Bridgman Method for Growing Metal Halide Single Crystals: A Review
Abstract
:1. Introduction
1.1. Basic Principle
1.2. Experimental Setup for the Bridgman Method
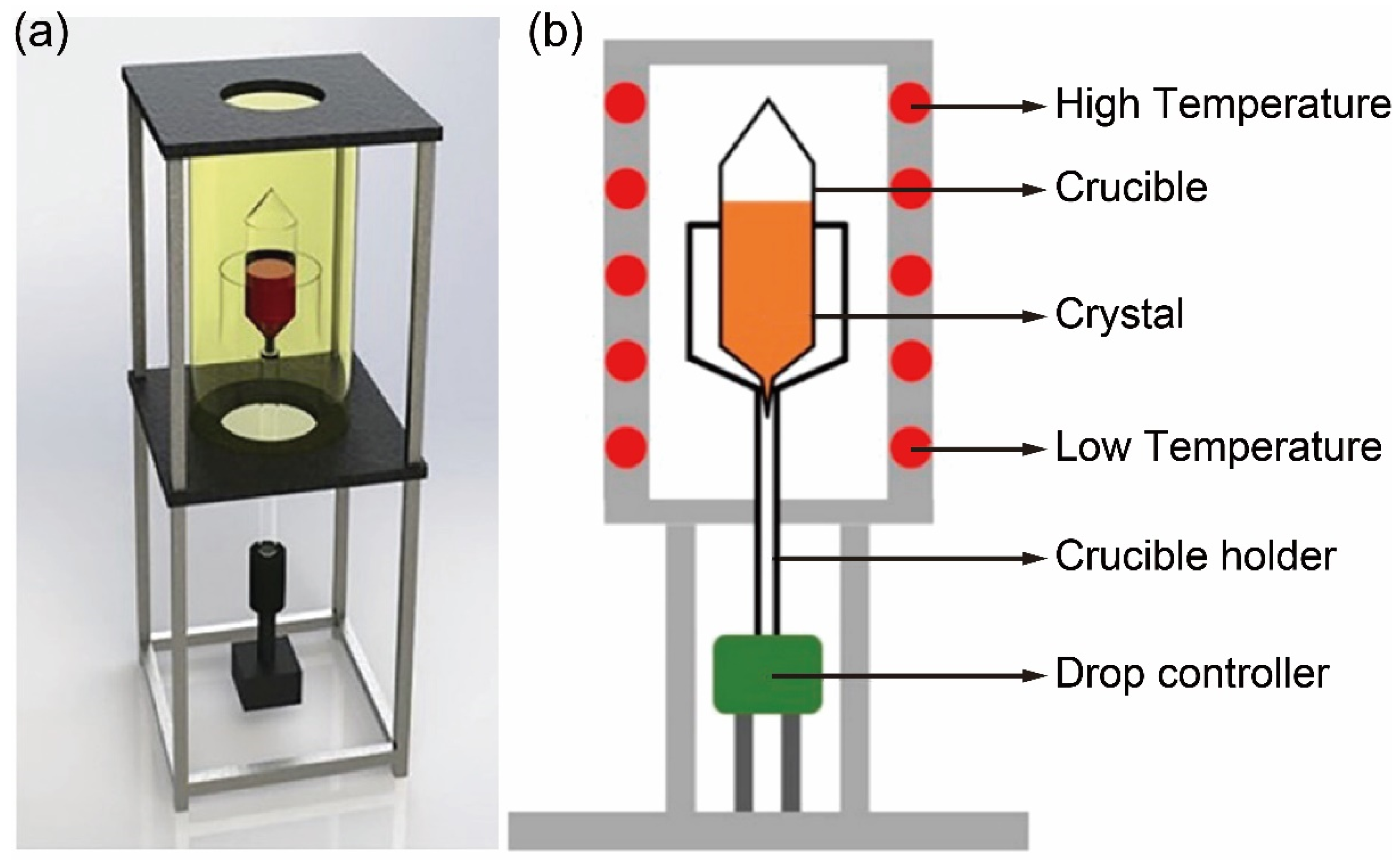
1.3. Growth Process
1.4. Advantages of the Bridgman Method
1.5. Current Mainstream Applications
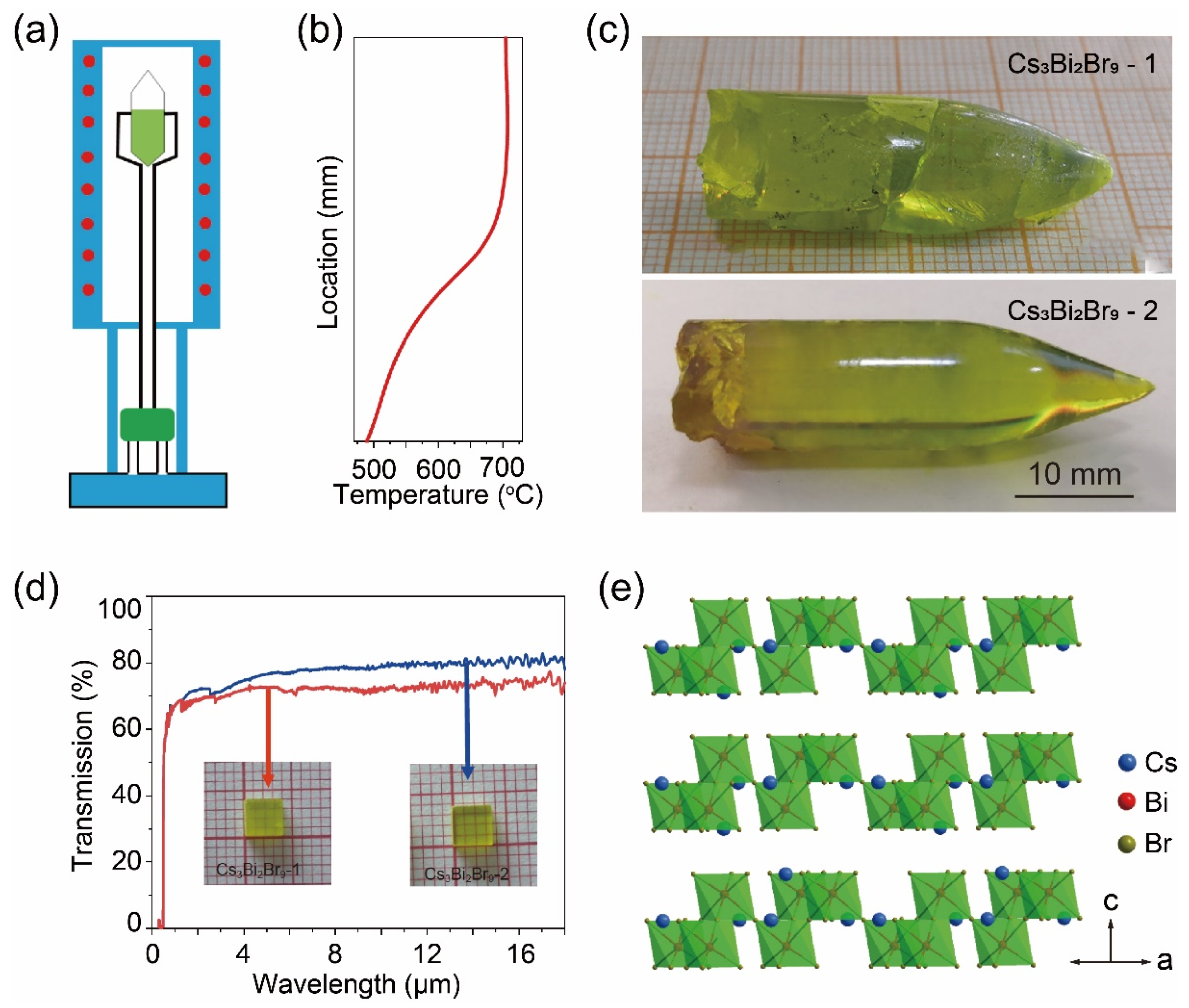
2. Bridgman Method for Growing Three-Dimensional Metal Halide Single Crystals
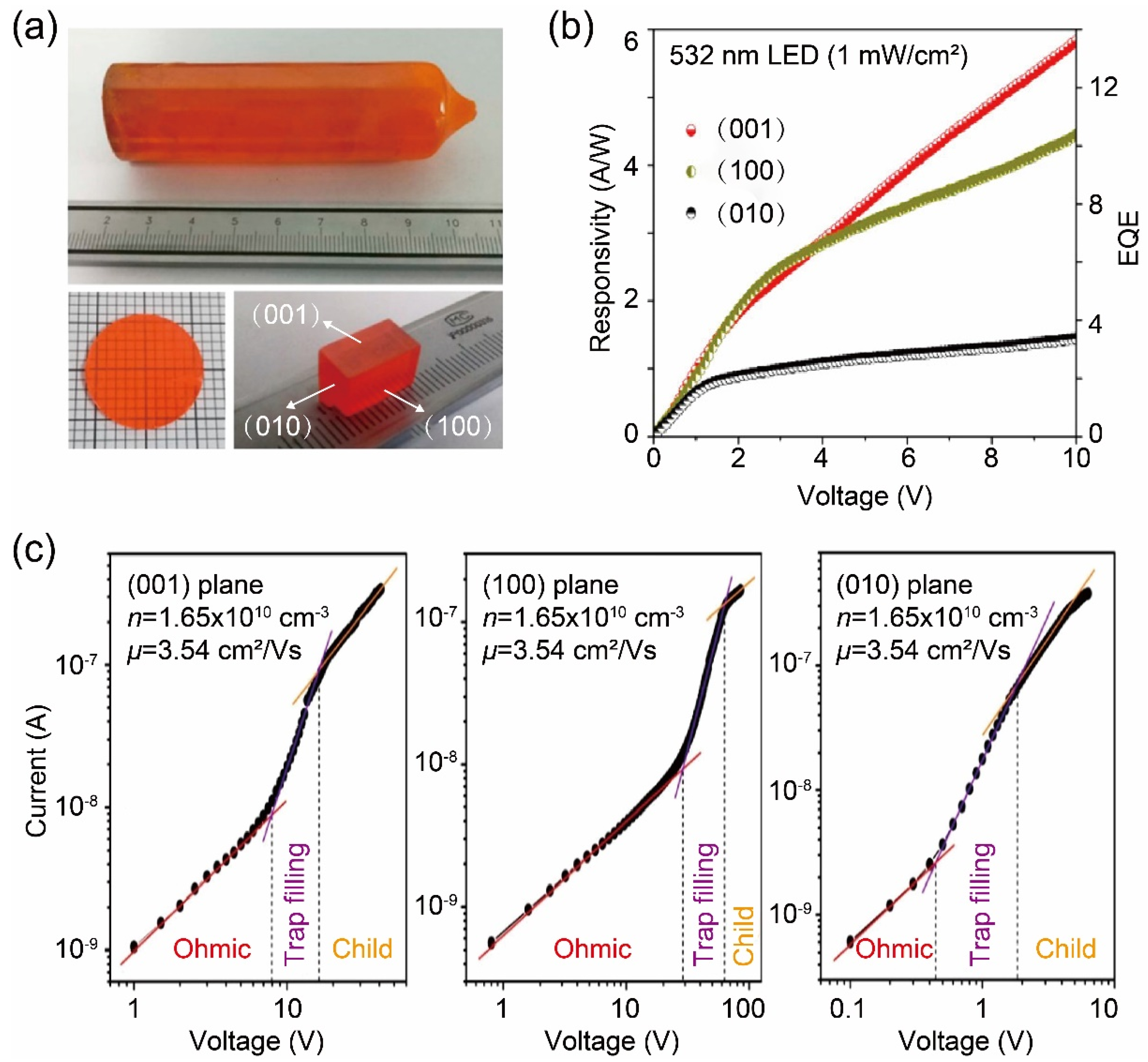
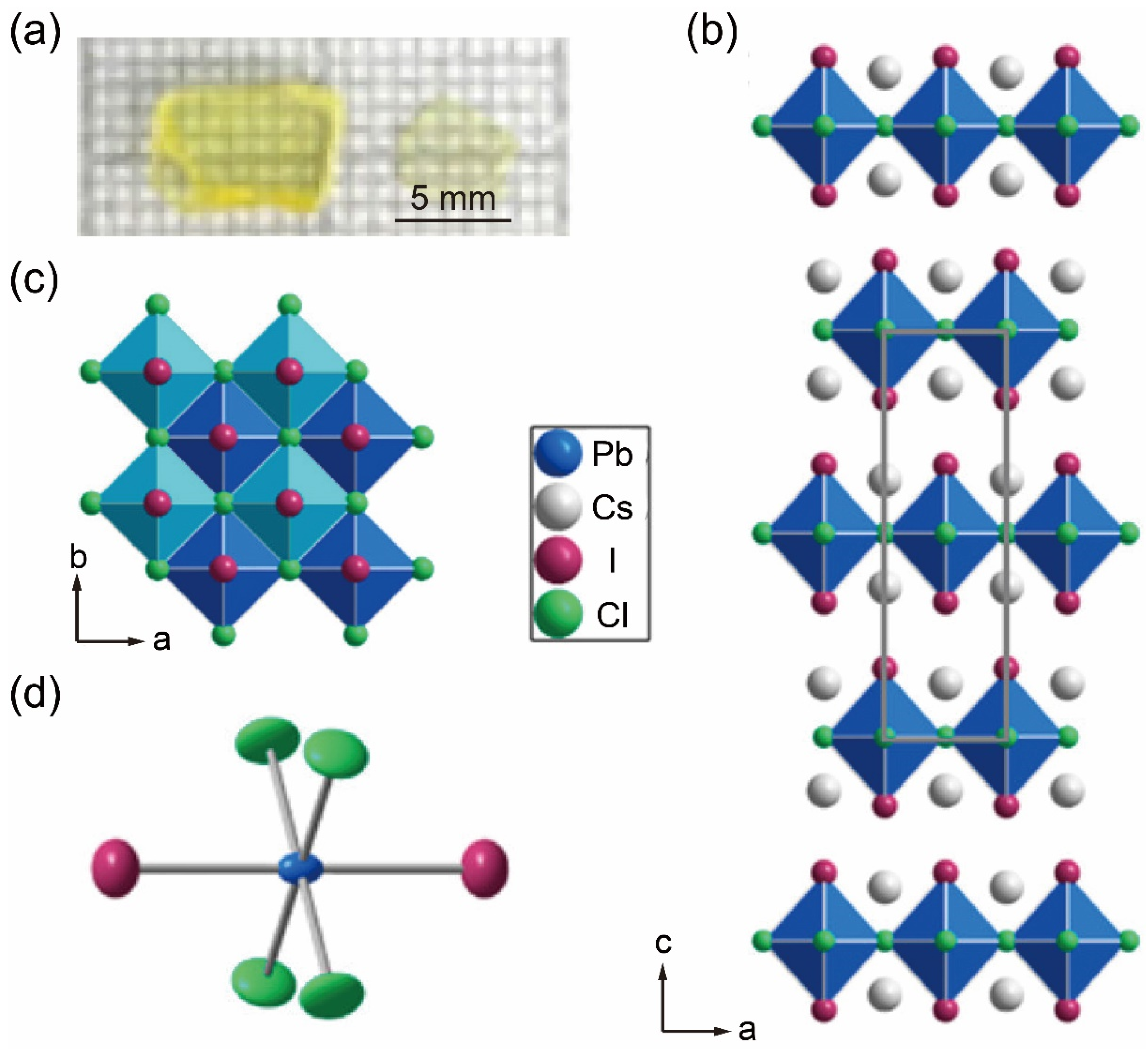
3. Bridgman Method for Growing Two-Dimensional Metal Halide Single Crystals
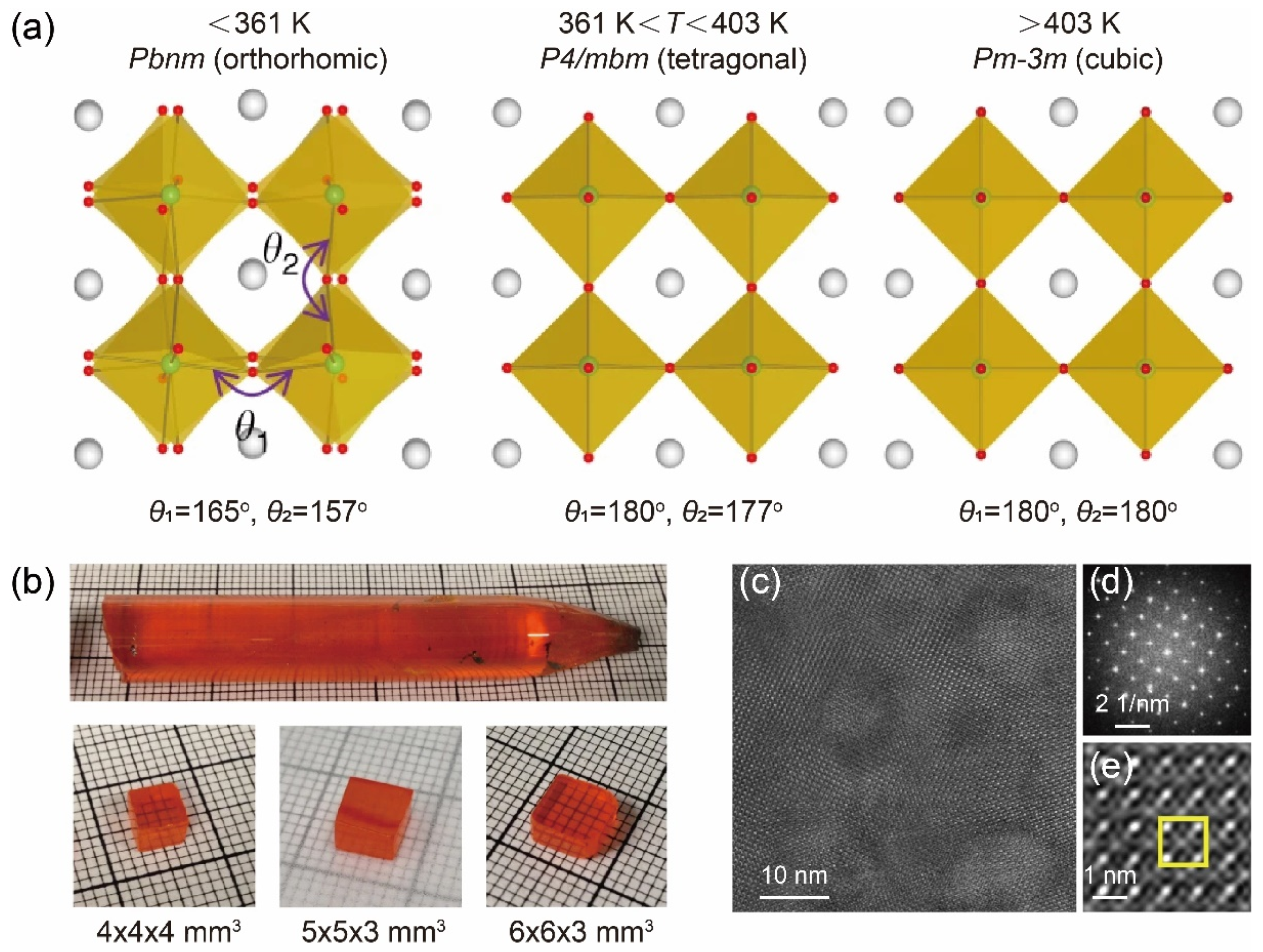
4. Bridgman Method for Growing Zero-Dimensional Metal Halide Single Crystals
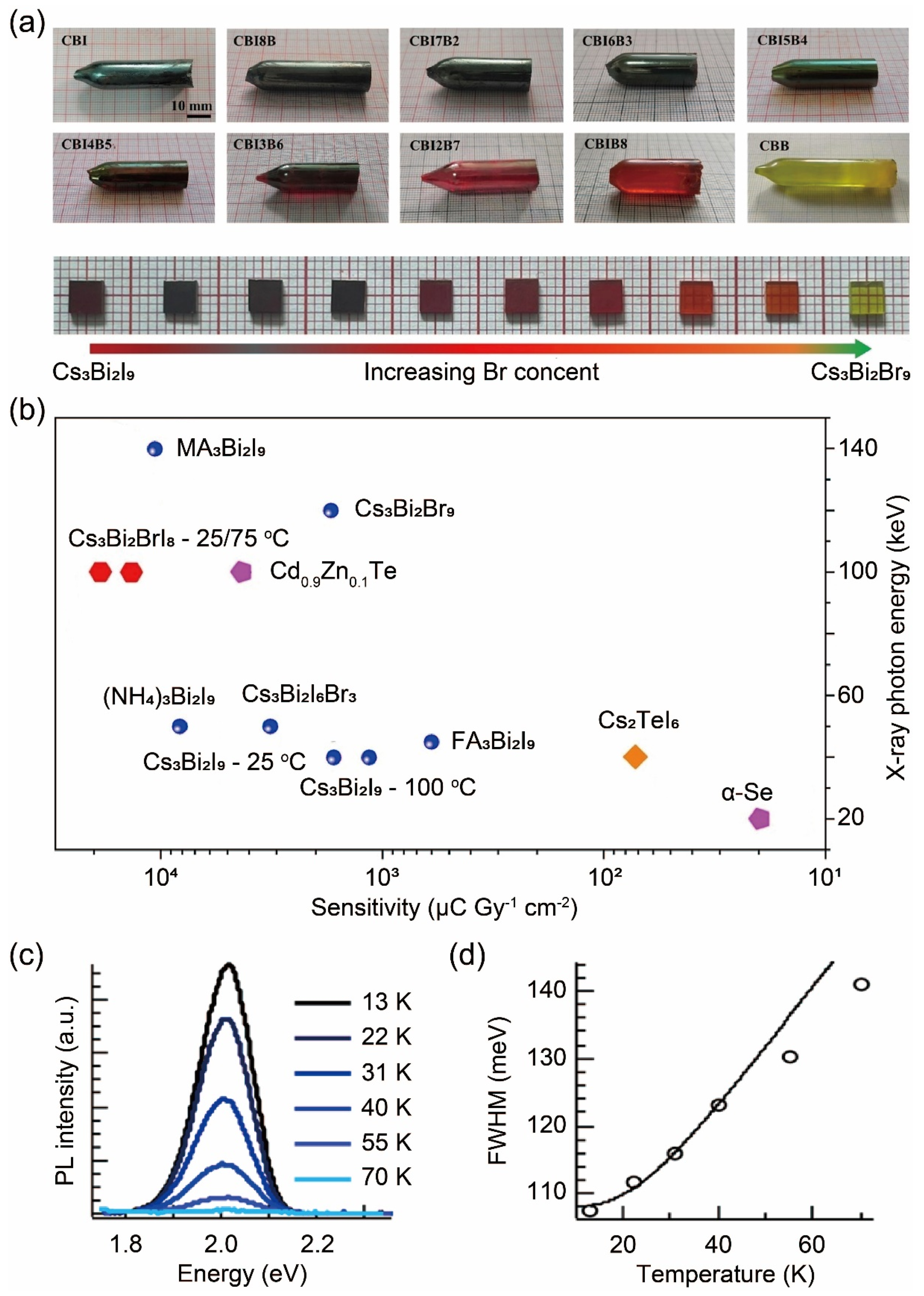
5. Bridgman Method for Growing Novel Metal Halide Single Crystals
5.1. Cs3Cu2I5 Single Crystals
5.2. Tl-Containing Single Crystals
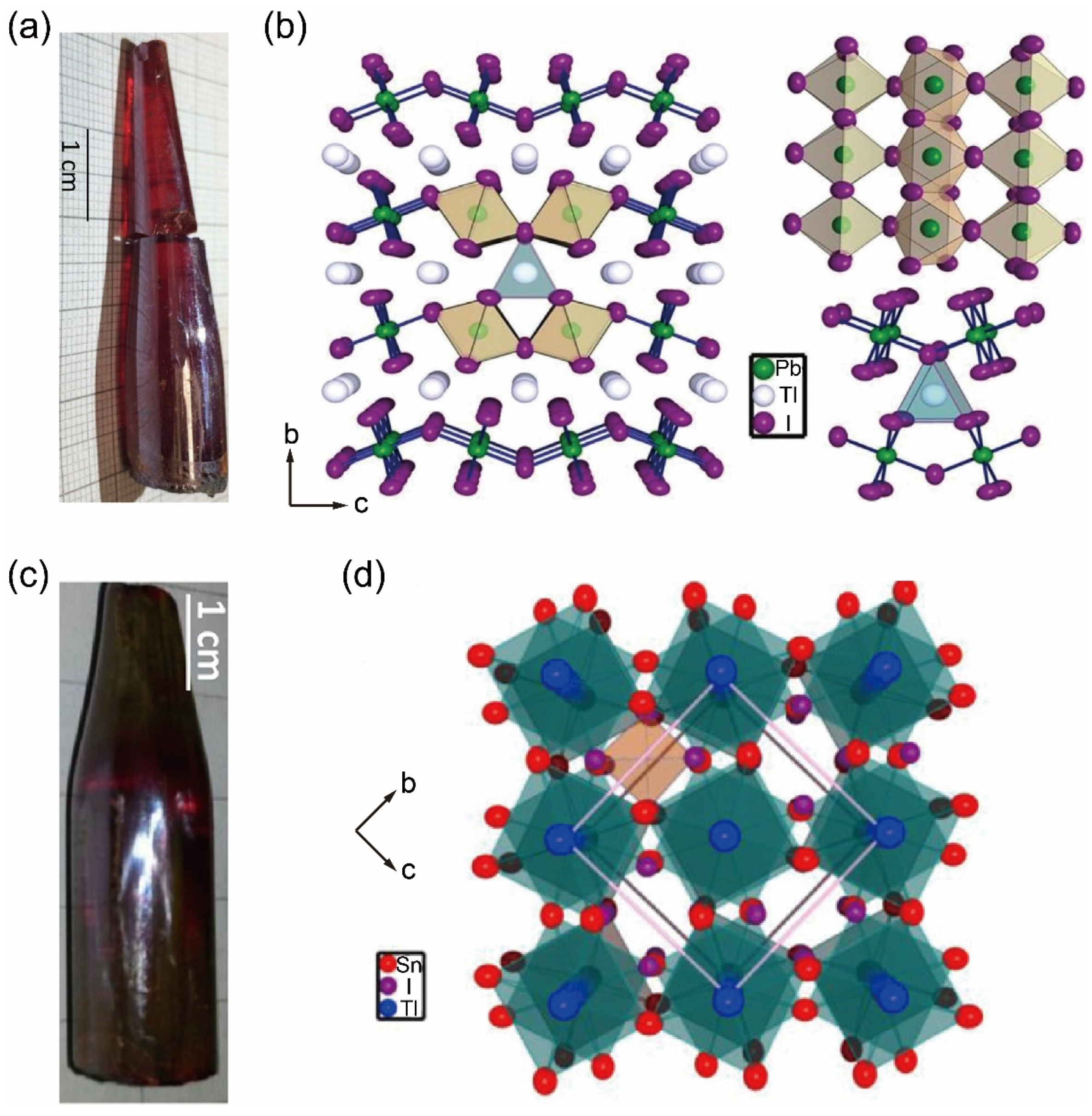
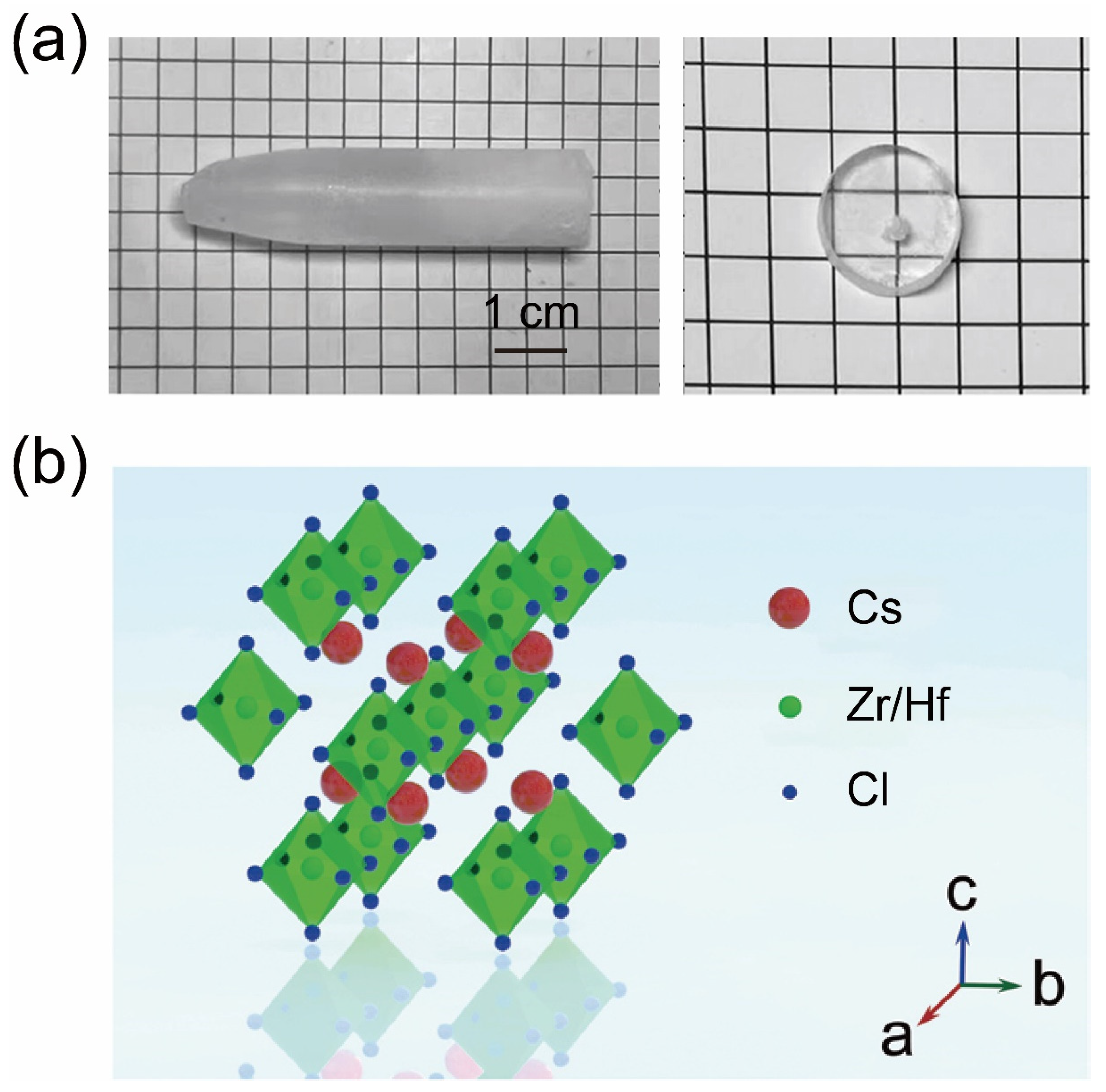
5.3. Zero-Dimensional A2BX6 Perovskite Single Crystals
6. Conclusions and Perspectives
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bisson, P.; Groenzin, H.; Barnett, I.L.; Shultz, M.J. High Yield, Single Crystal Ice via the Bridgman Method. Rev. Sci. Instrum. 2016, 87, 034103. [Google Scholar] [CrossRef] [PubMed]
- Derby, J.J.; Yeckel, A. Heat Transfer Analysis and Design for Bulk Crystal Growth: Perspectives on the Bridgman Method. In Handbook of Crystal Growth; Elsevier: Amsterdam, The Netherlands, 2015; pp. 793–843. ISBN 978-0-444-63303-3. [Google Scholar]
- Rudolph, P.; Kiessling, F. The Horizontal Bridgman Method. Cryst. Res. Technol. 1988, 23, 1207–1224. [Google Scholar] [CrossRef]
- Gaxandet, J.P.; Alboussire, T. Bridgman Growth: Modelling and Experiments. Prog. Cryst. Growth Charact. Mater. 1999, 38, 133–159. [Google Scholar] [CrossRef]
- Murugesan, M.; Sidden, C.; Paulraj, R. Growth, Optical and Thermal Properties of Triphenylamine Single Crystal Grown by Modified Vertical Bridgman Method for Optical Application. Solid. State Sci. 2022, 132, 106991. [Google Scholar] [CrossRef]
- Fedyushkin, A.I.; Burago, N.G.; Puntus, A.A. Convective Heat and Mass Transfer Modeling under Crystal Growth by Vertical Bridgman Method. J. Phys. Conf. Ser. 2020, 1479, 012029. [Google Scholar] [CrossRef]
- Yu, P.; Jiang, B.; Chen, Y.; Zheng, J.; Luan, L. Study on In-Doped CdMgTe Crystals Grown by a Modified Vertical Bridgman Method Using the ACRT Technique. Materials 2019, 12, 4236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hua, Y.; Li, X.; Zhang, L.; Liu, L.; Li, R.; Zhang, G.; Tao, X. Filter-Free Color Image Sensor Based on CsPbBr3−3n X3n (X = Cl, I) Single Crystals. J. Mater. Chem. C 2021, 9, 2840–2847. [Google Scholar] [CrossRef]
- Gille, P.; Mühlberg, M.; Parthier, L.; Rudolph, P. Crystal Growth of PbTe and (Pb, Sn)Te by the Bridgman Method and by THM. Cryst. Res. Technol. 1984, 19, 881–891. [Google Scholar] [CrossRef]
- Zhang, G.; Tao, X.; Wang, S.; Shi, Q.; Ruan, H.; Chen, L. Growth Improvement and Quality Evaluation of ZnGeP2 Single Crystals Using Vertical Bridgman Method. J. Cryst. Growth 2012, 352, 67–71. [Google Scholar] [CrossRef]
- Wang, C.; Xia, H.; Feng, Z.; Zhang, Z.; Jiang, D.; Gu, X.; Zhang, Y.; Chen, B.; Jiang, H. Infrared Luminescent Properties of Na5Lu9F32 Single Crystals Co-Doped Er3+/Yb3+ Grown by Bridgman Method. J. Alloys Compd. 2016, 686, 816–822. [Google Scholar] [CrossRef]
- Mooney, R.P.; McFadden, S.; Gabalcová, Z.; Lapin, J. An Experimental–Numerical Method for Estimating Heat Transfer in a Bridgman Furnace. Appl. Therm. Eng. 2014, 67, 61–71. [Google Scholar] [CrossRef]
- He, X.; Li, L.; He, X.; Xie, C. Multi-Physical Field Simulation of Cracking during Crystal Growth by Bridgman Method. Materials 2023, 16, 3260. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Jie, W.; Wang, T.; Zheng, X.; Xu, Y.; Luan, L. Solution Growth of In-Doped CdMnTe Crystals by the Vertical Bridgman Method with the ACRT Technique. J. Cryst. Growth 2012, 355, 33–37. [Google Scholar] [CrossRef]
- Sheng, M.; Wang, S.; Zhu, H.; Liu, Z.; Zhou, G. Computational Applications for the Discovery of Novel Antiperovskites and Chalcogenide Perovskites: A Review. Front. Chem. 2024, 12, 1468434. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Zhu, H.; Wang, S.; Liu, Z.; Zhou, G. Accelerated Discovery of Halide Perovskite Materials via Computational Methods: A Review. Nanomaterials 2024, 14, 1167. [Google Scholar] [CrossRef] [PubMed]
- Wojakowska, A.; Krzy, E. Phase Diagram for the RbBr–CuBr System. J. Therm. Anal. Calorim. 2011, 65, 491–495. [Google Scholar] [CrossRef]
- Kanno, R.; Takeda, Y.; Masuyama, Y.; Yamamoto, O. Phase Diagram and High Copper Ion Conductivity of the Copper(l) Chloride-Rubidium Chloride System. Solid State Ion. 1983, 11, 221–226. [Google Scholar] [CrossRef]
- Takahashi, T. Solid-State Ionics—The CuCI-CuI-RbCl System. Solid State Ion. 1981, 3, 283–287. [Google Scholar] [CrossRef]
- Malakhovskaya-Rosokha, T.A.; Barchii, I.E.; Pogodin, A.I.; Kokhan, A.P.; Stercho, I.P.; Peresh, E.Y. Interaction of Components in the RbI-CsI-CuI Quasi-Ternary System. Russ. J. Inorg. Chem. 2013, 58, 577–580. [Google Scholar] [CrossRef]
- Matsui, T.; Wagner, J.B. Investigations on a High Conductivity Solid Electrolyte System, RbCl + CuCl. J. Electrochem. Soc. 1977, 124, 941–944. [Google Scholar] [CrossRef]
- Gut, R.; Iberson, E.; Gruen, D.M. Compounds in the Binary Phase Diagrams of the Monovalent Chlorides with the Multivalent Chlorides; Report number ANL-6803; OSTI ID: 4117130; Argonne National Laboratory: Lemont, IL, USA, 1963. [Google Scholar]
