Abstract
Dentists used silver-containing solutions for deep cavity disinfection before restoration. This review aims to identify the silver-containing solutions reported in the literature for deep cavity disinfection and summarize their effects on dental pulp. An extensive search was performed using the search words “(silver) AND (dental pulp OR pulp)” in ProQuest, PubMed, SCOPUS, and Web of Science to identify English publications on silver-containing solutions for cavity conditioning. The pulpal response to the included silver-containing solutions was summarized. The initial search identified 4112 publications and 14 publications met the inclusion criteria. Silver fluoride, silver nitrate, silver diamine nitrate, silver diamine fluoride, and nano-silver fluoride were used in deep cavities for antimicrobial purposes. Indirect silver fluoride application induced pulp inflammation and reparative dentine in most cases, and pulp necrosis in some cases. Direct silver nitrate application caused blood clots and a wide inflammatory band in the pulp, whilst indirect silver nitrate application caused hypoplasia in shallow cavities and partial pulp necrosis in deep cavities. Direct silver diamine fluoride application induced pulp necrosis, while indirect silver diamine fluoride application induced a mild inflammatory response and reparative dentine formation. No evidence of the dental pulpal response to silver diamine nitrate or nano-silver fluoride was available in the literature.
1. Introduction
Dental caries remains one of the most prevalent diseases worldwide [1]. As the carious lesions progress, dental hard tissues are destroyed [2]. In advanced stages, the dentine–pulp complex can be involved [3]. Restorative treatment is indicated for carious teeth with moderate to advanced lesions.
Restorative treatment of carious teeth requires removing the carious tissues and restoring them with dental filling materials [4]. Because caries removal in deep lesions during restorative treatment implies a higher risk of pulp exposure or pulp vitality loss, the concept of selective or partial caries removal is now commonly adopted [4,5,6]. Selective caries removal made the “extension for prevention” concept, by G.V. Black, and the conventional non-selective caries removal obsolete in preserving the natural tooth structure and pulp vitality [7]. In the selective caries removal technique, the inner part of the caries lesion is preserved while the superficial part of the caries lesion is removed and restored with restoration [8]. This concept is based on the histological finding that carious dentine can be divided into two structurally distinct zones [9]. The superficial layer is known as infected dentine, which is irreversibly demineralised dentine consisting of cariogenic microorganisms, metabolic by-products, and degraded collagen networks due to proteolytic degradation [10]. The inner layer is known as the affected dentine, which is reversibly demineralised dentine consisting of acid-demineralized minerals and a repairable collagen network [11].
However, it is almost impossible to confirm that all the infected dentine-containing cariogenic microorganisms are removed in the clinical situation. The infected dentine and the affected dentine are difficult to differentiate clinically. Currently, the most common strategy to differentiate the infected and affected dentine is to use tactile sensations to determine the hardness during caries excavation, which is liable to subjective judgement [12]. In some cases, a dense portion of the microorganisms might be left in the cavity after excavation, resulting in recurrent caries.
The elimination of the microorganisms in the cavity is the key to the success of selective caries removal and the subsequent restoration. The elimination of the microorganisms can be achieved by an ideal sealing of the cavity with the restorative materials. The sealed microorganisms would be isolated from the oral environment without nutrients and a biofilm would not form [13]. However, ideal sealing can be difficult to achieve or maintain with current restorative materials. Using antimicrobial agents for cavity conditioning or lining can kill the cariogenic bacteria and inhibit the further development of cariogenic biofilm in the cavity, reducing the risk of secondary caries and potentially improving the longevity of the restoration.
Silver is a broad-spectrum antimicrobial agent that can be used for caries management [14]. Resistance to the antimicrobial action of silver has seldom been found in bacteria [15,16]. The effect of silver-containing solutions in caries control has been confirmed in previous studies [17,18,19]. The mechanism of silver’s antimicrobial action is closely related to its interaction with the thiol (sulfhydryl) groups in the cariogenic bacteria [20]. Silver interacts with the enzymes and proteins in the cell by combining with the thiol groups, which are key functional groups in the enzymes [21]. The silver ions can release the potassium ions from the bacterial cytoplasmic membrane, which is a site associated with numerous enzymes [20]. Moreover, silver can cause the marked inhibition of cell growth and damage the cell envelope as silver granules are deposited in the vacuoles and cell walls [22]. Structural abnormalities and an increase in bacterial cell size have also been found. Finally, it can interact with the bases in the deoxyribonucleic acid (DNA) [23].
