Pyridinesilver Tetraoxometallate Complexes: Overview of the Synthesis, Structure, and Properties of Pyridine Complexed AgXO4 (X = Cl, Mn, Re) Compounds
Abstract
:1. Introduction
2. Synthesis and Composition of AgPynXO4 (X = Mn, Cl, and Re) Complexes
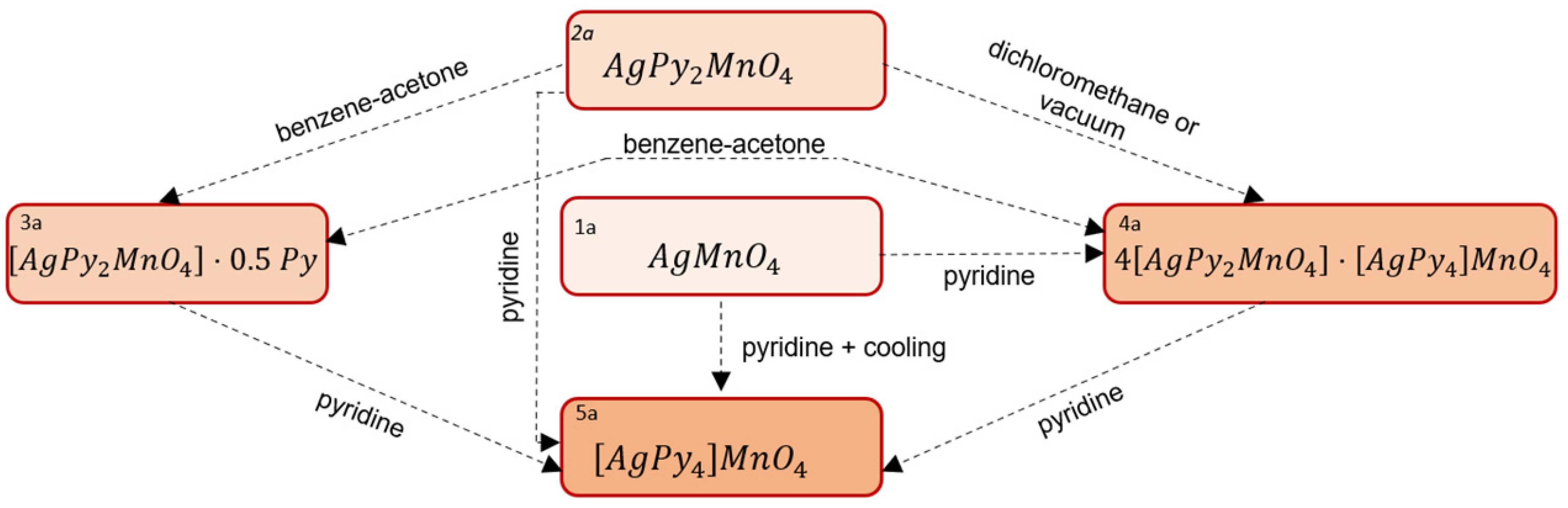
2.1. Pyridine Complexes of Silver Permanganate
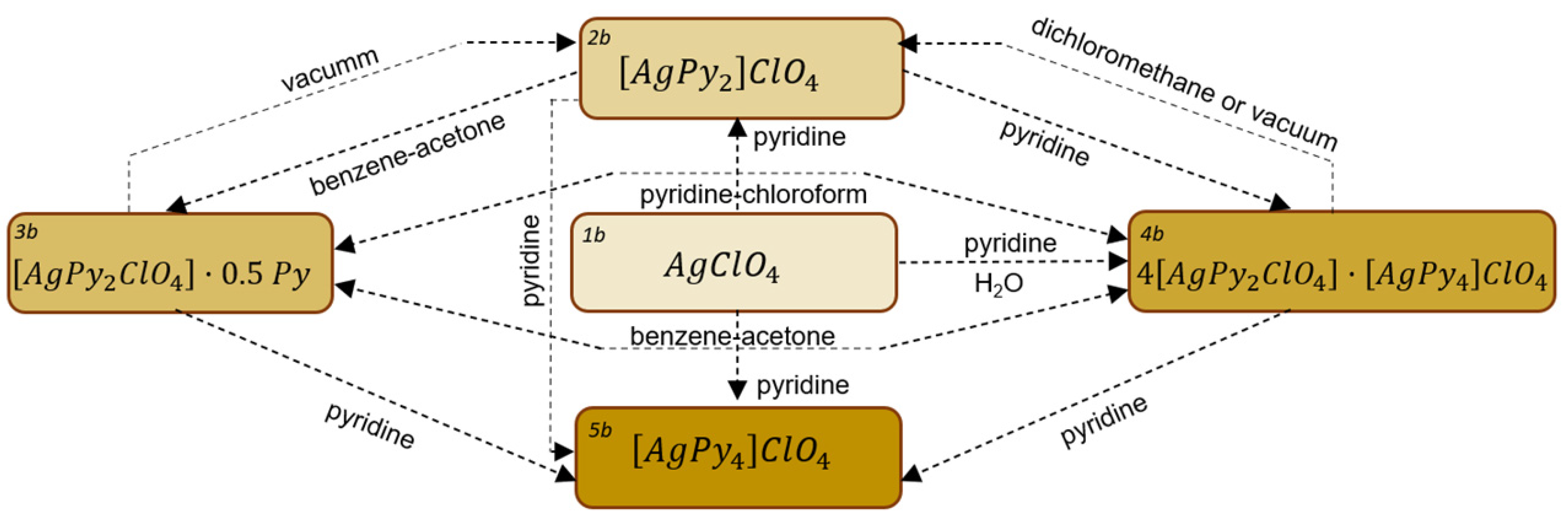
2.2. Pyridine Complexes of Silver Perchlorate
2.3. Pyridine Complexes of Silver Perrhenate
3. Thermal Analysis of Pyridine Complexes of AgXO4 Compounds (X = Mn, Cl, and Re)
3.1. Pyridine Complexes of AgMnO4
3.2. Pyridine Complexes of AgClO4
3.3. Pyridine Complexes of AgReO4
4. Crystallographic Structure of Pyridine Complexes of AgXO4 Compounds (X = Mn, Cl, and Re)
5. Spectroscopic Properties of Pyridine Complexes of AgXO4 Compounds (X = Mn, Cl, and Re)
5.1. Infrared and Raman Spectra
5.2. UV-Vis Spectra
6. Organic Oxidation Reactions with Pyridine-Silver Permanganate Complexes
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klobb, T. Combinaisons de la pyridine avec les permanganates. C. R. Chim. 1886, 118, 1271–1273. [Google Scholar]
- Klobb, T. Compounds of pyridine and permanganates. Bull. Soc. Chim. Paris 1894, 11, 604–609. [Google Scholar]
