Abstract
UV-activated catalytic hydrosilation is a low-temperature crosslinking process that has attracted attention for its high efficiency and lower energy demand relative to thermal curing. In this study, formulations comprising industrially relevant model silanes and Pt photocatalysts trimethyl(methylcyclopentadienyl)platinum(IV) and trimethyl(pentamethylcyclopentadienyl)platinum(IV) (MeCpPtMe3 and Cp*PtMe3, respectively) were prepared with and without a photosensitizer (PS) and assessed for catalytic performance by a novel strategy. Photopolymerizations were initiated using different wavelengths from LEDs and monitored in real-time using an Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) “well” strategy to track the degree of cure in ultra-thin films by consumption of hydride via the disappearance of the Si-H bending absorption band at 915 cm−1. Irradiation of formulations with 365 nm excitation showed higher conversions relative to 400 nm light and improvements to calculated initial reaction rates by incorporation of a PS suggested increased sensitization to 365 nm irradiation. To the best of our knowledge, this is the first study to report catalytic performance, electronic absorption spectroscopic data, and the crystal structure of Cp*PtMe3.
1. Introduction
Silicone materials possess an impressive range of advantageous properties, including electrical insulation, chemical stability, weatherability, and tolerance to extreme temperatures, owing in part to the unique chemistry of the characteristic Si-O bond [1,2,3]. These physiologically inert materials have medical and food processing applications due to their low toxicity and are widely used in electrical, construction, aerospace, automotive, and release coatings industries [1,2,3,4]. The curing of silicone polymers can be accomplished by a high-temperature reaction involving the addition of a silane across an olefin known as hydrosilation. This thermopolymerization reaction is generally regarded as a time- and energy-intensive process, particularly in the production of release coatings otherwise known as siliconized paper. In this case, the manufacturing process is heated and runs continuously [5]. Karstedt’s catalyst and simple hydrido-/vinyl-functional silicone fluids (with inhibitor) are coated on paper and processed at high speeds using conveyance equipment with a long processing hot section/oven to achieve sufficient residence time for complete cure on paper in a continuous line process [6]. Photopolymerization has emerged as an attractive alternative in recent decades for hydrosilation. Photocuring can occur at room temperature, may continue after a period of irradiation in the absence of light (i.e., dark curing), and requires the use of a transition metal (e.g., Pt, Rh, Co, Ru, Pd, Ni, Cu) or lanthanide catalyst, of which platinum complexes are regarded as the most active photoinitiators [7]. However, high dosages of Pt are currently required for this method to obtain acceptable production line speed and release characteristics of release coatings on paper substrate. Continued improvements to syntheses of new catalysts and ligand scaffolds are necessary to bring photo cure systems on a cost-competitive scale with respect to release coatings prepared via thermal cure. The innovation potential of hydrosilation catalysts has not been fully explored, yet the field of hydrosilation is considered to be among the largest-scale applications of homogenous catalysis [8].
The incorporation of certain photosensitizers (PS), including naphthalene and a variety of other polycyclic aromatic hydrocarbons, has been shown to accelerate conversion and reduce the amount of Pt catalyst required for photocuring [9]. Efficient energy transfer from naphthalene to a trimethyl(methylcyclopentadienyl)platinum(IV) (MeCpPtMe3) photocatalyst resulted in complete crosslinking of vinyl-terminated polydimethylsiloxane (PDMS-Vi) with trimethylsilyl-terminated polymethylhydrosiloxane (PMHS), in contrast to the 70% conversion achieved without the PS [9]. In our paper, the kinetics of photo-activated hydrosilation reactions were monitored using Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) spectroscopy using a novel strategy to evaluate and benchmark platinum photocatalysts as we embark on exploring the innovation potential of new hydrosilation catalysts under investigation in our lab that feature aryl-functionalized light-harvesting Cp ligands [10]. This strategy allowed for the analysis of reaction kinetics using an interesting unobscured Si-H bending mode at 915 cm−1, which offered an acceptable signal-to-noise ratio, easy setup, and an efficient process that enabled consecutive experiments with small sample volumes. The impact of different Pt catalysts, wavelengths of irradiation, and the presence of PS on the rate of conversion was investigated through dark curing hydrosilation after the initial LED excitation. Finally, electronic absorption spectroscopic data and the X-ray crystal structure of trimethyl(pentamethylcyclopentadienyl)platinum(IV) (Cp*PtMe3) are reported.
