Abstract
This work describes the synthesis of a polysilane (PSH)–barium titanate (BT) ferroelectric polymer composite that keeps stable in the presence of ultraviolet light (UV). To evaluate the stability in the presence of UV radiation and the mechanism of interaction between the PSH matrix and BT, FTIR measurements were carried out. The UV/VIS absorption measurement reveals that PSH absorbs strongly in the ultraviolet range, while the composite behaves similarly to BT. Although PSH is a semiconductor, the dielectric spectrometry analysis determined that BT is a ferroelectric material due to its high dielectric constant and low dielectric losses. In contrast to the polymer matrix, the composite polymer has a greater dielectric constant and a lower loss permittivity. PSH is a semiconductor, as indicated by its electrical conductivity of 10−5 S/cm; nevertheless, the UV-irradiated polymer has antistatic properties (10−8 S/cm). Irradiated or not, the polymer composite is a semiconductor, with conductivity of 10−6 S/cm, significantly lower than that of PSH. The interaction with electromagnetic radiation indicates electromagnetic shielding behavior for both BT (highest absorption magnitude of −57 dB) and the polymer composite (maximum absorption magnitudes range from 8.4 to −15.2 dB). Based on these research results, the novel composite with specific characteristics may be used in electronic applications in UV-irradiated conditions.
1. Introduction
Composites represent a class of heterogeneous materials with at least two components, varying physical and/or chemical properties, and significant structural barriers. The components complement each other, resulting in a new material with superior properties.
Barium titanate, BaTiO3 (BT), is a ferroelectric material with a perovskite structure, a high permittivity (dielectric constant above 1000), and a resistivity higher than 108 Ω∙cm. It is probably the most well-known and widely used ferroelectric material in the electrical and electronics industries (ceramic condensers, multi-layer condensers, piezoelectric appliances, pyroelectric sensors, thermistors, electromagnetic shields with electric field absorption, and biomedical applications) [1,2,3].
Polysilanes (in the scientific literature they are also called organo-polysilanes, polysilylenes, or catena-silicon polymers) were first synthesized in 1920 by Kipping through condensation of dichlorosilanes using molted sodium metal dispersion in toluene. At that time, the highly crystalline material had little practical interest as it was insoluble and non-fusible. After 1975, Fujima and coworkers restarted research and expanded their interest in polysilanes. They discovered uses in optoelectronics such as photoconductor polymers, electric charge transport, and a precursor to generate heat-resistant materials (β-SiC) [4,5,6]. The interest in optoelectronics resulted from the σ electron delocalization effect along the main chain, which leads to the high mobility of the electric charge carriers, similar to the semiconductive polyenes. S. Nespurek et al. reported values of mobility of about 106 m2/V∙s [6]. A common issue with this kind of macromolecular compound is its structural instability (degradation) caused by the scission of the Si-Si bond under UV radiation. For polysilanes, the UV light absorption is stronger in the near ultraviolet region within 300–400 nm. Moreover, excitation of polysilanes within this range usually results in strong emission at wavelengths that are sometimes located beyond 400 nm depending on the electronic character of the substituents attached to the silicon atoms in the main chain. Polysilanes can be considered a collection of cromophores in trans-planar conformation. Usually, the UV absorption maximum shifts toward a greater wave length if the macromolecular chain has more than 40 atoms [6,7,8]. The temperature affects the maximum wave length, which shifts to higher values as the temperature drops (a process known as thermochromism). Various types of photostabilizing agents can improve the stability of polysilanes under UV exposure. Common photostabilizing additives include Hostavin PR 25 (C13H14O5), Sanduvor EPU (C22H28N2O3), and Tinuvin 1577 (C27H27N3O2) [6].
