The Impact of Laser Thermal Effect on Histological Evaluation of Oral Soft Tissue Biopsy: Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
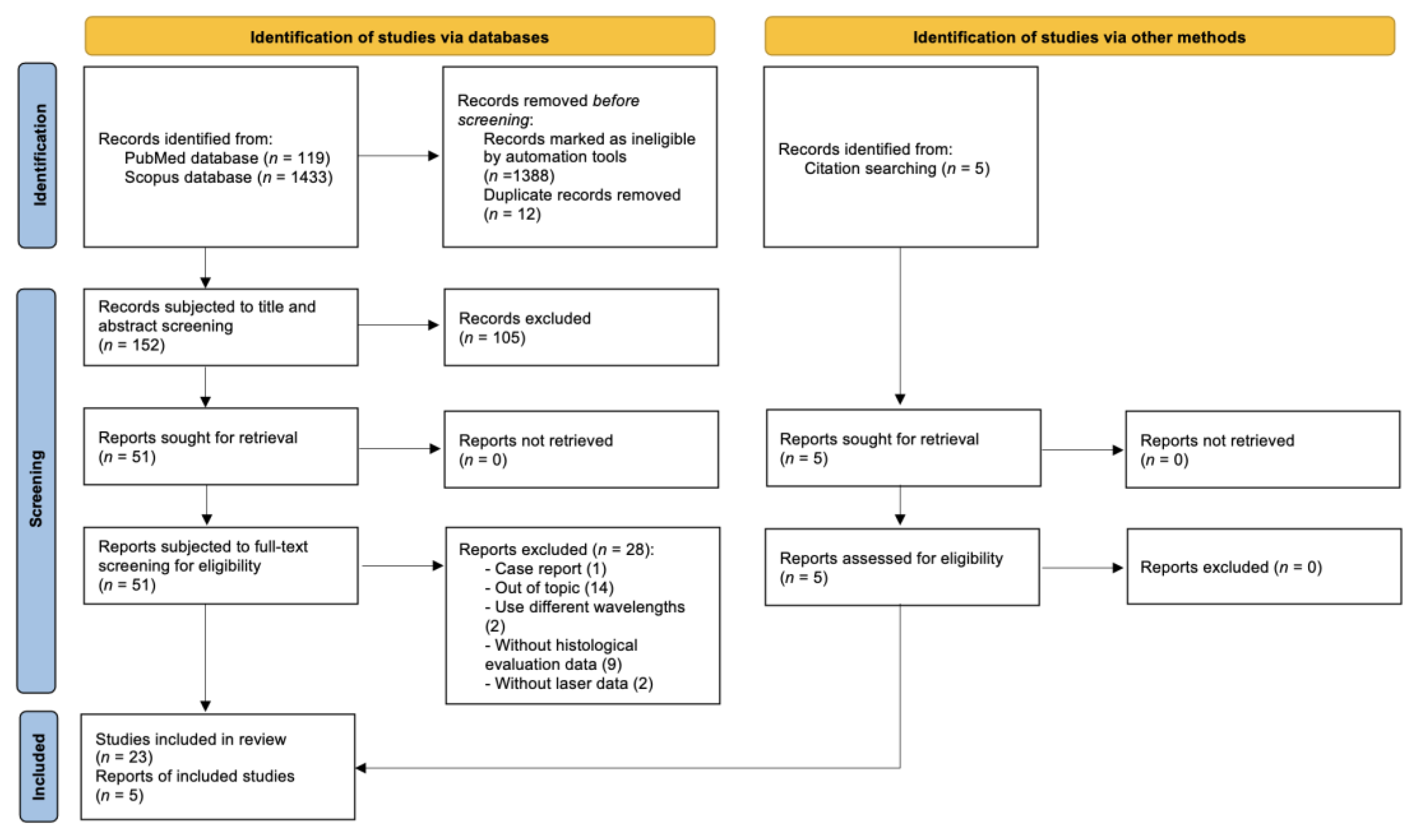
2.2. Search Strategy
2.3. Study Selection
2.4. Extraction and Synthesis of Data
2.5. Assessment of Quality and Bias
3. Results
3.1. Animal Studies
3.2. Clinical Studies
3.3. Laser Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saini, R.; Saini, S.; Sharma, S. Oral biopsy: A dental gawk. J. Surg. Tech. Case Rep. 2010, 2, 93. [Google Scholar] [CrossRef] [PubMed]
- Avon, S.L.; Klieb, H.B. Oral soft-tissue biopsy: An overview. J. Can. Dent. Assoc. 2012, 78, c75. [Google Scholar] [PubMed]
- Cercadillo-Ibarguren, I.; España-Tost, A.; Arnabat-Domínguez, J.; Valmaseda-Castellón, E.; Berini-Aytés, L.; Gay-Escoda, C. Histologic evaluation of thermal damage produced on soft tissues by CO2, Er,Cr:YSGG and diode lasers. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e912–e918. [Google Scholar] [CrossRef] [Green Version]
- Pick, R.M.; Colvard, M.D. Current status of lasers in soft tissue dental surgery. J. Periodontol. 1993, 64, 589–602. [Google Scholar] [CrossRef]
- Palaia, G.; Del Vecchio, A.; Impellizzeri, A.; Tenore, G.; Visca, P.; Libotte, F.; Russo, C.; Romeo, U. Histological ex vivo evaluation of peri-incisional thermal effect created by a new-generation CO2 superpulsed laser. Sci. World J. 2014, 2014, 345685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Convissar, R.A. Laser biopsy artifact. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 84, 458. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [Green Version]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Romeo, U.; Del Vecchio, A.; Ripari, F.; Palaia, G.; Chiappafreddo, C.; Tenore, G.; Visca, P. Effects of different laser devices on oral soft tissue: In vitro experience. J. Oral. Laser Appl. 2007, 7, 155–159. [Google Scholar]
- Romeo, U.; Palaia, G.; Del Vecchio, A.; Tenore, G.; Gambarini, G.; Gutknecht, N.; De Luca, M. Effects of KTP laser on oral soft tissues. An in vitro study. Lasers Med. Sci. 2010, 25, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Romeo, U.; Libotte, F.; Palaia, G.; Del Vecchio, A.; Tenore, G.; Visca, P.; Nammour, S.; Polimeni, A. Histological in vitro evaluation of the effects of Er:YAG laser on oral soft tissues. Lasers Med. Sci. 2012, 27, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, A.S.; Monteiro, L.S.; Ferreira, F.; Delgado, M.L.; Garcês, F.; Carreira, S.; Martins, M.; Suarez-Quintanilla, J. In vitro histological evaluation of the surgical margins made by different laser wavelengths in tongue tissues. J. Clin. Exp. Dent. 2016, 8, e388–e396. [Google Scholar] [CrossRef] [PubMed]
- Palaia, G.; Impellizzeri, A.; Tenore, G.; Caporali, F.; Visca, P.; Del Vecchio, A.; Galluccio, G.; Polimeni, A.; Romeo, U. Ex vivo histological analysis of the thermal effects created by a 445-nm diode laser in oral soft tissue biopsy. Clin. Oral. Investig. 2020, 24, 2645–2652. [Google Scholar] [CrossRef] [Green Version]
- Palaia, G.; Renzi, F.; Pergolini, D.; Del Vecchio, A.; Visca, P.; Tenore, G.; Romeo, U. Histological Ex Vivo Evaluation of the Suitability of a 976 nm Diode Laser in Oral Soft Tissue Biopsies. Int. J. Dent. 2021, 2021, 6658268. [Google Scholar] [CrossRef]
- Prado, M.C.O.; Nwizu, N.N.; Patel, S.A.; Streckfus, C.F.; Zezell, D.M.; Barros, J. Thermal damage and excision time of micro and super pulsed diode lasers: A comparative ex vivo analysis. Clin. Exp. Dent. Res. 2022. ahead of print. [Google Scholar] [CrossRef]
- Fornaini, C.; Merigo, E.; Rocca, J.P.; Lagori, G.; Raybaud, H.; Selleri, S.; Cucinotta, A. 450 nm Blue Laser and Oral Surgery: Preliminary ex vivo Study. J. Contemp. Dent. Pract. 2016, 17, 795–800. [Google Scholar] [CrossRef]
- Braun, A.; Kettner, M.; Berthold, M.; Wenzler, J.S.; Heymann, P.G.B.; Frankenberger, R. Efficiency of soft tissue incision with a novel 445-nm semiconductor laser. Lasers Med. Sci. 2018, 33, 27–33. [Google Scholar] [CrossRef]
- Rizoiu, I.M.; Eversole, L.R.; Kimmel, A.I. Effects of an erbium, chromium: Yttrium, scandium, gallium, garnet laser on mucocutanous soft tissues. Oral Surg Oral Med. Oral Pathol. Oral Radiol. Endod. 1996, 82, 386–395. [Google Scholar] [CrossRef]
- Seoane, J.; Caballero, T.G.; Urizar, J.M.; Almagro, M.; Mosquera, A.G.; Varela-Centelles, P. Pseudodysplastic epithelial artefacts associated with oral mucosa CO2 laser excision: An assessment of margin status. Int. J. Oral Maxillofac. Surg. 2010, 39, 783–787. [Google Scholar] [CrossRef]
- Kawamura, R.; Mizutani, K.; Lin, T.; Kakizaki, S.; Mimata, A.; Watanabe, K.; Saito, N.; Meinzer, W.; Iwata, T.; Izumi, Y.; et al. Ex Vivo Evaluation of Gingival Ablation with Various Laser Systems and Electroscalpel. Photobiomodul. Photomed. Laser Surg. 2020, 38, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Suter, V.G.; Altermatt, H.J.; Dietrich, T.; Reichart, P.A.; Bornstein, M.M. Does a pulsed mode offer advantages over a continuous wave mode for excisional biopsies performed using a carbon dioxide laser? J. Oral Maxillofac. Surg. 2012, 70, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Suter, V.G.A.; Altermatt, H.J.; Bornstein, M.M. A randomized controlled clinical and histopathological trial comparing excisional biopsies of oral fibrous hyperplasias using CO2 and Er:YAG laser. Lasers Med. Sci. 2017, 32, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Suter, V.G.A.; Altermatt, H.J.; Bornstein, M.M. A randomized controlled trial comparing surgical excisional biopsies using CO2 laser, Er:YAG laser and scalpel. Int. J Oral. Maxillofac. Surg. 2020, 49, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Suzuki, H.; Usami, Y.; Hattori, M.; Komoro, T. Histological evaluation of artifacts in tongue tissue produced by the CO2 laser and the electrotome. Photomed. Laser Surg. 2008, 26, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Vescovi, P.; Corcione, L.; Meleti, M.; Merigo, E.; Fornaini, C.; Manfredi, M.; Bonanini, M.; Govoni, P.; Rocca, J.P.; Nammour, S. Nd:YAG laser versus traditional scalpel. A preliminary histological analysis of specimens from the human oral mucosa. Lasers Med. Sci. 2010, 25, 685–691. [Google Scholar] [CrossRef]