- Zhang, P.; Zhang, G.; Liu, L.; Ju, D.; Zhang, L.; Cheng, K.; Tao, X. Anisotropic Optoelectronic Properties of Melt-Grown Bulk CsPbBr3 Single Crystal. J. Phys. Chem. Lett. 2018, 9, 5040–5046. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Huang, C.; Ni, Y.; Wang, Z.; Wu, H.; Hu, Q.; Liu, G.; Zhou, Q.; Wei, L. Optical Property and Judd-Ofelt Analysis of Dy3+, Na+:PbGa2S4 Crystal Grown by Bridgman Method. J. Lumin. 2023, 262, 119951. [Google Scholar] [CrossRef]
- Wang, L.-H.; Chang, L.-S. Thermoelectric Properties of P-Type Ba8Ga16Ge30 Type-I Clathrate Compounds Prepared by the Vertical Bridgman Method. J. Alloys Compd. 2017, 722, 644–650. [Google Scholar] [CrossRef]
- Zayachuk, D.M.; Ilyina, O.S.; Pashuk, A.V.; Mikityuk, V.I.; Shlemkevych, V.V.; Csik, A.; Kaczorowski, D. Segregation of the Eu Impurity as Function of Its Concentration in the Melt for Growing of the Lead Telluride Doped Crystals by the Bridgman Method. J. Cryst. Growth 2013, 376, 28–34. [Google Scholar] [CrossRef]
- Lai, J.; Zhang, J.; Wang, L.; Min, J.; Wu, W.; Shen, M.; Liang, W. Comparison of Cadmium Manganese Telluride Crystals Grown by Traveling Heater Method and Vertical Bridgman Method. Cryst. Res. Technol. 2015, 50, 817–822. [Google Scholar] [CrossRef]
- Xiong, W.; Chen, L.; Huang, L.; Guo, F.; Zhou, Y.; Yuan, H. Bridgman Growth and Characterization of Birefringent Crystal NaNO3. Cryst. Res. Technol. 2015, 50, 250–254. [Google Scholar] [CrossRef]
- Jin, M.; Shen, H.; Fan, S.; He, Q.; Xu, J. Industrial Growth and Characterization of Si-doped GaAs Crystal by a Novel Multi-crucible Bridgman Method. Cryst. Res. Technol. 2017, 52, 1700052. [Google Scholar] [CrossRef]
- Yang, C.; Wen, Y.; Xiong, J.; Zhou, H.; Pan, S.; Chen, H.; Pan, J. Crystal Growth and Photoelectric Performance of γ-CuCl by the Vertical Bridgman Method. J. Cryst. Growth 2022, 579, 126463. [Google Scholar] [CrossRef]
- Gul, R.; Roy, U.N.; Camarda, G.S.; Hossain, A.; Yang, G.; Vanier, P.; Lordi, V.; Varley, J.; James, R.B. A Comparison of Point Defects in Cd1−xZnxTe1−ySey Crystals Grown by Bridgman and Traveling Heater Methods. J. Appl. Phys. 2017, 121, 125705. [Google Scholar] [CrossRef]
- Roy, U.N.; Bolotnikov, A.E.; Camarda, G.S.; Cui, Y.; Hossain, A.; Lee, K.; Lee, W.; Tappero, R.; Yang, G.; Gul, R.; et al. High Compositional Homogeneity of CdTexSe1−x Crystals Grown by the Bridgman Method. APL Mater. 2015, 3, 026102. [Google Scholar] [CrossRef]
- Colombier, E.; Braithwaite, D. Simple Adaptation of the Bridgman High Pressure Technique for Use with Liquid Media. Rev. Sci. Instrum. 2007, 78, 093903. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, P.; Li, Z.; Chen, Z. Thermal Field Simulation and Optimization of PbF2 Single Crystal Growth by the Bridgman Method. Crystals 2024, 14, 473. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, X.; Zhao, T.; Chen, C.; Han, J.; Pan, S.; Pan, J. Growth and Luminescence Properties of γ-CuBr Single Crystals by the Bridgman Method. CrystEngComm 2023, 25, 1669–1674. [Google Scholar] [CrossRef]
- Zayachuk, D.M.; Mikityuk, V.I.; Shlemkevych, V.V.; Kaczorowski, D. Complex Formation and Background Impurity of Oxygen in the PbTe: Eu Doped Crystals Grown from Melt by the Bridgman Method. J. Cryst. Growth 2012, 338, 35–41. [Google Scholar] [CrossRef]
- Toufanian, R.; Swain, S.; Becla, P.; Motakef, S.; Datta, A. Cesium Lead Bromide Semiconductor Radiation Detectors: Crystal Growth, Detector Performance and Polarization. J. Mater. Chem. C 2022, 10, 12708–12714. [Google Scholar] [CrossRef]
- Dahlen, A.; Fattah, A.; Hanke, G.; Karthaus, E. Bridgman and Czochralski Growth of Ge Si Alloy Crystals. Cryst. Res. Technol. 1994, 29, 187–198. [Google Scholar] [CrossRef]
- Kliemt, K.; Krellner, C. Crystal Growth by Bridgman and Czochralski Method of the Ferromagnetic Quantum Critical Material YbNi4P2. J. Cryst. Growth 2016, 449, 129–133. [Google Scholar] [CrossRef]
- Hurle, D.T.J. A Mechanism for Twin Formation during Czochralski and Encapsulated Vertical Bridgman Growth of III–V Compound Semiconductors. J. Cryst. Growth 1995, 147, 239–250. [Google Scholar] [CrossRef]
- Tonn, J.; Danilewsky, A.N.; Cröll, A.; Matuchova, M.; Maixner, J. Czochralski Growth of Lead Iodide Single Crystals: Investigations and Comparison with the Bridgman Method. J. Cryst. Growth 2011, 318, 558–562. [Google Scholar] [CrossRef]
- Akasaka, M.; Iida, T.; Matsumoto, A.; Yamanaka, K.; Takanashi, Y.; Imai, T.; Hamada, N. The Thermoelectric Properties of Bulk Crystalline N- and p-Type Mg2Si Prepared by the Vertical Bridgman Method. J. Appl. Phys. 2008, 104, 013703. [Google Scholar] [CrossRef]
- Lyubimova, T.P.; Croell, A.; Dold, P.; Khlybov, O.A.; Fayzrakhmanova, I.S. Time-Dependent Magnetic Field Influence on GaAs Crystal Growth by Vertical Bridgman Method. J. Cryst. Growth 2004, 266, 404–410. [Google Scholar] [CrossRef]
- Shimizu, A.; Nishizawa, J.; Oyama, Y.; Suto, K. InP Single Crystal Growth by the Horizontal Bridgman Method under Controlled Phosphorus Vapor Pressure. J. Cryst. Growth 2001, 229, 119–123. [Google Scholar] [CrossRef]
- Li, F.; Cabral, M.J.; Xu, B.; Cheng, Z.; Dickey, E.C.; LeBeau, J.M.; Wang, J.; Luo, J.; Taylor, S.; Hackenberger, W.; et al. Giant Piezoelectricity of Sm-Doped Pb(Mg1/3Nb2/3)O3-PbTiO3 Single Crystals. Science 2019, 364, 264–268. [Google Scholar] [CrossRef]
- Li, F.; Lin, D.; Chen, Z.; Cheng, Z.; Wang, J.; Li, C.; Xu, Z.; Huang, Q.; Liao, X.; Chen, L.-Q.; et al. Ultrahigh Piezoelectricity in Ferroelectric Ceramics by Design. Nat. Mater. 2018, 17, 349–354. [Google Scholar] [CrossRef]
- Qiu, C.; Wang, B.; Zhang, N.; Zhang, S.; Liu, J.; Walker, D.; Wang, Y.; Tian, H.; Shrout, T.R.; Xu, Z.; et al. Transparent Ferroelectric Crystals with Ultrahigh Piezoelectricity. Nature 2020, 577, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liang, Z.; Luo, L.; Ke, Y.; Shen, Q.; Xia, Z.; Jiang, C.; Pan, J. Bridgman Growth, Crystallographic Characterization and Electrical Properties of Relaxor-Based Ferroelectric Single Crystal PIMNT. J. Alloys Compd. 2012, 518, 63–67. [Google Scholar] [CrossRef]