In this research, we study the possibility of replacing such photostabilizing additives with ferroelectric barium titanate, which is known to act as an electromagnetic shield against UV radiation. The electromagnetic shielding property of a material is related to its capacity to refract, adsorb, or reflect the electromagnetic field. From this point of view, there are three kinds of electromagnetic shields: shields with reflection of radiation, shields with absorption of electromagnetic radiation, and shields with multiple reflective properties. The first class, representing shielding by reflecting radiation, consists of materials that contain charge carriers (electrons or holes) that will interact with the electromagnetic field’s electric component. In the second case, the shielding material is usually dielectric and has high electric and/or magnetic dipoles (ferroelectric and/or ferromagnetic materials). Absorption loss occurs when the electromagnetic waves traversing the medium induce electronic cloud shifting, which in turn produces ohmic losses and heating (heat loss). Multiple reflective shields involve the presence of large specific surfaces (porous foam materials) or a large interface (nanofibers, nanotubes, or nanometric pyramidal structures) [9,10,11]. Common examples include graphene, multi-walled carbon nanotubes, carbon fibers for multiple reflective shields, nickel foam, aluminum foam, carbon-based foam, NiO, Fe3O4 nanocrystals, and ferroelectric materials for shields with reflection and absorption of electromagnetic radiation [12,13].
2. Results and Discussion
2.1. Fourier-Transform Infrared (FTIR) Spectroscopy
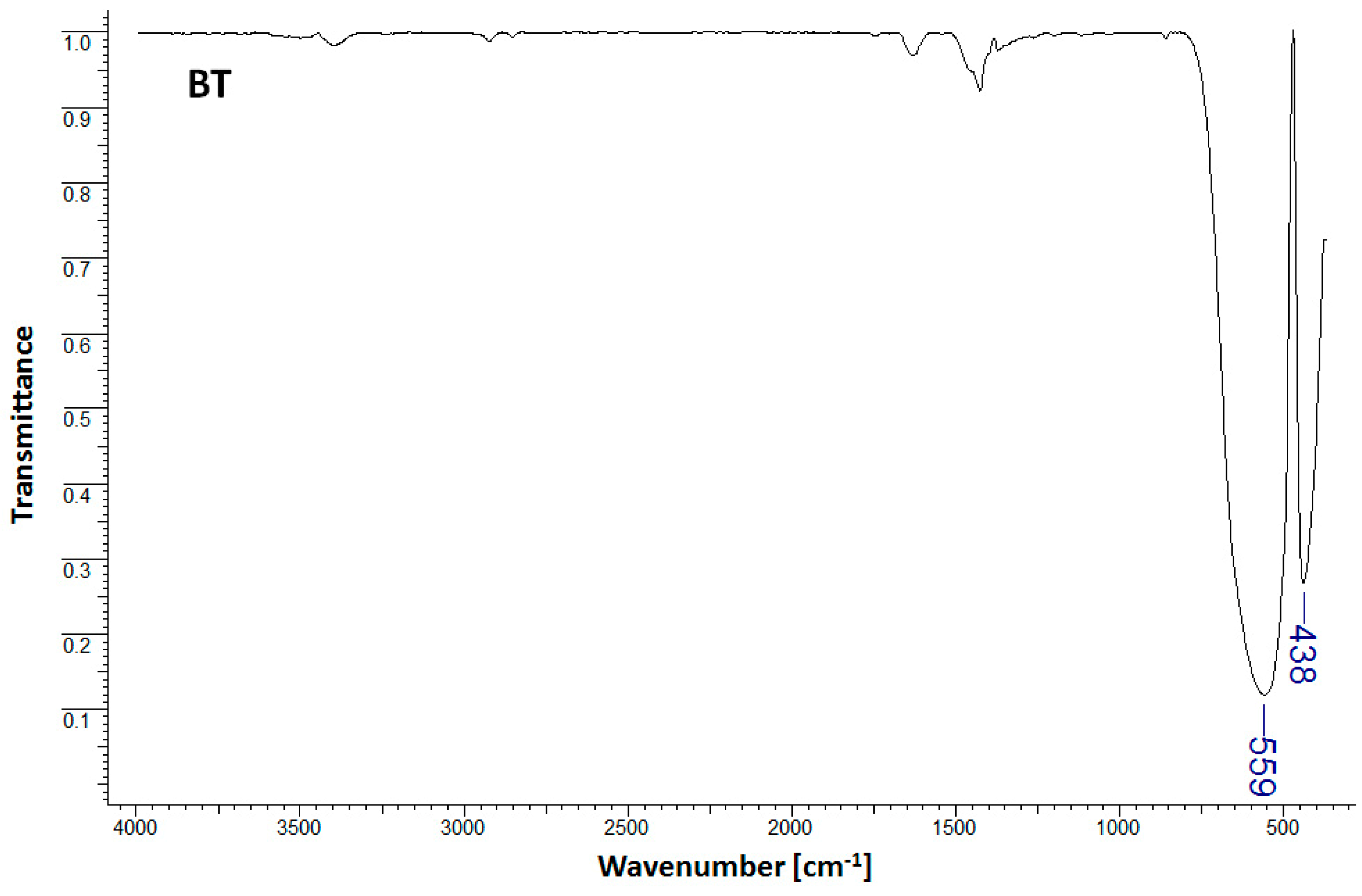
As observed in Figure 1, the infrared spectrum of the BT sample shows characteristic bands for pure barium titanate at 438 cm−1 and 559 cm−1 [1]. The band observed within the lower wavelength range (438 cm−1) is characteristic of barium titanate, and was assigned to the Ba-O and Ti-O bond vibrations in metal oxides [1]. The presence of the band within the higher wavelength region (559 cm−1) and assigned to Ti-O bond stretching normal vibration is representative of the formation of the BaTiO3 tetragonal phase with direct consequences for the dielectric properties (high dielectric constant) [1,14]. Figure 2 presents the FTIR spectra for PSH and PSH/BT composites.
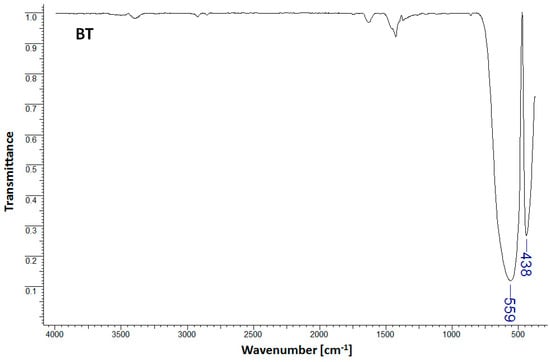
Figure 1.
FTIR spectra for BT.
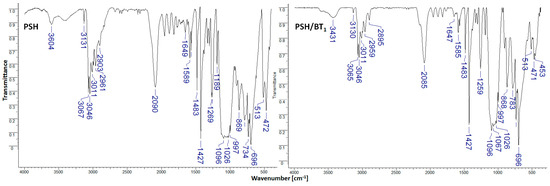
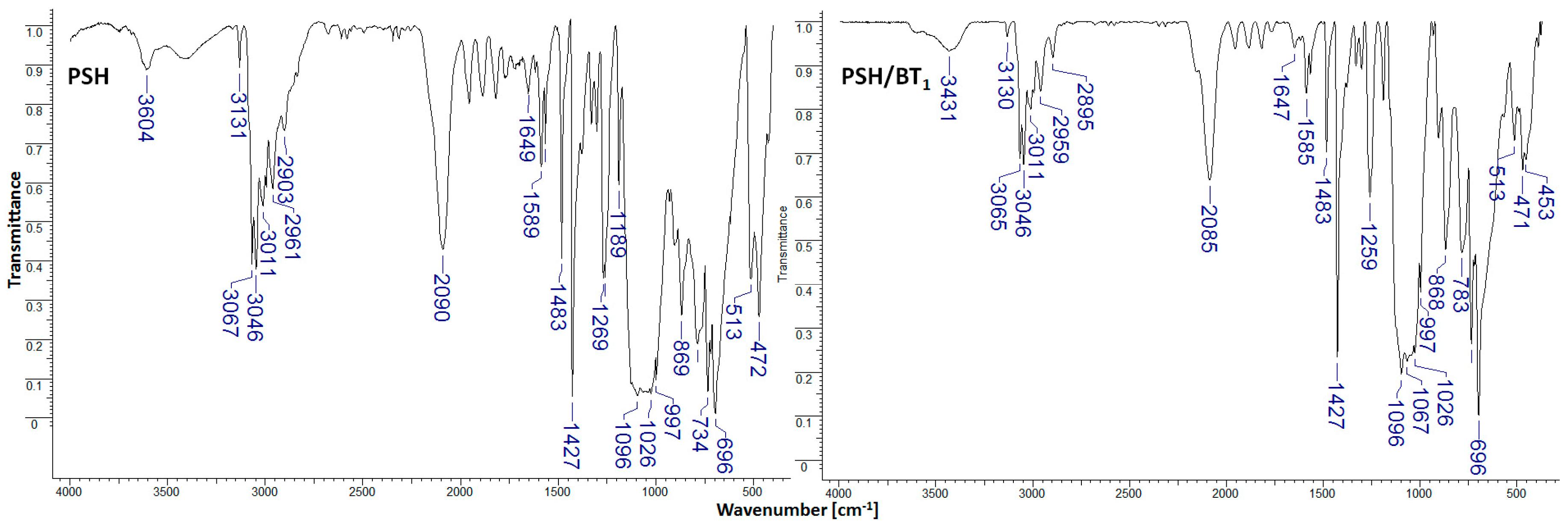
Figure 2.
FTIR spectra for PSH and PSH/BT1 composites.
The infrared spectra for PSH present characteristic bands at 3604 cm−1 (OH vibrations), 3067 and 3046 cm−1 (C-H aromatic), 2961 and 2903 cm−1 (C-H aliphatic), 2090 (Si-H vibrations), 1649 cm−1 (OH vibrations), 1427 and 1096 cm−1 (Si-C6H5 vibrations), 1269 cm−1 (Si-CH3 vibrations), 1026 cm−1 (Si-O-C vibrations), 734 cm−1 (C-C aromatic), 696 cm−1 (Si-C vibrations), and 472 cm−1 (Si-Si vibrations) [7,8]. In PSH/BT1 and PSH/BT2 composites, the addition of barium titanate does not change the polysilane’s chemical structure. In general, the wave numbers stay the same, but the spectral intensities change.
