Compositional and Fabrication Cycle Optimization of Ceria-Zirconia-Supported Mo-Based Catalysts for NH3-SCR NOx Reduction
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
- Cerium and zirconium precursors are dissolved in deionized water to achieve a total cation concentration of 0.1M; parallelly, a diluted ammonia solution (1M NH3) is prepared.
- Both solutions are stirred vigorously for 15 min to ensure complete dissolution and homogenization of the different precursors.
- An appropriate amount of the ammonia solution is gradually added to the cations-containing solution, either at room temperature in the case of sole support (doped/undoped ceria) or in a reflux setup at around 90 °C in the case of precursors containing molybdenum. This latter step is performed to ensure a significant excess of the base at a high temperature, leading to the formation of a Mo-containing powder precursor.
- After precipitation, the resulting suspensions are aged for a short period (several minutes), filtered, and thoroughly washed with deionized water and ethanol (to favor the complete elimination of Cl ions).
- Finally, all the co-precipitates are dried overnight at 80 °C and subsequently calcined at 450 °C for 1 h.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, S.W.; Heckel, A.; McKeen, S.A.; Frost, G.J.; Hsie, E.Y.; Trainer, M.K.; Richter, A.; Burrows, J.P.; Peckham, S.E.; Grell, G.A. Satellite-observed US power plant NOx emission reductions and their impact on air quality. Geophys. Res. Lett. 2006, 33, L22812. [Google Scholar] [CrossRef]
- Wang, X.; Lei, Y.; Yan, L.; Liu, T.; Zhang, Q.; He, K. A unit-based emission inventory of SO2, NOx and PM for the Chinese iron and steel industry from 2010 to 2015. Sci. Total Environ. 2019, 676, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Ashok, B. NOx Emission Control Technologies in Stationary and Automotive Internal Combustion Engines: Approaches toward NOx Free Automobiles; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Zhang, S.; Zhao, P.; He, L.; Yang, Y.; Liu, B.; He, W.; Cheng, Y.; Liu, Y.; Liu, S.; Hu, Q.; et al. On-board monitoring (OBM) for heavy-duty vehicle emissions in China: Regulations, early-stage evaluation and policy recommendations. Sci. Total Environ. 2020, 731, 139045. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Peng, Q.; Jiaqiang, E.; Xie, B.; Wei, J.; Yin, R.; Fu, G. Mechanism, performance and modification methods for NH3-SCR catalysts: A review. Fuel 2023, 331, 125885. [Google Scholar] [CrossRef]