- Pogrel, M.A.; McCracken, K.J.; Daniels, T.E. Histologic evaluation of the width of soft tissue necrosis adjacent to carbon dioxide laser incisions. Oral Surg. Oral Med. Oral Pathol. 1990, 70, 564–568. [Google Scholar] [CrossRef]
- Angiero, F.; Parma, L.; Crippa, R.; Benedicenti, S. Diode laser (808 nm) applied to oral soft tissue lesions: A retrospective study to assess histopathological diagnosis and evaluate physical damage. Lasers Med. Sci. 2012, 27, 383–388. [Google Scholar] [CrossRef]
- Tenore, G.; Palaia, G.; Mohsen, A.; Ambrogiano, S.; Gioia, C.R.T.D.; Dominiak, M.; Romeo, U. Could the super-pulsed CO2 laser be used for oral excisional biopsies? Adv. Clin. Exp. Med. 2019, 28, 1513–1517. [Google Scholar] [CrossRef] [Green Version]
- Palaia, G.; Pergolini, D.; D’Alessandro, L.; Carletti, R.; Del Vecchio, A.; Tenore, G.; Di Gioia, C.R.T.; Romeo, U. Histological Effects of an Innovative 445 Nm Blue Laser During Oral Soft Tissue Biopsy. Int. J. Envrion. Res. Public Health 2020, 17, 2651. [Google Scholar] [CrossRef]
- Palaia, G.; D’Alessandro, L.; Pergolini, D.; Carletti, R.; Di Gioia, C.R.T.; Romeo, U. In vivo clinical and histological thermal effect of a 445 nm diode laser on oral soft tissues during a biopsy. J. Oral. Sci. 2021, 63, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Romeo, U.; Russo, C.; Palaia, G.; Lo Giudice, R.; Del Vecchio, A.; Visca, P.; Migliau, G.; De Biase, A. Biopsy of different oral soft tissues lesions by KTP and diode laser: Histological evaluation. Sci. World J. 2014, 2014, 761704. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, M.; Bussani, R.; Perinetti, G.; Rupel, K.; Bevilaqua, L.; Ottaviani, G.; Biasotto, M. Blue diode laser versus traditional infrared diode laser and quantic molecular resonance scalpel: Clinical and histological findings after excisional biopsy of benign oral lesions. J. Biomed. Opt. 2017, 22, 121602, Erratum in J. Biomed. Opt. 2019, 24, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, L.; Delgado, M.L.; Garcês, F.; Machado, M.; Ferreira, F.; Martins, M.; Salazar, F.; Pacheco, J.J. A histological evaluation of the surgical margins from human oral fibrous-epithelial lesions excised with CO2 laser, Diode laser, Er:YAG laser, Nd:YAG laser, electrosurgical scalpel and cold scalpel. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e271–e280. [Google Scholar] [CrossRef]
- Gill, K.; Sandhu, S.V.; Sethi, N.; Bhandari, R. Biopsy of oral soft tissue lesions by 808 nm and 980 nm diode laser: A morphological and histochemical evaluation. Laser Dent. Sci. 2021, 5, 207–222. [Google Scholar] [CrossRef]



| Databases | Search Strategy |
|---|---|
| PubMed | {“laser s”(All Fields) OR “lasers”(MeSH Terms)OR “lasers”(All Fields) OR “laser”(All Fields) OR “lasered”(All Fields) OR “lasering”(All Fields)} AND {“mouth”(MeSH Terms) OR “mouth”(All Fields) OR “oral”(All Fields)} AND {“biopsie”(All Fields) OR “biopsy”(MeSH Terms) OR “biopsy”(All Fields) OR “biopsied”(All Fields) OR “biopsies”(All Fields) OR “biopsy s”(All Fields) OR “biopsying”(All Fields) OR “biopsys”(All Fields) OR “pathology”(MeSH Subheading) OR “pathology”(All Fields)} AND {“thermal”(All Fields) OR “thermalization”(All Fields) OR “thermalize”(All Fields) OR “thermalized”(All Fields) OR “thermalizes”(All Fields) OR “thermalizing”(All Fields) OR “thermally”(All Fields) OR “thermals”(All Fields)} AND {“effect”(All Fields) OR “effecting”(All Fields) OR “effective”(All Fields) OR “effectively”(All Fields) OR “effectiveness”(All Fields) OR “effectivenesses”(All Fields) OR “effectives”(All Fields) OR “effectivities”(All Fields) OR “effectivity”(All Fields) OR “effects”(All Fields)} |
| Scopus | laser AND oral AND biopsy AND thermal AND effect AND {LIMIT-TO (SUBJAREA, “DENT”)} AND {LIMIT-TO (DOCTYPE, “ar”)} AND {LIMIT-TO (LANGUAGE, “English”)} |
| Author, Year | Laser | Sample | Sample (n) | Type of Microscope | Magnification | Method of Evaluation | Nr. of Pathologists | Main Outcomes | Conclusions |
|---|---|---|---|---|---|---|---|---|---|
| 1. Rizoiu et al., 1996 [19] | Er,Cr:YSGG | Ventral tongue mucosa of New Zealand white rabbits | 1 | Optical microscope | 13× | Histological evaluation of artifactual changes, marginal coagulation zones of hyalinization, and degree of inflammation. | - | - Minimal edge coagulation and carbonization artifacts were observed. - No vacuolization changes on contiguous epithelial or connective tissues. | This laser can be used for the collection of diagnostic biopsy specimens. |
| 2. Romeo et al., 2007 [10] | Diode lasers (808 nm, 980 nm), Nd:YAG, Er,Cr:YSGG | Pig tongues | 36 | Optical microscope | 10× or 25× | Histological evaluation and determination of the exact extent of peripheral thermal damage and comparing among different lasers. | 2 | - The 808 nm in PW 1 and the Er,Cr:YSGG showed the best results. - Deeper thermal effect in the CT 2 with Nd:YAG even if the laser worked with lower fluence than diode lasers in CW 3. | These lasers can be employed in the biopsy investigations of dysplastic lesions with the extension of margins to ensure a safe histological evaluation. |
| 3. Cercadillo-Ibarguren et al., 2010 [3] | Er,Cr:YSGG, CO2 | Porcine oral mucosa | 90 | Optical microscope | 40× | Macroscopic and histological evaluation through the measurement of the extension of the hyalinized or coagulated tissue adjacent to the irradiation edge. | 1 | - A wide range of thermal damage was observed with significant differences among different lasers and parameters. - The lowest thermal effect was observed with Er,Cr:YSGG laser using water/air spray followed by CO2 laser. | - The extent of thermal effect on soft tissues may be determined by the emission parameters of each laser type. - The wavelength remains the determinant of the absorption rate characteristics of every tissue and the thermal effect. |
| 4. Romeo et al., 2010 [11] | KTP | Pig cadaver tongue | 45 | Optical microscope | 10× | Histological analysis was performed by assigning a score from 0 to 3 to the peripheral thermal damage according to the following scale: 0—no damage, 1—little damage, 2—moderate damage, and 3—severe damage. | 2 | - The cut edges of the incision of all the specimens were free from histological artefacts. - No statistical differences were observed among the groups with a slight increase of thermal effect on groups with the higher power. | KTP laser showed surgical effectiveness and caused little peripheral damage to the cut edges. |
