NiSe4@NC (Figure 3d) exhibit two peaks at 710.9 and 724.5 eV, corresponding to the Fe2+ 2p3/2 and 2p1/2 orbitals [28], respectively, with two satellite peaks (marked with “Sat.”) [29,30]. The Co 2p of Fe2CoSe4@NC (Figure 3b) exhibits peaks at 780.3 and 796.3 eV, which are attributed to the Co2+ 2p3/2 and 2p1/2 orbitals, accompanied with two satellite peaks at 785.9 and 802.8 eV, respectively [31,32,33,34,35]. As depicted in Figure 3e, the high-resolution XPS spectrum of Ni2+ 2p comprises a pair of spin-orbit doublets at 854.6 and 873.2 eV, as well as two satellite peaks [36,37].
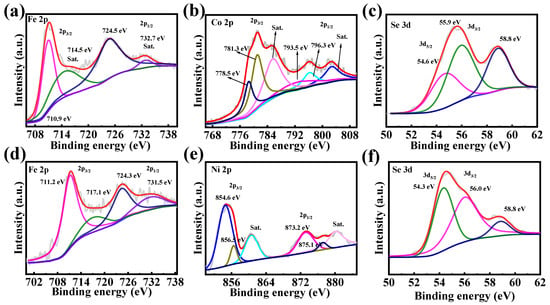
Figure 3.
High-resolution XPS spectra of (a) Fe 2p, (b) Co 2p, and (c) Se 3d for Fe2CoSe4@NC, and (d) Fe 2p, (e) Co 2p, and (f) Se 3d for Fe2NiSe4@NC.
The spectra of Se 3d (Figure 3c,f) for Fe2CoSe4@NC and Fe2NiSe4@NC are well fitted, with two peaks corresponding to Se 3d3/2 and 3d5/2. The two peaks at 54.6 eV and 55.9 eV corresponds to Se 3d5/2 and Se 3d3/2, respectively. An additional peak located at 58.8 eV may be attributed to SeOx species [38], consistent with previous reports. The XPS analysis above evidently indicate that the Fe2CoSe4@NC sample contains Fe2+, Co2+, and Se2−, while Fe2NiSe4@NC comprises species of Fe2+, Ni2+, and Se2−, consistent with the literature [39,40,41].
The Raman bands at 1350 and 1576 cm–1 are indexed to amorphous carbon (D band) and graphitic carbon (G band) [42]. The ID/IG values for Fe2CoSe4@NC and Fe2NiSe4@NC were 0.97 and 0.94, respectively (Figure S4), indicating higher graphitization of Fe2CoSe4@NC than Fe2NiSe4@NC [43].
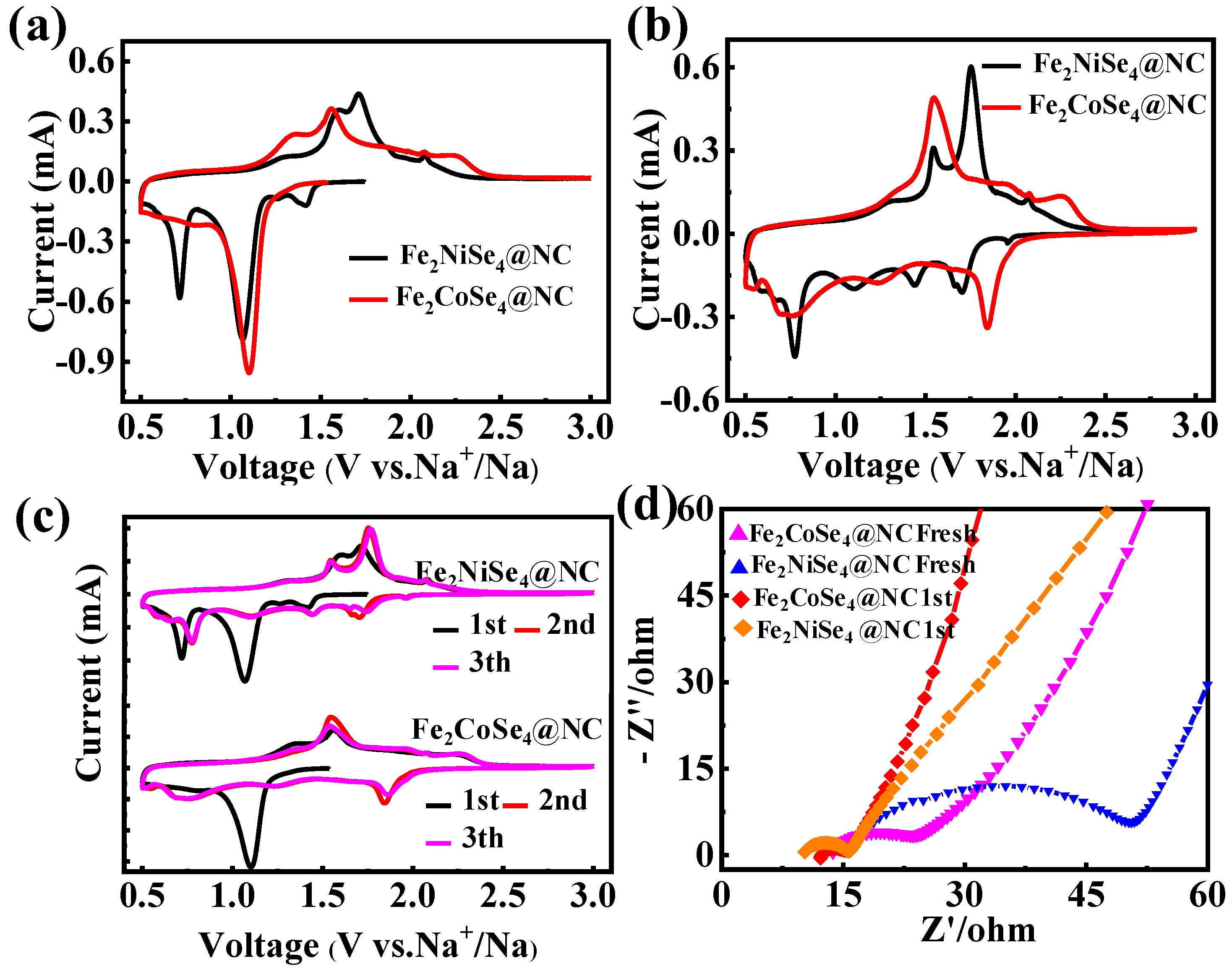
The storage properties of the sodium-ion of the Fe2CoSe4@NC and Fe2NiSe4@NC anodes were assessed through cyclic voltammetry (CV) in a half-cell configuration, wherein CR2032-type cells were assembled with Na foil serving as a counter electrode. Figure 4a illustrates the first CV cycle of Fe2CoSe4@NC and Fe2NiSe4@NC as an anode of SIBs within the voltage range of 0.5~3.0 V, which was conducted at a scan rate of 0.2 mV s−1. The significant peak near 1.10 V in the cathodic scans for the Fe2CoSe4@NC electrode, which was not observed in the subsequent scan, and can be mainly attributed to electrolyte decomposition and the formation of a solid electrolyte interphase (SEI), as well as the conversion of Fe2CoSe4@NC to Na2Se and NaxFe2CoSe4−y, as described in Equations (1)–(3) [35,44].