- Wu, T.; Guo, R.T.; Li, C.F.; You, Y.H.; Pan, W.G. Recent advances in core-shell structured catalysts for low-temperature NH3-SCR of NOx. Chemosphere 2023, 333, 138942. [Google Scholar] [CrossRef] [PubMed]
- Damma, D.; Ettireddy, P.R.; Reddy, B.M.; Smirniotis, P.G. A review of low temperature NH3-SCR for removal of NOx. Catalysts 2019, 9, 349. [Google Scholar] [CrossRef]
- Inomata, Y.; Hata, S.; Mino, M.; Kiyonaga, E.; Morita, K.; Hikino, K.; Yoshida, K.; Kubota, H.; Toyao, T.; Shimizu, K.-I.; et al. Bulk vanadium oxide versus conventional V2O5/TiO2: NH3–SCR catalysts working at a low temperature below 150 °C. ACS Catal. 2019, 9, 9327–9331. [Google Scholar] [CrossRef]
- Yan, Z.; Shan, W.; Shi, X.; He, G.; Lian, Z.; Yu, Y.; Shan, Y.; Liu, J.; He, H. The way to enhance the thermal stability of V2O5-based catalysts for NH3-SCR. Catal. Today 2020, 355, 408–414. [Google Scholar] [CrossRef]
- Lee, K.J.; Kumar, P.A.; Maqbool, M.S.; Rao, K.N.; Song, K.H.; Ha, H.P. Ceria added Sb- V2O5/TiO2 catalysts for low temperature NH3 SCR: Physico-chemical properties and catalytic activity. Appl. Catal. B 2013, 142, 705–717. [Google Scholar] [CrossRef]
- Zhao, S.; Peng, J.; Ge, R.; Yang, K.; Wu, S.; Qian, Y.; Xu, T.; Gao, J.; Chen, Y.; Sun, Z. Poisoning and regeneration of commercial V2O5-WO3/TiO2 selective catalytic reduction (SCR) catalyst in coal-fired power plants. Process Saf. Environ. Prot. 2022, 168, 971–992. [Google Scholar] [CrossRef]
- Jeon, S.W.; Song, I.; Lee, H.; Kim, J.; Byun, Y.; Koh, D.J.; Kim, D.H. Enhanced SO2 resistance of V2O5-WO3/TiO2 catalyst physically mixed with alumina for the selective catalytic reduction of NOx with NH3. Chem. Eng. J. 2022, 433, 133836. [Google Scholar] [CrossRef]
- Zhu, H.; Song, L.; Li, K.; Wu, R.; Qiu, W.; He, H. Low-temperature SCR catalyst development and industrial applications in China. Catalysts 2022, 12, 341. [Google Scholar] [CrossRef]