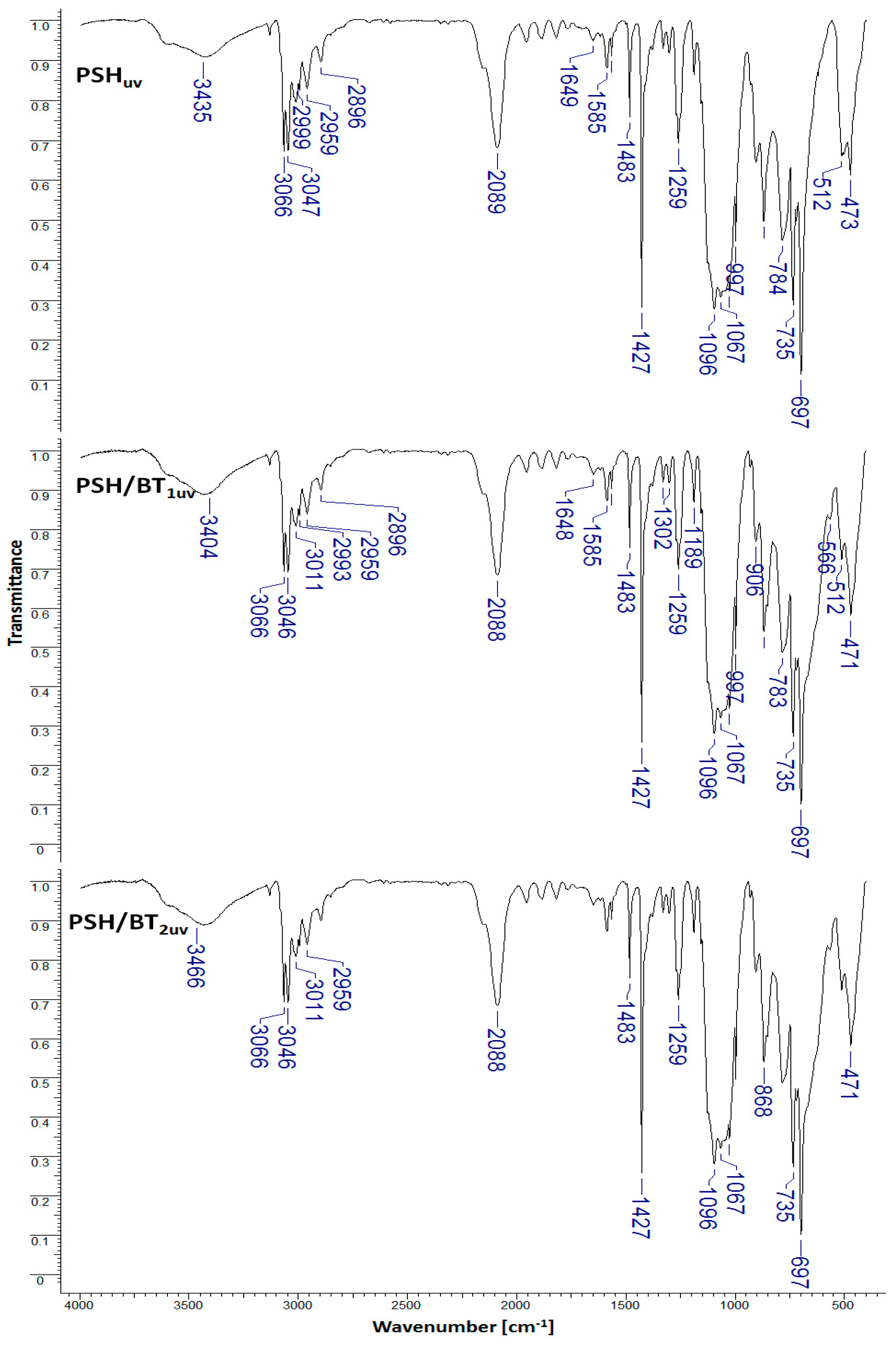
To observe the influence of barium titanate particles in the degradation of polysilanes under UV radiation, PSH, PSH/BT1, and PSH/BT2 samples were subjected to ultraviolet irradiation. Figure 3 shows the IR spectra of the UV-irradiated samples (PSHuv, PSH/BT1uv, and PSH/BT2uv).
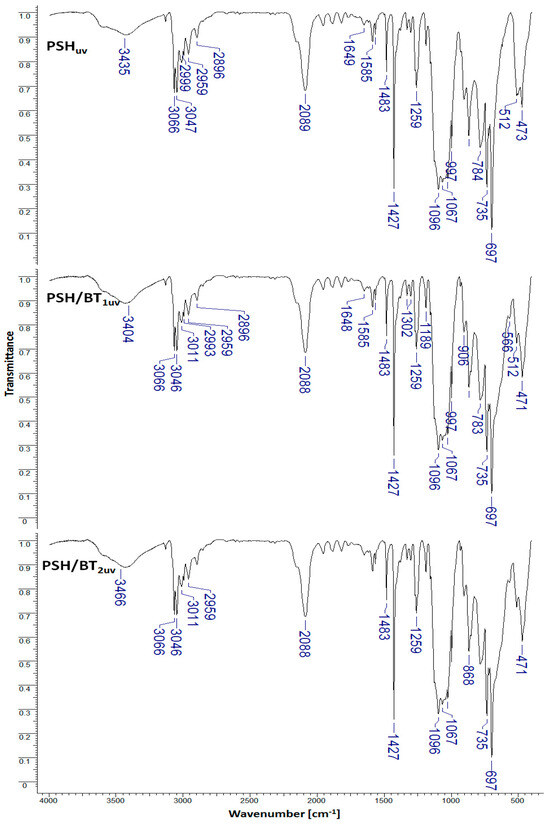
Figure 3.
FTIR spectra for UV irradiated samples (PSHuv, PSH/BT1uv, and PSH/BT2uv).
According to Miller [15], the degradation of polysilanes under UV radiation leads to a reduction in the number of Si-Si bonds while the number of Si-O-C and/or Si-O-Si bonds increases. Quantitative investigations were conducted using the IR absorption band ratios [7]. The representative IR absorption band ratios for the studied system are shown in Table 1.

Table 1.
The representative IR absorption band ratios.
In the case of the irradiated polymer (PSHuv), a drastic reduction in the Si-Si/Si-O-C ratio compared to the non-irradiated polymer (PSH) from 0.78 to 0.36 was observed. This result shows a decrease in the vibration energy of the Si-Si bond compared to the Si-O-C bond. This reduction is strongly attenuated in the case of the PSH/BT1uv composite (from 0.74 to 0.69) and almost insignificant for the composite with a high amount of barium titanate, PSH/BT2uv (from 0.76 to 0.74). The same phenomenon was observed for the Si-Si/Si-H, Si-Si/Si-CH3, Si-Si/Si-C6H5, and Si-H/Si-O-C ratios. This behavior suggests that BT acts as a protective UV electromagnetic shield.