Because of their long-lasting antimicrobial effect, silver-containing solutions have been used for cavity disinfection after caries removal [24]. However, the effect of silver-containing solutions on dental pulp beneath deep cavities has never been elucidated. Therefore, this scoping review aims to identify and provide a comprehensive overview of the available silver-containing solutions for deep cavity disinfection and summarize their effects on dental pulp.
2. Materials and Methods
This review was prepared according to the PRISMA checklist for scoping reviews [25]. An extensive search was performed using the search terms “(silver) AND (dental pulp OR pulp)” in four databases (ProQuest, PubMed, SCOPUS, and Web of Science). The included studies were limited to articles published in English on or before 1 November 2022. Duplicate articles; clinical, in vivo, or in vitro studies not related to silver-containing materials; abstracts; conference papers; literature reviews; and systematic reviews were excluded. For a study to be included, it had to evaluate the pulpal response (clinical, in vivo, and ex vivo) or the cell’s pulpal response (in vitro) to silver-containing materials used as cavity conditioners. Two authors participated in the study selection process (A.Z. & O.Y.Y.), and in case of a disagreement, they referred to a third author (C.-H.C.). Important information was collected on separate spreadsheets by the investigators. All the reference lists of the included studies were checked for potential studies. The study selection process is presented in the flow diagram below (Figure 1). A summary of all the included studies can be found in Table 1.
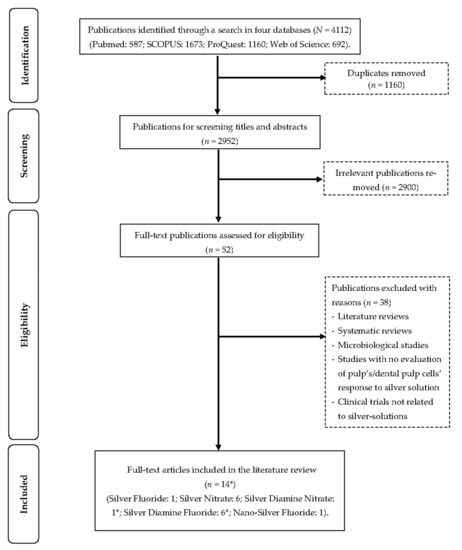
Figure 1.
Flow diagram of literature search. * One study has both silver diamine nitrate and silver diamine fluoride.

Table 1.
Summary of all included studies.
3. Silver-Containing Solutions for Deep Caries Management
The main properties of various silver-containing solutions are summarized in Table 2.
3.1. Silver Fluoride
The 40% silver fluoride solution was used for deep caries management. The 40% silver fluoride solution contained 34% silver and 6% fluoride. According to this percentage, the fluoride content was supposed to be 59,900 ppm [40]. The chemical analysis of the components of two silver fluoride products found that the fluoride content was higher than expected, ranging from 78,000 to 120,000 ppm [40,41]. As a result of these findings, Gotjamanos & Afonso [41] recommended that the use of silver fluoride in paediatric dentistry be discontinued because these unacceptably high fluoride levels pose a potential risk of toxicity to children and, if it enters the bloodstream, it might result in fluorosis, especially in the case of an undetected pulp exposure [42]. Silver fluoride has antibacterial and remineralising properties. The silver ions present in the silver fluoride act in two different ways: (1) their bactericidal/bacteriostatic effect on the microorganisms present in the carious lesion; (2) mechanical sealing of the carious and sound dentinal tubules [26]. Silver fluoride is used as a cavity conditioner in conjunction with glass ionomer cement as an atraumatic restorative method. After partial caries excavation, silver fluoride is applied, and then the cavity is sealed with a glass ionomer cement restoration. Silver fluoride showed a 100% success rate in 400,000 cases treated at the Dentistry School of Western Australia University [26]. The treatment success was based solely on the absence of symptoms.