- Consentino, L.; Pantaleo, G.; Parola, V.L.; Migliore, C.; Greca, E.L.; Liotta, L.F. NH3-NO SCR catalysts for engine exhaust gases abatement: Replacement of toxic V2O5 with MnOx to improve the environmental sustainability. Top. Catal. 2023, 66, 850–859. [Google Scholar] [CrossRef]
- Reddy, B.M.; Ganesh, I.; Reddy, E.P. Study of dispersion and thermal stability of V2O5/TiO2− SiO2 catalysts by XPS and other techniques. J. Phys. Chem. B 1997, 101, 1769–1774. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Y.; Yuan, H. Recycling strategies of spent V2O5-WO3/TiO2 catalyst: A review. Resour. Conserv. Recycl. 2020, 161, 104983. [Google Scholar] [CrossRef]
- Ding, L.; Wang, Y.; Qian, L.; Qi, P.; Xie, M.; Long, H. Flue gas deNOxing spent V2O5-WO3/TiO2 catalyst: A review of deactivation mechanisms and current disposal status. Fuel 2023, 338, 127268. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Tian, J.; Zhong, Y.; Zou, Z.; Dong, R.; Gao, S.; Xu, W.; Tan, D. The effects of Mn-based catalysts on the selective catalytic reduction of NOx with NH3 at low temperature: A review. Fuel Process. Technol. 2022, 230, 107213. [Google Scholar] [CrossRef]
- Mohan, S.; Dinesha, P.; Kumar, S. NOx reduction behaviour in copper zeolite catalysts for ammonia SCR systems: A review. Chem. Eng. J. 2020, 384, 123253. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, B.; Liu, B.; Sun, S. A review of Mn-containing oxide catalysts for low temperature selective catalytic reduction of NOx with NH3: Reaction mechanism and catalyst deactivation. RCS Adv. 2017, 7, 26226–26242. [Google Scholar] [CrossRef]
- Bonelli, B.; Tammaro, O.; Martinovic, F.; Nasi, R.; Dell’Agli, G.; Rivolo, P.; Giorgis, F.; Ditaranto, N.; Deorsola, F.A.; Esposito, S. Reverse Micelle Strategy for the Synthesis of MnOx–TiO2 Active Catalysts for NH3-Selective Catalytic Reduction of NOx at Both Low Temperature and Low Mn Content. ACS Omega 2021, 6, 24562–24574. [Google Scholar] [CrossRef]
- Kumar, M.S.; Alphin, M.S.; Kumar, P.S.; Raja, S. A review on zeolite catalyst for de NOx performance in ammonia–selective catalytic reduction. Fuel 2023, 334, 126828. [Google Scholar] [CrossRef]
- Theis, J.R. The poisoning and desulfation characteristics of iron and copper SCR catalysts. SAE Int. J. Fuels Lubr. 2009, 2, 324–331. [Google Scholar] [CrossRef]