- Macy, R. The ternary system: Silver perchlorate, pyridine and water. J. Am. Chem. Soc. 1925, 47, 1031–1036. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Vessal, B.; Naderi, M. Bispyridinesilver permanganate [Ag(C5H5N)2]MnO4: An efficient oxidizing reagent for organic substrates. Tetrahedron Lett. 1982, 23, 1847–1850. [Google Scholar] [CrossRef]
- Nilson, K.; Oskarsson, A. The crystal structure of tetrapyridine copper (I) perchlorate and tetrapyridine silver (I) perchlorate at 260 K. Acta Chem. Scand. Ser. A Phys. Inorg. Chem. 1982, 37, 605–610. [Google Scholar] [CrossRef]
- Kovács, G.B.; May, N.V.; Bombicz, P.A.; Klébert, S.; Németh, P.; Menyhárd, A.; Novodárszki, G.; Petrusevski, V.; Franguelli, F.P.; Magyari, J.; et al. An unknown component of a selective and mild oxidant: Structure and oxidative ability of a double salt-type complex having κ1O-coordinated permanganate anions and three- and four-fold coordinated silver cations. RSC Adv. 2019, 9, 28387–28398. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.G. Bis(pyridine) silver(I) Permanganate. In Encyclopedia of Reagents for Organic Synthesis; John Wiley and Sons Inc.: New York, NY, USA, 2001; Print ISBN: 9780471936237|Online ISBN: 9780470842898. [Google Scholar] [CrossRef]
- Kótai, L.; Sajó, I.; Fodor, J.; Szabó, P.; Jakab, E.; Argay, G.; Holly, S.; Gács, I.; Banerji, K.K. Reasons for and Consequences of the Mysterious Behaviour of Newly Prepared Hemipyridine Solvate of Bis(pyridine)silver(I) Permanganate, Agpy2MnO4*0.5py. Trans. Met. Chem. 2005, 30, 939–943. [Google Scholar] [CrossRef]