3.2. Silver Nitrate
The first use of silver nitrate was reported around 1846 [41]. The results, reported by Stebbins in 1891, shed light on the clinical significance of silver nitrate [43]. It was mainly used to arrest dental caries and sterilize cavities because of its escharotic, dehydrating, and sclerosing properties [16]. Silver nitrate is acidic in nature, and it causes protein coagulation [44]. In 1917, Howe introduced ammoniacal silver nitrate by adding ammonium hydroxide to silver nitrate [45]. The addition of the ammonium hydroxide converted the acidic silver nitrate into a solution with an alkaline nature, limiting its irritating action [41]. The new compound, which contained 25% silver nitrate, was later known as Howe’s solution and was widely used by practitioners.
The nature of dental caries as a noncommunicable disease rather than an infectious disease made the idea of the sole application of silver nitrate debatable. The results of various studies regarding the efficacy of silver nitrate in the prevention of dental caries have not revealed any significant reduction in caries incidence compared to no treatment [46,47,48]. Other studies examined the efficacy of silver nitrate in arresting carious lesions and reported that it can arrest caries on both permeant and primary dentition [48,49]. The use of silver nitrate in preventing/arresting dental caries decreased significantly after fluoride was introduced [16]. In 2015, silver nitrate was used in conjugation with sodium fluoride, and the reported results exhibited effectiveness in arresting dental caries [50].
3.3. Silver Diamine Nitrate
Silver diamine nitrate was developed from silver nitrate. The 48% silver diamine nitrate solution was proposed to act as a less expensive alternative to silver diamine fluoride in regions with lower and middle-income economies as it lacks the fluoride component. There is a delay in the mineral induction time of silver nitrate, which affects its remineralization potential [51]. The addition of the diamine group to silver nitrate stabilizes the silver ions, and it is expected to enhance its mineralization potential [33]. When the silver diamine nitrate is applied to a dentine surface, it reacts with the ions present in the environment and produces silver phosphate and silver oxide. The silver compounds may readily react with the chloride in the environment, resulting in the formation of less soluble silver chloride [52,53]. In the presence of silver salts, demineralized dentine becomes harder and dentinal tubules become blocked, preventing any further progress of the acidic by-products responsible for demineralization [52]. The efficacy of silver diamine nitrate in caries control has not yet been reported.
3.4. Silver Diamine Fluoride
Introduced by Dr Nishino and Dr Yamaga in the 1950s, silver diamine fluoride (SDF) has been used for arresting caries, preventing secondary caries, and decreasing hypersensitivity [54]. Commercial SDF products are available in various concentrations (3.8%, 10%, 12%, 30%, and 38%). Solutions in all of these concentrations are suitable for use as anticaries solutions, with the exception of the 3.8% solution, which is intended for root canal therapy. Silver diamine fluoride is an alkaline solution with a high concentration of fluoride and silver ions [1]. Fluoride changes the hydroxyapatite into fluorapatite, which is more resistant to acid dissolution, so it can remineralise the caries-affected dental hard tissues [15]. In addition, it has an antimicrobial effect on the plaque biofilm [55]. Silver, the other main component of silver diamine fluoride, is well known for its strong antimicrobial effect which helps in sterilizing the infected tissues, and it blocks the dentinal tubules to seal them from microorganisms and their by-products [56]. Combing both silver and fluoride in one solution so they can perform their actions synergistically resulted in the superior results achieved by the silver diamine fluoride. Despite its effectiveness, silver diamine fluoride usage for arresting caries is still an off-label usage in many countries, where it is used only as a desensitizing agent. Silver diamine fluoride requires only topical application to achieve its effect. This ease of use has led to its widespread use among practitioners recently, especially during the COVID-19 pandemic [57]. The World Health Organization’s Center for Quality Improvement and Evidence-Based Dentistry introduced safer aerosol-free emergent dentistry as a protocol for caries management [58]. Silver diamine fluoride was the first choice in this protocol for the treatment of caries and toothache related to caries, as its use can prevent cross-infection. The effectiveness of silver diamine fluoride in caries control has been proved in many previous studies [59,60,61].
