Tunable Electronic and Magnetic Properties of 3d Transition Metal Atom-Intercalated Transition Metal Dichalcogenides: A Density Functional Theory Study
Abstract
:1. Introduction
2. Results and Discussion
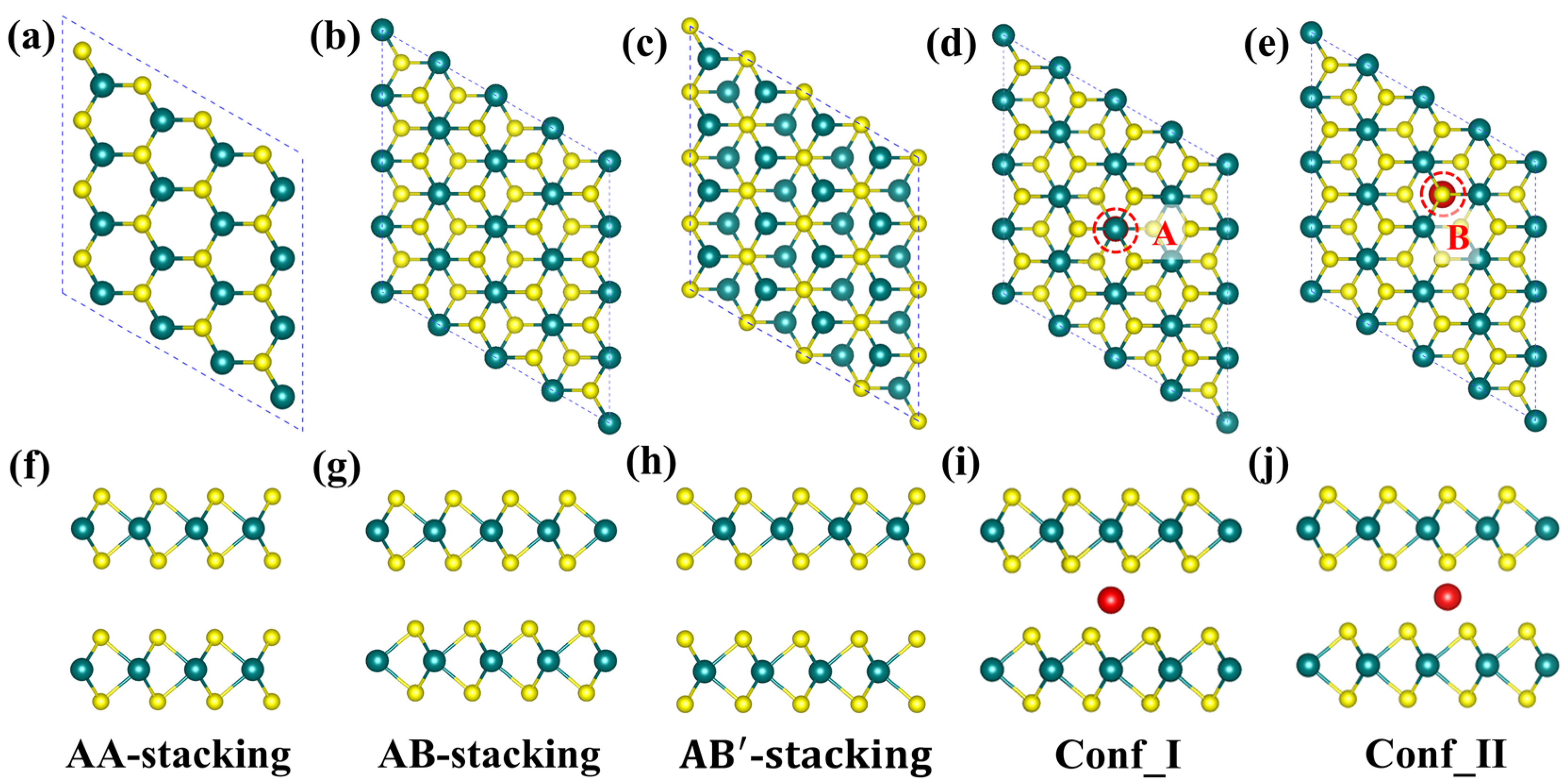
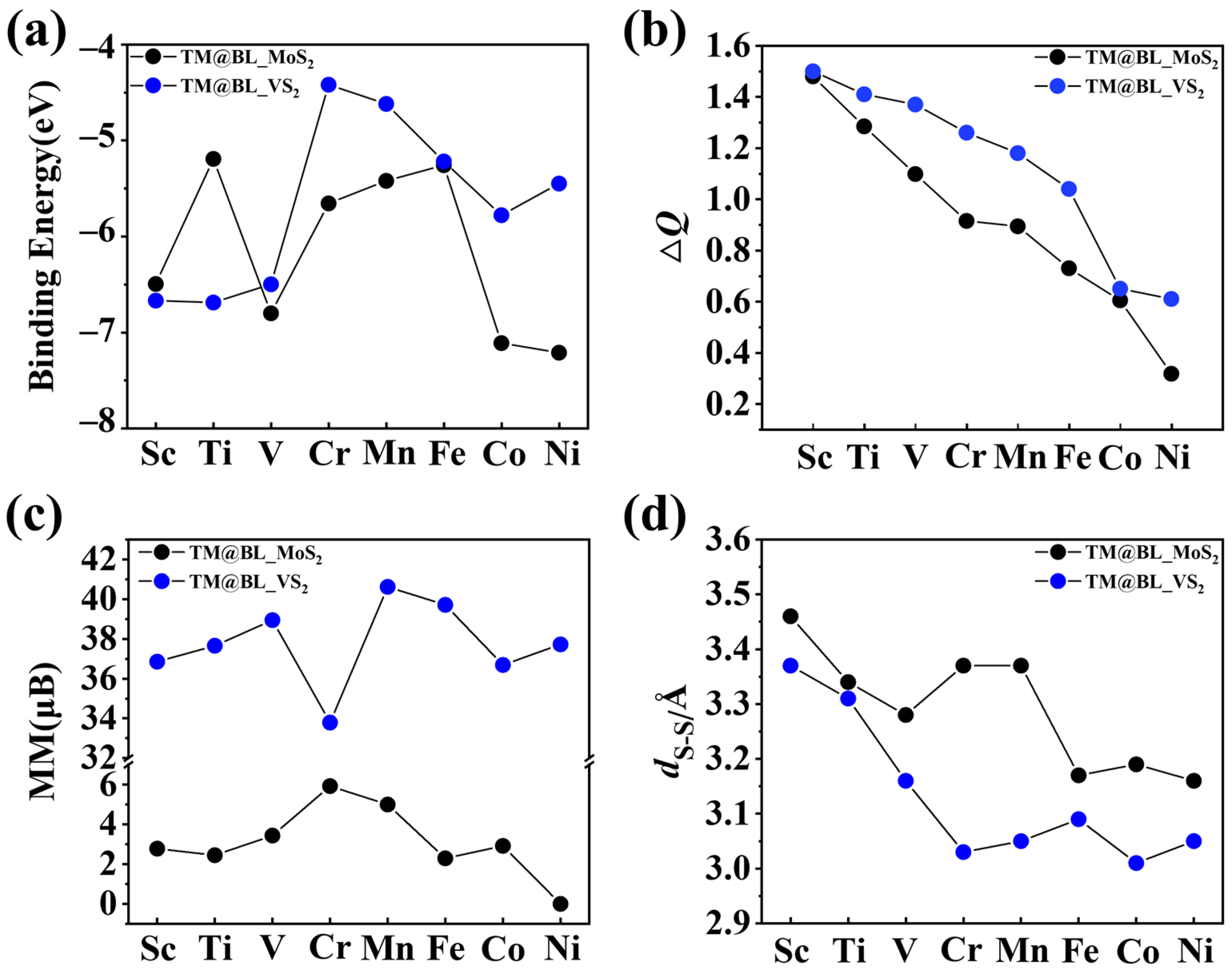
2.1. Structure and Stability of TM@BL_MS2 (TM = Sc–Ni, M = Mo, V)
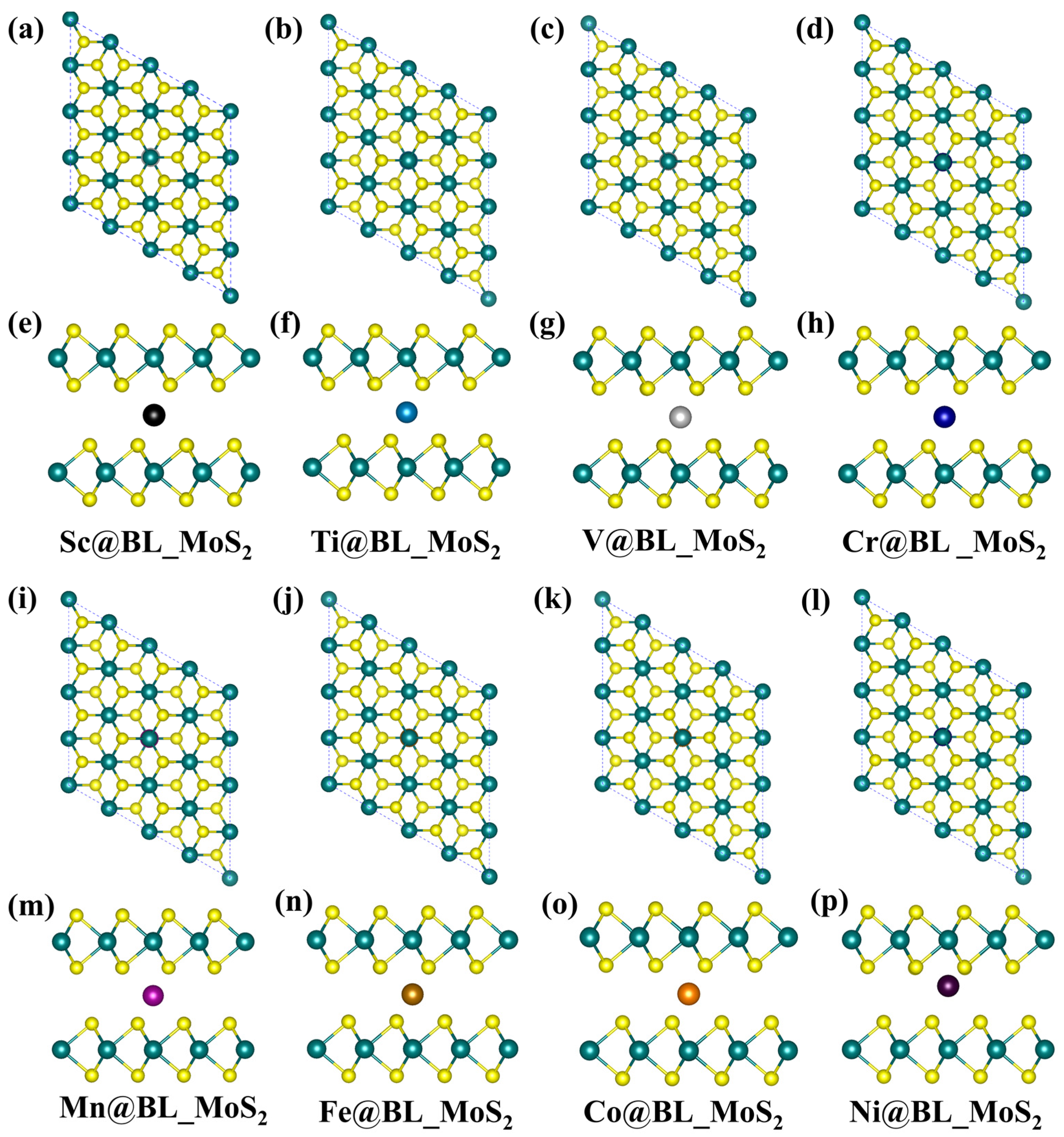
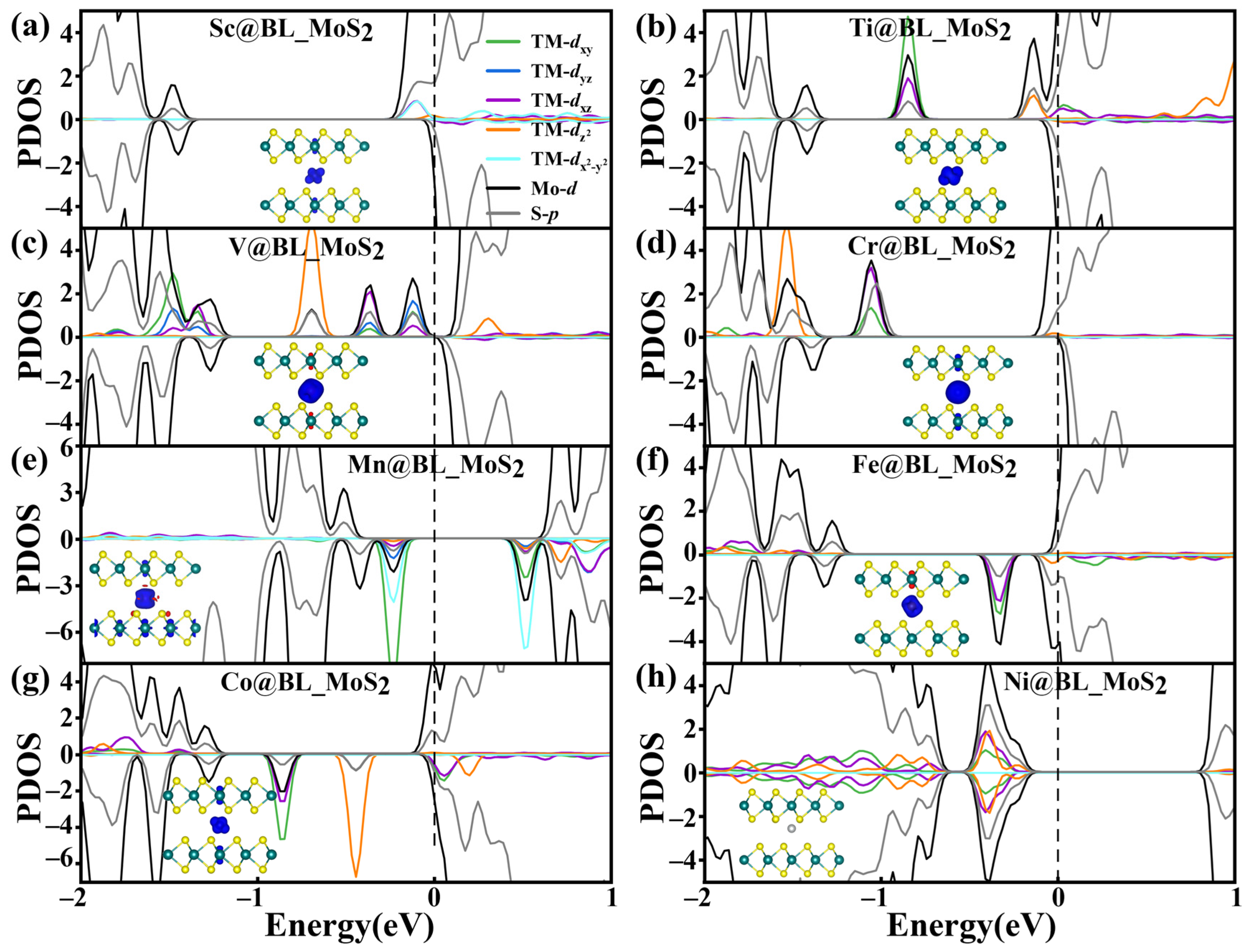
2.2. Electronic and Magnetic Properties of TM@BL_MS2 (TM = Sc–Ni, M = Mo, V)
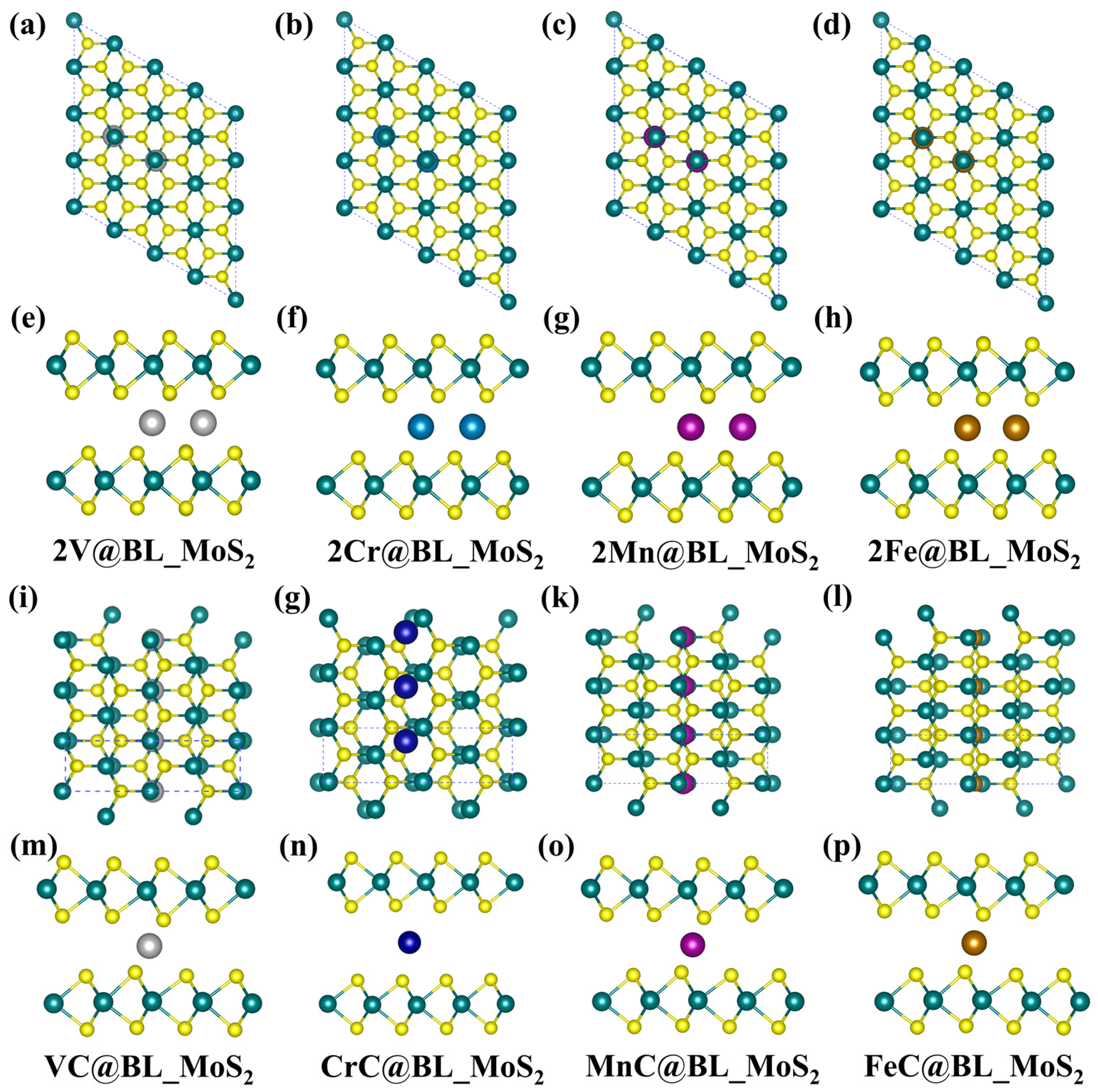
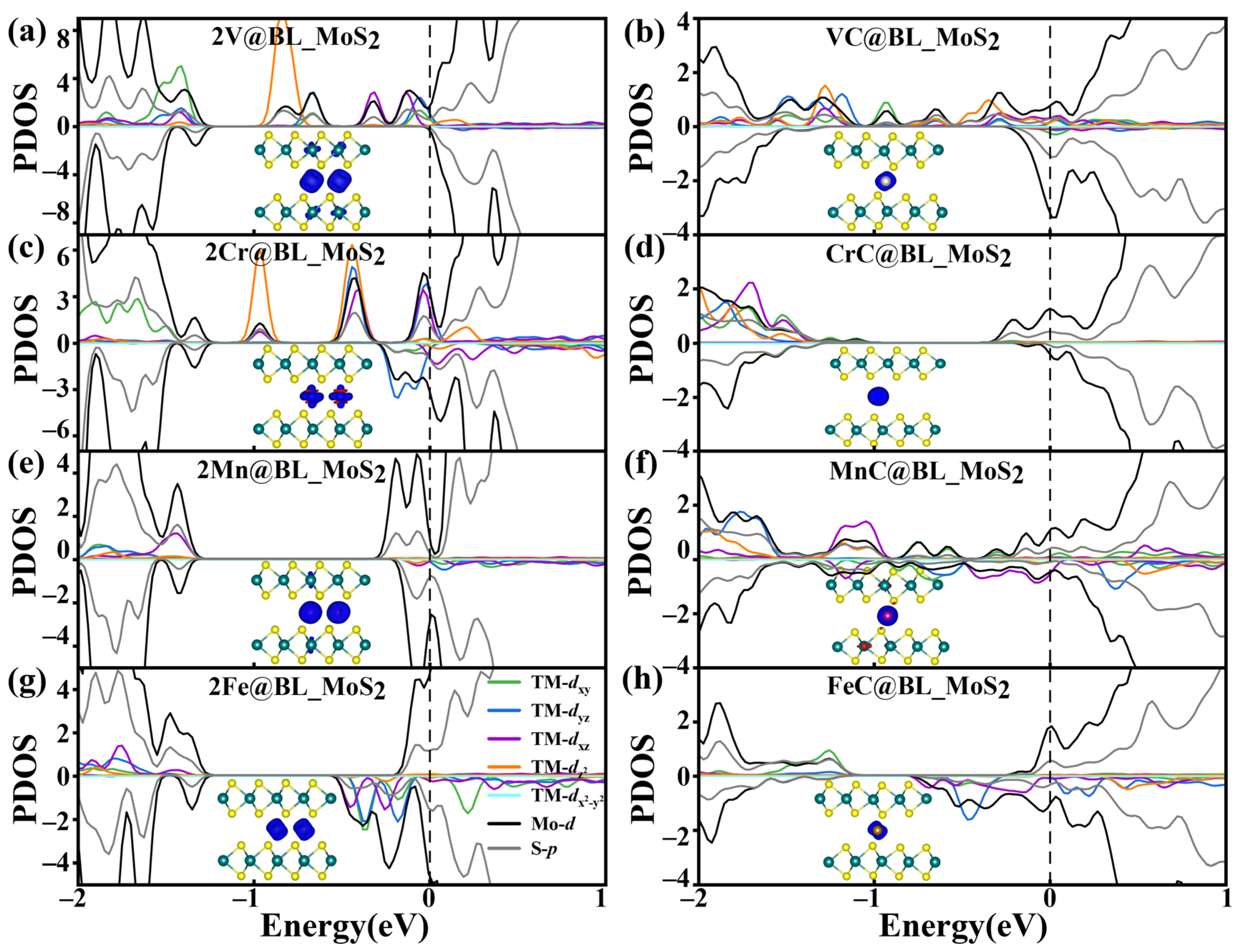
2.3. Effect of TM Intercalation Concentration on the TM BL_MS2 (TM = V, Cr, Mn, Fe, M = Mo)
3. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Chen, S.; Shi, G.Q. Two-dimensional materials for halide perovskite-based optoelectronic devices. Adv. Mater. 2017, 29, 1605448. [Google Scholar] [CrossRef]
- Geim, A.K.; Grigorieva, I.V. Van der Waals heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef]
- Williams, T.J.; Aczel, A.A.; Lumsden, M.D.; Nagler, S.E.; Stone, M.B.; Yan, J.Q.; Mandrus, D. Magnetic correlations in the quasi-two-dimensional semiconducting ferromagnet CrSiTe3. Phys. Rev. B 2015, 92, 144404. [Google Scholar] [CrossRef]
- Jiang, S.W.; Li, L.Z.; Wang, Z.F.; Mak, K.F.; Shan, J. Controlling magnetism in 2D CrI3 by electrostatic doping. Nat. Nanotechnol. 2018, 13, 549–553. [Google Scholar] [CrossRef]
- Son, S.; Coak, M.J.; Lee, N.; Kim, J.; Kim, T.Y.; Hamidov, H.; Cho, H.; Liu, C.; Jarvis, D.M.; Brown, P.A.C.; et al. Bulk properties of the van der Waals hard ferromagnet VI3. Phys. Rev. B 2019, 99, 041402. [Google Scholar] [CrossRef]
- Gong, C.; Li, L.; Li, Z.L.; Ji, H.W.; Stern, A.; Xia, Y.; Cao, T.; Bao, W.; Wang, C.Z.; Wang, Y.A.; et al. Discovery of intrinsic ferromagnetism in two-dimensional van der Waals crystals. Nature 2017, 546, 265–269. [Google Scholar] [CrossRef]