2. Results and Discussion
2.1. Kinetics of Polymerization
Photopolymerization of bis(trimethylsiloxy)methylsilane (MDHM) and vinyl-terminated polydimethylsiloxane (DMS-V05) was studied in real-time by ATR-FTIR, although alternative methods [9,10,11,12,13,14,15,16,17,18,19] were evaluated in our lab based on their suitability for monitoring this system. Transmission FTIR spectroscopy was considered due to the high signal-to-noise ratio of the Si-H stretch of commercially relevant silicone fluids but was suboptimal in that it entailed lengthy preparation and cleaning processes between runs and the Si-H bending mode peak was obscured by overlapping peaks. In situ ReactIR™ (Mettler Toledo, Columbus, OH, USA) FTIR spectroscopy allowed for easier irradiation of samples but was not compatible with other commercial mixtures such as SilForce™ 6100/6020 due to gelation/solidification on the probe and obscured the Si-H stretching absorption band because of absorptions from the composite diamond optical window and noise from the HgCdTe detector in this region. In addition, ReactIR™ as a strategy consumes a much greater volume of silicone fluids since the probe tip needs to be submerged in a vessel containing the sample. The novel ATR strategy reported herein allowed for the analysis of reaction kinetics in significantly thinner films and unobscured monitoring of the Si-H bending mode referenced to an unchanging Si-C stretching mode that is present in both silicone fluids. Our strategy offered an acceptable signal-to-noise ratio, easy setup, and a more efficient process that enabled consecutive experiments. As described later in detail (Section 3.3), an exceedingly shallow sample “well” encircling the ATR crystal was constructed where 10 μL (total volume) formulations were dispensed to monitor hydrosilation of single-digit micron film thickness. This approach is industrially relevant in the context of ultra-thin release coatings and is a different strategy as compared to following reaction kinetics of thicker films (≥100 μm) by transmission mode FTIR on KBr plates [9].
The disappearance of the Si-H bending absorption band at 915 cm−1 of MDHM was monitored [10] to track the consumption of hydride due to diamond absorption (spectral artifacts) in the wavelength region occupied by the Si-H stretching peak and was referenced to the unchanging Si-C stretching vibration at 1260 cm−1 (see Figure 1). Percent conversion as a function of time was calculated using the following equation [20]:
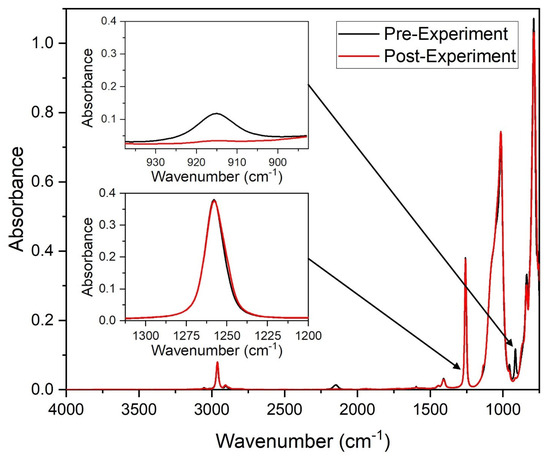
Figure 1.
Example IR spectra pre- and post-experiment showing the unchanging Si-C stretching absorption band (1260 cm−1) and decreasing Si-H bending absorption band (915 cm−1) proportional to hydride consumption.
The terms A0 and At refer to the area under the Si-H bending absorption band before irradiation and at time t, respectively. Similarly, Ar0 and Art are terms associated with the area under the internal reference Si-C stretching vibration peak.
In Figure 2, percent conversion curves as a function of dark cure hydrosilation reaction time are reported for experiments in which catalyst type and irradiation wavelength were varied. Error bars represent the standard deviation of the % conversion values of replicate experiments at each time t. Hydrosilation reactions between MDHM and DMS-V05 proceeded in darkness (post-irradiation) and in the presence of oxygen to indicate that an ‘addition cure’ mechanism occurred [21]. Irradiation of formulations containing catalysts with 365 nm light appeared to have a more productive effect on conversion relative to 400 nm light, as near-complete conversion was observed for experiments using 365 nm LED irradiation. Overall conversion for formulations containing the MeCpPtMe3 catalyst improved significantly when irradiated with 365 nm light as opposed to 400 nm light, and an increase from 34.7% to 97.0% was observed. To the best of our knowledge, this is the first study in which data pertaining to the catalytic performance of Cp*PtMe3 have ever been reported.
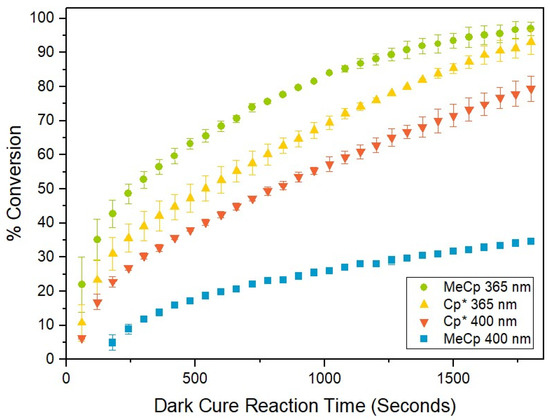
Figure 2.
Plot of percent conversion as a function of dark cure reaction time for experiments in which catalyst type and irradiation wavelength were varied.