| 5. Seoane et al., 2010 [20] | CO2 | Sprague rat tongue | 20 | Optical microscope | 20× and 40× | Histological evaluation included epithelial features, number of artefacts, and width of thermal damage. | 2 | - The histological artefacts were mainly localized in the basal and suprabasal layers of the oral epithelium. - No significant difference in the number and width of thermal damage was observed among the experimental groups (with low and high powers). | The epithelial damage produced by CO2 laser (low and high powers) was similar to light dysplasia features that may cause erroneous therapy. |
| 6. Romeo et al., 2012 [12] | Er:YAG | Swine cadaver tongues | 45 | Optical microscope | 10× | Histological analysis by assigning a score from 0 to 3 to each sample indicating the degree of peripheral thermal damage (TDS) and obtaining the average TDS 4. | 2 | - Less thermal damage was observed with intermediate parameters (at 80 and 100 mJ and 28 and 35 J/cm2). - No difference in the peripheral thermal damage was observed among the groups and the histological evaluation was possible in all the specimens. | Er:YAG laser can be safely employed for oral biopsy investigations with a successful histological evaluation. |
| 7. Palaia et al., 2014 [5] | CO2 | Pig cadaver tongue | 30 | Optical microscope | 40× | Histological evaluation through assessing the TDS (from 0 to 3), and determining the width of peripheral thermal effects. | 1 | In all the samples, the histological readability was optimal and the thermal damage was negligible. | CO2 laser showed surgical effectiveness with little peripheral thermal damage, allowing a safe histological diagnosis. |
| 8. Azevedo et al., 2016 [13] | Diode laser 980 nm, Nd:YAG, Er:YAG, CO2 | Pig cadaver tongue | 100 | Optical microscope | - | - Macroscopic evaluation based on the criteria of Cercadillo-Ibarguren (a scale of 0 to 4). - Histologic evaluation including epithelial, CT, vascular changes, incision morphology, and overall width of tissue modifications. | 2 | - The lasers can be ordered according to the resulted thermal tissue damage extension as follows (from the greatest to the lowest): Nd:YAG laser, diode 980 nm (3.5 W and boost PW), CO2 laser (7 W in CW), CO2 laser (7 W in PW), diode 980 nm (3.5 W in PW), CO2 laser (3.5 W in PW), and Er:YAG laser. - The presence of an association between the tissue damage extension and the degree of carbonization was observed, and an association between the tissue damage extension and regularity of the incision. | - These lasers can be employed safely for oral biopsies with a successful histological analysis with taking into consideration the biological effects of each laser type. - Er:YAG laser and CO2 laser at 3.5 W in pulsed mode presented the best lasers for ensuring the successful histological evaluation. |
| 9. Fornaini et al., 2016 [17] | Diode lasers (808 nm, 450 nm), Nd:YAG, KTP | Dorsal surface of bovine tongue | 4 | Low- and high-power microscope | - | Histological evaluation through assigning a cut quality score (from 0 to 5), where “5” is the highest with cold blade. | 1 | - The best quality of cut was observed with 450 nm laser. - The cut quality score for 450 nm and KTP was 3, 2 for 808 nm laser, and 1 for Nd:YAG. | Based on the results of this study, blue diode laser (450 nm) is suggested to be used in oral surgery in daily practice. |
| 10. Braun et al., 2018 [18] | Diode lasers 970 nm, 445 nm | Pig cadavers oral mucosa | 30 | Optical microscope at 35-fold magnification | - | Histological evaluation of tissue denaturation including the measurement of MDD 5 and DA 6. | - | - The highest mean values of MDD were observed with the 970 nm laser in CW (with significance). - No significant difference was observed among different lasers in regard to the DAs. - The highest incision depth with the lowest amount of soft tissue denaturation was observed in the group of the 445 nm laser in contact mode (with statistical significance). | The 445 nm laser shows a higher cutting efficiency when compared to the 970 nm laser. |
| 11. Kawamura et al., 2019 [21] | Diode laser 808 nm, Nd:YAG, Er,Cr:YSGG, Er:YAG, CO2 | Porcine gingiva | 56 (8 samples for each treatment set) | Optical microscope | - | Histological and histometric analysis of the coagulated layers and thermally affected layers at the ablation bottom. | 2 | - Sharp and deep grooves were detected with Er:YAG, Er,Cr:YSGG, and CO2, while the grooves were flatter with diode and Nd:YAG. - The minimal thickness of the coagulated layer and thermally affected layer were observed with Er:YAG and Er,Cr:YSGG. Then, they were followed by CO2, diode, and Nd:YAG, respectively. - The use of water spray with Er:YAG and Er,Cr:YSGG reduced significantly the coagulated layer and prevented the thermal effect for both. | Er:YAG laser demonstrated the most efficient tool among the other lasers for gingival ablation with minimal thermal effect in particular with the use of water spray. |
| 12. Palaia et al., 2020 [14] | Blue diode (445 nm) | Pig cadaver tongues | 30 | Optical microscope | 10× and 40× | Histological evaluation including the measurement of the width of thermal damage in the peri-incisional epithelium and CT. | 1 | - The thermal effect on epithelium was lower than on the CT in all the groups with significance. - The mean thermal effect in groups of PW was significantly lower than that of CW. - Evaluating groups with the same parameters and groups that differ in the mode of transmission (CW and PW), some of them showed significant difference toward the PW. | The 445 nm diode laser is suggested for biopsy investigations with excellent surgical properties. |