- Meng, L.J.; Zhou, Z.; Xu, M.Q.; Yang, S.Q.; Si, K.P.; Liu, L.X.; Wang, X.G.; Jiang, H.N.; Li, B.X.; Qin, P.X.; et al. Anomalous thickness dependence of Curie temperature in air-stable two-dimensional ferromagnetic 1T-CrTe2 grown by chemical vapor deposition. Nat. Commun. 2021, 12, 809. [Google Scholar] [CrossRef]
- Qin, J.; Yang, C.; Yakushi, K.; Nakazawa, Y.; Ichimura, K.; Liu, D. Synthesis and ferromagnetism of a new intercalation compound: Mn0.86PS3(bipy)0.56. Synth. Met. 1997, 85, 1673–1674. [Google Scholar] [CrossRef]
- Chu, H.; Roh, C.J.; Island, J.O.; Li, C.; Lee, S.; Chen, J.J.; Park, J.G.; Young, A.F.; Lee, J.S.; Hsieh, D. Linear magnetoelectric phase in ultrathin MnPS3 probed by optical second harmonic generation. Phys. Rev. Lett. 2020, 124, 027601. [Google Scholar] [CrossRef] [PubMed]
- Chittari, B.L.; Park, Y.; Lee, D.; Han, M.; MacDonald, A.H.; Hwang, E.; Jung, J. Electronic and magnetic properties of single-layer MPX3 metal phosphorous trichalcogenides. Phys. Rev. B 2016, 94, 184428. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, D.; Zheng, W.; Ma, Y.; Chen, C. Global structural optimization of tungsten borides. Phys. Rev. Lett. 2013, 110, 136403. [Google Scholar] [CrossRef]
- Sharma, A.; Rangra, V.S.; Thakur, A. Synthesis, properties, and applications of MBenes (two-dimensional metal borides) as emerging 2D materials: A review. J. Mater. Sci. 2022, 57, 12738–12751. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, J.; Sun, Z. MBenes: Progress, challenges and future. J. Mater. Chem. A 2022, 10, 15865–15880. [Google Scholar] [CrossRef]