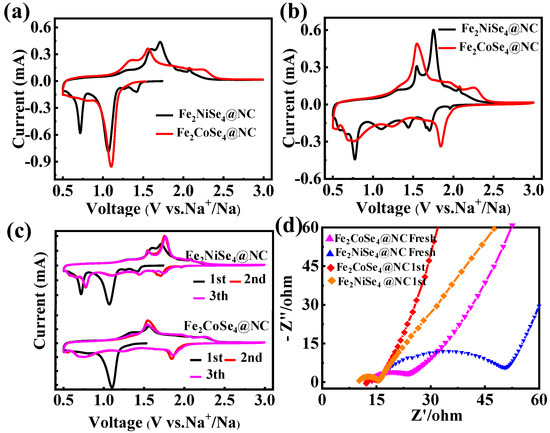
Figure 4.
Na-storage properties of the Fe2CoSe4@NC and Fe2NiSe4@NC electrodes as anodes: (a) the first and (b) second cycle of CV curves for Fe2CoSe4@NC and Fe2NiSe4@NC at 0.2 mV s−1 within 0.5~3.0 V; (c) the first three cycles of the CV curves for Fe2CoSe4@NC and Fe2NiSe4@NC; and (d) the EIS spectra of Fe2CoSe4@NC and Fe2NiSe4@NC electrodes before and after cycling at 0.1 A g−1.
- Discharge procedure of Fe2CoSe4@NC:Fe2CoSe4 + xNa+ + xe− → NaxFe2CoSe4NaxFe2CoSe4 + 2yNa+ + 2ye− → NaxFe2CoSe4−y + yNa2SeNaxFe2CoSe4−y + (8 − 2y − x)Na+ + (8 − 2y − x) e−
→ 2Fe + Co + (4 − y) Na2Se
- Discharge procedure of Fe2NiSe4@NC:Fe2 NiSe4 + xNa+ + xe− → NaxFe2NiSe4NaxFe2NiSe4 + (2 − x)Na+ + (2 − x)e− → 2FeSe + NiSe + Na2Se2FeSe + 4Na+ + 4e− → 2Fe + 2Na2SeNiSe + 2Na+ + 2e− → Ni + Na2Se
In contrast to that of Fe2CoSe4@NC, Fe2NiSe4@NC exhibits two prominent cathodic peaks at 1.07 V and 0.71 V in the first cycle, respectively, which can be attributed to electrolyte decomposition, the formation of a solid electrolyte interphase (SEI), and the conversion of Fe2NiSe4@NC to NaxSe, FexSe, and NixSe, as well as the generation of Ni (NiSe + 2Na+ + 2e− → Na2Se + Ni) (Equations (4)–(7)) [26,45]. Meanwhile, the additional cathodic peak for Fe2NiSe4@NC might indicate that the surface reaction and formation of SEI film on Fe2NiSe4@NC is more complicated than that of the Fe2CoSe4@NC electrode.
In the anodic scan of the first cycle for the Fe2CoSe4@NC electrode, a peak and shoulder were observed at 1.350 V and 1.562 V, respectively, which are indexed to the conversion reaction of Fe2CoSe4 + 8Na+ + 8e− → 4Na2Se + 2Fe + Co. The anodic peak potential for Fe2CoSe4@NC, which emerges at 1.71 and 1.60 V, is less positive than that of Fe2NiSe4@NC, suggesting a higher oxidation potential for the reaction of Fe2NiSe4 + 8Na+ + 8e− → 4Na2Se + 2Fe + Ni.
In the second cycle of the CV scan, Fe2CoSe4@NC (Figure 4b) exhibits three cathodic peaks at 1.84, 1.24, and 0.75 eV, respectively, corresponding to sodiation reactions [8]. It should be noticed that the cathodic peak intensity at 1.10 V in the first scan was much decreased in the second scan, due to the stable formation SEI film in the first scan. In the second cycle of Fe2NiSe4@NC (Figure 4b), four distinct cathodic peaks are observed at 1.70, 1.43, 1.01, and 0.77 eV, respectively, corresponding to the naturalization reactions (4)–(7), respectively [28,45]. The cathodic peak at 0.77 eV remains very intense, indicating more complicated reactions involved in the discharging process of the Fe2NiSe4@NC electrode than the Fe2CoSe4@NC electrode. The second and third CV scans (Figure 4c) overlap very well with each other for both the Fe2CoSe4@NC and Fe2NiSe4@NC electrodes, suggesting a reversible and stable cycling performance.
To further investigate the interfacial charge transfer kinetics of the Fe2CoSe4@NC and Fe2NiSe4@NC electrodes, electrochemical impedance spectroscopy (EIS) was conducted within a frequency range of 0.1 Hz to 100 kHz. As depicted in Figure 4d, the Nyquist plots of both fresh electrodes exhibit a semicircle in the high-frequency region and a slanted line in the low-frequency region, corresponding to charge transfer resistance (Rct) at the electrode–electrolyte interface and Na+ diffusion process in the electrode, respectively. Fresh Fe2CoSe4@NC exhibits a significantly smaller Rct state (10.56 Ω) than that of fresh Fe2NiSe4@NC (35.25 Ω) due to the higher percentage of the smaller pore size and more complete electrolyte penetration for Fe2CoSe4@NC than the Fe2NiSe4@NC electrode, as revealed using a BET measurement (Figure 1c,d) [46]. After the first cycle, the Rct values of Fe2CoSe4@NC and Fe2NiSe4@NC decreased to 4.63 and 6.52 Ω, respectively, and a much larger variation of the Rct values for Fe2NiSe4@NC was observed, indicating a more robust structure of Fe2CoSe4@NC than Fe2NiSe4@NC. This observation is in agreement with the results obtained from CV scans. On the other hand, the slope of the slanted line observed in the low-frequency region represents the Warburg impedance (Zw) associated with the diffusion of Na+ [47,48]. It is apparently observed that the slope of Fe2CoSe4@NC is much higher than that of Fe2NiSe4@NC after the first charge/discharge cycle. This indicates a faster Na+ diffusion rate in the former electrode than the latter one, and also that the crystalline structure of Fe2CoSe4@NC is more conductive for Na+ diffusion than Fe2NiSe4@NC, which is probably due to the larger interplanar distance of Fe2CoSe4@NC than Fe2NiSe4@NC, as observed in HRTEM.