The results of experiments using MDHM/DMS-V05 formulations and MeCpPtMe3 catalyst that also incorporated naphthalene in a 50:1 ratio of PS to Pt catalyst by mass are displayed in Figure 3. The data show that photopolymerization proceeded more efficiently than in the absence of PS. For all experiments, plots of ln[SiH] vs. t are linear (an example is shown in Figure S1 of the Supplementary Information), suggesting that these hydrosilation reactions obey first-order reaction kinetics [9]. An exponential regression was applied to plots of [SiH] versus t (examples in the Supplementary Information, Figures S2 and S3), which allowed for the calculation of kinetic parameters such as initial reaction rate (Ratei), rate constant (k), and half-life time (t1/2). With reference to Table 1, the computed Ratei and k values are greater for formulations that include PS, indicating an initial acceleration of conversion and quicker overall reaction rate, even when irradiation time was reduced by 50% (150 s). Experiments performed by Xi and coworkers that involved the MeCpPtMe3 catalyst, but utilized different strategies and formulations, determined that incorporation of naphthalene lowers the activation energy required for hydrosilation to occur [9].
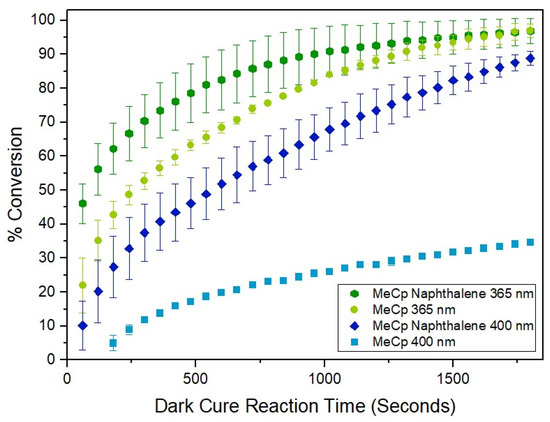
Figure 3.
Plot of percent conversion as a function of dark cure reaction time for experiments in which naphthalene was incorporated into formulations containing the trimethyl(methylcyclopentadienyl)platinum(IV) (MeCpPtMe3) catalyst and irradiated with 365 and 400 nm light.

Table 1.
Kinetic data for hydrosilation reactions catalyzed by MeCpPtMe3, including initial reaction rate (Ratei), rate constant (k), half-life time (t1/2), and final % conversion.
Similar results were obtained when naphthalene was incorporated into MDHM/DMS-V05 formulations containing the catalyst Cp*PtMe3 in a 50:1 ratio of PS to catalyst. Improvements in Ratei, t1/2, k, and final % conversion are shown in Table 2. Förster resonance energy transfer (FRET), known to occur primarily within the UV and visible regions (200–800 nm) [22], is hypothesized to be responsible for the observed phenomena [23]. For FRET to occur, the intermolecular distance between a properly oriented catalyst and PS molecules must be between 1 and 10 nm [24,25]. As discussed below, Pt catalysts are sensitized to 365 nm by naphthalene, which facilitates photo-activation and more efficient hydrosilation catalysis.

Table 2.
Kinetic data for hydrosilation reactions catalyzed by trimethyl(pentamethylcyclopentadienyl)platinum(IV) (Cp*PtMe3), including initial reaction rate (Ratei), half-life time (t1/2), rate constant (k), and final % conversion.
UV-vis spectroscopic data for the Cp*PtMe3 catalyst and naphthalene in cyclohexane is included in Figure 4, along with the results of an X-ray diffraction study that resulted in the crystal structure of Cp*PtMe3. The catalyst’s absorption spectrum features a high energy band at 261 nm (ε = 13,400 M−1cm−1) and a weak shoulder at approximately 294 nm, which are associated with the Cp* → Pt and σ bond (PtCH3) → Pt charge transfer transitions, respectively [14]. An increase in molar absorptivity was observed for the sample solution containing both Cp*PtMe3 and naphthalene (Cp*PtMe3/naphthalene, ε = 18,700 M−1cm−1). This finding is consistent with MeCpPtMe3 data obtained by Xi and coworkers (MeCpPtMe3 in 1,3-dioxolane: ε = 10,400 M−1cm−1, MeCpPtMe3/naphthalene in 1,3-dioxolane: ε = 13,400 M−1cm−1), who determined that quantum efficiency at 365 nm was increased by naphthalene sensitization through calculations that involve these values [9]. Considering the striking similarities in spectra, structures (see next section), and electronic properties between MeCpPtMe3 and its permethylated derivative, including enhanced photo-activation by naphthalene, suggests a quantum efficiency improvement at 365 nm for the Cp*PtMe3/naphthalene system as well. Electronic absorption spectra of Cp*PtMe3 and naphthalene in any neat (or combined) silicone fluid(s) were not obtained in the present study, nor was cyclohexane used in the study of polymerization kinetics.