- Sajó, I.E.; Kovács, G.B.; Pasinszki, T.; Bombicz, P.A.; May, Z.; Szilágyi, I.M.; Jánosity, A.; Banerji, K.K.; Kant, R.; Kótai, L. The chemical identity of “[Ag(py)2]MnO4” organic solvent soluble oxidizing agent and new synthetic routes for the preparation of [Ag(py)n]XO4 (X = Mn, Cl, and Re, n = 2–4) complexes. J. Coord. Chem. 2018, 71, 2884–2904. [Google Scholar] [CrossRef]
- Kauffman, G.B.; Pinnell, R.P.; Stone, R.D. Dipyridinesilver(I) Perchlorate. In Inorganic Synthesis, 6th ed.; McGraw-Hill: New York, NY, USA, 1925. [Google Scholar]
- Dyason, J.; Healy, P.; Engelhardt, L.; White, A. Lewis-Base Adducts of Group 1B Metal(I) Compounds. XXII. Crystal Structure of ’Bis(pyridine)silver(I) Perchlorate. Aust. J. Chem. 1985, 38, 1325–1328. [Google Scholar] [CrossRef]
- Bowmaker, G.A.; Effendy, M.S.; Skelton, B.W.; White, A.H. Syntheses, structures and vibrational spectroscopy of some 1:1 and 1:2 adducts of silver(I) oxyanion salts with 2,2′-bis(pyridine) chelates. Inorg. Chim. Acta 2005, 358, 4371–4388. [Google Scholar] [CrossRef]
- Holló, B.B.; Petrusevski, V.M.; Kovács, G.B.; Franguelli, F.P.; Farkas, A.; Menyhárd, A.; Lendvay, G.; Sajó, I.E.; Nagy-Bereczki, L.; Pawar, R.P.; et al. Thermal and spectroscopic studies on a double-salt-type pyridine–silver perchlorate complex having κ1-O coordinated perchlorate ions. J. Therm. Anal. Calorim. 2019, 138, 1193–1205. [Google Scholar] [CrossRef] [Green Version]