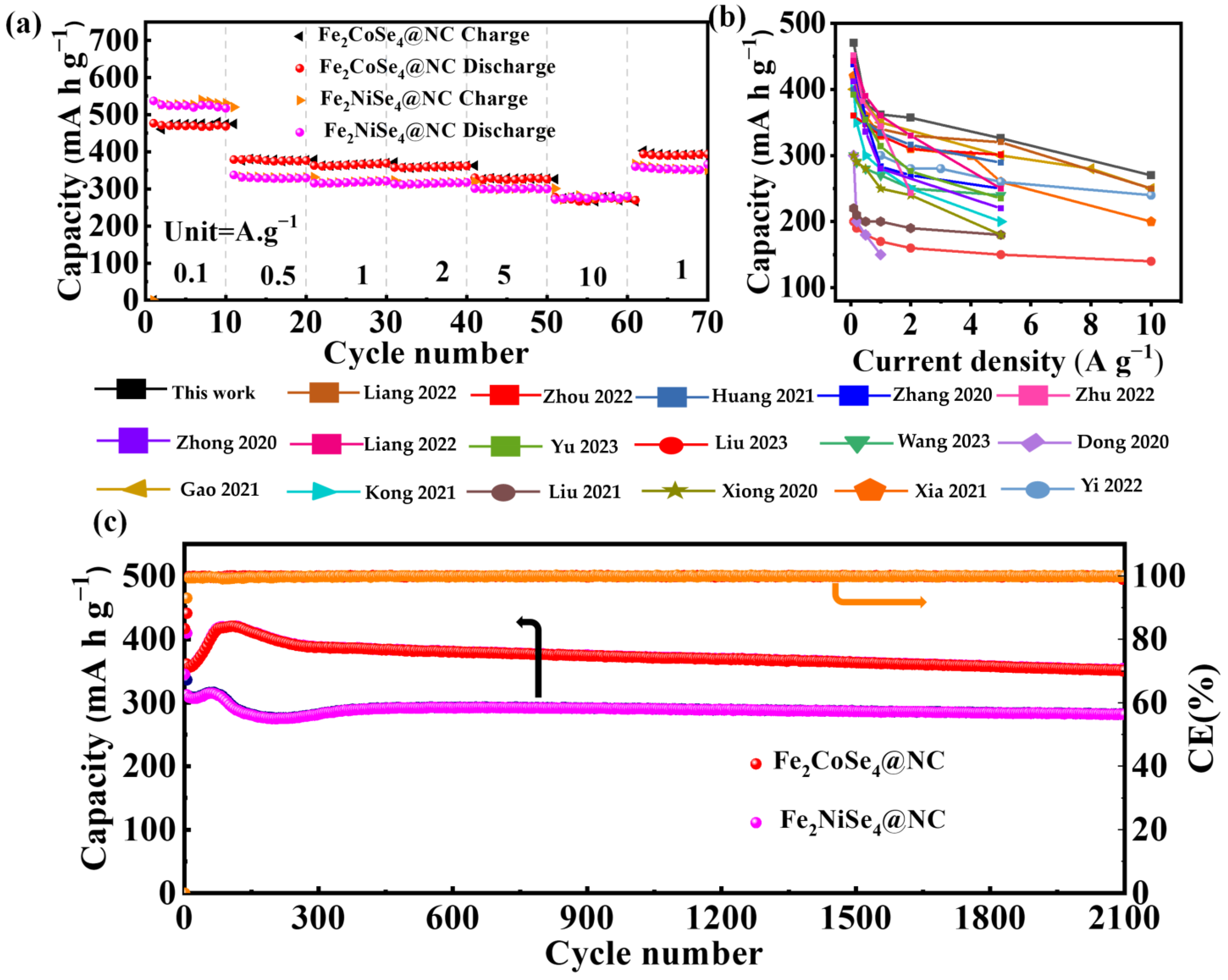
The rate capability of Fe2CoSe4@NC and Fe2NiSe4@NC was evaluated by varying the current densities from 0.1 to 10 A g−1, as depicted in Figure 5a. Fe2CoSe4@NC demonstrates a reversible specific capacity of 470, 378, 362, 357, 326, and 270 mAh g−1 at a current density of 0.1, 0.5, 1, 2, 5, and 10 A g−1, respectively. Similarly, Fe2NiSe4@NC exhibits rate capability, delivering 530, 330, 318, 315, 300, and 270 mAh g−1-specific capacities at the current densities of 0.1, 0.5, 1.0, 2, 5, and 10, respectively. It is observed that Fe2CoSe4@NC exhibits a lower reduction in capacity than Fe2NiSe4@NC with an increase in current density, possibly due to the more robust structure of the former than the latter, which surpasses that of most single transition metal selenides reported in the literature, as illustrated in Figure 5b and Table S1.
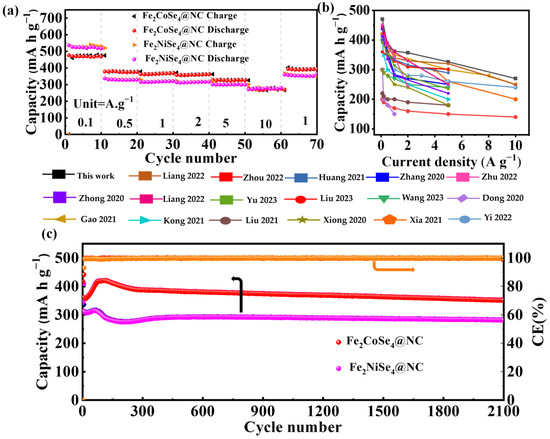
Figure 5.
Electrochemical performance of Fe2CoSe4@NC and Fe2NiSe4@NC. (a) Rate performances at current densities from 0.1 to 10 A g−1 and (c) cycling performances at a current density of 1 A g−1 of Fe2CoSe4@NC and Fe2NiSe4@NC.The Coulombic efficiency (CE, orange line) is closing to 100%, as shown in the right axis and directed by the orange arrow. (b) Rate capability of Fe2CoSe4@NC and those of reported single transition metal selenide electrodes, as cited in supporting information [29,42,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63].
As illustrated in Figure 5c, the Fe2CoSe4@NC electrode delivers a specific discharge capacity of 352 mA h g−1 after 2100 cycles at 1.0 A g−1, much higher than that of Fe2NiSe4@NC (282.2 mA h g−1). Fe2CoSe4@NC and Fe2NiSe4@NC maintain capacities of 380 and 340 mA h g−1 (Figure S5) after 1500 cycles with a current density of 2 A g−1 (Figure S5). At a current density of 4 A g−1, the capacities of the Fe2CoSe4@NC and Fe2NiSe4@NC electrodes retain 370 and 310 mA h g−1 after 900 cycles, respectively (Figure S6). This indicates that the Fe2CoSe4@NC electrode possesses a higher energy density and cycle stability than Fe2NiSe4@NC.