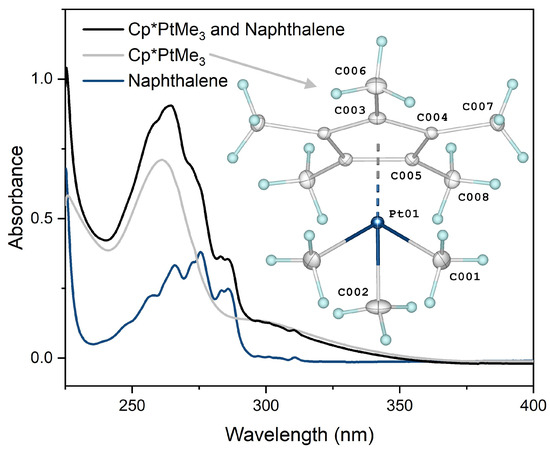
Figure 4.
UV-visible absorption spectra of Cp*PtMe3 and naphthalene (4.84 × 10−5 M, 7.09 × 10−5 M), Cp*PtMe3 (5.32 × 10−5 M), and naphthalene (7.09 × 10−5 M) in cyclohexane, and an ORTEP of the crystal structure of Cp*PtMe3.
2.2. X-ray Crystallography
Despite the commercial availability of Cp*PtMe3 as an organometallic catalyst and precursor for chemical deposition processes, a crystal structure has never been reported. We determined the molecular structure of Cp*PtMe3 and displayed an ORTEP alongside an electronic absorption spectrum in Figure 4. CCDC 2352755 contains the supplementary crystallographic data that can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, accessed on 18 July 2024 (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk). A concise summary of crystal data for C13H24Pt (M = 375.41 g/mol) is as follows: orthorhombic, space group Pnma (no. 62), a = 9.7206(5) Å, b = 11.8506(6) Å, c = 11.6699(5) Å, V = 1344.31(11) Å3, Z = 4, T = 100.00(10) K, μ(MoKα) = 10.404 mm−1, Dcalc = 1.855 g/cm3, 8322 reflections measured (4.898° ≤ 2Θ ≤ 58.79°), and 1768 unique (Rint = 0.0386, Rσ = 0.0326), which were used in all calculations. The final R1 was 0.0183 (I > 2σ(I)) and wR2 was 0.0416 (all data).
A complete geometrical analysis of Cp*PtMe3 was performed using a Mogul geometry check [26] examining all fragment types, e.g., bond length, valence angle, torsion angle, and ring. After searching the Cambridge Structural Database [27], no unusual bond lengths or angles were identified. The known compounds Cp*PtMe2Br (Refcode: JETHEV) [28], Cp*PtMe2Cl (Refcode: SOWTED) [29], Cp*PtMe2(S-pTol) (Refcode: VAQFUO) [30], and MeCpPtMe3 (Refcode: KEFMEN) [31] were identified from the search as structurally very similar to Cp*PtMe3 (Figure 4). A further search of the database for any “trimethyl-Cp*-transition-metal” fragment identified only nine (9) mononuclear hits [32,33,34,35,36,37,38,39], the closest being tetramethyl(pentamethylcyclopentadienyl)molybdenum(V) (Refcode: ZELPIP) [32], tetramethyl(pentamethylcyclopentadienyl)tungsten(V) (ZELPOV) [32], and the tetramethyl(ethyltetramethylcyclopentadienyl)tungsten(VI) cation (TENMII) [33]; all other hits feature additional non-CH3 ligands as part of the transition metal’s primary coordination sphere. We note in the structure of Cp*PtMe3 that four (4) atoms of the total nine (9) non-hydrogen atoms in the asymmetric unit are sitting on an element of symmetry (a mirror plane), including Pt, C2, C3, and C6. In addition, a glide plane passes between C4 and C5 of the pentamethylcyclopentadienyl ring carbons. A high-resolution ORTEP of Cp*PtMe3 is provided in Figure S4 of the Supplementary Information.
3. Materials and Methods
3.1. Materials
The following materials were purchased from Gelest (Morrisville, PA, USA) and used as received: bis(trimethylsiloxy)methylsilane (MDHM, Mw 222.51 g/mol) and vinyl-terminated polydimethylsiloxane (DMS-V05, Mw 800 g/mol). Further, the catalysts MeCpPtMe3 and Cp*PtMe3 were purchased from Strem Chemicals (Newburyport, MA, USA) (see Figure 5). Naphthalene was purchased from Sigma-Aldrich (St. Louis, MO, USA).
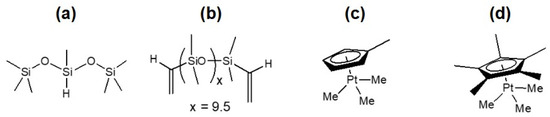
Figure 5.