- Wang, H.; Huang, B.; Yu, C.; Lu, M.; Huang, H.; Zhou, Y. Research progress, challenges and perspectives on the sulfur and water resistance of catalysts for low temperature selective catalytic reduction of NOx by NH3. Appl. Catal. A Gen. 2019, 588, 117207. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, J.; Zhang, J.; Zhu, H.; Feng, Y.; Jin, J. Effect of ceria doping on the catalytic activity and SO2 resistance of MnOx/TiO2 catalysts for the selective catalytic reduction of NO with NH3 at low temperatures. Aerosol Air Qual. Res. 2020, 20, 477–488. [Google Scholar] [CrossRef]
- Wang, L.; Ren, Y.; Yu, X.; Peng, C.; Yu, D.; Zhong, C.; Hou, J.; Yin, C.; Fan, X.; Zhao, Z.; et al. Novel preparation method, catalytic performance and reaction mechanisms of PrxMn1− xOδ/3DOM ZSM-5 catalysts for the simultaneous removal of soot and NOx. J. Catal. 2023, 417, 226–247. [Google Scholar] [CrossRef]
- Mu, J.; Liu, J.; Qin, J.; Li, X.; Liu, B. Unveiling remarkable resistance to Pb poisoning over an Fe–Mo catalyst for low-temperature NH3-SCR: Poison transforms into a promoter. Catal. Sci. Technol. 2022, 12, 4388–4400. [Google Scholar] [CrossRef]
- Mosrati, J.; Atia, H.; Eckelt, R.; Vuong, T.H.; Rabeah, J.; Mhamdi, M.; Armbruster, U. Ta and Mo oxides supported on CeO2-TiO2 for the selective catalytic reduction of NOx with NH3 at low temperature. J. Catal. 2021, 394, 325–339. [Google Scholar] [CrossRef]
- Kwon, D.W.; Choi, J.; Nam, K.B.; Ha, H.P. New insight into the role of Mo–Sb addition towards VMoSbTi catalysts with enhanced activity for selective catalytic reduction with NH3. Chem. Eng. J. 2022, 428, 132078. [Google Scholar] [CrossRef]
- Park, Y.J.; Kim, D.H.; Lee, J.H.; Ha, H.P.; Kwon, D.W. Unravelling the promotional effect of Nb and Mo on VOx-based catalysts for NOx reduction with NH3. Appl. Surf. Sci. 2023, 614, 156072. [Google Scholar] [CrossRef]
- Yu, J.; Wang, C.; Wang, Y.; Qiu, L.; Yin, Y.; Li, X.; Zhao, F.; Chang, H. Poisoning Effects of HCl on MOx-WO3/TiO2 (M= Mn, Ce and V) Catalysts for Selective Catalytic Reduction of NOx by NH3. ChemCatChem 2023, 15, e202201093. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, D.; Bao, X. Catalysis for selected C1 chemistry. Chem 2020, 6, 2497–2514. [Google Scholar] [CrossRef]