- Wilke-Dörfurt, E.; Gunzert, T. Über Neue Salze der Perrheniumsäure. Z. Anorg. Allg. Chem. 1933, 215, 369–387. [Google Scholar] [CrossRef]
- Woolf, A.A. A comparison of silver perrhenate with silver perchlorate. J. Less Common Met. 1978, 61, 151–160. [Google Scholar] [CrossRef]
- Kótai, L.; Fodor, J.; Jakab, E.; Sajó, I.E.; Sazbó, P.; Lónyi, F.; Valyon, J.; Gács, I.; Argay, G.; Banerji, K.K. A Thermally Induced Low-temperature Intramolecular Redox Reaction of bis(pyridine)silver(I) Permanganate and its hemipyridine Solvate. Trans. Met. Chem. 2006, 31, 30–34. [Google Scholar] [CrossRef]
- Buck, R.P.; Singhadeja, S.; Rogers, L.B. Ultraviolet Absorption Spectra of Some Inorganic Ions in Aqueous Solutions. Anal. Chem. 1954, 26, 1240–1242. [Google Scholar] [CrossRef]
- Larbi, T.; Doll, K.; Manoubi, T. Density functional theory study of ferromagnetically and ferrimagnetically ordered spinel oxide Mn3O4. A quantum mechanical simulation of their IR and Raman spectra. J. Alloys Compd. 2016, 688, 692–698. [Google Scholar] [CrossRef]
- Solymosi, F. The Thermal Stability and Some Physical Properties of Silver Chlorite, Chlorate and Perchlorate. Z. Phys. Chem. 1968, 57, 1–18. [Google Scholar] [CrossRef]