Structures of (a) bis(trimethylsiloxy)methylsilane (MDHM), (b) vinyl-terminated polydimethylsiloxane (DMS-V05), (c) MeCpPtMe3, and (d) Cp*PtMe3.
3.2. Sample Preparation
MDHM and DMS-V05 were combined to produce a formulation with a nearly equimolar ratio that is slightly hydride-rich (~1.02–1.03:1 Si-H/Si-Vi moieties). Naphthalene was dissolved through stirring and gentle heating in the absence of Pt catalysts; vortexing was employed to dissolve the Pt catalysts in the silicone fluids. The dosage of Pt was held constant across all formulations at approximately 550 ppm. Naphthalene was added in a 50:1 ratio of PS to Pt catalyst, which has been reported to be an optimal ratio under different experimental conditions [9].
The following volumetric UV-vis solutions were prepared in darkness: (1) 4.84 × 10−5 M Cp*PtMe3 and 7.09 × 10−5 M naphthalene in cyclohexane; (2) 5.32 × 10−5 M Cp*PtMe3 in cyclohexane; and (3) 7.09 × 10−5 M naphthalene in cyclohexane.
3.3. Characterization
The kinetics of the photohydrosilation reactions were monitored by ATR-FTIR spectroscopy on a Spectrum 3 FTIR-NIR spectrometer (PerkinElmer, Waltham, MA, USA). A sample “well” encircling the ATR crystal was constructed using six layers of electrical tape (WarriorWrap Yellow Vinyl) (NSI Industries, Huntersville, NC, USA) and one layer of double-sided tape (3M Scotch, Saint Paul, MN, USA) with a hole punched through the tape layers. Formulations were dispensed into the well atop the ATR crystal (10 μL total volume) and a UV-transparent quartz microscope slide (Thermo Fisher Scientific, Waltham, MA, USA) was placed on top of the well to avoid evaporation. Kinetics of hydrosilation/polymerization of films were monitored in real-time by this technique (on the order of 2–3 μm thickness), initiated by an OmniCure LX500 UV LED spot-curing system controller in conjunction with either 365 or 400 nm UV LED spot-curing heads (Excelitas Technologies Corp., Waltham, MA, USA) to irradiate sample formulations for 300 s at a fixed distance of 2 cm in darkness. Platinum(II) β-diketonate complexes have been reported to catalytically hydrosilate a vinyl and hydride-functional siloxane mixture using LED (455 nm) irradiation [40]. However, these catalysts were not evaluated by our strategy due to (1) insolubility in our formulation without a cosolvent such as 1,2-dichloroethane to aid dissolution and (2) our desire to employ siloxane formulations that mirror commercially relevant, solventless release coating offerings. The surface of the samples experienced a light intensity of ~500 mW/cm2, which was measured using a PM100D module (ThorLabs, Newton, NJ, USA) interfaced with an S120VC sensor (ThorLabs, Newton, NJ, USA) at a fixed distance from the LEDs outfitted with a mounting clamp/heat sink (019-00087R) (Excelitas Technologies Corp., Waltham, MA, USA).
Electronic absorption spectra were collected using a U-2910 UV–Visible Spectrophotometer (Hitachi, Tokyo, Japan). Spectra of samples were measured in quartz cuvettes and spanned a wavelength range of 200–400 nm.
A suitable crystal of Cp*PtMe3 grown by sublimation inside the reagent bottle (dimensions 0.159 × 0.110 × 0.081 mm3) was selected, attached to a nylon loop, and mounted on a XtaLAB Synergy-S Dualflex diffractometer (Rigaku, Tokyo, Japan), equipped with a HyPix 6000-HE HPC detector (Rigaku, Tokyo, Japan) and a Cryostream 800 low-temperature cryostat (Oxford Cryosystems, Oxford, United Kingdom). For bond length and angle determinations, the crystal was kept at 100 K during data collection, which used MoKα radiation (λ = 0.71073 Å). Using Olex2-1.5 as a GUI [41], the structure was solved with the SHELXT structure solution program by intrinsic phasing [42] and refined with the SHELXL refinement package using least squares minimization [43].