3.5. Nano Silver Fluoride
Silver nanoparticles have been introduced in dentistry for multiple applications, including caries management [62]. The antibacterial effect of silver nanoparticles relies on their large contact area with the microorganisms due to their nanometric particle size [63]. Silver ions can be continuously released by the silver nanoparticles [64]. This disrupts the permeability and respiration of the bacteria by binding to the sulphur proteins in the cytoplasmic membrane and cell wall [65,66,67]. A further instance of this is the detection of reactive oxygen species inside bacterial cells. Reactive oxygen species elevate the oxidative stresses and damage the deoxyribonucleic acid (DNA) [67,68]. In addition, the interaction of the DNA’s sulphur and phosphorus, with silver ions, can create issues with DNA replication and cell reproduction, and can even cause microorganism death [69]. The denaturation of the cytoplasmic proteins is another effect of silver ions [70]. Moreover, silver nanoparticles can kill bacteria themselves by accumulating in the pits that form the cell wall after attaching to the cell surface [71]. Furthermore, silver nanoparticles can disrupt bacterial signal transduction, resulting in a decrease in proliferation and cell apoptosis [72]. Fluoride solutions, combined with silver nanoparticles, proved the capability to remineralise carious lesions [73]. Therefore, silver nanoparticles, in the form of nano-silver fluoride, have been developed for caries management [74]. The effectiveness of nano-silver fluoride as a cariostatic agent was investigated by dos Santos et al. [75]. They found that active caries decreased by 50% at 12 months after a single application of nano-silver fluoride [75]. Nagireddy et al. conducted another study in which nano-silver fluoride was applied to dentinal caries, and 65.21% of the carious lesions were arrested after a 1-year follow-up [76].

Table 2.
Properties of silver-containing solutions for deep caries management.
Table 2.
Properties of silver-containing solutions for deep caries management.
| Solution [Ref.] | Wt.% (Concentration According to Manufacturer(s)) | Anticaries Properties |
|---|---|---|
| Silver fluoride [40,42] | 40% (Ag: 340,000 ppm; F: 60,000 ppm) |
|
| Silver nitrate [77] | 25% (Ag: 151,130 ppm) |
|
| Silver diamine nitrate [33] | 48% (Ag: 319,914 ppm *) |
|
| Silver diamine fluoride [78] | 12% (Ag: 80,170 ppm; F: 14,150 ppm) |
|
| 30% (Ag: 200,400 ppm; F: 35,400 ppm) | ||
| 38% (Ag: 253,900 ppm; F: 44,800 ppm) | ||
| Nano-silver fluoride [39] | 1.05% * (Ag: 399.33 ppm; F: 10,147 ppm) |
|
* By calculation.
4. Dental Pulpal Response to Silver-Containing Solutions
Dental pulpal response to different silver-containing solutions is summarized in Table 3.

Table 3.
Dental pulpal response to different silver-containing solutions.
4.1. Silver Fluoride
Only one study reported the dental pulpal response to silver fluoride. In the study, by Gotjamanos [26], the 40% silver fluoride was applied as a cavity liner on the surface of residual caries in the deep proximal cavities. Fifty-five teeth were included in this study, and only five exhibited an unfavourable pulpal response. These five teeth displayed signs of acute and chronic inflammation, internal resorption, and necrosis. In contrast, the pulps of the other 40 teeth in the same study exhibited a favourable pulpal response with the presence of reparative dentine, a continuous odontoblast layer, no signs of tissue disarrangement, and no necrotic foci. The dental pulp response to various silver-containing solutions is summarized in Table 3.
4.2. Silver Nitrate
Six studies reported the dental pulpal response to silver nitrate [27,28,29,30,31,32]. In the study by Englander et al. [30], the indirect application of the silver nitrate on the dental pulp resulted in inflammatory changes, atrophy, and the destruction of the odontoblastic layer beneath the area of application. Many of the degenerating odontoblasts were found filled with silver. In addition, black silver particles were found inside the pulp, and haemorrhaging and oedema were observed in areas of dense silver accumulation. Despite these changes, the pulp tissue was normal in the deeper portions. In the same study, silver nitrate was applied directly to exposed areas of the dental pulp. The direct application of silver nitrate caused a large superficial haemorrhage that subsequently formed a blood clot. Large black globules of free silver were present on the surface of this blood clot, while the deeper portion of the clot was free of any globules. A wide inflammatory band was present beneath the clot, but the pulp beneath the area of inflammation was normal.