- Du, M.; Li, D.; Liu, S.; Yan, J. Transition metal phosphides: A wonder catalyst for electrocatalytic hydrogen production. Chin. Chem. Lett. 2023, 34, 108156. [Google Scholar] [CrossRef]
- Zhang, N.; Amorim, I.; Liu, L. Multimetallic transition metal phosphide nanostructures for supercapacitors and electrochemical water splitting. Nanotechnology 2022, 33, 432004. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, C.; Wang, W.; Xiong, W.; Zeng, G.; Huang, D.; Zhang, C.; Song, B.; Xue, W.; Li, X.; et al. Recent advances in application of transition metal phosphides for photocatalytic hydrogen production. Chem. Eng. J. 2021, 405, 126547. [Google Scholar] [CrossRef]
- Sun, L.; Leong, W.S.; Yang, S.; Chisholm, M.F.; Liang, S.-J.; Ang, L.K.; Tang, Y.; Mao, Y.; Kong, J.; Yang, H.Y. Concurrent synthesis of high-performance monolayer transition metal disulfides. Adv. Funct. Mater. 2017, 27, 1605896. [Google Scholar] [CrossRef]
- Cheng, J.; Jin, Y.; Zhao, J.; Jing, Q.; Gu, B.; Wei, J.; Yi, S.; Li, M.; Nie, W.; Qin, Q.; et al. From VIB- to VB-group transition metal disulfides: Structure engineering modulation for superior electromagnetic wave absorption. Nano-Micro Lett. 2023, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qi, G.; Tang, C.; Chen, M.; Chen, Y.; Shu, Z.; Xiang, H.; Jin, Y.; Wang, S.; Li, H.; et al. Growth of large-area homogeneous monolayer transition-metal disulfides via a molten liquid intermediate process. ACS Appl. Mater. 2020, 12, 13174–13181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Y.; Jin, C.H.; Wu, J.L.; Ji, W. Magnetism in molybdenum disulphide monolayer with sulfur substituted by 3d transition metals. J. Appl. Phys. 2016, 120, 144305. [Google Scholar] [CrossRef]