| 13. Palaia et al., 2021 [15] | Diode laser 976 nm | Pig cadaver tongues | 30 | Optical microscope | 5× and 10×. | Histological evaluation including the measurement of the width of thermal damage in the peri-incisional epithelium and CT. | 1 | - Readable cut margins were shown in all the samples with a small damaged area. - The damage extension was always smaller in the epithelium than in CT. - The highest epithelium damage was 689.6 μm in a sample taken at 6 W in PW, while the highest CT damage was 964.24 μm in a sample taken at 5 W in CW. | The 976 nm diode laser can be considered as a good surgical tool and allowed a safe histological diagnosis. |
| 14. Prado et al., 2022 [16] | Diode laser 940 nm | Fresh pig tongues | 90 | Optical microscope | 40× | Histological assessment of quantitative measures of thermal damage through calculating the thermal damage depth and the thermal damage area (which is the difference between the total area of each specimen and its total area of damage). | - | - No statistical differences in thermal damage depth were observed between different laser groups. - Only a statistical difference was observed between group 3 and other 2 groups at the level of the thermal damage area. | - The cutting efficiency of the super pulsed diode laser is comparable to traditional blade. - Appropriate parameters of this laser can produce surgical outcomes with less collateral damage. |
| Author, Year | Laser | Pathological Perspectives | Sample (n) | Type of Microscope | Magnification | Method of Evaluation | Nr. of Pathologists | Main Outcomes | Conclusions | |
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical | Histopathological (n) | |||||||||
| 1. Pogrel et al., 1990 [27] | CO2 | Oral soft tissue lesions | - | 23 | Optical microscope | 100× | Histological evaluation through measuring the mean of tissue necrosis in microns for five different soft tissues (epithelium, connective tissue “loose and dense”, muscle, and salivary gland epithelium). | - | - The width of tissue necrosis was different among the different examined tissues types, that were probably based on the water content of each tissue type. - The dense fibrous tissue, mucosal epithelium, and muscle showed the widest tissue necrosis. | The relative narrow width of tissue necrosis with this tool and protocol may provide a superior properties on the wound healing when compared to those created by electrosurgery. |
| 2. Matsumoto et al., 2008 [25] | CO2 | Excised different tongue lesions, including malignant, precancerous, and benign lesions | SCC 1 (4), oral leucoplakia (5), fibroma (5), mucocele (4), granuloma (1), hemangioma (1) | 20 | Optical microscope | 40× or 100× | Assessment and comparison histologically of the resulted artifactual patterns of thermal injury by the CO2 in (PW 2 and CW 3) laser and the electrotome. In addition, comparing the distances from the borders of the biopsy specimen to the thermal artifacts produced by each method. | - | - Thermal denaturation including carbonization, vacuolar degeneration, and elongation of nuclei, were reported at the peripheral margins for all the methods. - CO2 laser in PW reduced the amount of thermal denaturation. | CO2 laser, particularly in PW, was better than the electrotome. |
| 3. Vescovi et al., 2010 [26] | Nd:YAG | Excised benign exophytic oral lesions (cheek and buccal mucosa) | Hyperplastic fibro-epithelial lesions (denture-induced fibrous hyperplasia, fibroma, and fibro-papilloma) | 15 | Low- and high power light microscope | 40× and 100× | Histological evaluation including epithelial, CT 4, vascular changes, incision morphology, and overall width of tissue modifications. | 1 | - No significant difference with regard to stromal changes, vascular stasis, and regularity of incision was observed between specimens removed with two different parameters of Nd:YAG laser. - Specimens with a mean size less than 7 mm showed significant Epithelial and stromal changes. - Serious thermal effects in small specimens (mean size less than 7 mm) were observed independently from the frequency and power employed. - Specimens obtained with higher frequency and lower power showed better quality of incision and less width of overall tissue injuries. | - Nd:YAG laser may cause serious thermal effects in small specimens (less than 7 mm) independently from the frequency and power employed. - Better quality of incision and less width of overall tissue injuries can be obtained with higher frequency and lower power - No statistical difference was observed between the two employed different parameters of the laser. |
| 4. Suter et al., 2012 [22] | CO2 | Excised benign exophytic lesions on the buccal mucosa (1 to 2 cm in diameter) | Fibrous hyperplasia (60) | 60 | Optical microscope | - | Histological evaluation including the measurement of the maximum width of the collateral thermal damage zone. | 1 | Similar medians of the histopathologic collateral damage zones were observed in the CW and PW groups. | - The CO2 laser can be safely used in both modes (CW and PW) for the excision of intraoral mucosal lesions. - For both modes, a 1 mm of safety margin is recommended. |
| 5. Angiero et al., 2012 [28] | Diode 808 nm | Different oral soft tissue lesions (Excision for benign lesions or lesions ≤5 mm of diameter “performed retrospectively”) | Hyperkeratosis (72), fibroma (63), mucocele (73), lichen planus (64), squamous papilloma (53), hyperplasia (36), hemangioma (32), pyogenic granuloma (24), SCC (20), ulcer (17), lichenoid lesion (16), amalgam tattoo (14), traumatic neuroma (14), schwannoma (12), salivary gland neoplasia (10), peripheral giant cell granuloma (8), epulis fissuratum (7), peripheral ossifying fibroma (6), unreadable specimen (60) | 608 | Optical microscope | 40× and 100× | Histological evaluation including epithelial, CT, vascular, cytological morphology changes, and thermal effect. | 2 | - Specimens larger than 3 mm were correctly diagnosed and did not show significant changes of all considered features. - Specimens under 3 mm showed frequently a significant epithelial, stromal and/or vascular changes where the diagnosis in 46.15% of these specimens was not achievable. | Diode laser (808 nm) can be safely used for the excision of oral lesions with a diameter larger than 3 mm. |