- Liao, F.; Zhao, Y.; Chen, Z.; Zheng, Y.; Chen, H. Bridgman Growth and Photoelectronic Property of Relaxor-Based Ferroelectric Single Crystal Pb(Sm1/2Nb1/2)O3-Pb(Mg1/3Nb2/3)O3-PbTiO3. Crystals 2021, 11, 402. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, X.; Liu, X. Luminescence Enrichment in Perovskite-Lanthanide Composites: Complexity and Complementarity. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2022; Volume 61, pp. 1–29. ISBN 978-0-323-98594-9. [Google Scholar]
- Liu, Z.; Qin, X.; Chen, Q.; Jiang, T.; Chen, Q.; Liu, X. Metal–Halide Perovskite Nanocrystal Superlattice: Self-Assembly and Optical Fingerprints. Adv. Mater. 2023, 35, 2209279. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wu, J.; Ou, X.; Huang, B.; Almutlaq, J.; Zhumekenov, A.A.; Guan, X.; Han, S.; Liang, L.; Yi, Z.; et al. All-Inorganic Perovskite Nanocrystal Scintillators. Nature 2018, 561, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qin, X.; Chen, Q.; Chen, Q.; Jing, Y.; Zhou, Z.; Zhao, Y.S.; Chen, J.; Liu, X. Highly Stable Lead-Free Perovskite Single Crystals with NIR Emission Beyond 1100 Nm. Adv. Opt. Mater. 2022, 10, 2201254. [Google Scholar] [CrossRef]
- Ma, W.; Qian, Q.; Qaid, S.M.H.; Zhao, S.; Liang, D.; Cai, W.; Zang, Z. Water-Molecule-Induced Reversible Fluorescence in a One-Dimensional Mn-Based Hybrid Halide for Anticounterfeiting and Digital Encryption–Decryption. Nano Lett. 2023, 23, 8932–8939. [Google Scholar] [CrossRef]
- Han, T.; Tan, S.; Xue, J.; Meng, L.; Lee, J.; Yang, Y. Interface and Defect Engineering for Metal Halide Perovskite Optoelectronic Devices. Adv. Mater. 2019, 31, 1803515. [Google Scholar] [CrossRef]
- Motti, S.G.; Meggiolaro, D.; Martani, S.; Sorrentino, R.; Barker, A.J.; De Angelis, F.; Petrozza, A. Defect Activity in Lead Halide Perovskites. Adv. Mater. 2019, 31, 1901183. [Google Scholar] [CrossRef]
- Ono, L.K.; Liu, S.; Qi, Y. Reducing Detrimental Defects for High-Performance Metal Halide Perovskite Solar Cells. Angew. Chem. Int. Ed. 2020, 59, 6676–6698. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Bodnarchuk, M.I.; Kershaw, S.V.; Kovalenko, M.V.; Rogach, A.L. Lead Halide Perovskite Nanocrystals in the Research Spotlight: Stability and Defect Tolerance. ACS Energy Lett. 2017, 2, 2071–2083. [Google Scholar] [CrossRef]
- Yang, F.; Jie, W.; Wang, M.; Sun, X.; Jia, N.; Yin, L.; Zhou, B.; Wang, T. Growth of Single-Crystal Cd0.9Zn0.1Te Ingots Using Pressure Controlled Bridgman Method. Crystals 2020, 10, 261. [Google Scholar] [CrossRef]
- Macpherson, S.; Doherty, T.A.S.; Winchester, A.J.; Kosar, S.; Johnstone, D.N.; Chiang, Y.-H.; Galkowski, K.; Anaya, M.; Frohna, K.; Iqbal, A.N.; et al. Local Nanoscale Phase Impurities Are Degradation Sites in Halide Perovskites. Nature 2022, 607, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Valueva, A.D.; Novikov, S.A.; Bledsoe, J.; Cai, Y.; Maksimova, A.A.; Locklin, J.; Zhao, Y.; Klepov, V.V. Cubic Halide Perovskites in the Cs(Pb1−xSnx)(Br3−yCly) Solid Solutions for Crack-Free Bridgman Grown Single Crystals. MRS Commun. 2024, 14, 942–948. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, Y.; Ma, Z.; Xiao, G.; Wang, K.; Zou, B. Structural Regulation and Optical Behavior of Three-Dimensional Metal Halide Perovskites under Pressure. J. Mater. Chem. C 2020, 8, 12755–12767. [Google Scholar] [CrossRef]
- Wong, W.P.D.; Yin, J.; Chaudhary, B.; Chin, X.Y.; Cortecchia, D.; Lo, S.-Z.A.; Grimsdale, A.C.; Mohammed, O.F.; Lanzani, G.; Soci, C. Large Polaron Self-Trapped States in Three-Dimensional Metal-Halide Perovskites. ACS Mater. Lett. 2020, 2, 20–27. [Google Scholar] [CrossRef]
- Huang, X.; Guo, Q.; Kang, S.; Ouyang, T.; Chen, Q.; Liu, X.; Xia, Z.; Yang, Z.; Zhang, Q.; Qiu, J.; et al. Three-Dimensional Laser-Assisted Patterning of Blue-Emissive Metal Halide Perovskite Nanocrystals inside a Glass with Switchable Photoluminescence. ACS Nano 2020, 14, 3150–3158. [Google Scholar] [CrossRef] [PubMed]
- Giappa, R.M.; Selivanov, N.I.; Samsonova, A.Y.; Pantousas, A.; Remediakis, I.N.; Kapitonov, Y.V.; Emeline, A.V.; Kopidakis, G.; Stoumpos, C.C. (3-CF3pyH)2(3-CF3Py)Pb3I8: A Three-Dimensional Metal Halide Inorganic Framework with Distinctive Kagomé Bands. Chem. Mater. 2024, 36, 11804–11813. [Google Scholar] [CrossRef]
- Krawczyk, R.P.; Hammerl, A.; Schwerdtfeger, P. Coinage Metal Halide Clusters: From Two-Dimensional Ring to Three-Dimensional Solid-State-Like Structures. ChemPhysChem 2006, 7, 2286–2289. [Google Scholar] [CrossRef]
- Zhang, W.; Eperon, G.E.; Snaith, H.J. Metal Halide Perovskites for Energy Applications. Nat Energy 2016, 1, 16048. [Google Scholar] [CrossRef]
- Lei, L.; Dong, Q.; Gundogdu, K.; So, F. Metal Halide Perovskites for Laser Applications. Adv Funct Mater. 2021, 31, 2010144. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, H.; Chen, J.; Hautzinger, M.P.; Zhu, X.-Y.; Jin, S. Metal Halide Perovskite Nanostructures for Optoelectronic Applications and the Study of Physical Properties. Nat. Rev. Mater. 2019, 4, 169–188. [Google Scholar] [CrossRef]
- Stranks, S.D.; Snaith, H.J. Metal-Halide Perovskites for Photovoltaic and Light-Emitting Devices. Nat. Nanotechnol. 2015, 10, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Pattanasattayavong, P.; Anthopoulos, T.D. Metal-Halide Perovskite Transistors for Printed Electronics: Challenges and Opportunities. Adv. Mater. 2017, 29, 1702838. [Google Scholar] [CrossRef]