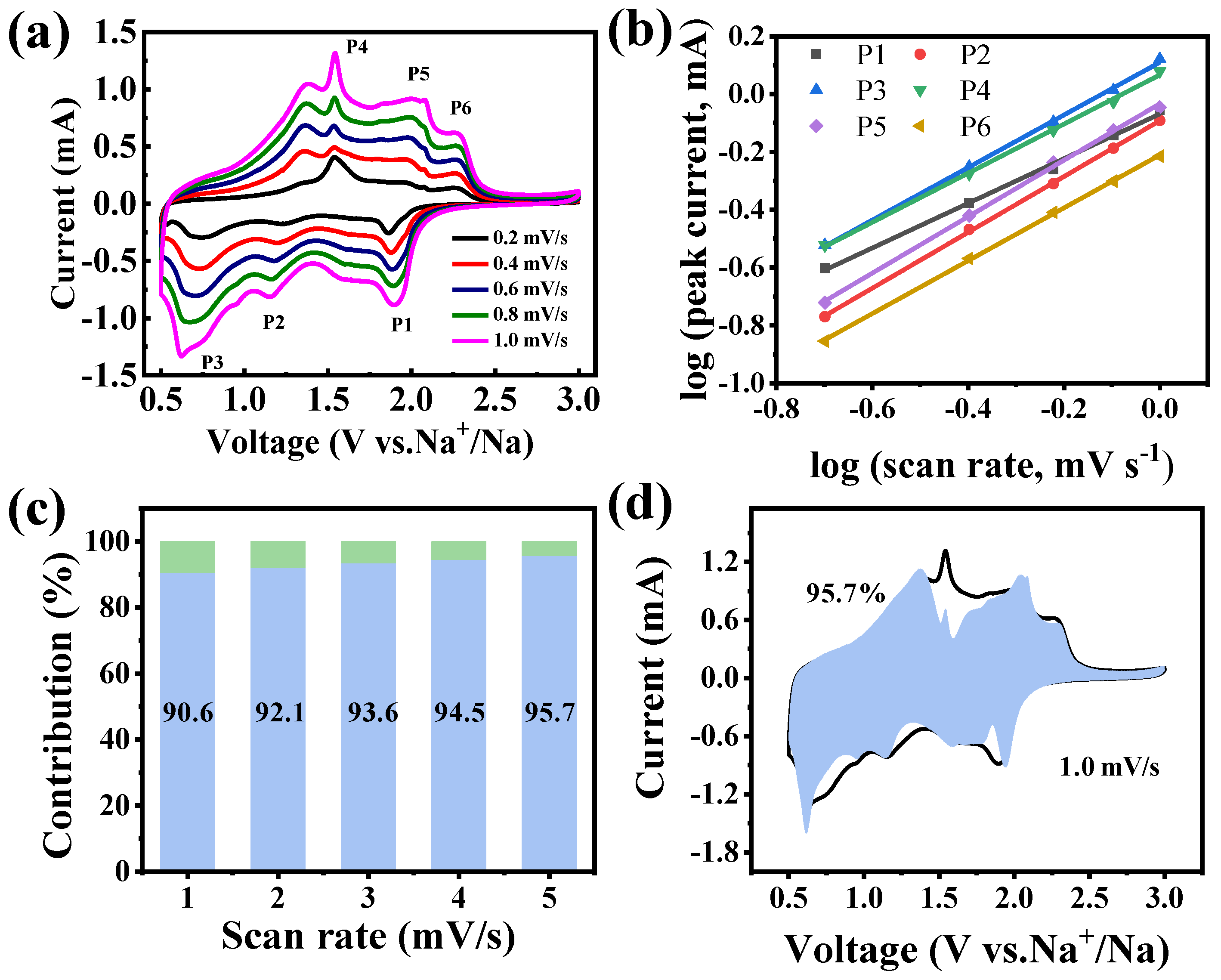
To further elucidate the exceptional rate performance of the Fe2CoSe4@NC electrode, CV scans at sweep rates from 0.2 to 1.0 mV s−1 were conducted to differentiate pseudocapacitive and diffusion-controlled contributions to energy storage capacity. As shown in Figure 6a, the CV profiles reveal two distinct pairs of cathodic and anodic peaks. The current response to the scan rate follows the relationship described below (Formulas (8) and (9)):
where a and b represent adjustable parameters. At b = 0.5, diffusion-controlled behavior prevails during the charge/discharge process, whereas when b = 1, the pseudocapacitive effect dominates. In the current study, the b values of the three reduction peaks (peaks 1, 2, and 3) and the corresponding oxidation peaks (peaks 4, 5, and 6) were determined to be 0.77, 0.97, 0.91, 0.85, 0.97, and 0.91, respectively, by log(i) versus log(v) plots (Figure 6b). These values are all between 0.6 to 1.0, indicating that the electrochemical reactions of the Fe2CoSe4@NC electrode are dominated by both diffusion-controlled and pseudocapacitive behaviors at a fixed voltage, which can be calculated following Equation (10):
where k1×v and k2×v1/2 represent the pseudocapacitive capacity and diffusion-controlled capacity, respectively. As summarized in Figure 6c, the pseudocapacitive contribution was estimated to be 90.6%, 92.1%, 93.6%, 94.5%, and 95.7% at the scan rates of 0.2, 0.4, 0.6, 0.8, and 1.0, respectively, showing a continuous increase in pseudocapacitive contribution to energy storage of Fe2CoSe4@NC with increasing scan rate. As expected, the pseudocapacitive contribution dominates the charge-storage capacity at higher scan rates, which is beneficial for fast Na+ transfer kinetics during the intercalation/extraction process [8]. Additionally, Figure 6d further illustrates the detailed pseudocapacitive portion (blue region) in comparison with the total current measured at a scan rate of 1.0 mV s−1.
i = avb
log(i) = b log(v) + log (a)
i(V) = k1 × v + k2 × v1/2
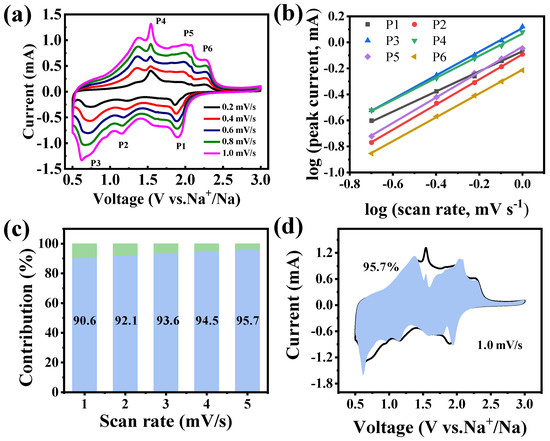
Figure 6.
(a) CV curves at scan rates from 0.2 to 1 mV s−1; (b) plots of log(v) versus log(i); (c) capacitive (light blue bars) and diffusion-controlled (light green bars) contribution at different scan rates; and (d) capacitive contribution (light blue shadow) at 1 mV s−1 for the Fe2CoSe4@NC electrode.