2.2. The UV/VIS Absorption Study in the Solid State
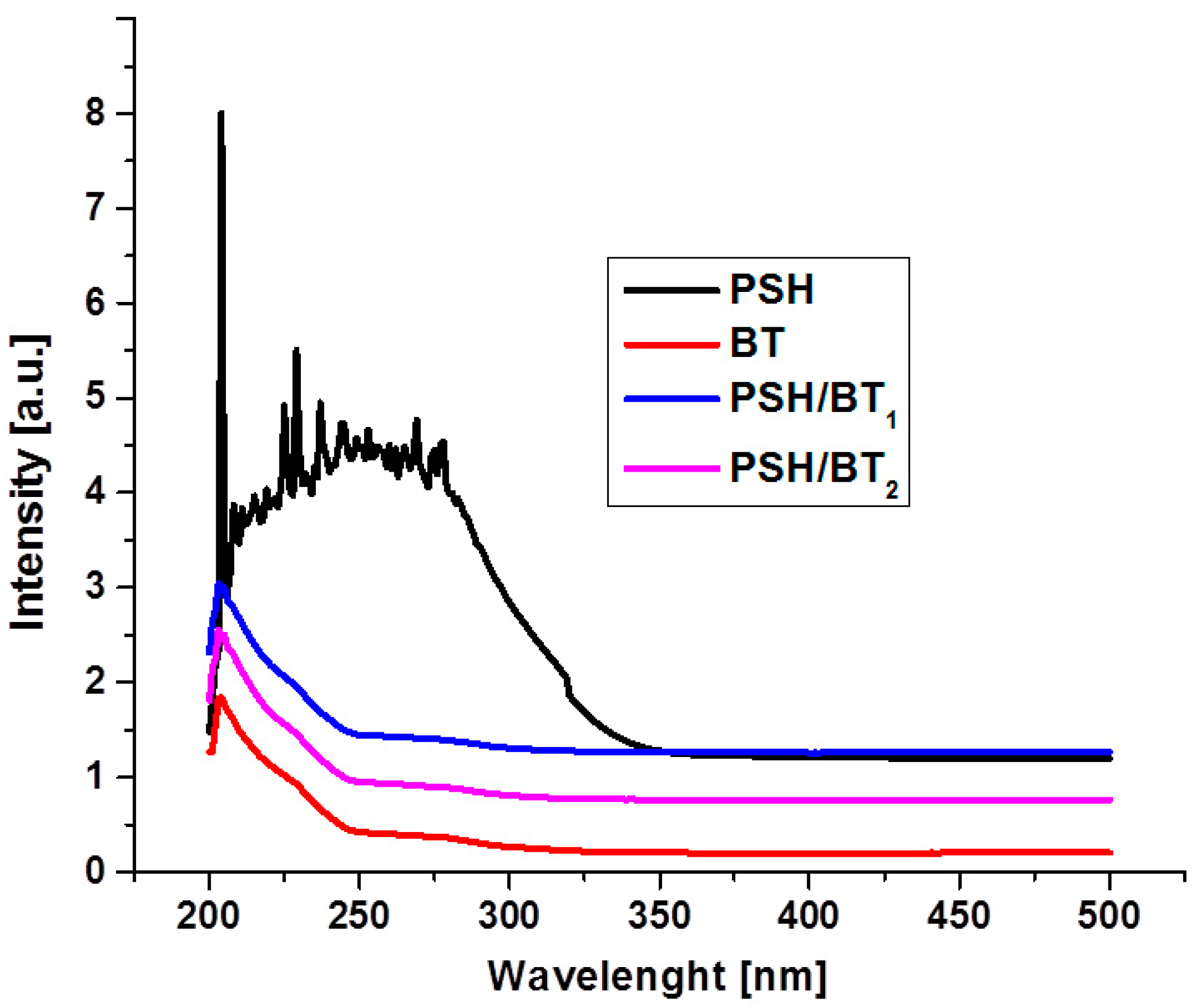
UV/VIS absorption spectroscopy was further used to study the behavior of polysilanes when exposed to UV light. The UV/VIS absorption spectra of PSH, BT, PSH/BT1, and PSH/BT2 are shown in Figure 4. PSH shows a strong absorption maximum in the ultraviolet spectrum. Due to the sample preparation (solid pellets), the intensity of the absorption band was outside the measurement range. The UV spectrum of BT displays a strong UV absorption band around 210 nm. The electron band structure of BaTiO3 has low-lying narrow conduction bands from Ti3+-3d states and valence bands from O2−-2p states. This is a normal behavior for ferroelectric structures [14]. Regarding the composites, it is evident (Figure 4) that their behavior is more like that of BT since the UV absorption band characteristic for PSH is no longer present.
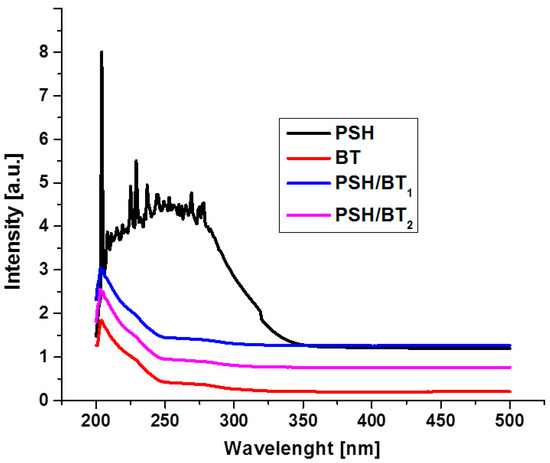
Figure 4.
UV/VIS absorption spectra for PSH, BT, and the composites.
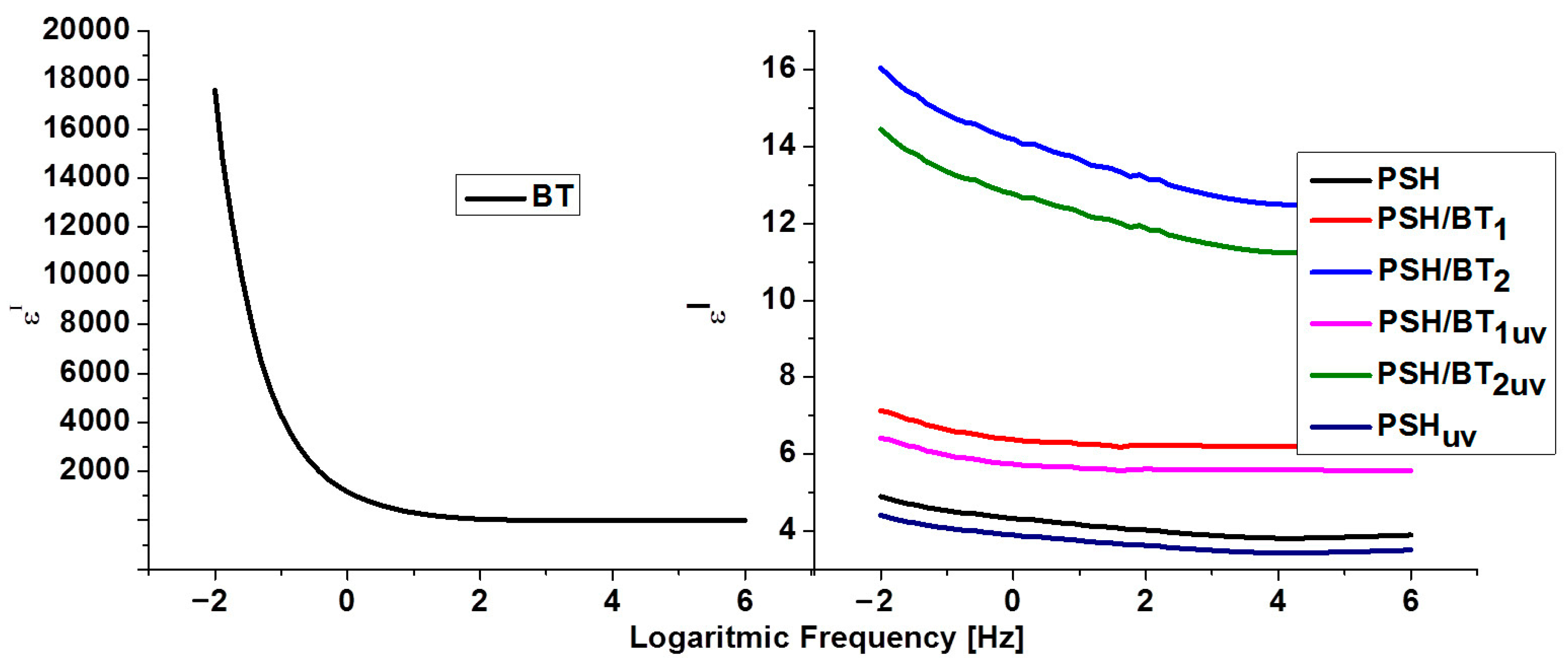
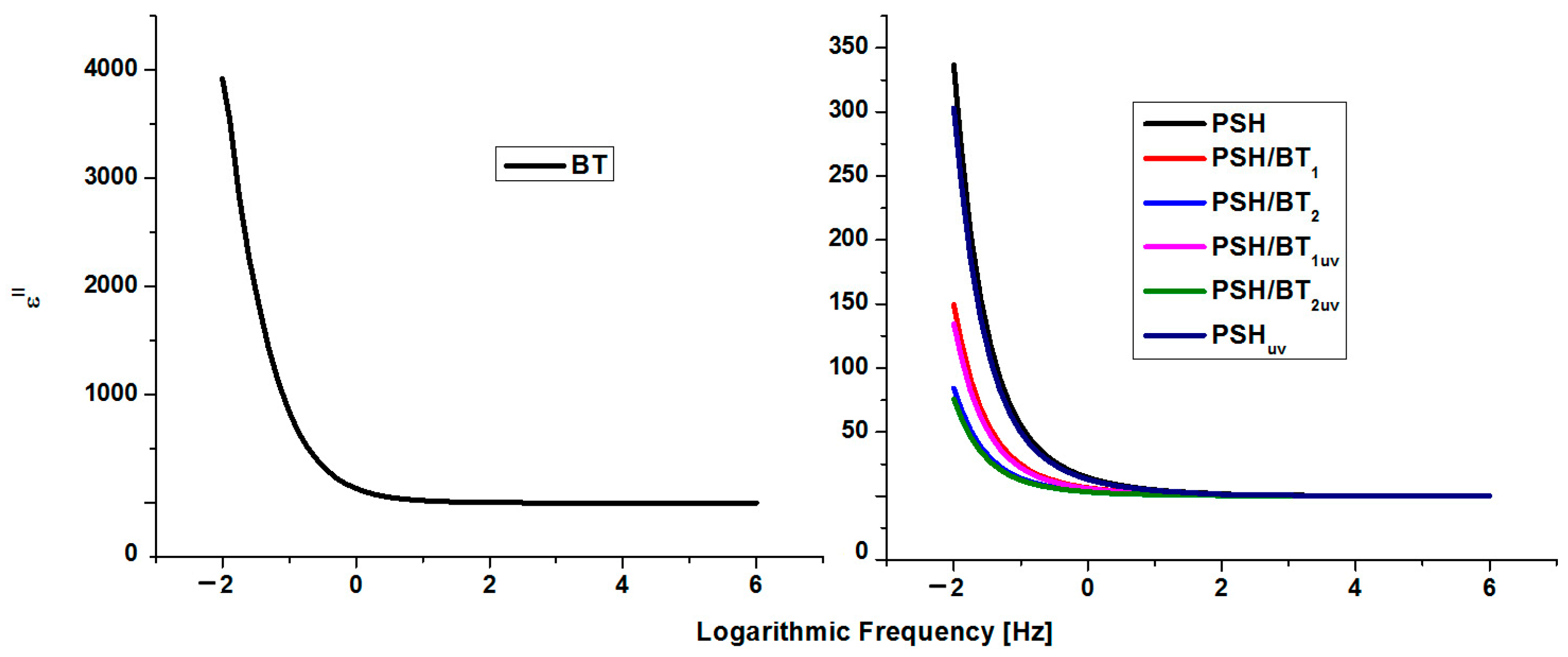
The components of the complex dielectric permittivity (ε′ and ε″) were measured in the frequency range of 10−2–106 Hz at room temperature. In general, ε′ and ε″ of the insulating materials are related to five types of polarizations of the electrons and molecules: electronic, vibrational (atomic), directional (dipolar), ionic, and interfacial. Electronic and vibrational polarizations are ideal for materials used as capacitors, while directional, ionic, and interfacial polarizations are perfect for materials used for electromagnetic shielding [1,16,17]. The variation in the dielectric constant and permittivity loss versus frequency for BT, PSH, and their composites is depicted in Figure 5 and Figure 6, respectively.
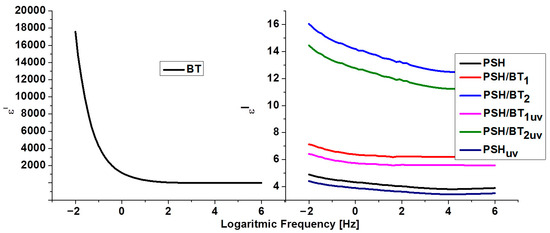