- Lopez-Bezanilla, A.; Lado, J.L. Defect-induced magnetism and Yu-Shiba-Rusinov states in twisted bilayer graphene. Phys. Rev. Mater. 2019, 3, 084003. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Xi, B.; Liu, Y.J.; Yao, X.J.; Wu, X.J. Antiferromagnetic semimetal in Ti-intercalated borophene heterobilayer. J. Phys. Chem. C 2020, 124, 4709–4716. [Google Scholar] [CrossRef]
- Wan, J.Y.; Lacey, S.D.; Dai, J.Q.; Bao, W.Z.; Fuhrer, M.S.; Hu, L.B. Tuning two-dimensional nanomaterials by intercalation: Materials, properties and applications. Chem. Soc. Rev. 2016, 45, 6742–6765. [Google Scholar] [CrossRef]
- Kumar, P.; Skomski, R.; Pushpa, R. Magnetically ordered transition-metal-intercalated WSe2. ACS Omega 2017, 2, 7985–7990. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Xu, W.X.; Dai, J.P.; Liu, Y.J. Role of embedded 3d transition metal atoms on the electronic and magnetic properties of defective bilayer graphene. Carbon 2017, 118, 376–383. [Google Scholar] [CrossRef]
- Chen, Z.; Leng, K.; Zhao, X.; Malkhandi, S.; Tang, W.; Tian, B.; Dong, L.; Zheng, L.; Lin, M.; Yeo, B.S.; et al. Interface confined hydrogen evolution reaction in zero valent metal nanoparticles-intercalated molybdenum disulfide. Nat. Commun. 2017, 8, 14548. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, C.; You, Y.; Xie, H.; Chi, H.; Sun, Y.; Liu, W.; Su, X.; Yan, Y.; Tang, X.; et al. Electron density optimization and the anisotropic thermoelectric properties of Ti self-intercalated Ti1+xS2 compounds. ACS Appl. Mater. 2018, 10, 32344–32354. [Google Scholar] [CrossRef]
- Li, F.; Hu, R.; Huang, Z.; Luo, S.; Qiao, H.; Zhong, J.; Qi, X. Properties tuning and applications for two dimension materials in electrochemical intercalation process. Appl. Mater. Today 2024, 36, 102069. [Google Scholar] [CrossRef]