3. Materials and Methods
3.1. Chemicals
Iron (III) nitrate (Fe(NO3)3·9H2O), cobalt nitrate hexa-hydrate (Co(NO3)3·6H2O) and nickel nitrate hexa-hydrate (Ni(NO3)3·6H2O) were purchased from Damao Chemical Reagents Factory (Tianjin, China). N, N-dimethylformamide (DMF), selenium powder, trimesic acid, polyvinylpyrrolidone (PVP), and ethanol were purchased from Energy Chemical Co (Beijing, China). H2/Ar (10 vol% H2) were obtained from Guangzhou Messer Gas (Guangzhou, China). Deionized (DI) water was obtained from a Barnstead Nanopure water purification system (18.3 MΩ∙cm, Thermo Fisher Scietific, MA, USA). All chemicals were used without further purification.
3.2. Synthesis of FeCo-MOFs and FeNi-MOFs
In a typical process, Fe(NO3)3·9H2O (4.5 mmol) and Co(NO3)3·6H2O, (1.5 mmol) were added to a mixed solution of ethanol, DMF, and DI water (20 mL, 20 mL, and 20 mL) under vigorous stirring for about 10 min. Simultaneously, 1.5 mmol of trimesic acid and 3 g of PVP were dissolved in the same mixed solution under vigorous stirring for about 30 min. The resulting solution was then transferred into a 100 mL Teflon-lined autoclave, heated to 150 °C, and maintained for 10 h. The product was cooled to room temperature and washed three times with ethanol and DMF to obtain a metal organic framework containing iron and cobalt metal centers (denoted as FeCo-MOFs). FeNi-MOFs was also prepared using a similar protocol, with the molar ratio of iron nitrate Fe(NO3)3·9H2O to Ni(NO3)3·6H2O being 1 mmol:1 mmol. All other conditions remained the same.
3.3. Synthesis of Fe2CoSe4@NC and Fe2NiSe4@NC
The FeCo-MOFs and FeNi-MOFs precursors were well ground with selenium powder (in weight ratio 1:2), placed in a silica-glazed ceramic boat, and covered with Cu foil. Selenization was carried out at 300 °C for 4 h under H2/Ar (10 vol% H2), with a ramping rate of 2 °C min−1. The temperature was heated to, and maintained at, 700 °C for 2 h for the carbonization of MOFs. Finally, the obtained samples are denoted as Fe2CoSe4@NC and Fe2NiSe4@NC.
3.4. Materials Characterization
X-ray diffraction (XRD) was performed on a Bruker D8 Advance (Billerica, MA, USA) using Cu-Kα radiation (λ = 1.5406Å). Thermal gravimetric analysis (TGA) was conducted on Mettler Toledo TGA/SDTA851 (Zurich, Switzerland) in an O2 atmosphere at a heating rate of 5 °C min−1. Nitrogen adsorption–desorption isotherms were acquired at 77 K with an Autosorb-iQ automatic volumetric instrument (Anton Paar, Graz, Austria). X-ray photoelectron spectroscopy (XPS) was acquired with a Phi X-tool instrument (Kanagawa, Japan). The microstructure and morphology of the samples were examined using field-emission scanning electron microscopy (SEM, Hitachi S-4800, Tokyo, Japan) and high-resolution transmission electron microscopy (HRTEM, (JEOL, JEM-2010), Tokyo, Japan).
3.5. Coin Cell Assembly
A slurry-coating procedure was adopted for the preparation of the working electrode as follows. Firstly, a homogeneous slurry was prepared by mixing Fe2CoSe4@NC and Fe2NiSe4@NC, carbon black (Super P, Timcal, Bironico, Switzerland), and sodium alginate binder at a mass ratio of 8:1:1, evenly pasted onto a copper foil by applying a film applicator, and was dried at 70 ºC in an electric oven overnight in order to remove the solvent. The mass loading of Fe2CoSe4@NC and Fe2NiSe4@NC was about 1.2–1.5 mg·cm−2 on each electrode.
The coin cells of CR2032-type were built in an argon-filled glove box (Vigor-LG2400/750TS, LTD, Suzhou, China), in which the oxygen and water contents were less than 1 ppm. SIBs were assembled with a sodium tablet and glass fiber as counterpart electrode and separator, respectively. A Celgard-2400 film was used as a separator. The recipe of the commercial electrolyte is 1.0 M NaCF3SO3 in diglyme.
3.6. Electrochemical Measurements
The electrochemical properties of Fe2CoSe4@NC and Fe2NiSe4@NC as anodes of SIBs was tested using CR2032 coin cells. The galvanostatic discharge/charge (GCD) measurements were tested on a battery analysis system (CT2001A, LAND). Cyclic voltammetry (CV) tested at a scan rate of 0.2 mV s−1, and electrochemical impedance spectra (EIS), conducted with the frequency range of 100 kHz to 0.01 Hz, were acquired with the CHI660 electrochemical workstation (Shanghai CH Instrument Co., Ltd., Shanghai, China).
4. Conclusions
In summary, the binary-metal selenides Fe2CoSe4@NC and Fe2NiSe4@NC were successfully synthesized with the preparation of a Fe/Co and Fe/Ni binary-metal organic framework using hydrothermal methods and sequential selenization binary Fe/Co and Fe/Ni MOF. The formation of Fe2CoSe4@NC and Fe2NiSe4@NC was evidenced using XRD, XPS, and HRTEM. Fe2CoSe4@NC demonstrates higher long-term cycling stability and rate performance than Fe2NiSe4@NC, maintaining capacities of 352 and 282.2 mAh g−1 after 2100 cycles at 1.0 A g−1, respectively. Such a higher electrochemical performance of Fe2CoSe4@NC than Fe2NiSe4@NC was ascribed to the higher portion of micropores and a higher diffusion rate of sodium-ions among the electrode composite. This study presents a novel method for synthesizing binary-metal selenides towards high-performance sodium-ion storage.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/inorganics12060165/s1, Figure S1: TGA patterns of (a) Fe2CoSe4@NC and (c) Fe2NiSe4@NC products of combustion in air; Figure S2: XRD spectra of (a) Fe2CoSe@NC and (b) Fe2NiSe4@NC products of combustion in air; Figure S3: Full XPS of (a) Fe2CoSe4@NC and (b) Fe2NiSe4@NC; Figure S4: Raman of Fe2CoSe4@NC and Fe2NiSe4@NC; Figure S5: Cycling performances of Fe2CoSe4@NC and Fe2NiSe4@NC at a current density of 2 A g−1; Figure S6: Cycling performances of Fe2CoSe4@NC and Fe2NiSe4@NC at a current density of 4 A g−1; Table S1: Electrochemical rate performances of already reported metal selenide anodes.