Figure 5.
Dielectric constant (ε′) for BT, PSH, and PSH/BT composite.
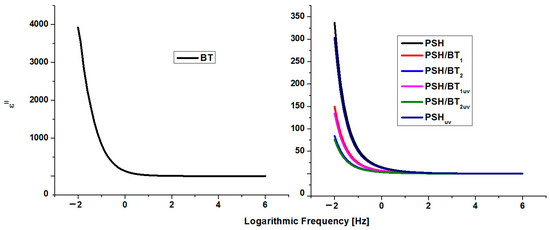
Figure 6.
Dielectric loss for BT, PSH, and PSH/BT composite.
It is obvious that in the case of BT, with a frequency increase, ε′ and ε″ decrease and remain constant at higher frequencies, indicating a dielectric dispersion (ionic and directional polarizabilities). This was assigned to dipole formation due to the changes in the valence states of cations and space-charge polarization [1,16,18]. The decrease for ε′ stops at frequencies higher than 102 Hz. Electronic polarization is always higher than ionic and directional polarization (ε′ > ε″). This behavior is normal for ferroelectric materials [1,19]. The values for ε′ ranged from 1.4 (for 106 Hz) to 17,604 (for 10−2 Hz), while ε″ registered values from 0.005 (for 106 Hz) to 3921 (for 10−2 Hz). These values were similar to values evidenced for barium titanate in the literature [20,21].
Polysilane shows a variation in the dielectric constant that is typical for dielectric polymers. However, the variation in the dielectric losses and the fact that they are higher than the dielectric constant could be related to the intrinsic semiconducting nature of the semiconductor materials [20]. Thus, ε′ varies from 3.8 to 4.8 in the frequency range 106–10−2, and ε″ varies from 0.11 to 336.5 in the same interval. In the case of polysilanes/barium titanate composites, the dielectric constant increases and the permittivity loss decreases. Thus, for the PSH/BT1 sample, ε′ varied from 6.18 to 7.12 and ε″ from 0.05 to 149.57 in the frequency range 106–10−2 Hz, and for the PSH/BT2, ε′ varied from 12.75 to 16.04 and ε″ from 0.02 to 84.13. For semiconductor materials, reducing the dielectric loss directly influences the electrical conductivity of the composite [22,23].