4. Conclusions
In this paper, photo-activated hydrosilation reaction kinetics in ultra-thin films (single-digit micron) were monitored by ATR-FTIR spectroscopy using a novel “well” strategy to benchmark platinum photocatalysts for evaluation against new hydrosilation catalysts featuring light-harvesting aryl-functionalized Cp ligands that are under investigation. This strategy allowed for analysis of reaction kinetics using an interesting unobscured Si-H bending mode at 915 cm−1, offered an acceptable signal-to-noise ratio, easy setup, and an efficient process that enabled consecutive experiments. LED excitation at 365 nm of liquid formulations showed higher conversions to ultra-thin films relative to 400 nm light with both Pt catalysts. Kinetic studies showcased the sensitization behavior of naphthalene as a PS. Formulations that included PS showed an acceleration in initial conversion and a quicker overall reaction rate based on Ratei, t1/2, and k values (computed kinetic parameters). Catalytic performance, electronic absorption spectroscopic data, and the crystal structure of Cp*PtMe3 have never been previously reported. Cp*PtMe3 featured a high energy band at 261 nm (ε = 13,400 M−1cm−1) and a weak shoulder at approximately 294 nm in cyclohexane. Vapor-deposited Cp*PtMe3 crystallized in the orthorhombic space group Pnma with four (4) atoms sitting on an element of symmetry (a mirror plane). Interestingly, a glide plane of symmetry passes between C4 and C5 of the pentamethylcyclopentadienyl ring carbons.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/inorganics12070197/s1, Figure S1: Plot of ln[SiH] vs. time (post-irradiation) for a formulation comprising naphthalene and the MeCpPtMe3 catalyst that was irradiated with 365 nm light for 150 s; Figure S2: Plot of [SiH] vs. time (post-irradiation) for a formulation comprising naphthalene and the MeCpPtMe3 catalyst that was irradiated with 365 nm light for 150 s with an exponential trendline; Figure S3: Plot of [SiH] vs. time (post-irradiation) for a formulation comprising naphthalene and the MeCpPtMe3 catalyst that was irradiated with 365 nm light with an exponential trendline; Figure S4: ORTEP drawing of Cp*PtMe3.
Author Contributions
Conceptualization, M.M., J.L. and P.J.B.J.; Methodology, M.M., J.L., and P.J.B.J.; Analysis, M.M.; Resources, P.J.B.J.; Data Curation, M.M.; Crystallography, P.J.B.J.; Writing—Original Draft Preparation, M.M. and P.J.B.J.; Writing—Review and Editing, M.M., J.L. and P.J.B.J.; Supervision, P.J.B.J.; Funding Acquisition, P.J.B.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funds but rather was supported using discretionary funds from P.J.B.J. The NSF is gratefully acknowledged for supporting the acquisition of an X-ray diffractometer (Award Number 2117596) through the Major Research Instrumentation program.
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Material, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shit, S.C.; Shah, P. A Review on Silicone Rubber. Natl. Acad. Sci. Lett. 2013, 36, 355–365. [Google Scholar] [CrossRef]
- de Buyl, F. Silicone Sealants and Structural Adhesives. Int. J. Adhes. Adhes. 2001, 21, 411–422. [Google Scholar] [CrossRef]
- Morari, C.; Balan, I.; Pintea, J.; Chitanu, E.; Iordache, I. Electrical Conductivity and Electromagnetic Shielding Effectiveness of Silicone Rubber Filled with Ferrite and Graphite Powders. Prog. Electromagn. Res. M 2011, 21, 93–104. [Google Scholar] [CrossRef]
- Owen, M.J. Interfacial Activity of Polydimethylsiloxane. In Surfactants in Solution; Springer: Boston, MA, USA, 1986; pp. 1557–1569. [Google Scholar] [CrossRef]
- Ekeland, R.A.; Tonge, J.S.; Gordon, G.V. Release Force Understanding-Recent Findings; Dow Corning Corporation: Midland, MI, USA, 2005; Available online: https://www.semanticscholar.org/paper/Release-Force-Understanding-%E2%80%93-Recent-Findings-Ekeland-Tonge/ae177a6d1e45cd12e36271a555a46d31999d2f71 (accessed on 18 July 2024).
- Eckberg, R.P. Coatings Technology Handbook, 3rd ed.; Tracton, A.A., Ed.; CRC Press Taylor Fr. Group: New York, NY, USA, 2005; Chapter 92 Silicone Release Coatings. [Google Scholar] [CrossRef]
- Marciniec, B.; Guliński, J. Recent Advances in Catalytic Hydrosilylation. J. Organomet. Chem. 1993, 446, 15–23. [Google Scholar] [CrossRef]
- Tondreau, A.M.; Atienza, C.C.H.; Weller, K.J.; Nye, S.A.; Lewis, K.M.; Delis, J.G.P.; Chirik, P.J. Iron Catalysts for Selective Anti-Markovnikov Alkene Hydrosilylation Using Tertiary Silanes. Science 2012, 335, 567–570. [Google Scholar] [CrossRef]