Author Contributions
Conceptualization, X.H., K.M. and H.X.; methodology, X.H.; validation, H.X., S.Z., and Z.H.; formal analysis, H.X., W.Z.; investigation, X. H, W.Z. and K.M.; resources, S.Z.. and C.W.; data curation, X. H, K.M., Z.H.; writing—original draft preparation, X. H, and H.X.; writing—review and editing, K.M. and X.K.; supervision, X.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Guangdong Province (No. 2024A1515010030).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Hangxuan Xie, Wei Zhang, Chao Wang, Shangcheng Zhao, and Zhentao Hao are employed by China Southern Power Grid Technology Co., Ltd.. The remaining authors of the paper declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Zhao, Y.; Kang, Y.; Wozny, J.; Lu, J.; Du, H.; Li, C.; Li, T.; Kang, F.; Tavajohi, N.; Li, B. Recycling of sodium-ion batteries. Nature Rev. Mater. 2023, 8, 623–634. [Google Scholar] [CrossRef]
- Jia, M.; Jin, Y.; Zhao, P.; Zhao, C.; Jia, M.; Wang, L.; He, X. Hollow NiCoSe2 microspheres@N-doped carbon as high-performance pseudocapacitive anode materials for sodium ion batteries. Electrochim. Acta 2019, 310, 230–239. [Google Scholar] [CrossRef]
- Su, Q.; Cao, X.; Yu, T.; Kong, X.; Wang, Y.; Chen, J.; Lin, J.; Xie, X.; Liang, S.; Pan, A. Binding MoSe2 with dual protection carbon for high-performance sodium storage. J. Mater. Chem. A 2019, 7, 22871–22878. [Google Scholar] [CrossRef]
- Wang, B.; Chen, K.; Wang, G.; Liu, X.; Wang, H.; Bai, J. A multidimensional and hierarchical carbon-confined cobalt phosphide nanocomposite as an advanced anode for lithium and sodium storage. Nanoscale 2019, 11, 968–985. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Xu, F.; Xu, J.; Fang, J.; Sun, Z. Rambutan-pitaya-like structured TiO2@Co-CNT-NC nanocomposite as high-performance lithium ion battery anodes. Ceram. Int. 2019, 45, 22131–22137. [Google Scholar] [CrossRef]
- Yin, X.; Zhao, Y.; Zhang, J. Research Progress and Performance Improvement Strategies of Hard Carbon Anode Materials for Sodium-Ion Batteries. J. Electrochem. 2023, 29, 2204301. [Google Scholar] [CrossRef]
- Chen, Y.C.; Yang, M.L.; Yang, L.T.; Chen, Z.W.; Li, H.Y.; Woo, H.J.; Chi, S.S.; Xiao, Y.G.; Wang, J.; Wang, C.Y.; et al. Alkali and alkaline ions co-substitution of P2 sodium layered oxides for sodium ion batteries. Chin. J. Struct. Chem. 2023, 42, 100028. [Google Scholar] [CrossRef]
- Ali, Z.; Asif, M.; Huang, X.; Tang, T.; Hou, Y. Hierarchically Porous Fe2CoSe4 Binary-Metal Selenide for Extraordinary Rate Performance and Durable Anode of Sodium-Ion Batteries. Adv. Mater. 2018, 30, 1802745. [Google Scholar] [CrossRef]
- Leibing, C.; Leistenschneider, D.; Neumann, C.; Oschatz, M.; Turchanin, A.; Balducci, A. Glyoxylic-Acetal-Based Electrolytes for Sodium-Ion Batteries and Sodium-Ion Capacitors. ChemSusChem 2023, 16, e202300161. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Yang, M.; Chen, W. High-safety separators for lithium-ion batteries and sodium-ion batteries: Advances and perspective. Energy Storage Mater. 2021, 41, 522–545. [Google Scholar] [CrossRef]
- Miao, K.; Jiang, W.; Chen, Z.; Luo, Y.; Xiang, D.; Wang, C.; Kang, X. Hollow-Structured and Polyhedron-Shaped High Entropy Oxide toward Highly Active and Robust Oxygen Evolution Reaction in a Full pH Range. Adv. Mater. 2024, 36, 2308490. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chang, Z.; Wu, Z.; Feng, Y.; Du, X.; Che, M.; Tian, J.; Xie, W.; Zhang, K. Nano-Confined Electrolyte for Sustainable Sodium-Ion Batteries. Adv. Funct. Mater. 2024, 34, 2314288. [Google Scholar] [CrossRef]
- Zhu, W.; Mao, Q.; Jia, Y.; Ni, J.; Gao, L. Dual-Carbon-Decorated Na3V2(PO4)3 Material for Sodium-Ion Batteries. J. Electron. Mater. 2023, 52, 836–846. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.; Sun, X.; Zhang, B.; He, S.; Li, L.; Wang, C. Preventing structural degradation from Na3V2(PO4)3 to V2(PO4)3: F-doped Na3V2(PO4)3/C cathode composite with stable lifetime for sodium ion batteries. J. Power Sources 2018, 378, 423–432. [Google Scholar] [CrossRef]
- Sam Oh, J.A.; Sun, Q.; Tian, C.; Song, X.; Chua, B.; Zeng, K.; Lu, L. Aerosol-deposited freestanding Na3V2(PO4)3 thin-film microbattery. Mater. Today Energy 2022, 27, 101006. [Google Scholar] [CrossRef]
- Du, J.; Zou, Z.; Liu, C.; Xu, C. Hierarchical Fe-doped Ni3Se4 ultrathin nanosheets as an efficient electrocatalyst for oxygen evolution reaction. Nanoscale 2018, 10, 5163–5170. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Li, Y.; Hu, C.; Huang, Z.; Wang, B.; Wang, Y.; Liu, Q.; Ye, F. A high-rate cathode material based on potassium-doped Na3V2(PO4)3 for high/low-temperature sodium-ion batteries. Mater. Today Chem. 2023, 30, 101506. [Google Scholar] [CrossRef]
- Liu, J.; Xie, J.; Dong, H.; Wei, H.; Sun, C.; Yang, J.; Geng, H. Iron doping of NiSe2 nanosheets to accelerate reaction kinetics in sodium-ion half/full batteries. Sci. China Mater. 2023, 66, 69–78. [Google Scholar] [CrossRef]
- Niu, F.; Yang, J.; Wang, N.; Zhang, D.; Fan, W.; Yang, J.; Qian, Y. MoSe2-Covered N,P-Doped Carbon Nanosheets as a Long-Life and High-Rate Anode Material for Sodium-Ion Batteries. Adv. Funct. Mater. 2017, 27, 1700522. [Google Scholar] [CrossRef]
- Hussain, I.; Sahoo, S.; Lamiel, C.; Nguyen, T.T.; Ahmed, M.; Xi, C.; Iqbal, S.; Ali, A.; Abbas, N.; Javed, M.S.; et al. Research progress and future aspects: Metal selenides as effective electrodes. Energy Storage Mater. 2022, 47, 13–43. [Google Scholar] [CrossRef]