- Sun, J.; Wu, J.; Tong, X.; Lin, F.; Wang, Y.; Wang, Z.M. Organic/Inorganic Metal Halide Perovskite Optoelectronic Devices beyond Solar Cells. Adv. Sci. 2018, 5, 1700780. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-K.; Xu, W.; Bai, S.; Jin, Y.; Wang, J.; Friend, R.H.; Gao, F. Metal Halide Perovskites for Light-Emitting Diodes. Nat. Mater. 2021, 20, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Liu, S.; Tress, W. A Review on the Stability of Inorganic Metal Halide Perovskites: Challenges and Opportunities for Stable Solar Cells. Energy Environ. Sci. 2021, 14, 2090–2113. [Google Scholar] [CrossRef]
- Cho, H.; Kim, Y.; Wolf, C.; Lee, H.; Lee, T. Improving the Stability of Metal Halide Perovskite Materials and Light-Emitting Diodes. Adv. Mater. 2018, 30, 1704587. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Song, J.; Li, X.; Xu, J.; Zeng, H.; Sun, H. Temperature Dependent Reflectance and Ellipsometry Studies on a CsPbBr3 Single Crystal. J. Phys. Chem. C 2019, 123, 10564–10570. [Google Scholar] [CrossRef]
- Cockroft, N.J.; Jones, G.D.; Nguyen, D.C. Dynamics and Spectroscopy of Infrared-to-Visible Upconversion in Erbium-Doped Cesium Cadmium Bromide (CsCdBr3:Er3+). Phys. Rev. B 1992, 45, 5187–5198. [Google Scholar] [CrossRef] [PubMed]
- Pilla, O.; Cazzanelli, E.; Blanzat, B.; Andraud, C.; Pelle, F. Comparative Raman Study of Phonon Linewidths in Pure and Lead-Doped CsCdBr3. Phys. Status Solidi (B) 1987, 144, 845–851. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Peters, J.A.; Liu, Z.; Sebastian, M.; Im, J.; Chasapis, T.C.; Wibowo, A.C.; Chung, D.Y.; Freeman, A.J.; et al. Crystal Growth of the Perovskite Semiconductor CsPbBr3: A New Material for High-Energy Radiation Detection. Cryst. Growth Des. 2013, 13, 2722–2727. [Google Scholar] [CrossRef]
- He, Y.; Matei, L.; Jung, H.J.; McCall, K.M.; Chen, M.; Stoumpos, C.C.; Liu, Z.; Peters, J.A.; Chung, D.Y.; Wessels, B.W.; et al. High Spectral Resolution of Gamma-Rays at Room Temperature by Perovskite CsPbBr3 Single Crystals. Nat. Commun. 2018, 9, 1609. [Google Scholar] [CrossRef] [PubMed]
- Dirin, D.N.; Cherniukh, I.; Yakunin, S.; Shynkarenko, Y.; Kovalenko, M.V. Solution-Grown CsPbBr3 Perovskite Single Crystals for Photon Detection. Chem. Mater. 2016, 28, 8470–8474. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hu, Z.; Hu, W.; Wangyang, P.; Yu, K.; Wen, M.; Zu, Z.; Liu, J.; Wang, M.; Chen, W.; et al. Two-Step Method for Preparing All-Inorganic CsPbBr3 Perovskite Film and Its Photoelectric Detection Application. Mater. Lett. 2017, 186, 243–246. [Google Scholar] [CrossRef]
- Tang, X.; Zu, Z.; Zang, Z.; Hu, Z.; Hu, W.; Yao, Z.; Chen, W.; Li, S.; Han, S.; Zhou, M. CsPbBr3/Reduced Graphene Oxide Nanocomposites and Their Enhanced Photoelectric Detection Application. Sens. Actuators B Chem. 2017, 245, 435–440. [Google Scholar] [CrossRef]
- Adinolfi, V.; Yuan, M.; Comin, R.; Thibau, E.S.; Shi, D.; Saidaminov, M.I.; Kanjanaboos, P.; Kopilovic, D.; Hoogland, S.; Lu, Z.; et al. The In-Gap Electronic State Spectrum of Methylammonium Lead Iodide Single-Crystal Perovskites. Adv. Mater. 2016, 28, 3406–3410. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Wang, L.-W. High Defect Tolerance in Lead Halide Perovskite CsPbBr3. J. Phys. Chem. Lett. 2017, 8, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Fu, Q.; Guo, P.; Chen, H.; Wang, M.; Luo, W.; Zheng, Z. Ionic Transport Characteristics of Large-Size CsPbBr3 Single Crystals. Mater. Res. Express 2019, 6, 115808. [Google Scholar] [CrossRef]
- Li, J.; Du, X.; Niu, G.; Xie, H.; Chen, Y.; Yuan, Y.; Gao, Y.; Xiao, H.; Tang, J.; Pan, A.; et al. Rubidium Doping to Enhance Carrier Transport in CsPbBr3 Single Crystals for High-Performance X-Ray Detection. ACS Appl. Mater. Interfaces 2020, 12, 989–996. [Google Scholar] [CrossRef]
- Lee, I.Y.; Deleplanque, M.A.; Vetter, K. Developments in Large Gamma-Ray Detector Arrays. Rep. Prog. Phys. 2003, 66, 1095–1144. [Google Scholar] [CrossRef]
- Liu, H.; Omura, T.; Watanabe, M.; Yamashita, T. Development of a Depth of Interaction Detector for γ-Rays. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2001, 459, 182–190. [Google Scholar] [CrossRef]
- Funk, S. Indirect Detection of Dark Matter with γ Rays. Proc. Natl. Acad. Sci. USA 2015, 112, 12264–12271. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Maxwell, A.; Wei, M.; Wang, Z.; Chen, B.; Zhu, T.; Sargent, E.H. Defect Tolerance of Mixed B-Site Organic–Inorganic Halide Perovskites. ACS Energy Lett. 2021, 6, 4220–4227. [Google Scholar] [CrossRef]
- Smart, T.J.; Takenaka, H.; Pham, T.A.; Tan, L.Z.; Zhang, J.Z.; Ogitsu, T.; Ping, Y. Enhancing Defect Tolerance with Ligands at the Surface of Lead Halide Perovskites. J. Phys. Chem. Lett. 2021, 12, 6299–6304. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Petrozza, A. Defect Tolerance and Intolerance in Metal-Halide Perovskites. Adv. Energy Mater. 2020, 10, 2001959. [Google Scholar] [CrossRef]
- He, Y.; Stoumpos, C.C.; Hadar, I.; Luo, Z.; McCall, K.M.; Liu, Z.; Chung, D.Y.; Wessels, B.W.; Kanatzidis, M.G. Demonstration of Energy-Resolved γ-Ray Detection at Room Temperature by the CsPbCl3 Perovskite Semiconductor. J. Am. Chem. Soc. 2021, 143, 2068–2077. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.I.; Young, K.F.; Chang, T.-T.; Brower, W.S. Phase Transitions in CsPbCl3. J. Appl. Phys. 1971, 42, 5267–5272. [Google Scholar] [CrossRef]
- Kobayashi, M.; Omata, K.; Sugimoto, S.; Tamagawa, Y.; Kuroiwa, T.; Asada, H.; Takeuchi, H.; Kondo, S. Scintillation Characteristics of CsPbCl3 Single Crystals. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2008, 592, 369–373. [Google Scholar] [CrossRef]
- Balvanz, A.; Bayikadi, K.S.; Liu, Z.; Ie, T.S.; Peters, J.A.; Kanatzidis, M.G. Unveiling the Monoclinic Phase in CsPbBr3−xClx Perovskite Crystals, Phase Transition Suppression and High Energy Resolution γ-Ray Detection. J. Am. Chem. Soc. 2024, 146, 31836–31848. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wu, R.; Wei, J.; Nie, W.; Mohite, A.D.; Brovelli, S.; Manna, L.; Li, H. Recent Progress in Halide Perovskite Radiation Detectors for Gamma-Ray Spectroscopy. ACS Energy Lett. 2022, 7, 1066–1085. [Google Scholar] [CrossRef]