Perreault et al. [29] applied the silver nitrate to cavities at different depths. Hypoplasia occurred in the pulp under the shallow cavities, whereas complete pulp necrosis was found in the moderate and deep cavities. Concerns about the adverse side effects on the pulp were raised due to its high penetration ability [28,30]. Some researchers consider the presence of a sound dentine barrier between the carious lesion and the pulp crucial to limiting the silver nitrate’s cytotoxic effects [28,41,79].
4.3. Silver Diamine Nitrate
Only one study reported the dental pulpal response to silver diamine nitrate. In the in vitro study by Srisomboon et al. [33], the silver diamine nitrate was applied directly to human dental pulp stem cells, and the results showed only a 13% reduction in vitality. According to ISO 10993–5:2009, a reduction in cell viability by more than 30% is considered a cytotoxic effect. Silver diamine nitrate is therefore considered a non-cytotoxic material, though it contains a high concentration of silver [80]. Studies reporting the pulp response to silver diamine nitrate are scarce. No clinical studies on pulp response to silver diamine nitrate are available.
4.4. Silver Diamine Fluoride
Five studies reported the dental pulpal response to silver diamine fluoride. The in vitro study by Hu et al. [38] evaluated the cytotoxicity of silver diamine fluoride on dental pulp stem cells with direct and indirect applications. In the case of direct application, SDF was found to be toxic even at a very low concentration of 0.001% (silver: 253.87 ppm, fluoride: 44.8 ppm). Additionally, in the case of indirect application, there was 100% cell death with dentine discs 0.5 and 1 mm thick. With a dentine disc 1.5 mm thick, only 30% of the cells were vital. On the contrary, another in vitro study reported that 92% of dental pulp stem cells remained vital after a direct contact test with the silver diamine fluoride [33].
The histological findings of the ex vivo study by Korwar et al. [35] showed that there were no inflammatory signs and the tertiary dentine was formed after using silver diamine fluoride as a cavity conditioner. Another ex vivo study used silver diamine fluoride for indirect pulp capping in both an animal and a human model [36]. The results in both models showed mild inflammatory signs and the tertiary dentine was formed. Moreover, a case study by Bimstein and Damm [37] confirmed the same findings. The results of these studies are all in agreement that there was no irreversible damage to the pulp tissue after the silver diamine fluoride was used as an indirect capping agent, and it even promoted tertiary dentine formation [35,36,37]. Hosoya et al. [34] applied silver diamine fluoride directly on the exposed pulp of dogs’ teeth. The pulp in that study exhibited suppurative inflammation, round cell infiltration, bleeding, hyperaemia, and partial necrosis.
The in vitro study by Luong et al. [81] evaluated ion penetration into the pulp chamber after the application of various silver diamine fluoride products. Their results showed that the amount of silver and fluoride that can reach the pulpal space is low, and it lies within the safety limit. The results of this study must be interpreted carefully, as it did not provide information such as the location of the SDF application within the tooth, the remaining dentine thickness, or the absence of carious lesions.
4.5. Nano Silver Fluoride
Only one study reported the biocompatibility of nano-silver fluoride in human erythrocytes; no study on dental pulp cells was found. Targino et al. [39] evaluated the nano-silver fluoride’s cytotoxicity based on its haemolytic activity in human erythrocytes in vitro. The nano-silver fluoride was not toxic at any tested concentration (100, 50, 25, 12.5 and 6.25 µg/mL) in this study. We did not find any studies that have evaluated the dental pulp cell’s response (e.g., odontoblasts, odontoblast-like cells, fibroblasts, and dental pulp stem cells) to nano-silver fluoride. Concerns still arise around the increased daily exposure to silver nanoparticles, as they are incorporated into various aspects of life. There are not enough in vitro biological studies to evaluate their effect on the pulpal cells on the molecular level.
5. Limitation
Only articles published in English or an English translation, which was provided by their authors, were included in this review. The silver-containing solutions have been commonly used in non-English-speaking countries, such as Argentina, Japan, and Brazil, for many years. It is important to note that most articles published in these countries concerning silver-containing solutions are in languages other than English and are not indexed in the English databases. Consequently, these articles were difficult to access, and the incorporation of silver-containing solutions into dental treatment protocols in other countries was delayed. As an example, it was not until 2014 that silver diamine fluoride was introduced to the United States of America, despite having been used in Japan since 1969 [24]. Additionally, there was a lack of information regarding the biological effect of some of the included materials due to either their limited availability in the literature or their novelty. Additionally, the scope of scoping reviews is generally broad in scope, which limits the depth of analysis, and they do not include the risk of bias or any other assessments of the studies included. It is worth noting that scoping reviews are not intended to provide a definitive answer to a research question. It is imperative that more research be conducted on these materials due to the recent expansion of their use in different countries and their inclusion in treatment protocols in recent years.