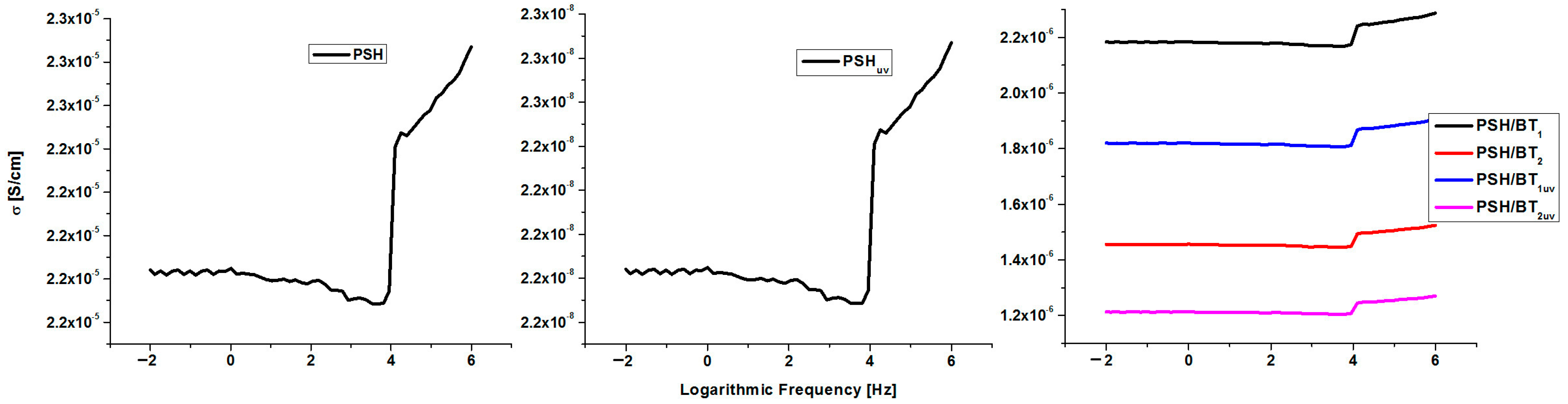
Figure 7 displays the variation in electrical conductivity (σ) of PSH, PSHuv, and the BT composites within the frequency range of 106–10−2 Hz (BT is not shown because it is an insulator and has insignificant electrical conductivity [1]).
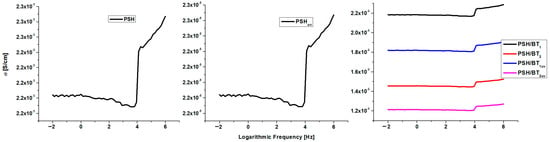
Figure 7.
Electrical conductivity (S/cm) for PSH, PSHuv, and BT/PSH samples.
The conductivity of polysilane slightly varies within the studied frequency range (10−2–106 Hz), with values between 2.18·× 10−5 and 2.28·× 10−5 S/cm. This range of conductivity variation indicates that polysilane belongs to the intrinsic semiconductor polymer class. Such materials could be used in the semiconductor industry to fabricate various devices (transistors, diodes, and photovoltaic cells) for electronic applications. The UV irradiation of polysilane strongly modifies the electrical conductivity to 10−8 S/cm, which is usual for polymeric electrical insulators.
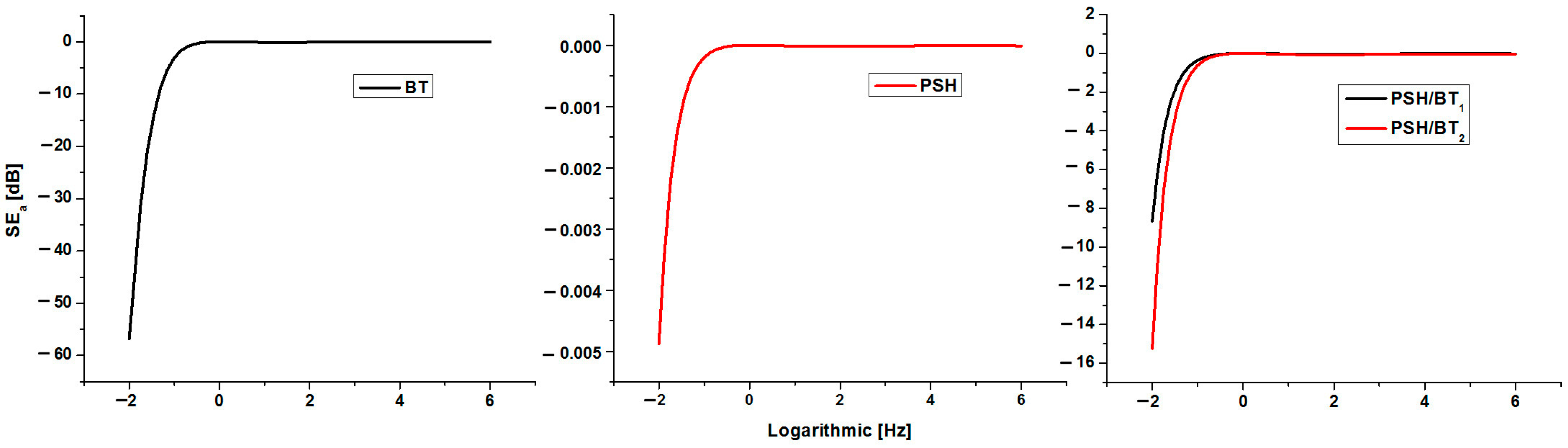
On the other hand, doping with BT lowers the conductivity of the PSH/BT composite to 10−6 S/cm. Furthermore, the concentration of BT within the composite modifies the electrical conductivity since σ PSH/BT1 > σ PSH/BT2. The UV irradiation of these composite samples leads to only a tempered decrease in electrical conductivity. From this point of view, both irradiated composites (PSH/BT1uv and PSH/BT2uv) remained in the semiconductor materials range, having conductivities around 10−6 S/cm. Therefore, BT acts as an electromagnetic shield that absorbs most of the UV radiation and protects polysilane from altering its chemical structure. In order to evidence the influence of UV radiation on polysilane and its composite with BT, the magnitude of the absorbance losses for these materials was calculated. Negative values indicate that the material behaves like an UV shield, absorbing the incident UV radiation through displacements of the electronic cloud [3,9]. The variation in the magnitude of the incident UV absorbance loss versus the frequency of the incident radiation is shown in Figure 8.
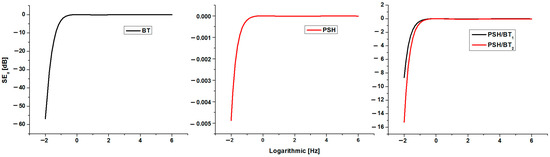
Figure 8.
Plot of the absorbance loss for BT, PSH, PSH/BT1, and PSH/BT2 versus frequency of the incident radiation.