- Chen, T.Y.; Zeng, J.Y.; Lee, H.M. Argentophilic interaction and anionic control of supramolecular structures in simple silver pyridine complexes. Inorg. Chem. Acta 2007, 360, 21–30. [Google Scholar] [CrossRef]
- Bando, Y.; Nagakura, S. The electronic structure and spectrum of the silver(I)perchlorate-pyridine complex. Theor. Chem. Acta 1968, 9, 210–221. [Google Scholar] [CrossRef]
- Boopalachandran, P.; Laane, J. Ultraviolet absorption spectra of pyridine-d0 and -d5 and their ring-bending potential energy function in the S1 (n,π*) state. Chem. Phys. Lett. 2008, 462, 178–182. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Sardarian, A.R. Facile oxidation od polycyclic arenes and acetylenic hydrocarbons with Bis(pyridine)silver permanganate and Bis(2,2’-bipyridil)copper(II)permanganate under mild and neutral conditions. Synth 1986, 946–948. [Google Scholar] [CrossRef]
- Besedin, D.V.; Gulevskaya, A.V.; Pozharskii, A.F. Reaction of 6,8-dimethylpyrimido[4,5- c]pyridazine-5,7(6 H,8 H)-dione with α,ω-diamines as the first example of tandem nucleophilic substitution in neutral azines. Mendeleev Commun. 2000, 10, 150–151. [Google Scholar] [CrossRef]
- Gulevskaya, A.V.; Besedin, D.V.; Pozharskii, A.F.; Starikova, Z.A. 6,8-Dimethylpyrimido[4, 5-c]pyridazine-5,7(6H,8H)-dione: A novel method of pyrrole-ring annulation to an azine nucleus based on a tandem SNH–SNH process. Tetrahedron Lett. 2001, 42, 5981–5983. [Google Scholar] [CrossRef]