| 6. Romeo et al., 2014 [32] | Diode 808 nm, KTP | Excised different oral soft tissue lesions (some lesions were incised because of site or size) | Fibroma (5), mucocele (3), hyperkeratotic lesion (4), oral lichen planus (3), giant cell granuloma (1), melanotic macula (1) | 17 | Optical microscope | 5× and 10× | Histological assessment to evaluate quantitatively and qualitatively the marginal alterations. | 1 | - Histological analysis was not influenced for all the samples, and a certainty diagnosis was possible. - Due to the morphological and structural characteristics of the various lesions that may influence the tissues’ response to the laser, peripheral damage was individually evaluated for each disease. | - The histologic diagnosis was achievable by both lasers. - An extension of margins of about 0.5 mm of biopsies is suggested in particular in inflammatory lesions. |
| 7. Gobbo et al., 2017 [33] | Diode 445 nm, 970 nm | Excised benign oral soft tissue lesions | Fibroma (35), angiofibroma (10), epulis (1), mucocele (8), papilloma (5), angioma (5), amalgam tattoo (1), papillae (1) | 66 | - | - | Histological evaluation and quantifying the maximum thermal damage along the cutting margin. | 1 | - The lowest thermal damage was observed in the blue laser group. - An obvious deference was observed between blue laser group and 970 nm laser group. | -All the tested instruments led to correct histological diagnosis. - Blue laser showed the minimal bleeding with limited thermal damage. |
| 8. Suter et al., 2017 [23] | Er:YAG, CO2 | Excised exophytic oral soft tissue lesions (with length and/or height from 5 mm to 20 mm) | Fibrous hyperplasia (31), non-identified lesion (1) | 32 | - | - | Histological evaluation and measurement of TDZ 5 (maximum and minimum). | 1 | Significant statistical difference was observed between CO2 laser group versus Er:YAG laser group in regard to the median of all minima, the median of all maxima, and the pooled median value (maxima and minima). | - The use of CO2 and Er:YAG lasers was valuable in excisional biopsy of oral soft tissues. - The Er:YAG laser showed the lower thermal effect which is considered an advantage for histopathological evaluation. |
| 9. Monteiro et al., 2019 [34] | Diode 980 nm, Nd:YAG, Er:YAG, CO2 | Excised benign oral soft tissue lesions (retrospectively) | Fibrous epithelial hyperplasia (95) | 95 | Optical microscope | 5×, 20×, and 40× | Histological evaluation including epithelial, CT, vascular changes, incision morphology, and overall width of tissue modifications. | 1 | - The use of any lasers employed did not hinder the histological evaluation in all the samples. - Among the employed lasers, the highest tissue damage extension was observed with the diode laser, followed by Nd:YAG, CO2, and Er:YAG. - The most regular incision was observed with CO2 laser, followed by Er:YAG laser, Nd:YAG laser, and diode laser. | - Lasers can be used for the excision of benign lesions without histological limitations. - Er:YAG laser showed few tissue damage extension and good incision regularity, and can be the instrument of choice for excision of these lesions. |
| 10. Suter et al., 2019 [24] | Er:YAG, CO2 | Excised exophytic oral soft tissue lesions (with length and/or height from 5 mm to 20 mm) | Fibrous hyperplasia (49) | 49 | - | - | Histological evaluation including the measurement of the TDZ. | 1 | The Er:YAG group was significantly lower than CO2 laser group with regard to the median of all minimum values, the median of all maximum values, and the median of all values of the TDZ | The Er:YAG and CO2 lasers can be used for excisional biopsy of small lesions. |
| 11. Tenore et al., 2019 [29] | CO2 | Excised oral soft tissue lesions | Carcinoma in situ (1), mucocele (2), focal fibrous hyperplasia (4), kaposiform hemangioendothelioma (1), peripheral giant cell granuloma (1), granular cell tumor (1) | 10 | Optical microscope | 100× | Histological evaluation including quantitatively and qualitatively assessing the thermal effect in both the epithelium and connective tissue. | 1 | - The thermal effects were limited to the surgical resection margins of all samples and did not hinder the histological diagnosis. - The thermal effect was greater in CT than that in epithelium in all samples except 1. - The most prominent thermal effect was observed on samples collected from attached gingiva. | Using CO2 laser for oral biopsy is valid and should be tailored with taking in consideration of different factors in addition to laser parameters. |
| 12. Palaia et al., 2020 [30] | Blue diode (445 nm) | Excised benign soft tissue lesions (≤ 2 cm in diameter) | Focal fibrous hyperplasia (4), squamous papilloma (3), pyogenic granuloma (2), giant cell granuloma (1) | 10 | Optical microscope | 100× | Histological evaluation including quantitative evaluation of the thermal effect at the peri-incisional margins for both the epithelium and CT. | 1 | - The resulted thermal effects did not hinder the histological diagnosis. - Almost all the cases showed thermal effect on both epithelium and CT lower than 1 mm except one case of focal fibrous hyperplasia with a thermal effect on epithelium more than 1 mm | The blue laser can be suggested as a safe tool for biopsies of clinically unsuspicious lesions. |
| 13. Gill et al., 2021 [35] | Diode 808 nm, 980 nm | Oral soft tissue lesions including premalignant disorders (PMDs) (leucoplakia, erythroplakia and lichen planus) | - | 70 | Optical microscope | 40× | Histological evaluation including quantitative evaluation of the LTD 6. | 2 | - Mean value of LTD in 808 nm group was five times that of 980 nm group. - The 808 nm has highest number of pathomorphological artefacts. - On histochemical evaluation, 80% of Giemsa-stained sections in scalpel group showed dense inflammation, 54.2% showed less dense in 808 nm group, and 34.2% sections in 980 nm group showed an absence of inflammation. | The 980 nm diode laser showed better results than the 808 nm. |