- Yoon, S.J.; Stamplecoskie, K.G.; Kamat, P.V. How Lead Halide Complex Chemistry Dictates the Composition of Mixed Halide Perovskites. J. Phys. Chem. Lett. 2016, 7, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Zhao, L.; Tran, N.L.; Lin, Y.L.; Silver, S.H.; Kerner, R.A.; Yao, N.; Kahn, A.; Scholes, G.D.; Rand, B.P. Mixed-Halide Perovskites with Stabilized Bandgaps. Nano Lett. 2017, 17, 6863–6869. [Google Scholar] [CrossRef]
- Knight, A.J.; Borchert, J.; Oliver, R.D.J.; Patel, J.B.; Radaelli, P.G.; Snaith, H.J.; Johnston, M.B.; Herz, L.M. Halide Segregation in Mixed-Halide Perovskites: Influence of A-Site Cations. ACS Energy Lett. 2021, 6, 799–808. [Google Scholar] [CrossRef]
- Zarick, H.F.; Soetan, N.; Erwin, W.R.; Bardhan, R. Mixed Halide Hybrid Perovskites: A Paradigm Shift in Photovoltaics. J. Mater. Chem. A 2018, 6, 5507–5537. [Google Scholar] [CrossRef]
- Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Grotevent, M.J.; Kovalenko, M.V. Fast Anion-Exchange in Highly Luminescent Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, D.; Yang, P. Ruddlesden–Popper Phase in Two-Dimensional Inorganic Halide Perovskites: A Plausible Model and the Supporting Observations. Nano Lett. 2017, 17, 5489–5494. [Google Scholar] [CrossRef]
- Pan, D.; Fu, Y.; Spitha, N.; Zhao, Y.; Roy, C.R.; Morrow, D.J.; Kohler, D.D.; Wright, J.C.; Jin, S. Deterministic Fabrication of Arbitrary Vertical Heterostructures of Two-Dimensional Ruddlesden–Popper Halide Perovskites. Nat. Nanotechnol. 2021, 16, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Guo, Y.; Wertz, E.; Shi, J. Merits and Challenges of Ruddlesden–Popper Soft Halide Perovskites in Electro-Optics and Optoelectronics. Adv. Mater. 2019, 31, 1803514. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Hao, M.; Zhang, Y.; Liu, M.; Zhou, Y. Layered 2D Halide Perovskites beyond the Ruddlesden–Popper Phase: Tailored Interlayer Chemistries for High-Performance Solar Cells. Angew. Chem. 2022, 134, e202112022. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Peng, J.; Tang, J.; Zheng, K.; Liang, Z. 2D Ruddlesden–Popper Perovskites for Optoelectronics. Adv. Mater. 2018, 30, 1703487. [Google Scholar] [CrossRef]
- Li, J.; Yu, Q.; He, Y.; Stoumpos, C.C.; Niu, G.; Trimarchi, G.G.; Guo, H.; Dong, G.; Wang, D.; Wang, L.; et al. Cs2PbI2Cl2, All-Inorganic Two-Dimensional Ruddlesden–Popper Mixed Halide Perovskite with Optoelectronic Response. J. Am. Chem. Soc. 2018, 140, 11085–11090. [Google Scholar] [CrossRef] [PubMed]
- Ruddlesden, S.N.; Popper, P. New Compounds of the K2NIF4 Type. Acta Cryst 1957, 10, 538–539. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Acharyya, P.; Ghosh, T.; Pal, K.; Kundu, K.; Singh Rana, K.; Pandey, J.; Soni, A.; Waghmare, U.V.; Biswas, K. Intrinsically Ultralow Thermal Conductivity in Ruddlesden–Popper 2D Perovskite Cs2PbI2Cl2: Localized Anharmonic Vibrations and Dynamic Octahedral Distortions. J. Am. Chem. Soc. 2020, 142, 15595–15603. [Google Scholar] [CrossRef] [PubMed]
- Delaire, O.; Ma, J.; Marty, K.; May, A.F.; McGuire, M.A.; Du, M.-H.; Singh, D.J.; Podlesnyak, A.; Ehlers, G.; Lumsden, M.D.; et al. Giant Anharmonic Phonon Scattering in PbTe. Nat. Mater. 2011, 10, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, P.D.; Yeom, K.M.; Sarkar, B.; Alessi, D.S.; Hou, D.; Rinklebe, J.; Noh, J.H.; Ok, Y.S. Environmental Impact of Metal Halide Perovskite Solar Cells and Potential Mitigation Strategies: A Critical Review. Environ. Res. 2023, 219, 115066. [Google Scholar] [CrossRef]
- Lyu, M.; Yun, J.; Chen, P.; Hao, M.; Wang, L. Addressing Toxicity of Lead: Progress and Applications of Low-Toxic Metal Halide Perovskites and Their Derivatives. Adv. Energy Mater. 2017, 7, 1602512. [Google Scholar] [CrossRef]
- Xiao, Z.; Song, Z.; Yan, Y. From Lead Halide Perovskites to Lead-Free Metal Halide Perovskites and Perovskite Derivatives. Adv. Mater. 2019, 31, 1803792. [Google Scholar] [CrossRef]
- Hu, H.; Dong, B.; Zhang, W. Low-Toxic Metal Halide Perovskites: Opportunities and Future Challenges. J. Mater. Chem. A 2017, 5, 11436–11449. [Google Scholar] [CrossRef]
- De Souza Carvalho, T.A.; Magalhaes, L.F.; Do Livramento Santos, C.I.; De Freitas, T.A.Z.; Carvalho Vale, B.R.; Vale Da Fonseca, A.F.; Schiavon, M.A. Lead-Free Metal Halide Perovskite Nanocrystals: From Fundamentals to Applications. Chem. Eur. J. 2023, 29, e202202518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, K.; Zou, B. Bismuth Halide Perovskite-Like Materials: Current Opportunities and Challenges. ChemSusChem 2019, 12, 1612–1630. [Google Scholar] [CrossRef]
- Ayscue, R.L.; Vallet, V.; Bertke, J.A.; Réal, F.; Knope, K.E. Structure–Property Relationships in Photoluminescent Bismuth Halide Organic Hybrid Materials. Inorg. Chem. 2021, 60, 9727–9744. [Google Scholar] [CrossRef] [PubMed]
- Creutz, S.E.; Liu, H.; Kaiser, M.E.; Li, X.; Gamelin, D.R. Structural Diversity in Cesium Bismuth Halide Nanocrystals. Chem. Mater. 2019, 31, 4685–4697. [Google Scholar] [CrossRef]
- Li, X.; Du, X.; Zhang, P.; Hua, Y.; Liu, L.; Niu, G.; Zhang, G.; Tang, J.; Tao, X. Lead-Free Halide Perovskite Cs3Bi2Br9 Single Crystals for High-Performance X-Ray Detection. Sci. China Mater. 2021, 64, 1427–1436. [Google Scholar] [CrossRef]
- Leng, M.; Yang, Y.; Zeng, K.; Chen, Z.; Tan, Z.; Li, S.; Li, J.; Xu, B.; Li, D.; Hautzinger, M.P.; et al. All-Inorganic Bismuth-Based Perovskite Quantum Dots with Bright Blue Photoluminescence and Excellent Stability. Adv. Funct. Mater. 2018, 28, 1704446. [Google Scholar] [CrossRef]