In general, BT, which has high dielectric constants and a maximum absorption of −57 dB, is currently a good absorber of electromagnetic radiation and is usually used as an electromagnetic shield for various materials [3,9]. On the other hand, polysilane has a very low absorption, the maximum being −0.0048 dB. The polysilane/BT composite proved to be a good absorber of electromagnetic radiation, having absorption maxima of −8.4 dB for PSH/BT1 and −15.2 dB for PSH/BT2. Materials with electromagnetic shielding properties caused by absorption cause a temporary displacement of the electronic cloud. The energy of the incident electromagnetic radiation is consumed in this way and, in the case of composites with polysilanes, electronic delocalization σ no longer occurs. This phenomenon is the explanation for the resistance to UV degradation of barium titanate composites compared to polysilane.
3. Materials and Methods
3.1. Materials
Barium carbonate (Sigma-Aldrich, St. Louis, MO, USA, 99% purity), titanium dioxide (Chemical Company, Iasi, Romania, 99% purity), diphenyldichlorosilane, methyl (hydrogen) dichlorosilane (Sigma-Aldrich, 99% purity), toluene (Lachner, Czech Republic, M = 92.14 g/mol), sodium (Sigma-Aldrich, 99% purity), and Milli-Q ultrapure distilled water were employed as dispersion mediums in ultrasonication experiments.
3.2. Methods
Synthesis of the BT and PSH/BT composites was performed using an ultrasound generator from Sonics Vibracell (750W, 20 kHz). A microwave oven (800 W electrical power, 2.5 GHz microwave frequency) and a Vulcan A 130 laboratory oven were used for BT synthesis. Irradiation of the polymer composite samples was conducted using a Spectroline-Spectronics lamp with two wavelengths of emission: short-wave (254 nm) and long-wave (365 nm).
The structures of the precursors and composites were investigated by FTIR spectroscopy on potassium bromide pellets using a Bruker Vertex 70 spectrometer (2 cm−1 resolution).
1H-NMR spectra were registered with a Bruker NMR instrument (Model DRX 400 MHz).
The molecular weight distribution of the polymers synthesized in this work was measured in THF on a Spectra Physics 8800 gel permeation chromatograph with two PL-gel packed columns. All molecular weights are reported versus the polystyrene standard.
To observe the influence of UV radiation, three samples (PSH, PSH/BT1, and PSH/BT2) were prepared as 13 mm pellets and then exposed to ultraviolet irradiation (365 nm wavelength) for 30 min (see Table 1). Specord 200 Analytik Jena UV/VIS (Jena, Germany) was used for the UV/VIS absorption study. The samples were studied in an ethyl alcohol solution at a 1% wt. concentration.
Barium titanate with a very high dielectric constant can act like an electromagnetic shield since it has the capacity to convert the incident electromagnetic radiation into thermal energy. For such materials, the absorption magnitude of the incident electromagnetic radiation can be calculated using Equation (1) [3,10]:
where t = material thickness; σ = total electrical conductivity of the material; ω = angular frequency (Hz), ω = 2πf, f = linear frequency (Hz), frequency of electromagnetic field; µr = relative magnetic permeability of the material.
SEA [dB] = −8.68(tσωµr/2)1/2
The dielectric measurements (dielectric constant, loss permittivity, and electrical conductivity) were performed on a Concept 40 Novocontrol Dielectric Spectrometer (Montabaur, Germany) at room temperature with silver electrodes in the 106–10−1 Hz frequency range. The contact surfaces with spectrometer electrodes were covered with silver conductive paste to avoid interfacial polarization and possible edge effects. BT, PSH, and the two analyzed composites (PSH/BT1 and PSH/BT2) were prepared in the form of pellets with a thickness of 3 mm by pressing with a Specac laboratory press.
For the calculation of SEA, the following working assumptions were taken into account: t = 3 mm, σ = ω·ε0·ε″ (ε0: free space permittivity: 8.854·10−12 F/m, and ε″ is the absorption or permittivity loss), and µr = 1, material without magnetic properties [3].
3.2.1. Barium Titanate (BT) Particle Preparation
The BT preparation followed a method described in previous work [1]. Briefly, a 1/2 TiO2/BaCO3 mixture dispersion in Milli-Q water was ultrasonicated for 60 min. The resulting powder was then separated, dried in a microwave oven for 10 min, and thermally treated at 500 °C for 3 h to obtain perovskite-like BT submicronic particles. The final product, BT, was obtained as a white, solid powder.
3.2.2. Poly(diphenyl-co-methyl(hydrogen))silane (PSH) Preparation
Poly(diphenyl-co-methyl(hydrogen))silane (PSH) was obtained by the conventional Wurtz coupling method [24] from diphenyldichlorosilane and methyl(H) dichlorosilane in the presence of molted sodium metal dispersion in toluene. The condensation reaction was carried out at reflux temperature. The reaction product, PSH, results in a white-yellowish solid powder.
1H-NMR (CDCl3, δ, ppm): 0.799-0.979 (Si-CH3); 3.22-4.00 (Si-H); 6.93-7.26 (Si-C6H5).
FT-IR (KBr, cm−1): 3065 and 3045 (C-Harom.); 2955 (C-Haliph.); 2085 (Si-H); 1482; 1426 and 1093 (Si-C6H5); 1257 and 869 (Si-CH3); 695 (Si-C); 468 (Si-Si).
GPC: Mw = 4495 g/mol; Mw/Mn= 1.5.
UV absorption (toluene): 310 nm.
3.2.3. PSH/BT Composite Preparation
Two samples of the PSH/BT composite were prepared with 10% (PSH/BT1) and 20% PSH/BT2 barium titanate.
For this purpose, 50 mL of Milli-Q water was mixed with 1 g of PSH, 0.1 g of BT (PSH/BT1), and 1 g of PSH, 0.2 g of BT (PSH/BT2). The resulting suspensions were ultrasonicated for 10 min at 20 kHz ultrasound frequency (Table 2) into a glass vial of 100 cc. These technical conditions were established in accordance with Santos [25] and other previous works [26,27,28] with the ultrasound probe immersed in liquid for at least 40% of its length and the distance to the vial walls as minimal as possible. The aqueous dispersions were then filtered, and the solid samples were vacuum dried overnight at 50 °C. The two composite samples resulted in solid yellowish-white powders.

Table 2.
Samples and the terms of the synthesis.
4. Conclusions
A composite polysilane–barium titanate was synthesized in two concentrations through an ultrasound homogenization procedure. Following the dielectric measurements, it was found that the composites have a dielectric constant that is much lower than that of the ferroelectric precursor (barium titanate) but higher than that of the polymer precursor (polysilane). Additionally, the new composite shows a degradation in the presence of ultraviolet radiation that is much more attenuated in comparison with the pristine polysilane. Following UV/VIS absorption measurements, it was found that PSH absorbs strongly in the ultraviolet range, while the composite behaves similarly to BT. Although PSH behaves almost like a semiconductor, the dielectric spectrometry analysis determined that BT is a ferroelectric material due to its high dielectric constant and low dielectric losses. In contrast to the polymer matrix, the composite polymer has a higher dielectric constant and a lower loss permittivity. Following these results, the new composite could be used in electronic applications used in UV-irradiated environments.