- Rajapakse, M.; Karki, B.; Abu, U.O.; Pishgar, S.; Musa, M.R.K.; Riyadh, S.M.S.; Yu, M.; Sumanasekera, G.; Jasinski, J.B. Intercalation as a versatile tool for fabrication, property tuning, and phase transitions in 2D materials. NPJ 2D Mater. Appl. 2021, 5, 30. [Google Scholar] [CrossRef]
- Guzman, R.; Liu, H.; Bian, C.; Bao, L.; Shen, C.-M.; Gao, H.-J.; Zhou, W. Temperature-induced structural evolution and magnetism in self-intercalated V1+xSe2 nanoplates. Adv. Funct. Mater. 2024, 2401304. [Google Scholar] [CrossRef]
- Zhao, X.X.; Song, P.; Wang, C.C.; Riis-Jensen, A.C.; Fu, W.; Deng, Y.; Wan, D.Y.; Kang, L.X.; Ning, S.C.; Dan, J.D.; et al. Engineering covalently bonded 2D layered materials by self-intercalation. Nature 2020, 581, 171–177. [Google Scholar] [CrossRef]
- Niu, J.; Yan, B.; Ji, Q.; Liu, Z.; Li, M.; Gao, P.; Zhang, Y.; Yu, D.; Wu, X. Anomalous hall effect and magnetic orderings in nanothick V5S8. Phys. Rev. B 2017, 96, 075402. [Google Scholar] [CrossRef]
- Matsuoka, H.; Barnes, S.E.; Ieda, J.I.; Maekawa, S.; Bahramy, M.S.; Saika, B.K.; Takeda, Y.; Wadati, H.; Wang, Y.; Yoshida, S.; et al. Spin–orbit-induced ising ferromagnetism at a van der Waals interface. Nano Lett. 2021, 21, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, Y.; Pardo-Almanza, M.; Garland, J.; Yamagami, K.; Zhu, X.; Chen, X.; Araki, K.; Takeda, T.; Kobayashi, M.; Takeda, Y.; et al. Tailoring magnetism in self-intercalated Cr1+δTe2 epitaxial films. Phys. Rev. Mater. 2020, 4, 114001. [Google Scholar] [CrossRef]
- Saha, R.; Meyerheim, H.L.; Göbel, B.; Hazra, B.K.; Deniz, H.; Mohseni, K.; Antonov, V.; Ernst, A.; Knyazev, D.; Bedoya-Pinto, A.; et al. Observation of Neel-type skyrmions in acentric self-intercalated Cr1+δTe2. Nat. Commun. 2022, 13, 3965. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, N.; Zhao, Y.; Jiang, X.; Zhou, S.; Zhao, J. Enhanced ferromagnetism of CrI3 bilayer by self-intercalation. Chin. Phys. Lett. 2020, 37, 107506. [Google Scholar] [CrossRef]