| 14. Palaia et al., 2021 [31] | Blue diode (445 nm) | Excised benign oral soft tissue lesions | Focal fibrous hyperplasia (23), squamous papilloma (7), pyogenic granuloma (4), keratosis with no dysplasia (2), peripheral giant cell granuloma (2), lymphoepithelial cyst (1), mucocele (1), ectopic lymphoid tissue (1), granulation tissue (1) | 42 | Optical microscope | 100× | Histological evaluation including quantitative evaluation of the thermal effect at the peri-incisional margins for both the epithelium and CT. | 1 | - The resulted thermal effects did not hinder the histological diagnosis. - The mean thermal effect extent was lower on CT than on epithelium. - The alteration extent was greater than 1 mm in only three specimens on the epithelium. | Blue diode laser has various intra- and postoperative advantages and is a safe tool for performing oral biopsy. |
| Author, Year | Study Type | Type of Emitter | Groups | Nr. of Samples (n) | Frequency/Energy | Air/H2O | Fiber/Spot Diameter (µm) | Fluence (J/cm2) | Irradiance (W/cm2) | Power (W) | Thermal Effects |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diode Laser (808 nm) | |||||||||||
| 1. Romeo et al., 2007 [10] | Animal | Diode | A | 3 | CW 1 | - | 320 | 2400 | 2400 | 2 | 3000 μm |
| B | 3 | CW | 1800 | 1800 | 1.5 | 1500 μm | |||||
| C | 3 | Ton 100 ms Toff 100 ms | 248 | 2400 | 2 | 8–10 rows of keratinocytes (<1000 μm) | |||||
| 2. Angiero et al., 2012 [28] | Clinical | Diode | - | 608 | CW | - | 320 | - | - | 1.6–2.7 (~2.5) | The width of thermal effect ranged from 260.7 μm to 321.4 μm, with an average of 282.8 μm. |
| 3. Romeo et al., 2014 [32] | Clinical | Diode | - | 15 | CW | - | 320 | 2400 | - | 2 | In mucocele: 245 ± 162 μm. In fibroma: 382 ± 149 μm. In hyperkeratotic lesions: 336 ± 106 μm. In OLP 2: 473 ± 105 μm. In giant cell granuloma: 182 μm. In melanotic macula: 149 μm. |
| 4. Fornaini et al., 2016 [17] | Animal | Diode | - | 1 | CW | - | 320 | - | - | 3 | Only the quality of cut score was reported and was 2. |
| 5. Kawamura et al., 2019 [21] | Animal | Diode | - | 8 | CW | - | 300 | - | 1415.4 | 1 | The mean thickness of coagulated layer was 137.9 ± 19.5 μm, and the thermally affected layer was 123.1 ± 33.1 μm. The total thickness was 261.0 ± 45.1 μm. |
| 6. Gill et al., 2021 [35] | Clinical | Diode | - | 35 | CW | - | - | - | - | 2.5 | The mean value of LTD 3 was 17.92 ± 12.495 μm |
| Diode Laser (980 nm) | |||||||||||
| 1. Romeo et al., 2007 [10] | Animal | Diode | D | 3 | CW | - | 320 | 2400 | 2400 | 2 | Collagen homogenization and dermoepithelial detachment. |
| E | 3 | CW | 1800 | 1800 | 1.5 | >1000 μm in epithelium and >1500 μm in CT 4. | |||||
| F | 3 | Ton 100 ms Toff 100 ms | 248 | 2400 | 2 | Larger peripheral damage (collagen homogenization and dermo-epithelial detachment). | |||||
| 2. Azevedo et al., 2016 [13] | Animal | Diode | 1 | 10 | PW 5 | - | - | - | - | 3.5 | The average ETTD 6 was 456.15 ± 108.513 μm. |
| 2 | 10 | Boost PW | - | - | - | 3.5 | The average ETTD was 626.82 ± 220.292 μm. | ||||
| 3. Gobbo et al., 2017 [33] | Clinical | Diode (970 nm) | 1 | 27 | 10 Hz | - | 320 | - | - | 2 | The mean thermal effect was 186.8 ± 82.7 μm. |
| 4. Braun et al., 2018 [18] | Animal | Diode (970 nm) | 1 | - | CW | - | 320 (contact mode) | - | - | 3 | Mean value of MDD 7 was 75 ± 41 μm. Mean value of DA 8 was 0.046 ± 0.043 mm2. |
| 5. Monteiro et al., 2019 [34] | Clinical | Diode | 1 | 21 | 50 Hz/70 mJ | - | 300 | 99.2 | 4957.5 | 3.5 | The mean epithelial damage distance was 913.73 ± 322.45 μm. The mean CT damage distance was 284.81 ± 110.56 μm. |
| 6. Gill et al., 2021 [35] | Clinical | Diode | 1 | 35 | PW (50 ms interval) | - | - | - | - | 5 | The mean value of LTD was 3.808 ± 5.852 μm. |
| 7. Palaia et al., 2021 [15] | Animal | Diode (976 nm) | A | 5 | CW | - | 400 | 3184 | - | 4 | The average epithelial peri-incisional effects was 0.2 ± 0.13 mm. The average CT peri-incisional effects was 0.3 ± 0.16 mm. |
| B | 5 | 3980 | 5 | ||||||||
| C | 5 | 4777 | 6 | ||||||||
| D | 5 | PW (ton–toff: 100 ms–100 ms) | 318.4 | 4 | The average epithelial peri-incisional effects was 0.1 ± 0.14 mm. The average CT peri-incisional effects was 0.3 ± 0.13 mm. | ||||||
| E | 5 | 398 | 5 | ||||||||
| F | 5 | 477.7 | 6 | ||||||||
| 8. Prado et al., 2022 [16] | Animal | Diode (940 nm) | G2 | 10 | CW | - | 300 | - | 1415.4 | 1 | - Median TDA 9 was 1.09 mm2 - Median TDD 10 was 606.4 μm |
| G3 | 10 | 2123.1 | 1.5 | - Median TDA was 0.91 mm2 - Median TDD was 655.8 μm | |||||||
| G4 | 10 | PW (40 μs pulse interval) “CP0” | 1415.4 | 1 | - Median TDA was 1.22 mm2 - Median TDD was 720.4 μm | ||||||
| G5 | 10 | 2123.1 | 1.5 | - Median TDA was 1.37 mm2 - Median TDD was 789.6 μm | |||||||
| G6 | 10 | PW (200 μs pulse interval) “CP1” | 1415.4 | 1 | - Median TDA was 1.53 mm2 - Median TDD was 669.3 μm | ||||||
| G7 | 10 | 2123.1 | 1.5 | - Median TDA was 1.93 mm2 - Median TDD was 1024.9 μm | |||||||
| G8 | 10 | PW (1 ms pulse interval) “CP2” | 1415.4 | 1 | - Median TDA was 1.50 mm2 - Median TDD was 666.4 μm | ||||||
| G9 | 10 | 2123.1 | 1.5 | - Median TDA was 1.97 mm2 - Median TDD was 831.1 μm | |||||||
| G10 | 10 | Super PW (0.01 ms–20 ms pulse interval) “STP” | 400 | 2547.7 | 3.2 | - Median TDA was 1.08 mm2 - Median TDD was 778.8 μm | |||||
| Diode Laser (445 nm) | |||||||||||
| 1. Fornaini et al., 2016 [17] | Animal | Blue diode (450 nm) | 1 | 1 | CW | - | 320 | - | - | 2 | Only the quality of cut score was reported and was 3. |
| 2. Gobbo et al., 2017 [33] | Clinical | Blue diode | 1 | 39 | Ton 20 ms, Toff 8 ms | - | 320 | - | - | 1.4 | The mean thermal effect was 71.3 ± 51.8 μm. |
| 3. Braun et al., 2018 [18] | Animal | Blue diode | 1 | - | CW | - | 320 (contact mode) | - | - | 2 | Mean value of MDD was 59 ± 18 μm. Mean value of DA was 0.050 ± 0.034 mm2. |
| 2 | - | 320 (non-contact mode (1 mm)) | - | - | 2 | Mean value of MDD was 65 ± 27 μm. Mean value of DA was 0.039 ± 0.017 mm2. | |||||
| 4. Palaia et al., 2020 [14] | Animal | Diode | A | 5 | CW | - | 320 | - | - | 2 | Epithelium was 137.5 ± 74.28 μm, CT was 416.25 ± 118.73 μm. |
| B | 5 | CW | - | - | 3 | Epithelium was 145.6 ± 101.88 μm, CT was 546.2 ± 359.49 μm | |||||
| C | 5 | CW | - | - | 4 | Epithelium was 85.2 ± 64.27 μm, CT was 321.6 ± 132.51 μm. | |||||
| D | 5 | 50 Hz | - | - | 2 | Epithelium was 69 ± 34 μm, CT was 328 ± 169.12 μm. | |||||
| E | 5 | 50 Hz | - | - | 3 | Epithelium was 80.2 ± 31 μm, CT was 250.6 ± 144.16 μm. | |||||