- Fang, S.; Bresser, D.; Passerini, S. Transition Metal Oxide Anodes for Electrochemical Energy Storage in Lithium- and Sodium-Ion Batteries. Adv. Energy Mater. 2020, 10, 1902485. [Google Scholar] [CrossRef]
- Wu, H.; Yuan, R.; Li, M.; Liu, L.; Liu, Y.; Song, Q.; Ai, W.; Du, H.; Du, Z.; Wang, K. Co0.85Se–Fe7Se8 nanocuboids embedded in reduced graphene oxides as cycle-stable anodes for sodium-ion batteries. Carbon 2022, 198, 171–178. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, S.; Chen, Y.; Wei, Y.; Zhang, M.; Li, C.; Sun, Y.; Zhang, S.; Jiang, Y. Epitaxial growth of Cu2-xSe on Cu (220) crystal plane as high property anode for sodium storage. Chin. Chem. Lett. 2023, 35, 108922. [Google Scholar] [CrossRef]
- Wang, S.; Xu, B.; Huo, W.; Feng, H.; Zhou, X.; Fang, F.; Xie, Z.; Shang, J.K.; Jiang, J. Efficient FeCoNiCuPd thin-film electrocatalyst for alkaline oxygen and hydrogen evolution reactions. Appl. Catal. B Environ. 2022, 313, 121472. [Google Scholar] [CrossRef]
- Ali, Z.; Asif, M.; Zhang, T.; Huang, X.; Hou, Y. General Approach to Produce Nanostructured Binary Transition Metal Selenides as High-Performance Sodium Ion Battery Anodes. Small 2019, 15, 1901995. [Google Scholar] [CrossRef]
- Huang, X.; Men, S.; Zheng, H.; Qin, D.-D.; Kang, X. Highly Porous NiCoSe4 Microspheres as High-Performance Anode Materials for Sodium-Ion Batteries. Chem. -Asian J. 2020, 15, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wang, L.; Qin, D.C.; Xu, Z.B.; Jin, M.Q.; Chen, Y.; Zeng, X.X.; Dai, Z.H. Constructing Heterostructured Bimetallic Selenides on an N-Doped Carbon Nanoframework as Anodes for Ultrastable Na-Ion Batteries. ACS Appl. Mater. Interf. 2022, 14, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Men, S.; Lin, J.; Zhou, Y.; Kang, X. N-doped porous carbon wrapped FeSe2 nanoframework prepared by spray drying: A potential large-scale production technique for high-performance anode materials of sodium ion batteries. J. Power Sources 2021, 485, 229310. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, X.; Zhang, L.; Wang, Q.; Yang, J.; Geng, H. Structural engineering of bimetallic selenides for high-energy density sodium-ion half/full batteries. Dalton Trans. 2022, 51, 16898–16905. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, H.; Sun, J.; Qin, F.; Yu, F.; Bao, J.; Yu, Y.; Chen, S.; Ren, Z. Cu nanowires shelled with NiFe layered double hydroxide nanosheets as bifunctional electrocatalysts for overall water splitting. Energy Environ. Sci. 2017, 10, 1820–1827. [Google Scholar] [CrossRef]
- Lin, J.J.; Zhou, Y.; Wen, J.B.; Si, W.J.; Gao, H.C.; Wang, G.M.; Kang, X.W. Pyrrole derivatives as interlayer modifier of Li-S batteries: Modulation of electrochemical performance by molecular perturbation. J. Energy Chem. 2022, 75, 164–172. [Google Scholar] [CrossRef]
- Wan, R.; Luo, M.; Wen, J.; Liu, S.; Kang, X.; Tian, Y. Pt-Co single atom alloy catalysts: Accelerated water dissociation and hydrogen evolution by strain regulation. J. Energy Chem. 2022, 69, 44–53. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, Y.; Xiang, D.; Qin, J.; Miao, K.; Wang, X.; Kang, X.; Tian, Y. Yolk-Shell Structured Zinc-Cobalt-Ruthenium Alloy Oxide Assembled with Ultra-Small Nanoparticles: A Superior Cascade Catalyst toward Oxygen Evolution Reaction. Adv. Funct. Mater. 2023, 33, 2214529. [Google Scholar] [CrossRef]
- Muralee Gopi, C.V.V.; Reddy, A.E.; Kim, H.-J. Wearable superhigh energy density supercapacitors using a hierarchical ternary metal selenide composite of CoNiSe2 microspheres decorated with CoFe2Se4 nanorods. J. Mater. Chem. A 2018, 6, 7439–7448. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Z.; Wang, X.; Zheng, B.; Zhang, W.; Chen, Y. Scalable synthesis of porous hollow CoSe2–MoSe2/carbon microspheres for highly efficient hydrogen evolution reaction in acidic and alkaline media. J. Mater. Chem. A 2018, 6, 12701–12707. [Google Scholar] [CrossRef]
- Park, J.-S.; Chan Kang, Y. Multicomponent (Mo, Ni) metal sulfide and selenide microspheres with empty nanovoids as anode materials for Na-ion batteries. J. Mater. Chem. A 2017, 5, 8616–8623. [Google Scholar] [CrossRef]
- He, Y.; Luo, M.; Dong, C.; Ding, X.; Yin, C.; Nie, A.; Chen, Y.; Qian, Y.; Xu, L. Coral-like NixCo1−xSe2 for Na-ion battery with ultralong cycle life and ultrahigh rate capability. J. Mater. Chem. A 2019, 7, 3933–3940. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Fan, H.; Hou, B.-H.; Rui, X.-H.; Ning, Q.-L.; Cui, Z.; Guo, J.-Z.; Yang, Y.; Wu, X.-L. Ni1.5CoSe5 nanocubes embedded in 3D dual N-doped carbon network as advanced anode material in sodium-ion full cells with superior low-temperature and high-power properties. J. Mater. Chem. A 2018, 6, 22966–22975. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Lv, L.; Tian, Y.; Li, Z.; Ao, X.; Lan, Y.; Jiang, J.; Wang, C. Rational Design of Cobalt–Iron Selenides for Highly Efficient Electrochemical Water Oxidation. ACS Appl. Mater. Interf. 2017, 9, 33833–33840. [Google Scholar] [CrossRef]
- Xiao, J.; Wan, L.; Yang, S.; Xiao, F.; Wang, S. Design Hierarchical Electrodes with Highly Conductive NiCo2S4 Nanotube Arrays Grown on Carbon Fiber Paper for High-Performance Pseudocapacitors. Nano Lett. 2014, 14, 831–838. [Google Scholar] [CrossRef]
- Shen, L.; Wang, J.; Xu, G.; Li, H.; Dou, H.; Zhang, X. NiCo2S4 Nanosheets Grown on Nitrogen-Doped Carbon Foams as an Advanced Electrode for Supercapacitors. Adv. Energy Mater. 2015, 5, 1400977. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Y.; Liu, X.; Wang, X.; Liu, P. Core–Shell Co, Zn Bimetallic Selenide Embedded Nitrogen-Doped Carbon Polyhedral Frameworks Assist in Sodium-Ion Battery Ultralong Cycle. ACS Sustain. Chem. Eng. 2020, 8, 8381–8390. [Google Scholar] [CrossRef]