- Kesenheimer, C.; Groth, U. Total synthesis of (-)-8-O-methyltetrangomycin (MM47755). Org. Lett. 2006, 8, 2507–2510. [Google Scholar] [CrossRef] [Green Version]
- Banerji, J.; Kótai, L.; Sharma, P.K.; Banerji, K.K. Kinetics and mechanisms of the oxidation of substituted benzaldehydes with bis(pyridine) silver permanganate. Eur. Chem. Bull. 2012, 1, 135–140. [Google Scholar]
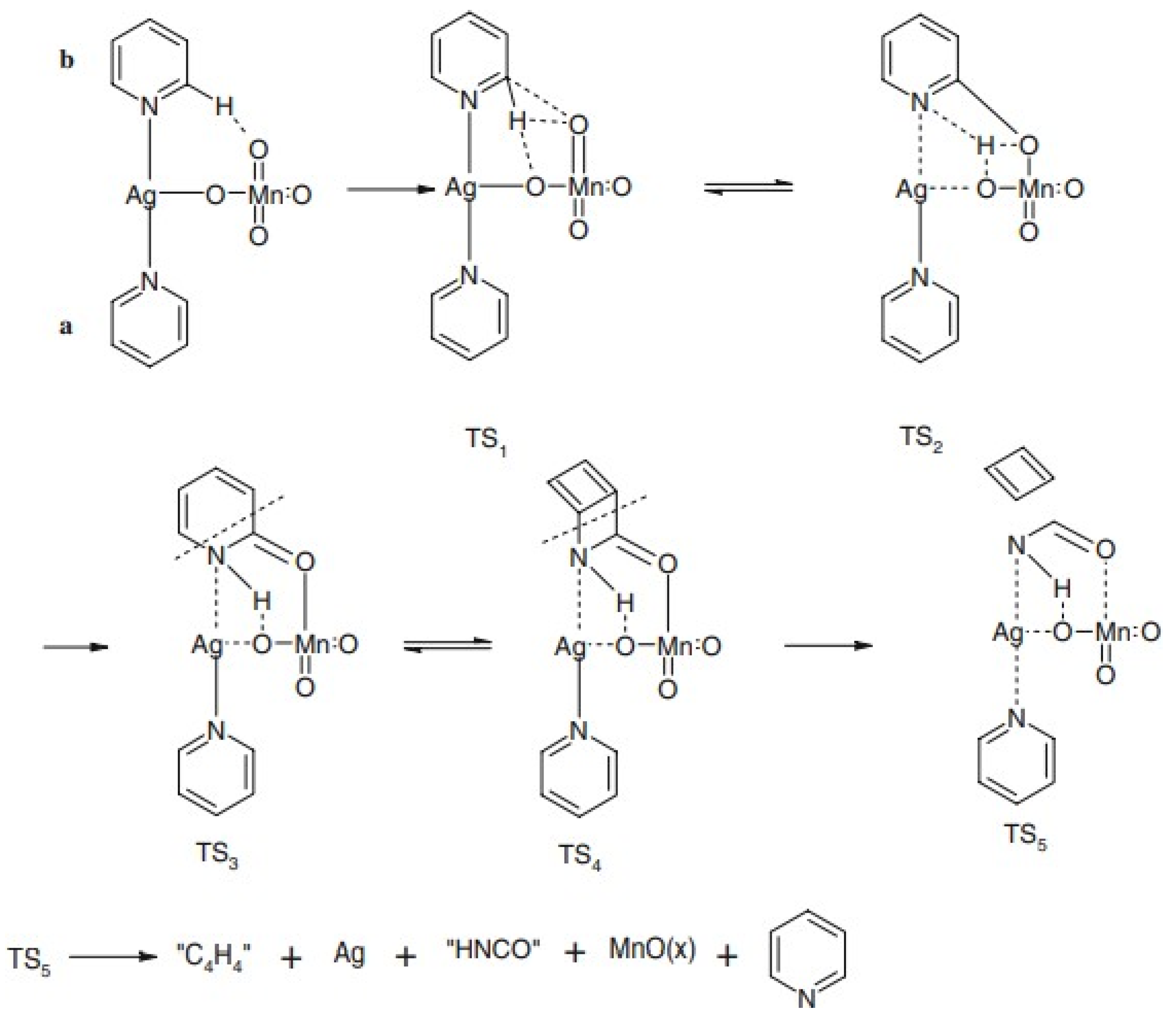

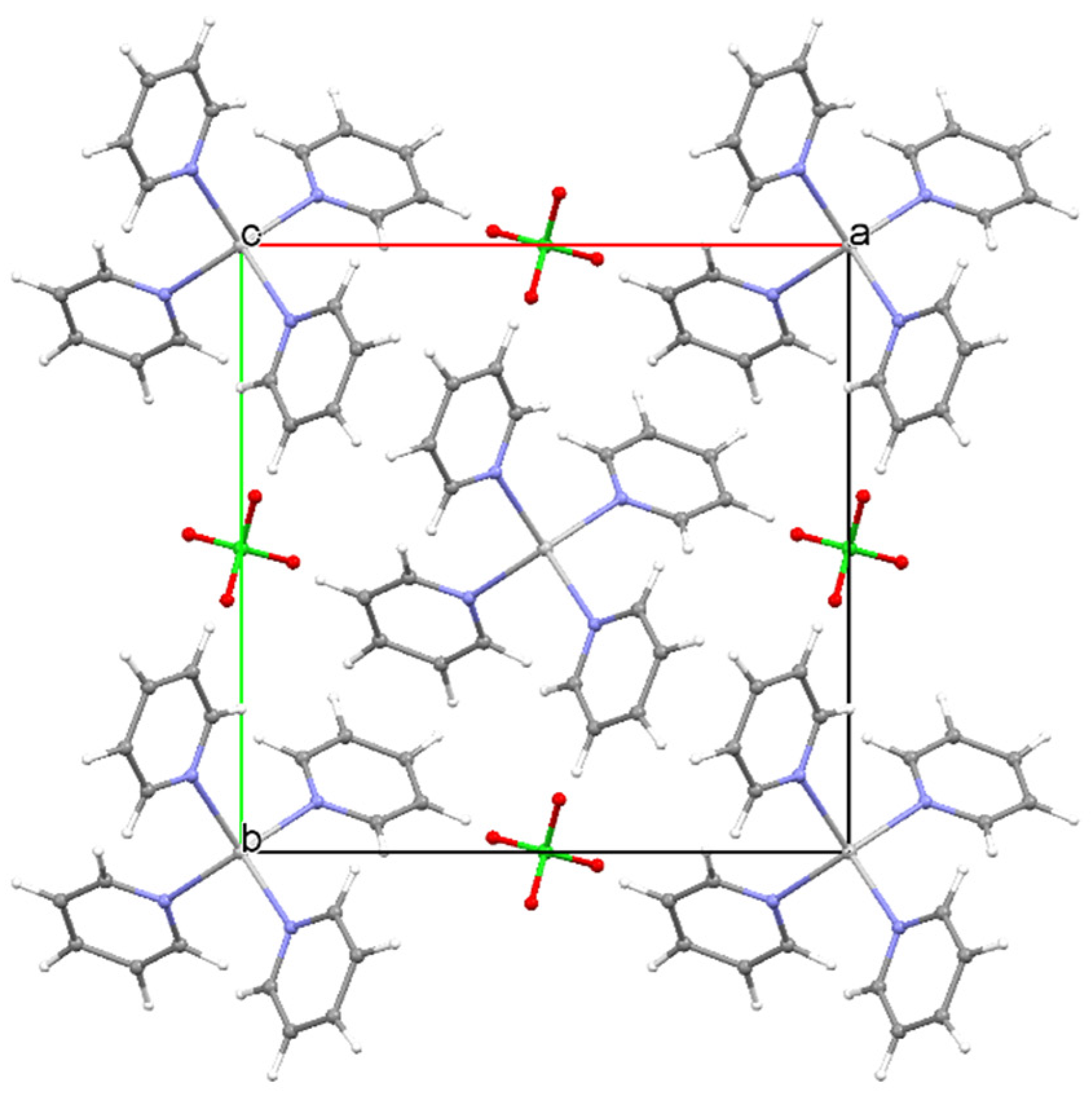

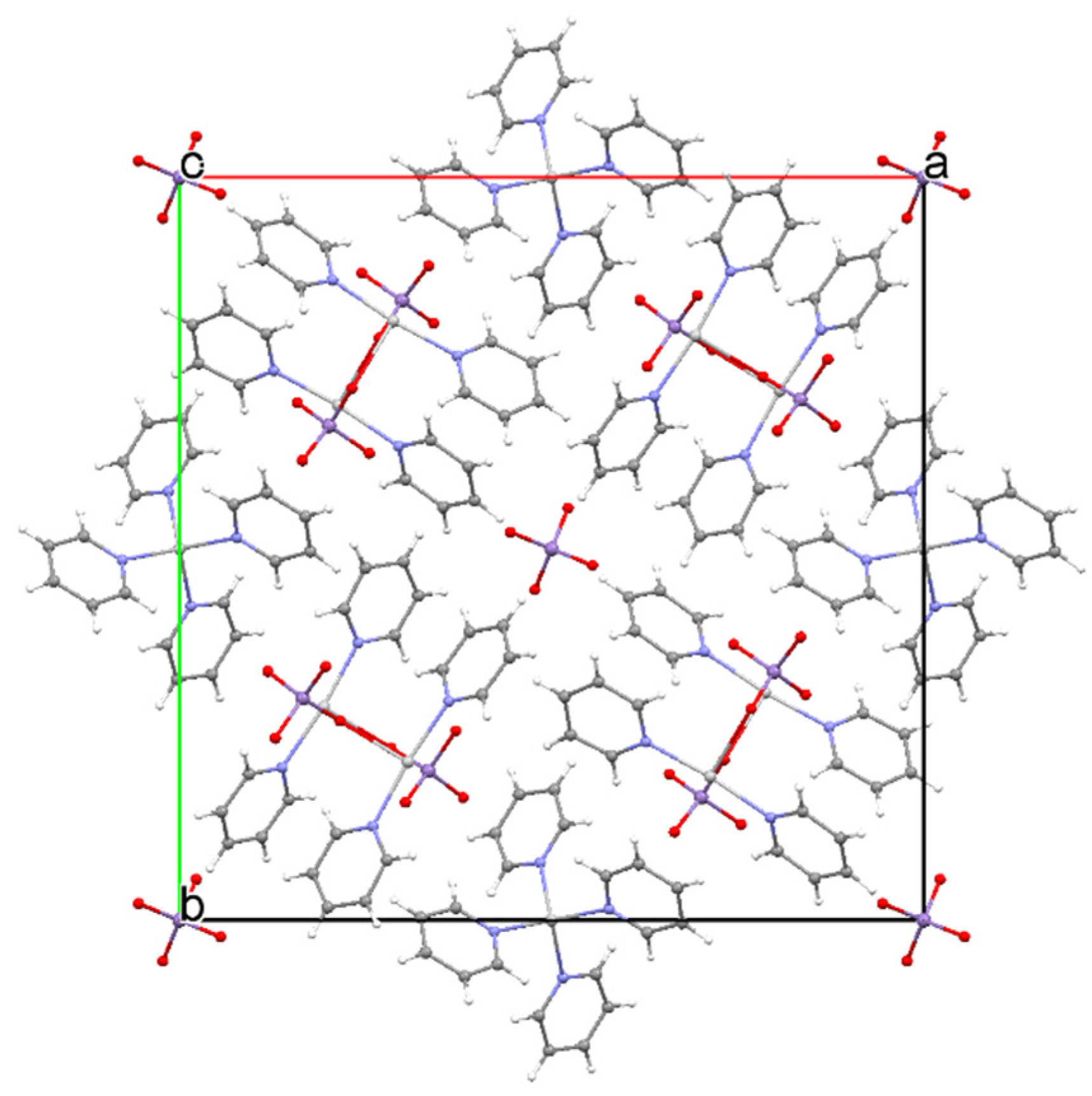
| Compound | Label | Py:Ag Ratio |
|---|---|---|
| AgMnO4 | 1a | - |
| AgClO4 | 1b | - |
| AgReO4 | 1c | - |
| AgPy2MnO4 | 2a | 2 |
| [AgPy2]ClO4 | 2b | 2 |
| [AgPy2]ReO4 | 2c | 2 |
| [AgPy2MnO4]·0.5Py | 3a | 2.5 |
| [AgPy2ClO4]·0.5Py | 3b | 2.5 |
| AgPy2ReO4·0.5Py | 3c | 2.5 |
| 4[AgPy2MnO4]·[AgPy4]MnO4 | 4a | 2.4 |
| 4[AgPy2ClO4]·[AgPy4]ClO4 | 4b | 2.4 |
| 4AgPy2ReO4·AgPy4ReO4 | 4c | 2.4 |
| [AgPy4]MnO4 | 5a | 4 |
| [AgPy4]ClO4 | 5b | 4 |
| [AgPy4]ReO4 | 5c | 4 |
| Empirical Formula | Label | Space Group | Unit Cell Dimensions, (Å or °) | Z | D (g·cm−3) | T (K) | V (Å)3 | Reference |
|---|---|---|---|---|---|---|---|---|
| Agpy2MnO4 | 2a | Cc | a = 22.875 b = 12.266 c = 20.225 β = 62.361 | 16 | 1.970 | 298 | 5191.2 | [9] |
| [Agpy2]ClO4 | 2b | Pnn2/Pnnm | a = 20.138 b = 12.694 c = 10.125 | 8 | 1.876 | 298 | 2588.3 | [9] |