- Iwasaki, M.; Dohmae, K.; Nagai, Y.; Sudo, E.; Tanaka, T. Experimental assessment of the bifunctional NH3-SCR pathway and the structural and acid-base properties of WO3 dispersed on CeO2 catalysts. J. Catal. 2018, 359, 55–67. [Google Scholar] [CrossRef]
- Can, F.; Berland, S.; Royer, S.; Courtois, X.; Duprez, D. Composition-Dependent Performance of CexZr1–xO2 Mixed-Oxide-Supported WO3 Catalysts for the NOx Storage Reduction–Selective Catalytic Reduction Coupled Process. ACS Catal. 2013, 3, 1120–1132. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Kang, M. Recent advances in heighten sulfur resistance of SCR catalysts: A review. Environ. Eng. Res. 2022, 27, 200642. [Google Scholar] [CrossRef]
- Chao, Y.; Zhang, W.; Zhou, P.; Chen, H.; Lu, S.; Li, M.; Zhang, Q.; Gu, L.; Guo, S. An in-situ NH4+-etched strategy for anchoring atomic Mo site on ZnIn2S4 hierarchical nanotubes for superior hydrogen photocatalysis. Sci. China Chem. 2021, 64, 1716–1722. [Google Scholar] [CrossRef]
- Du, P.; Hu, K.; Lyu, J.; Li, H.; Lin, X.; Xie, G.; Liu, X.; Ito, Y.; Qiu, H.-J. Anchoring Mo single atoms/clusters and N on edge-rich nanoporous holey graphene as bifunctional air electrode in Zn− air batteries. Appl. Catal. B 2020, 276, 119172. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.; Chen, X.; Rui, N.; Betancourt, L.E.; Lin, L.; Xu, W.; Sun, C.-J.; Abeykoon, A.M.M.; Rodriguez, J.A.; et al. Effects of Zr doping into ceria for the dry reforming of methane over Ni/CeZrO2 catalysts: In situ studies with XRD, XAFS, and AP-XPS. ACS Catal. 2020, 10, 3274–3284. [Google Scholar] [CrossRef]
- Das, S.; Jangam, A.; Jayaprakash, S.; Xi, S.; Hidajat, K.; Tomishige, K.; Kawi, S. Role of lattice oxygen in methane activation on Ni-phyllosilicate@Ce1-xZrxO2 core-shell catalyst for methane dry reforming: Zr doping effect, mechanism, and kinetic study. Appl. Catal. B 2021, 290, 119998. [Google Scholar] [CrossRef]
- Chen, J.; Carlson, B.D.; Toops, T.J.; Li, Z.; Lance, M.J.; Karakalos, S.G.; Choi, J.-S.; Kyriakidou, E.A. Methane Combustion Over Ni/ CexZr1–xO2 Catalysts: Impact of Ceria/Zirconia Ratio. ChemCatChem 2020, 12, 5558–5568. [Google Scholar] [CrossRef]
- Esposito, S.; Turco, M.; Bagnasco, G.; Cammarano, C.; Pernice, P. New insight into the preparation of copper/zirconia catalysts by sol–gel method. Appl. Catal. A Gen. 2011, 403, 128–135. [Google Scholar] [CrossRef]