| F | 5 | 50 Hz | - | - | 4 | Epithelium was 55.8 ± 33.51 μm, CT was 320.8 ± 191.82 μm. | |||||
| 5. Palaia et al., 2020 [30] | Clinical | Diode | 1 | 10 | CW | - | 320 | 3100 | - | 2.5 | The average thermal effect was 650.93 ±311.96 μm on epithelium, and 468.07 ± 264.23 μm on CT. |
| 6. Palaia et al., 2021 [31] | Clinical | Diode | 1 | 42 | CW | - | 320 | 3100 | - | 2.5 | The mean thermal effect was 507.08 ± 283.76 µm on epithelium, and 320.39 ± 209.04 µm on CT. |
| Nd:YAG Laser (1064 nm) | |||||||||||
| 1. Romeo et al., 2007 [10] | Animal | Nd:YAG | G | 3 | 40 Hz/120 mJ | - | 400 | 95.5 | 3800 | 4.8 | Wide epithelium detachment and basal layer damage. |
| H | 3 | 50 Hz/120 mJ | 95.5 | 4700 | 6 | At least 1500 μm of epithelial damage, and involving CT damage. | |||||
| I | 3 | 90 Hz/60 mJ | 47.7 | 4300 | 5.4 | The most damaged specimens, one of the specimens was so compromised with only <0.5 cm of the specimen was interpretable. | |||||
| 2. Vescovi et al., 2010 [26] | Clinical | Nd:YAG | 1 | 6 | 60 Hz with pulse width of 100 µs | - | 320 | - | 488.281 | 3.5 | 305.8 μm of epithelial changes, 376.6 μm of CT changes, and 151.6 μm of vascular changes. |
| 2 | 9 | 30 Hz with pulse width of 100 µs | - | 300.000 | 5 | 399.8 μm of epithelial changes, 521 μm of CT changes, and 183.5 μm of vascular changes. | |||||
| 3. Azevedo et al., 2016 [13] | Animal | Nd:YAG | 1 | 10 | 40 Hz | - | 300 | - | - | 6 | The average ETTD was 670.68 ± 251.85 μm. |
| 4. Fornaini et al., 2016 [17] | Animal | Nd:YAG | 1 | 1 | 30 Hz | - | 320 | - | - | 3 | Only the quality of cut score was reported and was 1. |
| 5. Kawamura et al., 2019 [21] | Animal | Nd:YAG | 1 | 8 | 20 Hz (with pulse duration of 100 µs)/50 mJ | - | 300 | 70.8 per pulse | - | 1 | The mean thickness of coagulated layer was 137.8 ± 16.3 μm, and the thermally affected layer was 161.8 ± 41.2 μm. The total thickness was 299.6 ± 49.6 μm. |
| 6. Monteiro et al., 2019 [34] | Clinical | Nd:YAG | 1 | 25 | 40 Hz/100 mJ | - | 300 | 141.6 | 5665.7 | 4 | The mean epithelial damage distance was 899.83 ± 327.75 μm. The mean CT damage distance was 310.85 ± 107.45 μm. |
| Er,Cr:YSGG Laser (2780 nm) | |||||||||||
| 1. Rizoiu et al., 1996 [19] | Animal | Er,Cr:YSGG | - | 1 (Not clear) | 20 Hz with pulse duration of 140 µs | - | 680 | - | - | 2 | The margins showed a 20–40 μm of carbonization and coagulation. |
| 2. Romeo et al., 2007 [10] | Animal | Er,Cr:YSGG | L | 3 | 20 Hz | 11/07 | 600 | 44.2 | 884 | 2.5 | Keratinocytes degeneration for 1500 μm. |
| M | 3 | 11/10 | 35 | 707 | 2 | Dermo-epithelial detachment for 2500 μm. | |||||
| N | 3 | 11/15 | 53 | 1000 | 3 | 1000 μm in both epithelium and CT. | |||||
| 3. Cercadillo-Ibarguren et al., 2010 [3] | Animal | Er,Cr:YSGG | 1 | 9 | 20 Hz | 11/07 | 600 | - | - | 1 | The mean thermal effect was 9.26 ± 2.05 μm. |
| 2 | 9 | without | - | - | 1 | The mean thermal effect was 23.38 ± 5.31 μm. | |||||
| 3 | 9 | 11/07 | - | - | 2 | The mean thermal effect was 14.88 ± 4.0 9 μm. | |||||
| 4 | 9 | without | - | - | 2 | The mean thermal effect was 55.67 ± 17.65 μm. | |||||
| 5 | 9 | 11/07 | - | - | 4 | The mean thermal effect was 13.42 ± 5.61 μm. | |||||
| 4. Kawamura et al., 2019 [21] | Animal | Er,Cr:YSGG | 1 | 8 | 20 Hz (with pulse duration of 140 µs)/50 mJ | without | 600 | 17.7 per pulse | - | 1 | The mean thickness of coagulated layer was 50.6 ± 6.7 μm, and the thermally affected layer was 65.7 ± 27.6 μm. The total thickness was 116.4 ± 31.7 μm. |
| 2 | 8 | with | - | 1 | The mean thickness of coagulated layer was 33.1 ± 1.4 μm, and the thermally affected layer was 0 μm. The total thickness was 33.1 ± 1.4 μm. | ||||||
| KTP Laser (532 nm) | |||||||||||
| 1. Romeo et al., 2010 [11] | Animal | KTP | 1 | 9 | Ton 50 ms Toff 50 ms | - | 300 | 141 | 2800 | 2 | Limited to only 2–3 lines of cells (<50 μm). |
| 2 | 9 | Ton 50 ms Toff 50 ms | 162 | 3200 | 2.3 | Signs of poor tissue coagulation both in the epithelium and CT (<50 μm). | |||||
| 3 | 9 | Ton 50 ms Toff 50 ms | 176 | 3500 | 2.5 | Highest amount of thermal damage over the epithelium, but the subepithelial corium was absolutely free from thermal alteration. | |||||
| 4 | 9 | Ton 50 ms Toff 50 ms | 191 | 3800 | 2.7 | Few signs of cellular hyperthermia near the cut edges, but they were always readable and showed clear details. | |||||
| 5 | 9 | Ton 50 ms Toff 50 ms | 212 | 4200 | 3 | Almost intact epithelium and signs of cellular damage were limited to 200 μm. | |||||
| 2. Romeo et al., 2014 [32] | Clinical | KTP | - | 2 | PW | - | 300 | 212 | - | 1.5 | In mucocele: 213 μm. In hyperkeratotic lesion: 196 μm. |
| 3. Fornaini et al., 2016 [17] | Animal | KTP | 1 | 1 | CW | - | 320 | - | - | 2 | Only the quality of cut score was reported and was 3. |
| Er:YAG (2940 nm) | |||||||||||
| 1. Romeo et al., 2012 [12] | Animal | Er:YAG | 1 | 9 | 30 Hz/60 mJ | Not used | 600 | 21 | - | 1.8 | Little peripheral thermal damage with clear and safe margins, with modifications confined to the lamina propria and muscle tissue (TDS 11 1.44). |
| 2 | 9 | 30 Hz/80 mJ | 28 | - | 2.4 | A very thin layer of cauterization (TDS 1.1). | |||||
| 3 | 9 | 30 Hz/100 mJ | 35 | - | 3 | Limited signs of thermal damage in both epithelium lamina propria, although the subepithelial chorion was free from thermal alteration (TDS 1.22). | |||||
| 4 | 9 | 30 Hz/130 mJ | 46 | - | 3.9 | Moderate damage localized to the epithelial margins (TDS 1.77). | |||||
| 5 | 9 | 30 Hz/150 mJ | 53 | - | 4.5 | Significant signs of peripheral thermal damage in the epithelium (TDS 2.44). | |||||
| 2. Azevedo et al., 2016 [13] | Animal | Er:YAG | 1 | 10 | 10 Hz/0.2 J (short pulse) | With | Not mentioned (non-contact) | - | - | 2 | The average ETTD was 68.39 ± 59.585 μm. |
| 2 | 10 | 10 Hz/0.2 J (short pulse) | Without | - | - | 2 | The average ETTD was 84.39 ± 51.363 μm. | ||||
| 3 | 10 | 10 Hz/0.4 J (short pulse) | With | - | - | 4 | The average ETTD was 66.34 ± 25.143 μm. | ||||
| 4 | 10 | 10 Hz/0.4 J (short pulse) | Without | - | - | 4 | The average ETTD was 79.54 ± 31.333 μm. | ||||
| 3. Suter et al., 2017 [23] | Clinical | Er:YAG | 1 | 16 | 35 Hz (pulse duration of 297 μs)/ 200 mJ | Air-water cooling (22.5 mL/min) | 400 (non-contact) | - | - | 7 | The median of all TDZ values was 34.0 μm. The median of all maxima TDZ was 56.8 μm. The median of all minima TDZ was 18.2 μm. |