- Bass, K.K.; Estergreen, L.; Savory, C.N.; Buckeridge, J.; Scanlon, D.O.; Djurovich, P.I.; Bradforth, S.E.; Thompson, M.E.; Melot, B.C. Vibronic Structure in Room Temperature Photoluminescence of the Halide Perovskite Cs3Bi2Br9. Inorg. Chem. 2017, 56, 42–45. [Google Scholar] [CrossRef]
- Li, X.; Zhang, G.; Hua, Y.; Cui, F.; Sun, X.; Liu, J.; Liu, H.; Bi, Y.; Yue, Z.; Zhai, Z.; et al. Dimensional and Optoelectronic Tuning of Lead-free Perovskite Cs3Bi2I9−nBrn Single Crystals for Enhanced Hard X-ray Detection. Angew. Chem. Int. Ed. 2023, 62, e202315817. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fei, C.; Uddin, M.A.; Zhao, L.; Ni, Z.; Huang, J. Self-Powered Perovskite Photon-Counting Detectors. Nature 2023, 616, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, T.; Ji, C.; Yao, Y.; Luo, J. In Situ Epitaxial Growth of Centimeter-Sized Lead-Free (BA)2CsAgBiBr7/Cs2 AgBiBr6 Heterocrystals for Self-Driven X-Ray Detection. J. Am. Chem. Soc. 2021, 143, 20802–20810. [Google Scholar] [CrossRef]
- McCall, K.M.; Stoumpos, C.C.; Kostina, S.S.; Kanatzidis, M.G.; Wessels, B.W. Strong Electron–Phonon Coupling and Self-Trapped Excitons in the Defect Halide Perovskites A3M2I9 (A = Cs, Rb; M = Bi, Sb). Chem. Mater. 2017, 29, 4129–4145. [Google Scholar] [CrossRef]
- McCall, K.M.; Liu, Z.; Trimarchi, G.; Stoumpos, C.C.; Lin, W.; He, Y.; Hadar, I.; Kanatzidis, M.G.; Wessels, B.W. α-Particle Detection and Charge Transport Characteristics in the A3M2I9 Defect Perovskites (A = Cs, Rb; M = Bi, Sb). ACS Photonics 2018, 5, 3748–3762. [Google Scholar] [CrossRef]
- Lei, X.-W.; Yue, C.-Y.; Zhao, J.-Q.; Han, Y.-F.; Yang, J.-T.; Meng, R.-R.; Gao, C.-S.; Ding, H.; Wang, C.-Y.; Chen, W.-D. Low-Dimensional Hybrid Cuprous Halides Directed by Transition Metal Complex: Syntheses, Crystal Structures, and Photocatalytic Properties. Cryst. Growth Des. 2015, 15, 5416–5426. [Google Scholar] [CrossRef]
- Cheng, P.; Sun, L.; Feng, L.; Yang, S.; Yang, Y.; Zheng, D.; Zhao, Y.; Sang, Y.; Zhang, R.; Wei, D.; et al. Colloidal Synthesis and Optical Properties of All-Inorganic Low-Dimensional Cesium Copper Halide Nanocrystals. Angew. Chem. 2019, 131, 16233–16237. [Google Scholar] [CrossRef]
- Zhang, P.; Yan, Z.; Li, C.; Du, Y.; Ma, L.; Wang, Z.; Lin, T.; Zhao, L.; Xiao, J. Low-Dimensional Hybrid Copper(I) Halides Single Crystals: Synthesis, Structures, and Tunable Photoluminescence. Chem. Eng. J. 2024, 496, 154106. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, C.; Wang, Z.; Sun, X.; Nikl, M.; OuYang, X.; Wu, Y. Achieving Efficient Neutron and Gamma Discrimination in a Highly Stable6 Li-Loaded Cs3Cu2I5 Perovskite Scintillator. J. Phys. Chem. Lett. 2022, 13, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wei, Q.; Tong, Y.; Xiang, P.; Cai, P.; Tang, G.; Shi, H.; Qin, L. A Novel Li+-Doped CsCu2I3 Single Crystal for Dual Gamma–Neutron Detection. CrystEngComm 2023, 25, 58–63. [Google Scholar] [CrossRef]
- Combes, C.M.; Dorenbos, P.; Van Eijk, C.W.E.; Krämer, K.W.; Güdel, H.U. Optical and Scintillation Properties of Pure and Ce3+-Doped Cs2LiYCl6 and Li3YCl6:Ce3+ Crystals. J. Lumin. 1999, 82, 299–305. [Google Scholar] [CrossRef]
- Glodo, J.; Hawrami, R.; Shah, K.S. Development of Cs2LiYCl6 Scintillator. J. Cryst. Growth 2013, 379, 73–78. [Google Scholar] [CrossRef]
- Cheng, S.; Nikl, M.; Beitlerova, A.; Kucerkova, R.; Du, X.; Niu, G.; Jia, Y.; Tang, J.; Ren, G.; Wu, Y. Ultrabright and Highly Efficient All-Inorganic Zero-Dimensional Perovskite Scintillators. Adv. Opt. Mater. 2021, 9, 2100460. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, Q.; Nikl, M.; Xiao, J.; Kucerkova, R.; Beitlerova, A.; Babin, V.; Prusa, P.; Linhart, V.; Wang, J.; et al. Highly Resolved X-Ray Imaging Enabled by In(I) Doped Perovskite-Like Cs3Cu2I5 Single Crystal Scintillator. Adv. Opt. Mater. 2022, 10, 2200304. [Google Scholar] [CrossRef]
- Belousov, M.P.; Gromyko, M.V.; Ignatyev, O.V. Scintillation γ Spectrometers for Use at Nuclear Power Plants (Review). Instrum. Exp. Tech. 2017, 60, 1–19. [Google Scholar] [CrossRef]
- Dorenbos, P. (INVITED) The Quest for High Resolution γ-Ray Scintillators. Opt. Mater. X 2019, 1, 100021. [Google Scholar] [CrossRef]
- Yuan, D. Air-Stable Bulk Halide Single-Crystal Scintillator Cs3Cu2I5 by Melt Growth: Intrinsic and Tl Doped with High Light Yield. ACS Appl. Mater. Interfaces 2020, 12, 38333–38340. [Google Scholar] [CrossRef]
- Tsuji, M.; Sasase, M.; Iimura, S.; Kim, J.; Hosono, H. Room-Temperature Solid-State Synthesis of Cs3Cu2I5 Thin Films and Formation Mechanism for Its Unique Local Structure. J. Am. Chem. Soc. 2023, 145, 11650–11658. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Xie, J.; Yang, Y.; Song, Y.; Xiao, G.; Fu, Y.; Zhang, L.; Fang, Y.; Yang, D.; et al. HCOO− Doping-Induced Multiexciton Emissions in Cs3Cu2I5 Crystals for Efficient X-Ray Scintillation. Small 2024, 20, 2309922. [Google Scholar] [CrossRef]
- Chen, H.; Pina, J.M.; Yuan, F.; Johnston, A.; Ma, D.; Chen, B.; Li, Z.; Dumont, A.; Li, X.; Liu, Y.; et al. Multiple Self-Trapped Emissions in the Lead-Free Halide Cs3 Cu2 I5. J. Phys. Chem. Lett. 2020, 11, 4326–4330. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Zheng, M.; Zhang, W.; Yin, L.; Du, X.; Zhang, P.; Zhang, X.; Gao, J.; Zhang, D.; Gao, L.; et al. Efficient and Reabsorption-Free Radioluminescence in Cs3Cu2I5 Nanocrystals with Self-Trapped Excitons. Adv. Sci. 2020, 7, 2000195. [Google Scholar] [CrossRef] [PubMed]
- Jun, T.; Sim, K.; Iimura, S.; Sasase, M.; Kamioka, H.; Kim, J.; Hosono, H. Lead-Free Highly Efficient Blue-Emitting Cs3Cu2I5 with 0D Electronic Structure. Adv. Mater. 2018, 30, 1804547. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; Yang, D.; Chen, X.; Geng, D.; Jiang, L.; Wu, Y.; Meng, C.; Zeng, H. Mn2+ Induced Significant Improvement and Robust Stability of Radioluminescence in Cs3Cu2I5 for High-Performance Nuclear Battery. Nat. Commun. 2021, 12, 3879. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H.; Shin, D.H.; Park, J.K.; Kim, D.H.; Lee, S.J.; Im, S.H. High-Performance Next-Generation Perovskite Nanocrystal Scintillator for Nondestructive X-Ray Imaging. Adv. Mater. 2018, 30, 1801743. [Google Scholar] [CrossRef]