- Xi, L.; Liu, Z.; Su, J.; Bei, Y.; Xiang, H.; Liu, X. UV-Activated Hydrosilylation of (Me-Cp)Pt(Me)3: Enhanced Photocatalytic Activity, Polymerization Kinetics, and Photolithography. J. Appl. Polym. Sci. 2019, 136, 48251. [Google Scholar] [CrossRef]
- Esteves, A.C.C.; Brokken-Zijp, J.; Laven, J.; Huinink, H.P.; Reuvers, N.J.W.; Van, M.P.; de With, G. Influence of Cross-Linker Concentration on the Cross-Linking of PDMS and the Network Structures Formed. Polymer 2009, 50, 3955–3966. [Google Scholar] [CrossRef]
- Yang, D.B. Kinetic Studies of Photopolymerization Using Real Time FT-IR Spectroscopy. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 199–208. [Google Scholar] [CrossRef]
- Mayer, T.; Burget, D.; Mignani, G.; Fouassier, J.P. Photohydrosilylation Reaction of Silicone Polymers. Platinum-Based Photocatalysts: Trimethyl(β-Dicarbonyl) Platinum IV Complexes. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 3141–3146. [Google Scholar] [CrossRef]
- Burget, D.; Mayer, T.; Mignani, G.; Fouassier, J.P. Kinetic Study of the Photoactivated Hydrosilylation of Some β-Dicarbonyl Complexes of Trialkylplatinum(IV). J. Photochem. Photobiol. A Chem. 1996, 97, 163–170. [Google Scholar] [CrossRef]
- Jakubek, V.; Lees, A.J. Quantitative Photochemistry of Cp‘Pt(CH3)3 (Cp‘ = η5-C5H4CH3) in Solution: A Highly Efficient Organometallic Photoinitiator for Hydrosilylation. Inorg. Chem. 2004, 43, 6869–6871. [Google Scholar] [CrossRef] [PubMed]
- Sangermano, M.; Marchi, S.; Meier, P.; Kornmann, X. UV-Activated Hydrosilation Reaction for Silicone Polymer Crosslinking. J. Appl. Polym. Sci. 2012, 128, 1521–1526. [Google Scholar] [CrossRef]
- Marchi, S.; Sangermano, M.; Meier, P.; Kornmann, X. A Comparison of the Reactivity of Two Platinum Catalysts for Silicone Polymer Cross-Linking by UV-Activated Hydrosilation Reaction. Macromol. React. Eng. 2015, 9, 360–365. [Google Scholar] [CrossRef]
- Marchi, S.; Sangermano, M.; Meier, P.; Kornmann, X. Visible Light-Activated Hydrosilation Reaction. J. Photochem. Photobiol. A Chem. 2015, 303–304, 86–90. [Google Scholar] [CrossRef]
- Marchi, S.; Sangermano, M.; Ligorio, D.; Meier, P.; Kornmann, X. Impressive Rate Raise of the Hydrosilation Reaction through UV-Activation: Energy and Time Saving. Macromol. Mater. Eng. 2016, 301, 610–613. [Google Scholar] [CrossRef]
- Hofmann, J. IR Spectroscopic Method for Determination of Silicone Cross-Linking; Pressure Sensitive Tape Council: Chicago, IL, USA, 2016. [Google Scholar]
- Xiang, H.; Wang, X.; Lin, G.; Xi, L.; Yang, Y.; Lei, D.; Dong, H.; Su, J.; Cui, Y.; Liu, X. Preparation, Characterization and Application of UV-Curable Flexible Hyperbranched Polyurethane Acrylate. Polymers 2017, 9, 552. [Google Scholar] [CrossRef]
- Eckberg, R.P. Photo-Initiated Addition Cure Silicone Release Coatings. In Proceedings of the Radtech Conference Proceeding, Disney Coronado Springs, Radtech UV+EB 2020, Orlando, FL, USA, 8–11 March 2020; Available online: https://radtech2020.com/wp-content/uploads/Papers/Materials%20II/Eckberg%20-%20Photo-Activated%20Hydrosilation%20-%20Prospects%20for%20UV%20Curable%20Silicone%20Coatings%20Applications.pdf (accessed on 8 July 2024).
- Jones, G.A.; Bradshaw, D.S. Resonance Energy Transfer: From Fundamental Theory to Recent Applications. Front. Phys. 2019, 7, 100. [Google Scholar] [CrossRef]
- Abdelhameed, M.; Martir, D.R.; Chen, S.; Xu, W.Z.; Oyeneye, O.O.; Chakrabarti, S.; Zysman-Colman, E.; Charpentier, P.A. Tuning the Optical Properties of Silicon Quantum Dots via Surface Functionalization with Conjugated Aromatic Fluorophores. Sci. Rep. 2018, 8, 3050. [Google Scholar] [CrossRef]
- Szabó, Á.; Szöllősi, J.; Nagy, P. Principles of Resonance Energy Transfer. Curr. Protoc. 2022, 2, e625. [Google Scholar] [CrossRef]
- Clegg, R.M. Fluorescence Resonance Energy Transfer. Curr. Opin. Biotechnol. 1995, 6, 103–110. [Google Scholar] [CrossRef]
- Bruno, I.J.; Cole, J.C.; Kessler, M.; Luo, J.; Motherwell, W.D.S.; Purkis, L.H.; Smith, B.R.; Taylor, R.; Cooper, R.I.; Harris, S.E.; et al. CCDC Mogul 2023.3.1 Retrieval of Crystallographically-Derived Molecular Geometry Information. Chem. Inf. Comput. Sci. 2004, 44, 2133–2144. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.; Ramamoorthy, V.; Sharp, P.R. Synthesis of Monomeric (η5-pentamethylcylopentadienyl)platinum(IV) Methyl and Bromo Complexes and of [Hydrotris(3,5-dimethyl-7-pyrazolyl)borato]trimethylplatinum. Inorg. Chem. 1990, 29, 3345–3349. [Google Scholar] [CrossRef]
- Rockensuss, W.; Roesky, H.W.; Gilje, J.W.; Noltemeyer, M. Chloro-dimethyl-(η5-pentamethyl-cyclopentadienyl)-platinum(IV), CSD Commun. 1992. Refcode: SOWTED. Available online: https://www.ccdc.cam.ac.uk/structures/Search?Ccdcid=SOWTED&DatabaseToSearch=Published (accessed on 8 July 2024).