- Tan, F.; Li, J.; Fang, X.; Guan, L. The optical properties of few-layer MoS2 by DFT calculations. Phys. E 2024, 155, 115813. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [PubMed]
- Szary, M.J. Adsorption of ethylene oxide on doped monolayers of MoS2: A DFT study. Mater. Sci. Eng. B 2021, 265, 115009. [Google Scholar] [CrossRef]
- Dai, J.-Q.; Yuan, J.; Ke, C. Synergistic magnetic proximity and ferroelectric field effect on a 2H-VS2 monolayer by ferromagnetic termination of a BiFeO3(0001) surface. J. Mater. Chem. C 2022, 10, 1498–1510. [Google Scholar] [CrossRef]
- Kan, M.; Wang, B.; Lee, Y.H.; Sun, Q. A density functional theory study of the tunable structure, magnetism and metal-insulator phase transition in VS2 monolayers induced by in-plane biaxial strain. Nano Res. 2015, 8, 1348–1356. [Google Scholar] [CrossRef]
- Karthick, K.; Bijoy, T.K.; Sivakumaran, A.; Mansoor Basha, A.B.; Murugan, P.; Kundu, S. Enhancing hydrogen evolution reaction activities of 2H-phase VS2 layers with palladium nanoparticles. Inorg. Chem. 2020, 59, 10197–10207. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Fan, W.; Liu, X.; Ren, J.; Gao, Y. Electronic conductivity of two-dimensional VS2 monolayers: A first principles study. Comp. Mater. Sci. 2021, 200, 110767. [Google Scholar] [CrossRef]
- Liu, X.; Pyatakov, A.P.; Ren, W. Magnetoelectric coupling in multiferroic bilayer VS2. Phys. Rev. Lett. 2020, 125, 247601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, Y.; Gao, W.; Lin, Y.; Zhao, X.; Wang, Q.; Yao, X.; He, M.; Ye, X.; Liu, Y. Sizable bandgaps of graphene in 3d transition metal intercalated defective graphene/WSe2 heterostructures. RSC Adv. 2019, 9, 18157–18164. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal—Amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Anasori, B.; Shi, C.; Moon, E.J.; Xie, Y.; Voigt, C.A.; Kent, P.R.C.; May, S.J.; Billinge, S.J.L.; Barsoum, M.W.; Gogotsi, Y. Control of electronic properties of 2D carbides (MXenes) by manipulating their transition metal layers. Nanoscale Horiz. 2016, 1, 227–234. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, J.; Li, R.; Zhang, C.; Zhang, H.; Han, P.; Fan, C. DFT + U predictions: Structural stability, electronic and optical properties, oxidation activity of BiOCl photocatalysts with 3d transition metals doping. J. Mater. Sci. 2018, 53, 4494–4506. [Google Scholar] [CrossRef]
- Liu, C.; Fu, B.; Yin, H.; Zhang, G.; Dong, C. Strain-tunable magnetism and nodal loops in monolayer MnB. Appl. Phys. Lett. 2020, 117, 103101. [Google Scholar] [CrossRef]
- Islam, M.F.; Canali, C.M.; Pertsova, A.; Balatsky, A.; Mahatha, S.K.; Carbone, C.; Barla, A.; Kokh, K.A.; Tereshchenko, O.E.; Jiménez, E.; et al. Systematics of electronic and magnetic properties in the transition metal doped Sb2Te3 quantum anomalous hall platform. Phys. Rev. B 2018, 97, 155429. [Google Scholar] [CrossRef]
| Metal | dS-S | dTM-S | μ (μB) | Eb (eV) | ΔQ (e−) | GS |
|---|---|---|---|---|---|---|
| TM@BL_MoS2 | ||||||
| Sc | 3.46 | 1.73 | 2.78 | −6.50 | 1.48 | M |
| Ti | 3.34 | 1.67 | 2.45 | −5.19 | 1.28 | M |
| V | 3.28 | 1.64 | 3.44 | −6.80 | 1.10 | HM |
| Cr | 3.37 | 1.69 | 5.93 | −5.66 | 0.92 | HM |
| Mn | 3.37 | 1.68 | 5.00 | −5.82 | 0.89 | SC |
| Fe | 3.17 | 1.58 | 2.29 | −5.42 | 0.73 | M |
| Co | 3.19 | 1.60 | 2.92 | −7.11 | 0.60 | M |
| Ni | 3.16 | 1.09 | 0 | −7.21 | 0.32 | SC |
| TM@BL_VS2 | ||||||
| Sc | 3.37 | 1.69 | 36.86 | −6.67 | 1.53 | HM |
| Ti | 3.31 | 1.66 | 37.65 | −6.69 | 1.41 | SC |