- Van Loef, E.V.; Ciampi, G.; Shirwadkar, U.; Soundara Pandian, L.; Shah, K.S. Crystal Growth and Scintillation Properties of Thallium-Based Halide Scintillators. J. Cryst. Growth 2020, 532, 125438. [Google Scholar] [CrossRef]
- Shirwadkar, U.; Loyd, M.; Du, M.-H.; Van Loef, E.; Ciampi, G.; Soundara Pandian, L.; Stand, L.; Koschan, M.; Zhuravleva, M.; Melcher, C.; et al. Thallium-Based Scintillators for High-Resolution Gamma-Ray Spectroscopy: Ce3+-Doped Tl2LaCl5 and Tl2LaBr5. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2020, 962, 163684. [Google Scholar] [CrossRef]
- Hawrami, R.; Ariesanti, E.; Buliga, V.; Burger, A. Thallium Strontium Iodide: A High Efficiency Scintillator for Gamma-Ray Detection. Opt. Mater. 2020, 100, 109624. [Google Scholar] [CrossRef]
- Kim, H.; Khan, A.; Daniel, J.; Rooh, G.; Vuong, P.Q. Thallium-Based Heavy Inorganic Scintillators: Recent Developments and Future Perspectives. CrystEngComm 2022, 24, 450–464. [Google Scholar] [CrossRef]
- Pan, L.; Sholom, S.; McKeever, S.W.S.; Jacobsohn, L.G. Magnesium Aluminate Spinel for Optically Stimulated Luminescence Dosimetry. J. Alloys Compd. 2021, 880, 160503. [Google Scholar] [CrossRef]
- Sakai, T.; Koshimizu, M.; Fujimoto, Y.; Nakauchi, D.; Yanagida, T.; Asai, K. Luminescence and Scintillation Properties of Tl- and In-Doped CsCl Crystals. Jpn. J. Appl. Phys. 2017, 56, 062601. [Google Scholar] [CrossRef]
- Zazubovich, S.; Voloshinovskii, A.; Stryganyuk, G. Luminescence of CsCl: Tl Crystal under Synchrotron Excitation. Phys. Status Solidi (B) 2002, 233, 238–249. [Google Scholar] [CrossRef]
- Nagirnyi, V.; Zazubovich, S.; Jaanson, N. Luminescence of CsCl: Tl Single Crystals. Phys. Status Solidi (B) 1993, 175, 155–164. [Google Scholar] [CrossRef]
- Shiran, N.V.; Gektin, A.V.; Boyarintseva, Y.; Vasyukov, S.; Boyarintsev, A.; Pedash, V.; Tkachenko, S.; Zelenskaya, O.; Kosinov, N.; Kisil, O.; et al. Eu Doped and Eu, Tl Co-Doped NaI Scintillators. IEEE Trans. Nucl. Sci. 2010, 57, 1233–1235. [Google Scholar] [CrossRef]
- Lin, W.; He, J.; McCall, K.M.; Stoumpos, C.C.; Liu, Z.; Hadar, I.; Das, S.; Wang, H.; Wang, B.; Chung, D.Y.; et al. Inorganic Halide Perovskitoid TlPbI3 for Ionizing Radiation Detection. Adv. Funct. Mater. 2021, 31, 2006635. [Google Scholar] [CrossRef]
- Rodi, F.; Babel, D. Ternäre Oxide der Übergangsmetalle. IV. Erdalkaliiridium(IV)-oxide: Kristallstruktur von CalrO3. Z. Anorg Allge Chem. 1965, 336, 17–23. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Kepenekian, M.; Guo, P.; Ke, W.; Even, J.; Katan, C.; Stoumpos, C.C.; Schaller, R.D.; Kanatzidis, M.G. Three-Dimensional Lead Iodide Perovskitoid Hybrids with High X-Ray Photoresponse. J. Am. Chem. Soc. 2020, 142, 6625–6637. [Google Scholar] [CrossRef]
- Vtyurina, D.N.; Kaurova, I.A.; Kuz’micheva, G.M.; Rybakov, V.B.; Chernyshov, D.Y.; Khramov, E.V.; Firstov, S.V.; Korchak, V.N. Structural Peculiarities, Point Defects and Luminescence in Bi-Doped CsCdX3 (X = Cl, Br) Single Crystals. J. Alloys Compd. 2019, 803, 912–921. [Google Scholar] [CrossRef]
- Vtyurina, D.N.; Eistrikh-Geller, P.A.; Kuz’micheva, G.M.; Rybakov, V.B.; Khramov, E.V.; Kaurova, I.A.; Chernyshov, D.Y.; Korchak, V.N. Influence of Monovalent Bi+ Doping on Real Composition, Point Defects, and Photoluminescence in TlCdCl3 and TlCdI3 Single Crystals. Sci. China Mater. 2017, 60, 1253–1263. [Google Scholar] [CrossRef]
- Yang, G.; Phan, Q.V.; Liu, M.; Hawari, A.; Kim, H. Material Defect Study of Thallium Lead Iodide (TlPbI3) Crystals for Radiation Detector Applications. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2020, 954, 161516. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, T.; Wang, Y.; Wang, C.; Zhang, P.; Sarvari, H.; Chen, Z.; Li, S. Electronic Properties of a New All-Inorganic Perovskite TlPbI3 Simulated by the First Principles. Nanoscale Res. Lett. 2017, 12, 232. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, F.; Wu, J.; Zhang, Z.; Wu, Z.; Cong, W.-Y.; Guan, C.; Lu, Y.-B. Evaluation of the Electronic Properties of Layered TlPbI3 Perovskites. Phys. B Condens. Matter 2023, 667, 415190. [Google Scholar] [CrossRef]
- Khyzhun, O.Y.; Fochuk, P.M.; Kityk, I.V.; Piasecki, M.; Levkovets, S.I.; Fedorchuk, A.O.; Parasyuk, O.V. Single Crystal Growth and Electronic Structure of TlPbI3. Mater. Chem. Phys. 2016, 172, 165–172. [Google Scholar] [CrossRef]
- Lin, W.; Stoumpos, C.C.; Liu, Z.; Das, S.; Kontsevoi, O.Y.; He, Y.; Malliakas, C.D.; Chen, H.; Wessels, B.W.; Kanatzidis, M.G. TlSn2I5, a Robust Halide Antiperovskite Semiconductor for γ-Ray Detection at Room Temperature. ACS Photonics 2017, 4, 1805–1813. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; He, Y.; Song, J.; Guo, K.; Pan, W.; Wei, H. Arising 2D Perovskites for Ionizing Radiation Detection. Adv. Mater. 2024, 36, 2309588. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Zhao, J.; Zhang, G.; Chen, Y.; Lian, H.; Lin, J. Synthesis of Pb-free Double-Perovskite Heterojunction (Cs2SnCl6-Bi3+&Te4+/Cs2GeF6-Mn4+) with White Emission for LED Application. Adv. Opt. Mater. 2023, 11, 2203029. [Google Scholar] [CrossRef]
- Zhou, J.; Luo, J.; Rong, X.; Wei, P.; Molokeev, M.S.; Huang, Y.; Zhao, J.; Liu, Q.; Zhang, X.; Tang, J.; et al. Lead-Free Perovskite Derivative Cs2 SnCl6−xBrxSingle Crystals for Narrowband Photodetectors. Adv. Opt. Mater. 2019, 7, 1900139. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, W.; Huang, P.; Zhang, M.; Zhang, W.; Deng, Z.; Yu, S.; Jin, M.; Chen, X. Efficient Near-Infrared Luminescence in Lanthanide-Doped Vacancy-Ordered Double Perovskite Cs2ZrCl6 Phosphors via Te4+ Sensitization. Angew. Chem. Int. Ed. 2022, 61, e202201993. [Google Scholar] [CrossRef]