| 4. Kawamura et al., 2019 [14] | Animal | Er:YAG | 1 | 8 | 20 Hz (with pulse duration of 200 µs)/50 mJ | without | 600 | 17.7 per pulse | - | 1 | The mean thickness of coagulated layer was 37.7 ± 9.6 μm, and the thermally affected layer was 0 μm. The total thickness was 37.7 ± 9.6 μm. |
| 2 | 8 | with | - | 1 | The mean thickness of coagulated layer was 17.9 ± 2.1 μm, and the thermally affected layer was 0 μm. The total thickness was 17.9 ± 2.1 μm. | ||||||
| 5. Monteiro et al., 2019 [34] | Clinical | Er:YAG | 1 | 22 | 20 Hz/200 mJ | - | 500 | 102 | 2040.8 | 4 | The mean epithelial damage distance was 166.47 ± 123.85 μm. The mean CT damage distance was 48.54 ± 26.09 μm. |
| 6. Suter et al., 2019 [24] | Clinical | Er:YAG | 1 | 25 | 35 Hz (with pulse duration of 297 µs)/200 mJ | 22.5 mL/min | 400 (non-contact) | - | - | 7 | The mean TDZ 12 was 41 μm. |
| CO2 Laser (10,600 nm) | |||||||||||
| 1. Pogrel et al., 1990 [27] | Clinical | CO2 | 1 | 23 | - | - | - | - | 2320 | 17.5 | Mean width of necrosis in epithelium was 85.9 ± 15.7 μm. Mean width of necrosis in muscle was 85.1 ± 12.8 μm. Mean width of necrosis in dense CT was 96.1 ± 22 μm. Mean width of necrosis in loose CT was 51.1 ± 14.9 μm. Mean width of necrosis in salivary gland was 41.5 ± 8 μm. |
| 2. Matsumoto et al., 2008 [25] | Clinical | CO2 | 1 | 10 | PW | - | 1000 | - | - | 3–4 | The range of thermal artifacts was from 210–320 μm, with lowest range of 269 ± 38.72 μm. |
| 2 | 10 | CW | - | - | 3–4 | The range of thermal artifacts was from 240–360 μm with lowest range of 306 ± 32.04 μm. | |||||
| 3. Cercadillo-Ibarguren et al., 2010 [3] | Animal | CO2 | 1 | 9 | CW | - | - | - | - | 1 | The mean thermal effect was 21.55 ± 8.24 μm. |
| 2 | 9 | CW | - | - | 2 | The mean thermal effect was 35.16 ± 15.93 μm. | |||||
| 3 | 9 | CW | - | - | 10 | The mean thermal effect was 20.44 ± 5.43 μm. | |||||
| 4 | 9 | CW | - | - | 20 | The mean thermal effect was 29.02 ± 13.56 μm. | |||||
| 5 | 9 | PW (with 100 msec pulse width and 200 msec interval) | - | - | 20 | The mean thermal effect was 20.30 ± 6.73 μm. | |||||
| 4. Seoane et al., 2010 [20] | Animal | CO2 | 1 | 5 | PW (0.05 s) | - | - | - | - | 3 | The mean width of thermal damage was 330 ± 237.7 μm. |
| 2 | 5 | - | - | 6 | The mean width of thermal damage was 300 ± 79.0 μm. | ||||||
| 3 | 5 | - | - | 9 | The mean width of thermal damage was 320 ± 119.1 μm. | ||||||
| 4 | 5 | - | - | 12 | The mean width of thermal damage was 245 ± 158.5 μm. | ||||||
| 5. Suter et al., 2012 [22] | Clinical | CO2 | 1 | 30 | CW | - | 200 | - | - | 5 | The mean of width of the collateral thermal damage zone was 247.2 μm. |
| 2 | 30 | 140 Hz (pulse duration of 400 μs)/ 33 mJ | - | - | 4.62 | The mean of width of the collateral thermal damage zone was 198.4 μm. | |||||
| 6. Palaia et al., 2014 [5] | Animal | CO2 | A | 5 | CW | - | 200/400 | - | - | 2 | Epithelium was 99 μm, CT was 169 μm. |
| B | 5 | CW | - | - | 3 | Epithelium was 68 μm, CT was 184 μm. | |||||
| C | 5 | CW | - | - | 4 | Epithelium was 90 μm, CT was 239 μm. | |||||
| D | 5 | 50 Hz | - | - | 3 | Epithelium was 74 μm, CT was 192 μm. | |||||
| E | 5 | 50 Hz | - | - | 3.5 | Epithelium was 77 μm, CT was 206 μm. | |||||
| F | 5 | 50 Hz | - | - | 4 | Epithelium was 71 μm, CT was 236 μm. | |||||
| 7. Azevedo et al., 2016 [13] | Animal | CO2 | 1 | 10 | 50 Hz | - | Not mentioned (non-contact) | - | - | 3.5 | The average ETTD was 306.19 ± 85.882 μm. |
| 2 | 10 | 50 Hz | - | - | 7 | The average ETTD was 485.45 ± 178.581 μm. | |||||
| 3 | 10 | CW | - | - | 7 | The average ETTD was 571.18 ± 183.216 μm. | |||||
| 8. Suter et al., 2017 [23] | Clinical | CO2 | 1 | 15 | 140 Hz (pulse duration of 400 μs)/ 33 mJ | - | 200 (non-contact) | - | - | 4.62 | The median of all TDZ values was 74.9 μm. The median of all maxima TDZ was 122.6 μm. The median of all minima TDZ was 49.9 μm. |
| 9. Kawamura et al., 2019 [21] | Animal | CO2 | 1 | 8 | CW | - | 400 (non-contact) | - | 796.2 | 1 | The mean thickness of coagulated layer was 163 ± 48.8 μm, and the thermally affected layer was 73.6 ± 23.4 μm. The total thickness was 236.6 ± 50.2 μm. |
| 10. Monteiro et al., 2019 [34] | Clinical | CO2 | 1 | 27 | 80 Hz/50 mJ | - | 500 | 40.8 | 2040.8 | 4 | The mean epithelial damage distance was 538.37 ± 170.50 μm. The mean CT damage distance was 201.69 ± 89.86 μm. |
| 11. Suter et al., 2019 [24] | Clinical | CO2 | 1 | 24 | 140 Hz (pulse duration of 400 μs)/33 mJ | with air cooling | 200 (non-contact) | - | - | 4.62 | The mean TDZ was 83.5 μm. |
| 12. Tenore et al., 2019 [29] | Clinical | CO2 | 1 | 10 | 80 Hz | - | 200–400 (non-contact) | - | - | 4.2 | The average thermal effect was 687 μm on epithelium, and 1470 μm on CT. The average total thermal effect was 2094 μm. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenore, G.; Mohsen, A.; Nuvoli, A.; Palaia, G.; Rocchetti, F.; Di Gioia, C.R.T.; Cicconetti, A.; Romeo, U.; Del Vecchio, A. The Impact of Laser Thermal Effect on Histological Evaluation of Oral Soft Tissue Biopsy: Systematic Review. Dent. J. 2023, 11, 28. https://doi.org/10.3390/dj11020028
Tenore G, Mohsen A, Nuvoli A, Palaia G, Rocchetti F, Di Gioia CRT, Cicconetti A, Romeo U, Del Vecchio A. The Impact of Laser Thermal Effect on Histological Evaluation of Oral Soft Tissue Biopsy: Systematic Review. Dentistry Journal. 2023; 11(2):28. https://doi.org/10.3390/dj11020028
Chicago/Turabian StyleTenore, Gianluca, Ahmed Mohsen, Alessandro Nuvoli, Gaspare Palaia, Federica Rocchetti, Cira Rosaria Tiziana Di Gioia, Andrea Cicconetti, Umberto Romeo, and Alessandro Del Vecchio. 2023. "The Impact of Laser Thermal Effect on Histological Evaluation of Oral Soft Tissue Biopsy: Systematic Review" Dentistry Journal 11, no. 2: 28. https://doi.org/10.3390/dj11020028
APA StyleTenore, G., Mohsen, A., Nuvoli, A., Palaia, G., Rocchetti, F., Di Gioia, C. R. T., Cicconetti, A., Romeo, U., & Del Vecchio, A. (2023). The Impact of Laser Thermal Effect on Histological Evaluation of Oral Soft Tissue Biopsy: Systematic Review. Dentistry Journal, 11(2), 28. https://doi.org/10.3390/dj11020028