- Esposito, S.; Turco, M.; Bagnasco, G.; Cammarano, C.; Pernice, P.; Aronne, A. Highly dispersed sol–gel synthesized Cu–ZrO2 materials as catalysts for oxidative steam reforming of methanol. Appl. Catal. A Gen. 2010, 372, 48–57. [Google Scholar] [CrossRef]
- Spiridigliozzi, L.; Dell’Agli, G.; Biesuz, M.; Sglavo, V.M.; Pansini, M. Effect of the precipitating agent on the synthesis and sintering behavior of 20 mol % Sm-doped ceria. Adv. Mater. Sci. Eng. 2016, 2016, 6096123. [Google Scholar] [CrossRef]
- Tammaro, O.; Paparo, R.; Chianese, M.; Ritacco, I.; Caporaso, L.; Camellone, M.F.; Esposito, S. Reverse micelle strategy for effective substitutional Fe-doping in small-sized CeO2 nanocrystals: Assessment of adsorption and photodegradation efficiency of ibuprofen under visible light. Chem. Eng. J. 2024, 479, 147909. [Google Scholar] [CrossRef]
- Dell’Agli, G.; Mascolo, G.; Mascolo, M.C.; Pagliuca, C. Drying effect on thermal behavior and structural modifications of hydrous zirconia gel. J. Am. Ceram. Soc. 2008, 91, 3375–3379. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Ma, X.; Wang, W.; Li, X.; Zhang, Z.; Qian, Y. Hydrothermal route to single crystalline α-MoO3 nanobelts and hierarchical structures. Solid State Commun. 2005, 136, 283–287. [Google Scholar] [CrossRef]
- Vegard, L. Die Röntgenstrahlen im dienste der erforschung der materie. Z. Für Krist.-Cryst. Mater. 1928, 67, 239–259. [Google Scholar] [CrossRef]
- Turco, R.; Bonelli, B.; Armandi, M.; Spiridigliozzi, L.; Dell’Agli, G.; Deorsola, F.A.; Esposito, S.; Di Serio, M. Active and stable ceria-zirconia supported molybdenum oxide catalysts for cyclooctene epoxidation: Effect of the preparation procedure. Catal. Today 2020, 345, 201–212. [Google Scholar] [CrossRef]
- Thommes, M. Physical adsorption characterization of nanoporous materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Thommes, M. Progress in the physisorption characterization of nanoporous gas storage materials. Engineering 2018, 4, 559–566. [Google Scholar] [CrossRef]