| [Agpy2]ClO4 | 2b | Pbcn | a = 19.958 (2) b = 10.0034 (13) c = 12.3082 (16) | 8 | 1.976 | 150 | 2457.3 (5) | [20] |
| [Agpy2]ReO4 | 2c | - | a = 7.140 b = 8.616 c = 10.827 α = 102.20 β = 96.25 γ = 105.58 | 2 | 2.655 | 298 | 645.85 | [9] |
| 4[Agpy2MnO4]· [Agpy4]MnO4 | 4a | a = 22.01 c = 7.6075 | 10 (1) | 1.877 | 298 | 3685.4 | [9] | |
| 4[Agpy2MnO4]· [Agpy4]MnO4 | 4a | a = 21.982 (3) b = 21.982 (3) c = 7.5974 (15) | 2 | 1.885 | 293 | 3671.13 | [6] | |
| 4[Agpy2ClO4]· [Agpy4]ClO4 | 4b | a = 21.95 (1) c = 7.6843 (3) | - | 1.78 | 295 | 3702 (2) | [11] | |
| 4[Agpy2ClO4]· [Agpy4]ClO4 | 4b | - | - | - | - | - | [13] | |
| [Agpy2MnO4]·0.5Py | 3a | C2/c | a = 19.410 (1) b = 7.788 (1) c = 21.177 (1) α = 90.00 β = 104.20 (1) γ = 90.00 | 4 | 1.817 | 293 | 3103.4 (5) | [9] |
| [Ag(py)4]MnO4 | 5a | P21 | a = 15.24 b = 13.89 c = 5.31 β = 84.13o | 2 | - | 298 | 1117 | [9] |
| [Ag(py)4]ClO4 | 5b | , | a = 12.874 (1) c = 6.748 (4) | 2 | 1.55 | 260 | 1118.4 | [5] |
| Compounds/Pace Group. | Label | Ag-Ag (Å) | Ag-N (Å) | α-CH…O-X/F-X (Å) | Reference |
|---|---|---|---|---|---|
| 4[Agpy2]ClO4·[Agpy4]ClO4, I-4 | 4b | 4.843 | 2.740 2.15 | 2.645 2.645 | [13] |
| 4[Agpy2]ClO4·[Agpy4]ClO4, I-4 | 4b | 4.843 | 2.16 2.30 | 2.753 2.781 | [11] |
| 4[Agpy2]MnO4·[Agpy4] MnO4, I-4 | 4a | 4.822 | 2.601 | 3.121 | [6] |
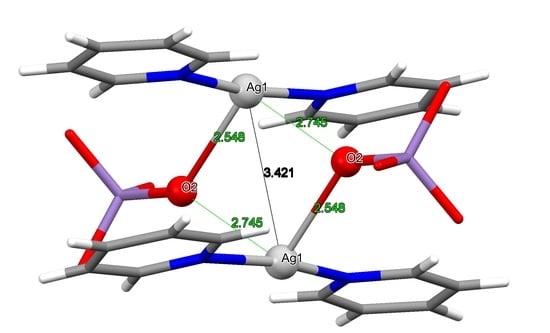