- Wang, A.; Yan, X.; Zhang, M.; Sun, S.; Yang, M.; Shen, W.; Pan, X.; Wang, P.; Deng, Z. Controlled Synthesis of Lead-Free and Stable Perovskite Derivative Cs2SnI6 Nanocrystals via a Facile Hot-Injection Process. Chem. Mater. 2016, 28, 8132–8140. [Google Scholar] [CrossRef]
- Scott, S.M.; Zhu, W.; Yao, T.; Vienna, J.D.; Ewing, R.C.; Lian, J. The Thermal Stability and Consolidation of Perovskite Variant Cs2SnCl6 Using Spark Plasma Sintering. J. Am. Ceram. Soc. 2018, 101, 2060–2065. [Google Scholar] [CrossRef]
- Chang, T.; Wei, Q.; Zeng, R.; Cao, S.; Zhao, J.; Zou, B. Efficient Energy Transfer in Te4+-Doped Cs2ZrCl6 Vacancy-Ordered Perovskites and Ultrahigh Moisture Stability via A-Site Rb-Alloying Strategy. J. Phys. Chem. Lett. 2021, 12, 1829–1837. [Google Scholar] [CrossRef] [PubMed]
- Qing, X.; Wu, C.; Han, X. Efficient Near-Infrared Luminescence Based on Double Perovskite Cs2SnCl6. Molecules 2023, 28, 3593. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Zheng, W.; Gong, Z.; Huang, P.; Li, R.; Xu, J.; Cheng, X.; Zhang, W.; Chen, X. Unraveling the Triplet Excited-State Dynamics of Bi3+ in Vacancy-Ordered Double Perovskite Cs2SnCl6 Nanocrystals. Nano Res. 2022, 15, 6422–6429. [Google Scholar] [CrossRef]
- Das Adhikari, S.; Echeverría-Arrondo, C.; Sánchez, R.S.; Chirvony, V.S.; Martínez-Pastor, J.P.; Agouram, S.; Muñoz-Sanjosé, V.; Mora-Seró, I. White Light Emission from Lead-Free Mixed-Cation Doped Cs2SnCl6 Nanocrystals. Nanoscale 2022, 14, 1468–1479. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, B.; Zhu, M.; Zhang, Y.; Chong, X.; Li, J.; Wang, J. Growth Process and Optical Properties of Cs2ZrCl6 Doped with Ce3+ and Li+ Crystals. Cryst. Growth Des. 2023, 23, 3589–3594. [Google Scholar] [CrossRef]
- Buryi, M.; Babin, V.; Ligthart, R.A.M.; Nagorny, S.S.; Mikhailik, V.B.; Vaněček, V.; Prouzová Prochazková, L.; Kandel, R.; Nahorna, V.V.; Wang, P. Correlation of Emission, Scintillation and Charge Trapping Properties in Cs2HfCl6 and Cs2ZrCl6 Single Crystals. J. Mater. Chem. C 2021, 9, 2955–2968. [Google Scholar] [CrossRef]
- Vanecek, V.; Kral, R.; Paterek, J.; Babin, V.; Jary, V.; Hybler, J.; Kodama, S.; Kurosawa, S.; Yokota, Y.; Yoshikawa, A.; et al. Modified Vertical Bridgman Method: Time and Cost Effective Tool for Preparation of Cs2HfCl6 Single Crystals. J. Cryst. Growth 2020, 533, 125479. [Google Scholar] [CrossRef]
- Jia, X.; Jiang, H.; Chong, X.; Ju, W.; Feng, T.; Zhao, Z.; Wei, H.; Li, J.; Cheng, S.; Wang, J. A Novel Co-Substitution Strategy for the Design, Growth, and Optoelectronic Performance Optimization of Cs2ZrCl6:Ce,Li Single Crystals. J. Mater. Chem. C 2025, 13, 2844–2852. [Google Scholar] [CrossRef]
- Song, J.; Cui, Q.; Li, J.; Xu, J.; Wang, Y.; Xu, L.; Xue, J.; Dong, Y.; Tian, T.; Sun, H.; et al. Ultralarge All-Inorganic Perovskite Bulk Single Crystal for High-Performance Visible–Infrared Dual-Modal Photodetectors. Adv. Opt. Mater. 2017, 5, 1700157. [Google Scholar] [CrossRef]




| Materials | Substance | Temperature Gradient (°C/cm) | Growth Rate | References |
|---|---|---|---|---|
| CsPbX3 | CsX, PbX2 | 10–30 | 0.2–1 mm/hour | [8] |
| CsPbBr3 | CsBr, PbBr2 | 30–40 | 1 mm/hour | [23] |
| CsPbBr3 | CsBr, PbBr2 | 50–70 | 0.2–0.6 mm/hour | [76,183] |
| CsPbBr3 | CsBr, PbBr2 | 5–20 | 0.5–2 mm/hour | [80] |
| CsPbCl3 | CsCl, PbCl2 | 5–20 | 0.1–1 mm/hour | [94] |
| γ-CuCl | CuCl | 20 | 8 mm/day | [30] |
| γ-CuBr | CuBr | 5 | 6 mm/day | [35] |
| CsPbBr3 | CsBr, PbBr2 | 10 | 3–30 mm/hour | [79] |
| PbI2 | PbI2 | 22 | 0.5–1.0 mm/hour | [41] |
| TlSn2I5 | Sn, I2, TlI | 23 | [168] | |
| Tl2HfX6 | TlX, HfX4 | / | 0.2–1 mm/hour | [150] |
| Tl2LaX5 | TlX, LaX3 | / | 0.2–1 mm/hour | [150] |
| A3M2I9 (A = Cs, Rb; M = Bi, Sb) | Cs3Sb2I9: Sb2O3, HI, Cs2CO3; Cs3Bi2I9: Bi2O3, HI, CsI; Rb3Bi2I9: BiI3, RbI | / | 2 mm/hour | [129,130] |
| TlPbI3 | TlI, PbI2 | 12 | 20 mm/day | [167] |
| TlPbI3 | Tl, Pb, I2 | 8 | 0.5 mm/hour | [159] |
| Eu-doped TlSr2I5 | TlI, SrI2, EuI2 | / | 10 mm/day | [152] |
| Bi-doped CsCdX3 | CsX, CdX2, BiX3 | / | 2 mm/hour | [162] |
| Sn-doped CsPbX3 | CsX, PbX2, SnX2 | / | 1.4 mm/hour | [61] |
| Bi-doped TlCdCl3 | TlCl, CdCl2, BiCl3 | / | 1 mm/hour | [163] |
| Cs2PbI2Cl2 | CsI, PbCl2 | / | 0.7 mm/hour | [109] |
| Cs2PbI2Cl2 | CsI, PbCl2 | / | 0.77 mm/hour | [113] |
| Cs3Bi2Br9 | CsBr, BiBr3 | 10–20 | 0.5–3.0 mm/hour | [123] |
| Cs3Bi2I8Br | CsI, BiBr3 | 15 | 1 mm/hour | [126] |
| 6Li-doped Cs3Cu2I5 | CsI, CuI, 6LiI | / | 0.4 mm/hour | [134] |
| Tl-doped Cs3Cu2I5 | CsI, CuI, TlI | 20–30 | 0.5–1.0 mm/hour | [138] |
| Ce,Li-doped Cs2ZrCl6 | CsCl, ZrCl4, CeCl3, LiCl | 15 | 0.7 cm/day | [179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Wang, S.; Sheng, M.; Shao, B.; He, Y.; Liu, Z.; Zhou, G. Bridgman Method for Growing Metal Halide Single Crystals: A Review. Inorganics 2025, 13, 53. https://doi.org/10.3390/inorganics13020053
Zhu H, Wang S, Sheng M, Shao B, He Y, Liu Z, Zhou G. Bridgman Method for Growing Metal Halide Single Crystals: A Review. Inorganics. 2025; 13(2):53. https://doi.org/10.3390/inorganics13020053
Chicago/Turabian StyleZhu, Hui, Suqin Wang, Ming Sheng, Bo Shao, Yu He, Zhuang Liu, and Guangtao Zhou. 2025. "Bridgman Method for Growing Metal Halide Single Crystals: A Review" Inorganics 13, no. 2: 53. https://doi.org/10.3390/inorganics13020053
APA StyleZhu, H., Wang, S., Sheng, M., Shao, B., He, Y., Liu, Z., & Zhou, G. (2025). Bridgman Method for Growing Metal Halide Single Crystals: A Review. Inorganics, 13(2), 53. https://doi.org/10.3390/inorganics13020053