- Grosman, A.; Ortega, C. Capillary condensation in porous materials. Hysteresis and interaction mechanism without pore blocking/percolation process. Langmuir 2008, 24, 3977–3986. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Sing, K.S.; Williams, R.T. Physisorption hysteresis loops and the characterization of nanoporous materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Manzoli, M.; Tammaro, O.; Marocco, A.; Bonelli, B.; Barrera, G.; Tiberto, P.; Allia, P.; Matéo-Vélez, J.-C.; Roggero, A.; Dantras, E.; et al. New insights in the production of simulated moon agglutinates: The use of natural zeolite-bearing rocks. ACS Earth Space Chem. 2021, 5, 1631–1646. [Google Scholar] [CrossRef]
- Loi, Q.K.; Tan, S.J.; Do, D.D.; Nicholson, D. Lower closure point for nitrogen or argon adsorption in mesoporous solids: Window-induced evaporation or surface-induced cavitation? Ind. Eng. Chem. Res. 2021, 60, 15343–15351. [Google Scholar] [CrossRef]
- Arfaoui, J.; Ghorbel, A.; Petitto, C.; Delahay, G. New MoO3-CeO2-ZrO2 and WO3-CeO2-ZrO2 nanostructured mesoporous aerogel catalysts for the NH3-SCR of NO from diesel engine exhaust. J. Ind. Eng. Chem. 2021, 95, 182–189. [Google Scholar] [CrossRef]
- Yang, Z.; Wei, Y.; Fu, Z.; Lu, Z.; Hermansson, K. Facilitated vacancy formation at Zr-doped ceria (111) surfaces. Surf. Sci. 2008, 602, 1199–1206. [Google Scholar] [CrossRef]
- Dell’Agli, G.; Mascolo, G. Agglomeration of 3 mol% Y–TZP powders synthesized by hydrothermal treatment. J. Eur. Ceram. Soc. 2001, 21, 29–35. [Google Scholar] [CrossRef]
- Biesuz, M.; Spiridigliozzi, L.; Frasnelli, M.; Dell’Agli, G.; Sglavo, V.M. Rapid densification of Samarium-doped Ceria ceramic with nanometric grain size at 900–1100 °C. Mater. Lett. 2017, 190, 17–19. [Google Scholar] [CrossRef]
- Pan, W.; He, J.; Huang, G.; Zhang, W.; Fang, D. Research Progress of the Selective Catalytic Reduction with NH3 over ZSM-5 Zeolite Catalysts for NOx Removal. Catalysts 2023, 13, 1381. [Google Scholar] [CrossRef]
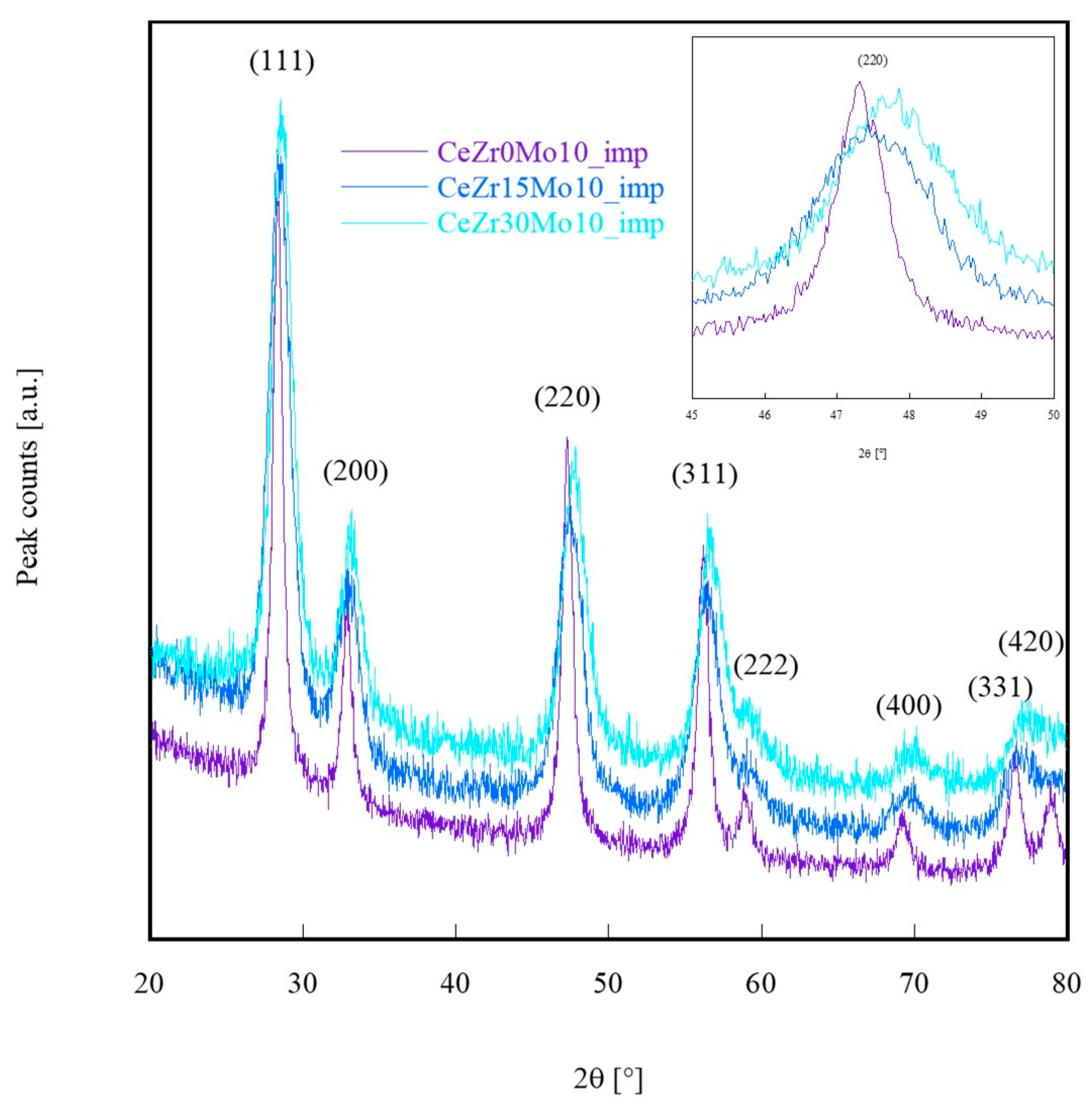
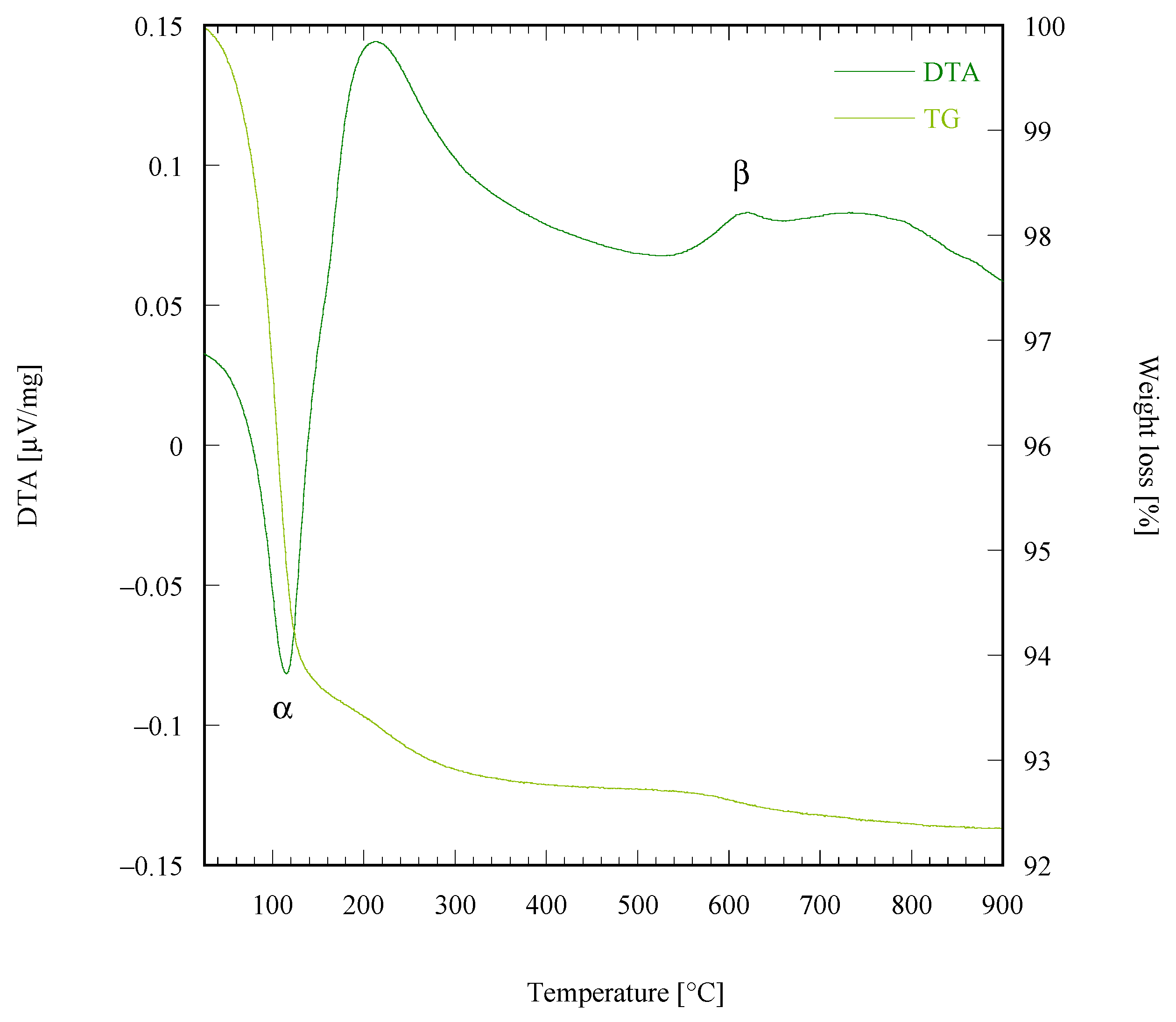
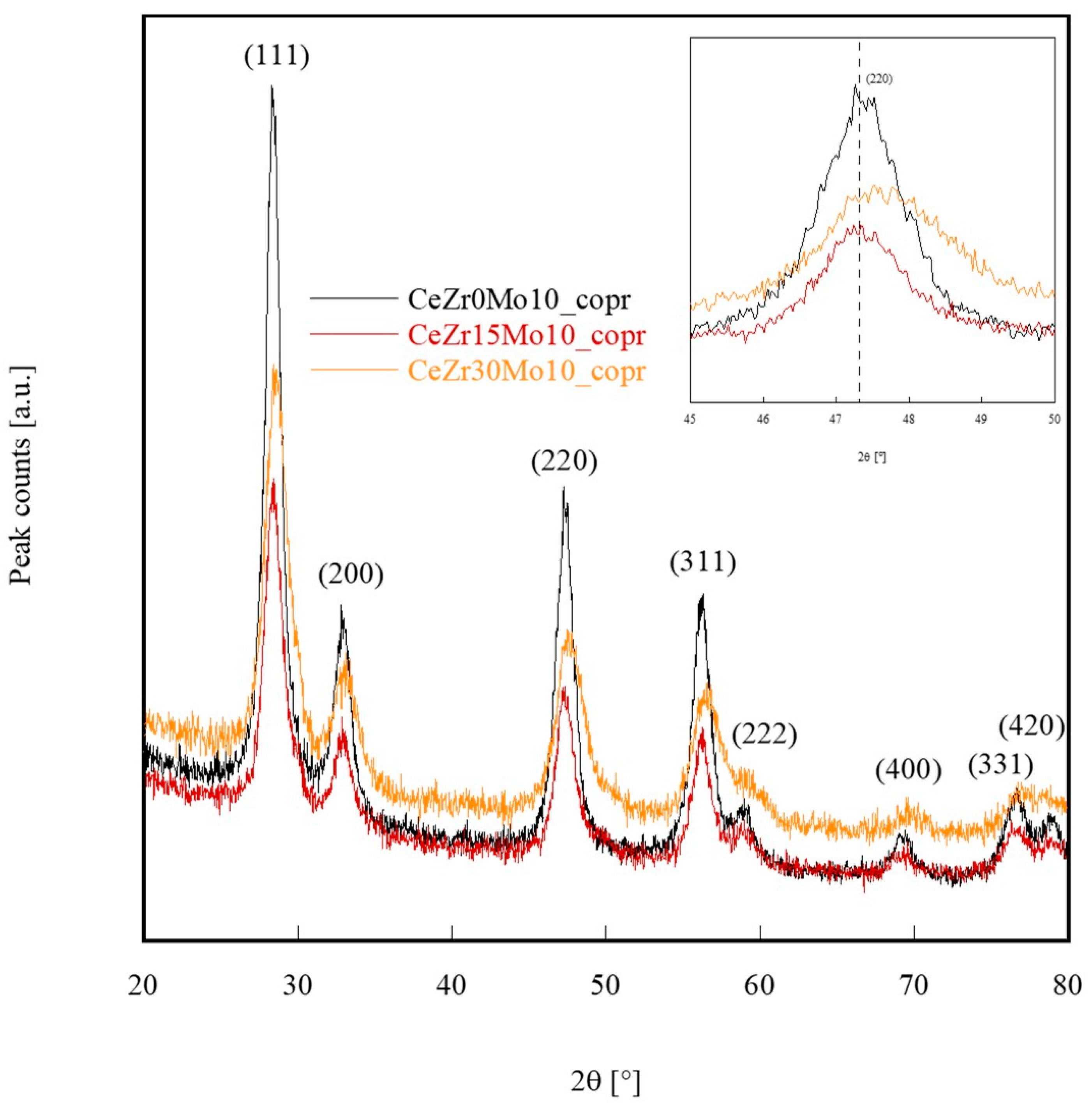
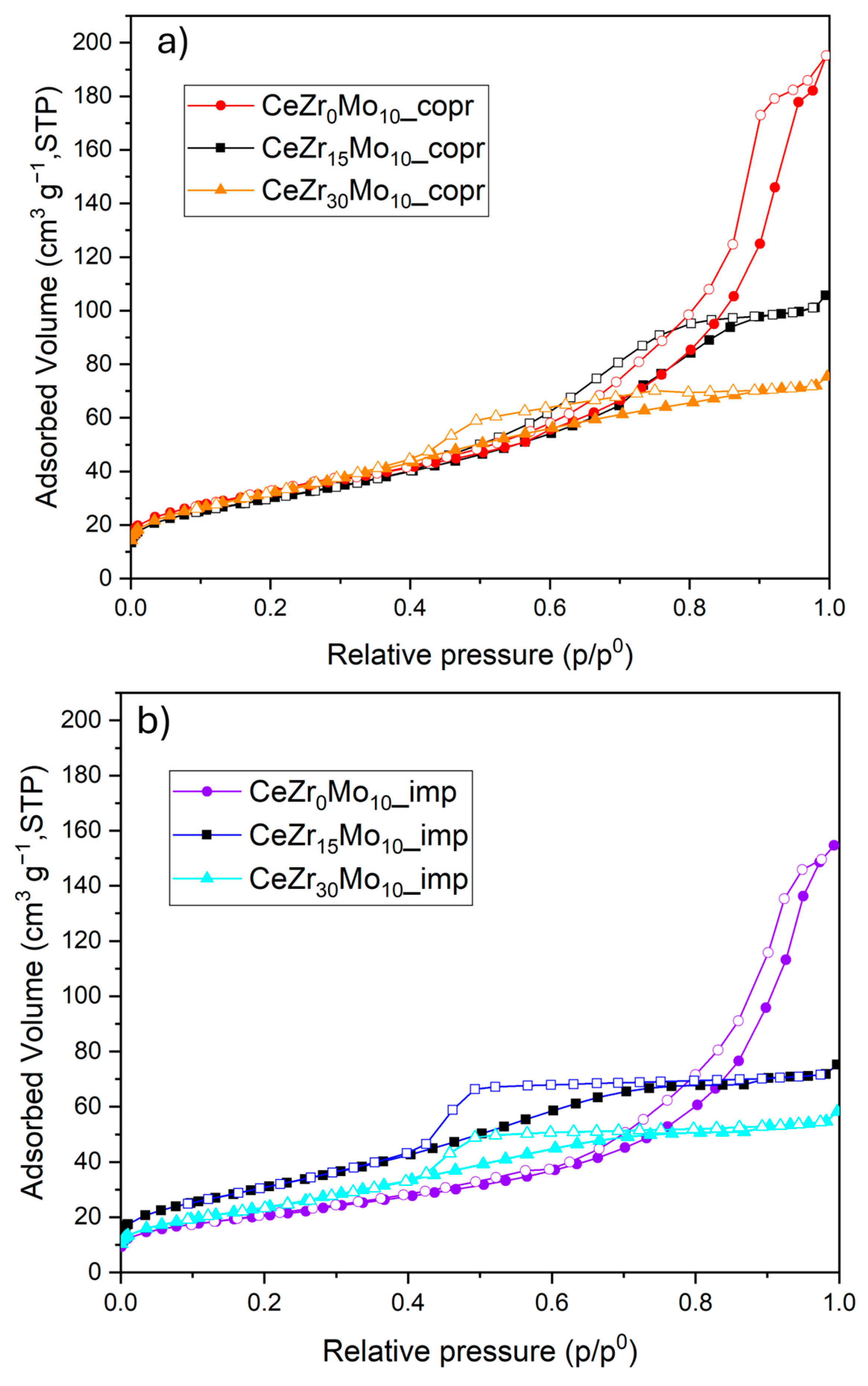
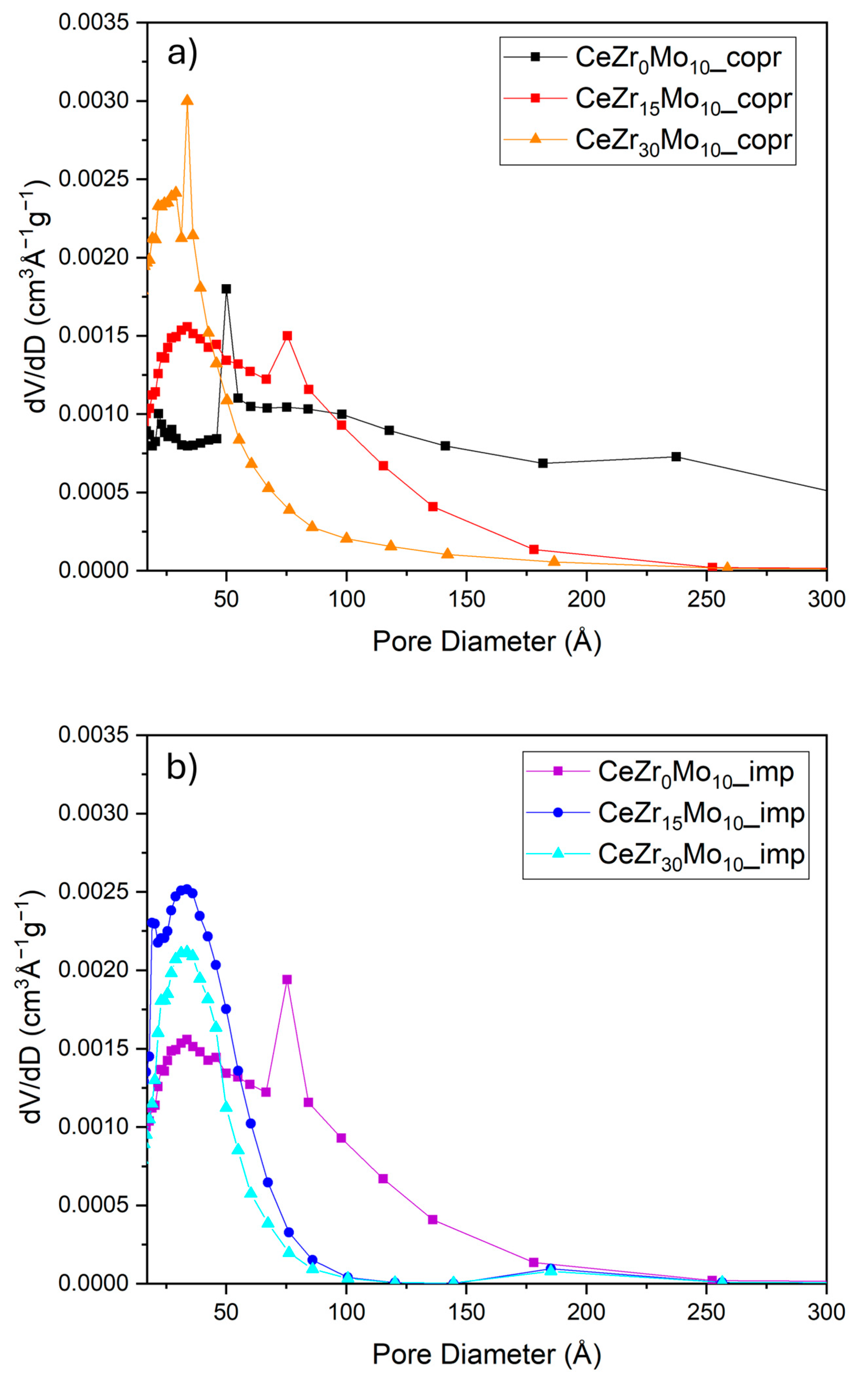
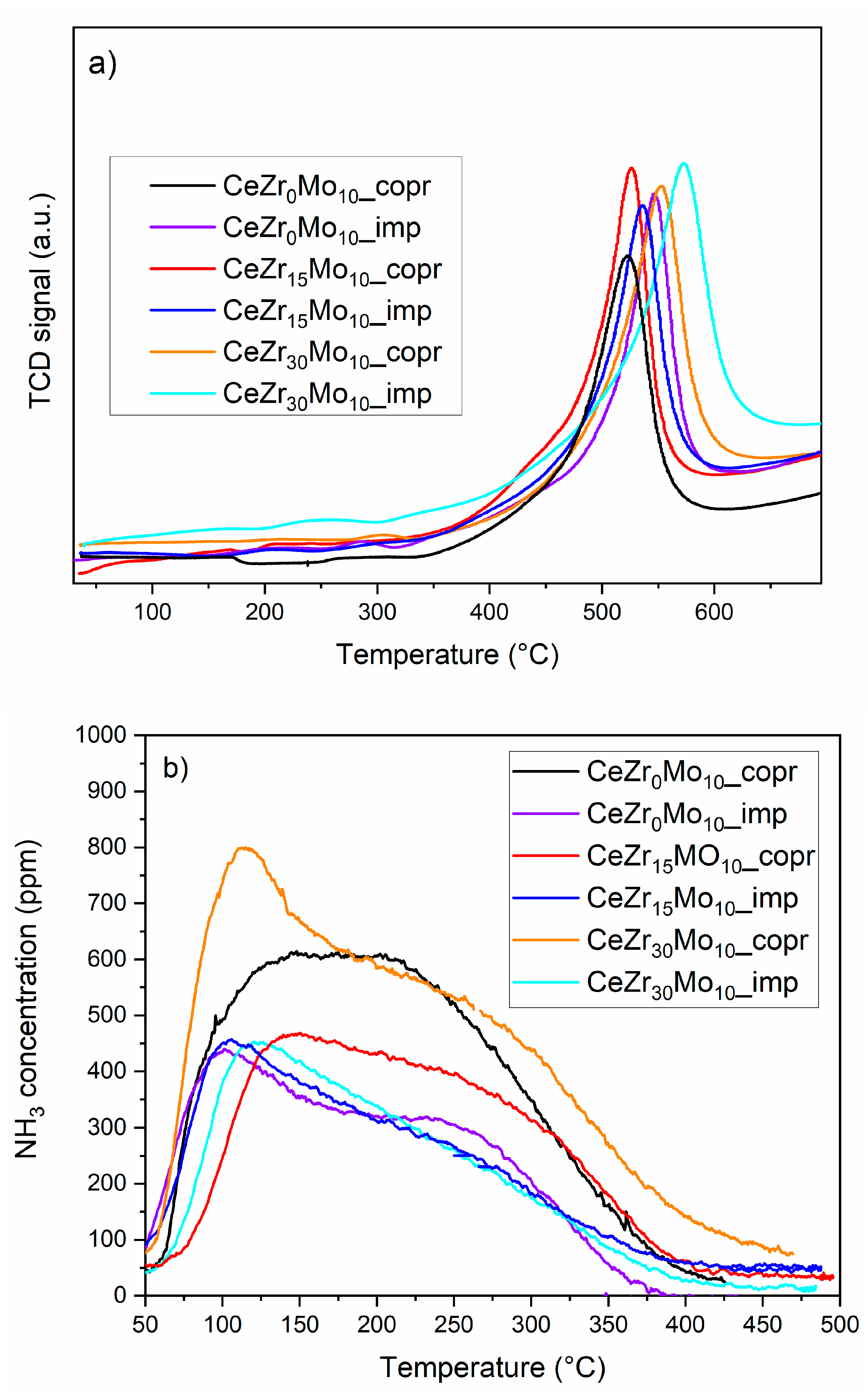
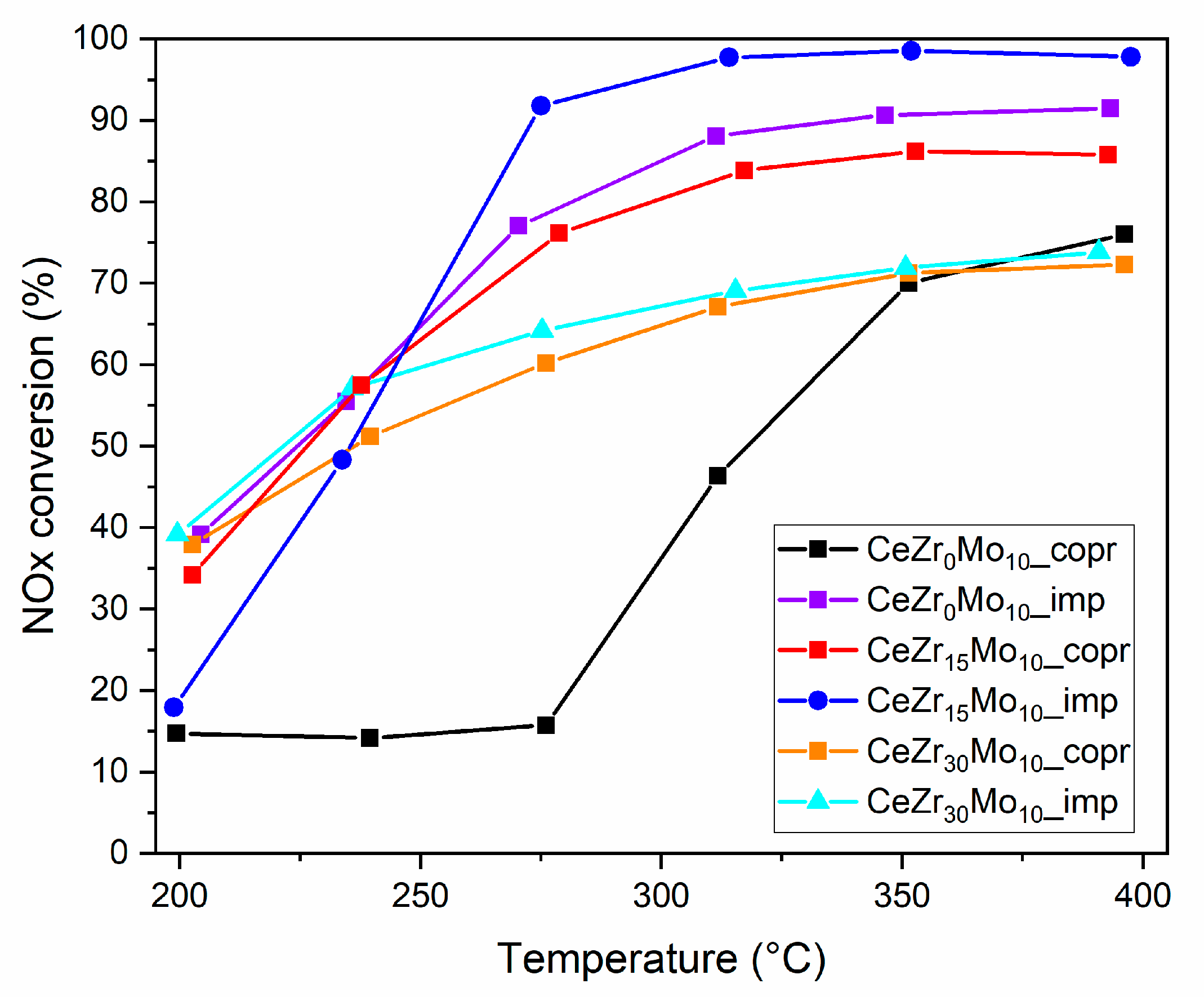
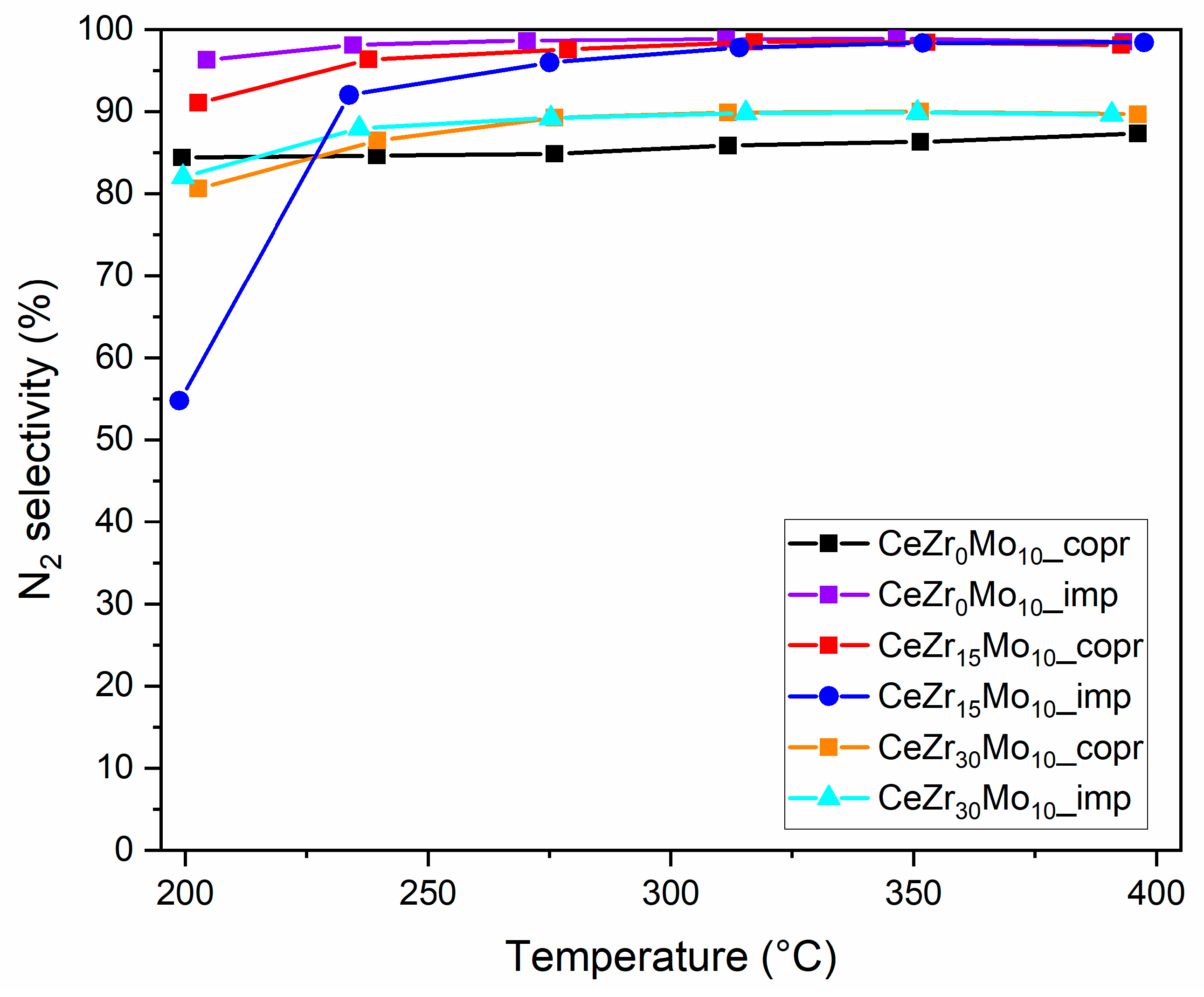
| Sample Labeling | Composition | Mo Addition | Precipitation Temperature |