- Howard, W.A.; Bergman, R.G. The Synthesis of Olefin, Carbonyl and Thiolate Complexes of Platinum(IV) in the [η5-C5Me5)Pt(IV)] System. Crystal and Molecular Structure of (η2-C5Me5)PtMe2[S(p-CH3(C6H4]. Polyhedron 1998, 17, 803–810. [Google Scholar] [CrossRef]
- Xue, Z.; Strouse, J.; Shuh, D.K.; Knobler, C.B.; Kaesz, H.D.; Hicks, R.F.; Williams, R.S. Characterization of (Methylcyclopentadienyl)trimethylplatinum and Low-temperature Organometallic Chemical Vapor Deposition of Platinum Metal. J. Am. Chem. Soc. 1989, 111, 8779–8784. [Google Scholar] [CrossRef]
- Kohler, K.; Steiner, A.; Roesky, H.W. Die Kristallstrukturen von (η5-C5Me5)MoMe4 und (η5-C5Me5)WMe4/The Crystal Structures of (η5-C5Me5)MoMe4 and (η5-C5Me5)WMe4. Z. für Naturforschung B 1995, 50, 1207–1209. [Google Scholar] [CrossRef]
- Maus, D.C.; Copie, V.; Sun, B.; Griffiths, J.M.; Griffin, R.G.; Luo, S.; Schrock, R.R.; Liu, A.H.; Seidel, S.W.; Davis, W.M.; et al. A Solid-State NMR Study of Tungsten Methyl Group Dynamics in [W(η5-C5Me5)Me4][PF6]. J. Am. Chem. Soc. 1996, 118, 5665–5671. [Google Scholar] [CrossRef]
- Decker, J.M.; Geib, S.J.; Meyer, T.Y. The Synthesis and Olefin Reactivity of Neutral and Cationic Tantalum Amidinate−pentamethylcyclopentadienyl Complexes. Organometallics 1999, 18, 4417–4420. [Google Scholar] [CrossRef]
- Schrock, R.R.; Liu, A.H.; O’Regan, M.B.; Finch, W.C.; Payack, J.F. Preparation and Characterization of Two Unsubstituted Hydrazido(1-) Complexes, W(η5-C5Me5)Me4(η2-NHNH2) and [W(η5-C5Me5)Me3(η2-NHNH2)]+[SO3CF3]−. Inorg. Chem. 1988, 27, 3574–3583. [Google Scholar] [CrossRef]
- Alaimo, P.J.; Bergman, R.G. Modeling the Proposed Intermediate in Alkane Carbon−hydrogen Bond Activation by Cp*(PMe3)Ir(Me)OTf: Synthesis and Stability of Novel Organometallic Iridium(V) Complexes. Organometallics 1999, 18, 2707–2717. [Google Scholar] [CrossRef]
- Glassman, T.E.; Vale, M.G.; Schrock, R.R. Synthesis and Structure of a Tungsten(IV) η2-dimethyldiazene Complex in which the Diazene Ligand Behaves as a Four-electron Donor. Inorg. Chem. 1992, 31, 1985–1986. [Google Scholar] [CrossRef]
- Vale, M.G.; Schrock, R.R. Synthesis and Reactions of Monomeric Hydrazine and Hydrazido Complexes that Contain the Cp*MoMe3 core. Inorg. Chem. 1993, 32, 2767–2772. [Google Scholar] [CrossRef]
- Schrock, R.R.; Glassman, T.E.; Vale, M.G.; Kol, M. High-Oxidation-State Pentamethylcyclopentadienyl Tungsten Hydrazine and Hydrazido Complexes and Cleavage of the Nitrogen-Nitrogen Bond. J. Am. Chem. Soc. 1993, 115, 1760–1772. [Google Scholar] [CrossRef]
- Moro, M.; Zardi, P.; Rossi, M.; Biffis, A. Evaluation of Heteroleptic Pt (II) β-Diketonate Complexes as Precatalysts for the Photoactivated Curing of Silicone Resins. Catalysts 2022, 12, 307. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal Structure Determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).