Author Contributions
Conceptualization, R.R. and L.S.; methodology, E.U. and M.E.F.; software, R.R. and L.S.; validation, R.R., M.E.F. and L.S.; formal analysis, E.U. and R.R.; investigation, R.R., E.U. and M.E.F.; resources, E.U.; data curation, M.E.F. and E.U.; writing—original draft preparation, R.R. and M.E.F.; writing—review and editing, R.R., E.U. and L.S.; visualization R.R. and M.E.F.; supervision, L.S. and M.E.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nowacki, B.; Mistewicz, K.; Hajra, S.; Kim, H.J. 3D printed triboelectric nanogenerator for underwater ultrasonic sensing. Ultrasonics 2023, 133, 107045. [Google Scholar] [CrossRef] [PubMed]
- Markovic, S.; Mitric, M.; Starcevic, G.; Uskokovic, D. Ultrasonic de-Agglomeration of Barium Titanate Powder. Ultrason. Sonochem. 2008, 15, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Patsidis, A.C. Barium titanate/polydimethylsiloxane nano/microcomposites fabrication, morphology, dielectric response and functionality. IET Nanodielectr. 2020, 1, 14–19. [Google Scholar] [CrossRef]
- Yajima, S.; Hayashi, J.; Okamura, K. Pyrolysis of a polyborodiphenylsiloxane. Nature 1977, 266, 521. [Google Scholar] [CrossRef]
- Miller, R.D.; Michl, J. Poly(di-n-hexyl-silane) in solid solutions: Experimental and theoretical studies of electronic excitations of a disordered linear chain. Chem. Rev. 1989, 89, 1359. [Google Scholar] [CrossRef]
- Nespurek, S.; Pospisil, J.; Kratochvilova, I.; Sworakowski, J. Polymeric Composites Based on Polysilanes for Plastic Electronics. Mol. Cryst. Liq. Cryst. 2008, 484, 265–631. [Google Scholar] [CrossRef]
- Feigl, A.; Bockholt, A.; Weis, J.; Rieger, B. Modern Synthetic and Application Aspects of Polysilanes: An Underestimated Class of Materials? In Silicon Polymers: Advances in Polymer Science; Muzafarov, A.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 235, pp. 1–31. [Google Scholar]
- Kumar, V.B.; Leitao, E.M. Properties and applications of polysilanes. Appl. Organomet. Chem. 2020, 34, e5402. [Google Scholar] [CrossRef]
- Qin, M.; Zhang, L.; Wu, H. Dielectric loss mechanism in electromagnetic wave absorbing materials. Adv. Sci. 2022, 9, 105553. [Google Scholar] [CrossRef] [PubMed]
- Sundar, U.; Lao, Z.; Cook-Chennault, K. Enhanced Dielectric Permittivity of Optimized Surface Modified of Barium Titanate Nanocomposites. Polymers 2020, 12, 827. [Google Scholar] [CrossRef]
- Fortună, M.E.; Ignat, M.; Tudorachi, N.; Ungureanu, E.; Rotaru, R.; Harabagiu, V. Hybrid Siloxane Materials Based on a Mutually Reactive Epoxy–Amine System: Synthesis, Structure, and Thermal Stability Investigations. Inorganics 2024, 4, 118. [Google Scholar] [CrossRef]
- Um, M.H.; Lee, C.T.; Kumasawa, H.J. Preparation and dielectric properties of ferroelectric barium titanate fine particles by hydrothermal method. J. Ind. Eng. Chem. 1997, 3, 251–256. [Google Scholar]
- Liu, P.; Hong Ng, V.M.; Yao, Z.; Zhou, J.; Lei, Y.; Yang, Z.; Lv, H.; Kong, L.B. Facile synthesis and hierarchical assembly of flowerlike NiO structures with enhanced dielectric and microwave absorption properties. ACS Appl. Mater. Interfaces 2017, 9, 16404–16416. [Google Scholar] [CrossRef]
- Liu, G.H.; Wei, C.Y.; Huang, T.; Wang, F.; Chang, J.F.; Sun, Q.; Zhang, X.H. Metal-catalyzed carbon foams synthesized from glucose as highly efficient electromagnetic absorbers. Materials 2024, 17, 3488. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.D.; Wallraff, G.; Clecak, N.; Sooriyakumaran, R.; Michl, J.; Karatsu, T.; McKinle, A.J.; Klingensmith, K.A.; Downing, J. Polysilanes: Solution Photochemistry and Deep-UV Lithography. Polym. Eng. Sci. 1989, 29, 882–886. [Google Scholar] [CrossRef]
- Rotaru, R.; Popescu, C.M.; Dascalu, A.; Timpu, D.; Asanduleasa, M.; Fortuna, M.E.; Harabagiu, V. Influence of Ultrasonic Treatment and Heating/Cooling Under Electric Field on High-K Cellulose-Barium Titanate Composites. Rev. Roum. Chim. 2023, 68, 175–185. [Google Scholar] [CrossRef]
- Astapenko, V. Polarization Bremsstrahlung on Atoms, Plasmas, Nanostructures and Solids, Chapter Polarization Bremsstrahlung on Nanostructures; Springer: Berlin/Heidelberg, Germany, 2013; pp. 207–243. ISBN 978-3-642-34082-6. [Google Scholar]
- Devan, R.S.; Chougule, B.K. Effect of composition on coupled electric, magnetic, and dielectric properties of two phase particulate magnetoelectric composite. J. Appl. Phys. 2007, 101, 014109. [Google Scholar] [CrossRef]
- Lines, M.E.; Glass, A.M. The Ferroelectric Perovskites—Barium Titanate. In Principles and Applications of Ferroelectric and Related Materials; Clarendon Press: Oxford, UK, 2001; pp. 241–245. [Google Scholar]
- Paunovic, V.; Mitic, V.V.; Kocic, L. Dielectric characteristic of donor-acceptor modified BaTiO3 ceramics. Ceram. Int. 2016, 42, 11692–11699. [Google Scholar] [CrossRef]
- Mitic, V.V.; Paunovic, V.; Kocic, L. Fractal approach to BaTiO3- ceramics micro-impedances. Ceram. Int. 2015, 41, 6566–6574. [Google Scholar] [CrossRef]
- Wang, Q.; Che, J.; Wu, W.; Hu, Z.; Liu, X.; Ren, T.; Chen, Y.; Zhang, J. Contributing factors of dielectric properties for polymer matrix composites. Polymers 2023, 15, 590. [Google Scholar] [CrossRef]
- Taherian, R. Electrical Conductivity in Polymer-Based Composites: Experiments, Modelling, and Applications; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Sacarescu, L.; Fortuna, M.; Soroceanu, M.; Cojocaru, C.; Sacarescu, G.; Simionescu, M.; Harabagiu, V. Computational Study of the Electronic Absorption Spectra of Polyhydrosilanes. Silicon 2015, 7, 343–349. [Google Scholar] [CrossRef]
- Santos, H.M.; Lodeiro, C.; Capelo-Martinez, J.L. The Power of Ultrasound. In Ultrasound in Chemistry: Analytical Applications; Capelo-Martinez, J.L., Ed.; Wiley-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2008; pp. 1–15. [Google Scholar]
- Shokrollahi, H. A review of the magnetic properties, synthesis methods and applications of maghemite. J. Magn. Magn. Mater. 2017, 426, 74–81. [Google Scholar] [CrossRef]
- Fatimah, I.; Fadillah, G.; Yudha, S.P. Synthesis of iron-based magnetic nanocomposites: A review. Arab. J. Chem. 2024, 17, 105405. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Seddik, U.; Okasha, N.; Imam, N.G. One-dimensional nanoferroic rods; synthesis and characterization. J. Mol. Struct. 2015, 1099, 330–339. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).