| V | 3.16 | 1.58 | 38.94 | −6.51 | 1.37 | HM |
| Cr | 3.03 | 1.51 | 33.79 | −4.23 | 1.26 | HM |
| Mn | 3.05 | 1.52 | 40.62 | −4.63 | 1.18 | SC |
| Fe | 3.09 | 1.55 | 39.71 | −5.22 | 1.04 | SC |
| Co | 3.01 | 1.50 | 36.69 | −5.78 | 0.65 | SC |
| Ni | 3.05 | 1.53 | 37.72 | −5.45 | 0.61 | SC |
| Metal | μ (μB) | Eb (eV) | ΔQ (e−) | GS | ||
|---|---|---|---|---|---|---|
| 2TM@BL_MoS2 | ||||||
| 2V | 3.44 | 1.72 | 7.85 | −4.21 | 1.13 | HM |
| 2Cr | 3.55 | 1.77 | 11.89 | −3.79 | 0.98 | M |
| 2Mn | 3.50 | 1.75 | 10.00 | −3.45 | 0.95 | M |
| 2Fe | 3.23 | 1.62 | 6.14 | −4.18 | 0.73 | M |
| TMC@BL_MoS2 | ||||||
| VC | 3.14 | 1.57 | 3.03 | −3.39 | 1.09 | M |
| CrC | 4.55 | 2.27 | 5.14 | −2.35 | 0.64 | M |
| MnC | 3.43 | 1.71 | 3.61 | −1.95 | 0.95 | M |
| FeC | 3.54 | 1.77 | 2.44 | −3.04 | 0.73 | M |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yang, G.; He, Z.; Wang, Y.; Niu, X.; Wang, S.; Liu, Y.; Zhang, X. Tunable Electronic and Magnetic Properties of 3d Transition Metal Atom-Intercalated Transition Metal Dichalcogenides: A Density Functional Theory Study. Inorganics 2024, 12, 237. https://doi.org/10.3390/inorganics12090237
Liu Y, Yang G, He Z, Wang Y, Niu X, Wang S, Liu Y, Zhang X. Tunable Electronic and Magnetic Properties of 3d Transition Metal Atom-Intercalated Transition Metal Dichalcogenides: A Density Functional Theory Study. Inorganics. 2024; 12(9):237. https://doi.org/10.3390/inorganics12090237
Chicago/Turabian StyleLiu, Yujie, Guang Yang, Zhiwen He, Yanbiao Wang, Xianghong Niu, Sake Wang, Yongjun Liu, and Xiuyun Zhang. 2024. "Tunable Electronic and Magnetic Properties of 3d Transition Metal Atom-Intercalated Transition Metal Dichalcogenides: A Density Functional Theory Study" Inorganics 12, no. 9: 237. https://doi.org/10.3390/inorganics12090237