|---|---|---|---|
| CeZr0Mo10_imp | 10MoO3/CeO2 | Impregnation | r.t. |
| CeZr15Mo10_imp | 10MoO3/Ce0.85Zr0.15O2 | Impregnation | r.t. |
| CeZr30Mo10_imp | 10MoO3/Ce0.70Zr0.30O2 | Impregnation | r.t. |
| CeZr0Mo10_copr | 10MoO3/CeO2 | Co-precipitation | 90 °C |
| CeZr15Mo10_copr | 10MoO3/Ce0.85Zr0.15O2 | Co-precipitation | 90 °C |
| CeZr30Mo10_copr | 10MoO3/Ce0.70Zr0.30O2 | Co-precipitation | 90 °C |
| Sample | a (Calculated) [nm] | a (Vegard’s Law) [nm] | Crystal Size [nm] |
|---|---|---|---|
| CeZr0Mo10_imp | 0.54266 | 0.54113 | 11.1 |
| CeZr15Mo10_imp | 0.54080 | 0.53690 | 5.3 |
| CeZr30Mo10_imp | 0.53795 | 0.53266 | 7.8 |
| Sample | a (Calculated) [nm] | a (Vegard’s Law) [nm] | Crystal Size [nm] |
|---|---|---|---|
| CeZr0Mo10_copr | 0.54225 | 0.54113 | 9.5 |
| CeZr15Mo10_copr | 0.54227 | 0.53690 | 5.4 |
| CeZr30Mo10_copr | 0.54047 | 0.53266 | 4.0 |
| Sample | Sbet (m2 g−1) a | Vp (cm3 g−1) | Adsorbed NH3 (µmol g−1) | H2 Uptake (µmol g−1) |
|---|---|---|---|---|
| CeZr0Mo10_copr | 113 | 0.302 | 203 | 1133 |
| CeZr15Mo10_copr | 108 | 0.164 | 171 | 1351 |
| CeZr30Mo10_copr | 118 | 0.117 | 174 | 1402 |
| CeZr0Mo10_imp | 75 | 0.239 | 140 | 955 |
| CeZr15Mo10_imp | 116 | 0.116 | 194 | 1081 |
| CeZr30Mo10_imp | 92 | 0.090 | 144 | 1374 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiridigliozzi, L.; Monfreda, V.; Esposito, S.; Tammaro, O.; Blangetti, N.; Deorsola, F.A.; Dell’Agli, G. Compositional and Fabrication Cycle Optimization of Ceria-Zirconia-Supported Mo-Based Catalysts for NH3-SCR NOx Reduction. Inorganics 2024, 12, 217. https://doi.org/10.3390/inorganics12080217
Spiridigliozzi L, Monfreda V, Esposito S, Tammaro O, Blangetti N, Deorsola FA, Dell’Agli G. Compositional and Fabrication Cycle Optimization of Ceria-Zirconia-Supported Mo-Based Catalysts for NH3-SCR NOx Reduction. Inorganics. 2024; 12(8):217. https://doi.org/10.3390/inorganics12080217
Chicago/Turabian StyleSpiridigliozzi, Luca, Viviana Monfreda, Serena Esposito, Olimpia Tammaro, Nicola Blangetti, Fabio Alessandro Deorsola, and Gianfranco Dell’Agli. 2024. "Compositional and Fabrication Cycle Optimization of Ceria-Zirconia-Supported Mo-Based Catalysts for NH3-SCR NOx Reduction" Inorganics 12, no. 8: 217. https://doi.org/10.3390/inorganics12080217