- Altaf, S.; Ajaz, H.; Imran, M.; Ul-Hamid, A.; Naz, M.; Aqeel, M.; Shahzadi, A.; Shahbaz, A.; Ikram, M. Synthesis and characterization of binary selenides of transition metals to investigate its photocatalytic, antimicrobial and anticancer efficacy. Appl. Nanosci. 2020, 10, 2113–2127. [Google Scholar] [CrossRef]
- Yu, W.; Kong, Y.; Lin, J.; Zheng, H.; Kang, X. Flower-like Spherical α-Ni(OH)2 Derived NiP2 as Superior Anode Material of Sodium-Ion Batteries. Chem. –Asian J. 2021, 16, 2100–2106. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Qiu, L.; Bao, J.; Zhou, Y. Hexagonal FeNi2Se4@C Nanoflakes as High Performance Anode Materials for Sodium-ion Batteries. Chem. Res. 2021, 37, 318–322. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Lu, Y.; Yu, P.; Du, W.; Ma, B.; Xie, M.; Yang, H.; Cheng, T. Multi-Scale Simulation Revealing the Decomposition Mechanism of Electrolyte on Lithium Metal Electrode. J. Electrochem. 2022, 28, 2105181. [Google Scholar] [CrossRef]
- Choi, J.H.; Park, S.-K.; Kang, Y.C. A Salt-Templated Strategy toward Hollow Iron Selenides-Graphitic Carbon Composite Microspheres with Interconnected Multicavities as High-Performance Anode Materials for Sodium-Ion Batteries. Small 2019, 15, 1803043. [Google Scholar] [CrossRef]
- Hu, X.; Liu, X.; Chen, K.; Wang, G.; Wang, H. Core–shell MOF-derived N-doped yolk–shell carbon nanocages homogenously filled with ZnSe and CoSe2 nanodots as excellent anode materials for lithium- and sodium-ion batteries. J. Mater. Chem. A 2019, 7, 11016–11037. [Google Scholar] [CrossRef]
- Liang, Z.; Li, Q.; Zhang, W.; Yu, D.; Zhang, W.; Wu, J.; Wang, G.; Fan, W.; Wang, J.; Huang, S. Pomegranate-inspired porous SnSe/ZnSe@C anode: A stress-buffer nanostructure for fast and ultrastable sodium-ion storage. J. Energy Chem. 2022, 75, 369–377. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, M.; Wang, L.; Huang, Q.; Su, Z.; Xu, P.; Zou, R.; Wang, X.; Zeng, C.; Ba, K. MOFs-Derived Flower-like Hierarchically Porous Zn-Mn-Se/C Composite for Extraordinary Rate Performance and Durable Anode of Sodium-Ion and Potassium-Ion Batteries. Small 2022, 18, 2203964. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, S.; Ying, H.; Zhang, Z.; Han, W. Few-layered Ti3C2 MXene anchoring bimetallic selenide NiCo2Se4 nanoparticles for superior Sodium-ion batteries. Chem. Eng. J. 2021, 417, 129161. [Google Scholar] [CrossRef]
- Zhong, W.; Ma, Q.; Tang, W.; Wu, Y.; Gao, W.; Yang, Q.; Yang, J.; Xu, M. Construction of a bimetallic nickel–cobalt selenide pompon used as a superior anode material for high performance sodium storage. Inorg. Chem. Front. 2020, 7, 1003–1011. [Google Scholar] [CrossRef]
- Liang, H.; Li, X.; Liu, Z.; Yang, W.; Liu, X.; Zhang, Y.; Fan, H. In situ etching strategy to construct yolk–shell CoSe2@NiCoSe4-NC heterostructures for high-performance sodium ion battery. Mater. Chem. Front. 2022, 6, 194–202. [Google Scholar] [CrossRef]
- Yu, D.; Liang, H.; Zhao, G.; Zhang, H.; Wei, X.; Zhao, D.; Yu, M.; Sun, Y. Bimetallic selenide nanocages covered by carbon layer deliver high rate performance for sodium ion storage. Mater. Today Energy 2023, 35, 101319. [Google Scholar] [CrossRef]
- Liu, J.; Leng, Z.; Dong, H.; Xu, X.; Lv, C.; Wei, H.; Yu, L.; Yang, J.; Geng, H. In situ interface engineering of NiSe with interlinked conductive networks for high energy density sodium-ion half/full batteries. Inorg. Chem. Front. 2023, 10, 4076–4086. [Google Scholar] [CrossRef]
- Wang, S.; Zou, R.; Liu, Q.; Chen, H. Bimetallic selenide Cu4Mo6Se8 nanosheet arrays grown on a carbon skeleton via MOF-derived with enhanced electrochemical kinetics for high-performance sodium-ion batteries. J. Mater. Chem. A 2023, 11, 8710–8718. [Google Scholar] [CrossRef]
- Dong, C.; Wu, L.; He, Y.; Zhou, Y.; Sun, X.; Du, W.; Sun, X.; Xu, L.; Jiang, F. Willow-Leaf-Like ZnSe@N-Doped Carbon Nanoarchitecture as a Stable and High-Performance Anode Material for Sodium-Ion and Potassium-Ion Batteries. Small 2020, 16, 2004580. [Google Scholar] [CrossRef]
- Gao, X.; Kuai, Y.; Xu, Z.; Cao, Y.; Wang, N.; Hirano, S.-i.; Nuli, Y.; Wang, J.; Yang, J. SnSe2/FeSe2 Nanocubes Capsulated in Nitrogen-Doped Carbon Realizing Stable Sodium-Ion Storage at Ultrahigh Rate. Small Methods 2021, 5, 2100437. [Google Scholar] [CrossRef]
- Kong, F.; Han, Z.; Tao, S.; Qian, B. Core–shell structured SnSe@C microrod for Na-ion battery anode. J. Energy Chem. 2021, 55, 256–264. [Google Scholar] [CrossRef]
- Liu, B.; Cao, J.; Li, J.; Li, L.; Chen, D.; Zhang, S.; Cai, D.; Han, W. Highly conductive Co3Se4 embedded in N-doped 3D interconnected carbonaceous network for enhanced lithium and sodium storage. J. Colloid Interface Sci. 2021, 586, 630–639. [Google Scholar] [CrossRef]
- Xiong, Z.; Sun, D.; Jia, X.; Zhou, J. Core/shell FeSe/carbon nanosheet-assembled microflowers with ultrahigh coulombic-efficiency and rate performance as nonpresodiate anode for sodium-ion battery. Carbon 2020, 166, 339–349. [Google Scholar] [CrossRef]
- Xia, L.; Yang, Z.; Tang, B.; Li, F.; Wei, J.; Zhou, Z. Carbon Nanofibers with Embedded Sb2Se3 Nanoparticles as Highly Reversible Anodes for Na-Ion Batteries. Small 2021, 17, 2006016. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Du, X.; Zhao, Z.; Liu, Y.; Guan, H.; Liu, X.; Pei, X.; Zhang, S.; Li, D. Coupling of Metallic VSe2 and Conductive Polypyrrole for Boosted Sodium-Ion Storage by Reinforced Conductivity Within and Outside. ACS Nano 2022, 16, 7772–7782. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).