| [Agpy2MnO4]·0.5Py C2/c | 3a | 3.421 | 2.166 2.174 | 2.602 2.770 | [9] |
| [Agpy2]ClO4, Pbcn | 2b | 2.999 | 2.126 2.133 | 2.672 2.581 2.700 2.566 | [20] |
| [Agpy4]ClO4, I-4 | 2b | 6.748 | 2.322 | 2.712 3.237 | [5] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franguelli, F.P.; Béres, K.A.; Kótai, L. Pyridinesilver Tetraoxometallate Complexes: Overview of the Synthesis, Structure, and Properties of Pyridine Complexed AgXO4 (X = Cl, Mn, Re) Compounds. Inorganics 2021, 9, 79. https://doi.org/10.3390/inorganics9110079
Franguelli FP, Béres KA, Kótai L. Pyridinesilver Tetraoxometallate Complexes: Overview of the Synthesis, Structure, and Properties of Pyridine Complexed AgXO4 (X = Cl, Mn, Re) Compounds. Inorganics. 2021; 9(11):79. https://doi.org/10.3390/inorganics9110079
Chicago/Turabian StyleFranguelli, Fernanda Paiva, Kende Attila Béres, and Laszló Kótai. 2021. "Pyridinesilver Tetraoxometallate Complexes: Overview of the Synthesis, Structure, and Properties of Pyridine Complexed AgXO4 (X = Cl, Mn, Re) Compounds" Inorganics 9, no. 11: 79. https://doi.org/10.3390/inorganics9110079
APA StyleFranguelli, F. P., Béres, K. A., & Kótai, L. (2021). Pyridinesilver Tetraoxometallate Complexes: Overview of the Synthesis, Structure, and Properties of Pyridine Complexed AgXO4 (X = Cl, Mn, Re) Compounds. Inorganics, 9(11), 79. https://doi.org/10.3390/inorganics9110079