6. Summary
Silver fluoride, silver nitrate, silver diamine nitrate, silver diamine fluoride, and nano-silver fluoride are silver solutions that can be used in carious cavities for antimicrobial purposes. Only the indirect silver diamine fluoride application induced a favourable pulpal response manifested as a mild inflammatory response and reparative dentine formation. No evidence of the dental pulpal response to silver diamine nitrate and nano-silver fluoride were available in the literature. This review underscores the importance of continued research in this area and the potential for future studies to contribute to our understanding. It also provides a guide in the clinical decision-making process. Although there is a growing interest in this topic, the insufficient number of studies makes it difficult to draw a definitive conclusion. Despite this, promising trends in the research indicate that data availability may increase in the future. Future investigations are warranted to explore the dental pulpal response to these silver solutions to support their usage as a cavity conditioner in case of deep cavities.
Author Contributions
Conceptualization, A.Z., O.Y.Y. and C.-H.C.; methodology, A.Z. and O.Y.Y.; validation, A.Z., O.Y.Y. and C.-H.C.; data curation, A.Z. and O.Y.Y.; writing—original draft preparation, A.Z.; writing—review and editing, O.Y.Y. and C.-H.C.; supervision, O.Y.Y. and C.-H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the General Research Fund of Research Grants Council of Hong Kong SAR, China (No. 17100820).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zaeneldin, A.; Yu, O.Y.; Chu, C.-H. Effect of silver diamine fluoride on vital dental pulp: A systematic review. J. Dent. 2022, 119, 104066. [Google Scholar] [CrossRef]
- Nyvad, B.; Fejerskov, O. Assessing the stage of caries lesion activity on the basis of clinical and microbiological examination. Community Dent. Oral Epidemiol. 1997, 25, 69–75. [Google Scholar] [CrossRef]
- Bjørndal, L.; Fransson, H.; Bruun, G.; Markvart, M.; Kjældgaard, M.; Näsman, P.; Hedenbjörk-Lager, A.; Dige, I.; Thordrup, M. Randomized Clinical Trials on Deep Carious Lesions: 5-Year Follow-up. J. Dent. Res. 2017, 96, 747–753. [Google Scholar] [CrossRef]
- Ricketts, D.; Innes, N.; Schwendicke, F. Selective Removal of Carious Tissue. Monogr. Oral Sci. 2018, 27, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Göstemeyer, G. Understanding dentists’ management of deep carious lesions in permanent teeth: A systematic review and meta-analysis. Implement. Sci. 2016, 11, 142. [Google Scholar] [CrossRef]
- Jurasic, M.M.; Gillespie, S.; Sorbara, P.; Clarkson, J.; Ramsay, C.; Nyongesa, D.; McEdward, D.; Gilbert, G.H.; Vollmer, W.M.; National Dental PBRN Collaborative Group. Deep caries removal strategies: Findings from The National Dental Practice-Based Research Network. JADA J. Am. Dent. Assoc. 2022, 153, 1078–1088.e7. [Google Scholar] [CrossRef] [PubMed]
- Osborne, J.; Summitt, J. Extension for prevention: Is it relevant today? Am. J. Dent. 1998, 11, 189–196. [Google Scholar] [PubMed]
- Banerjee, A.; Watson, T.; Kidd, E. Dentine Caries: Take It or Leave It? Dent. Update 2000, 27, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Fusayama, T. Two layers of carious dentin; diagnosis and treatment. Oper. Dent. 1979, 4, 63–70. [Google Scholar]
- Takahashi, N.; Nyvad, B. Ecological Hypothesis of Dentin and Root Caries. Caries Res. 2016, 50, 422–431. [Google Scholar] [CrossRef]
- Cate, J.M.T. Remineralization of Caries Lesions Extending into Dentin. J. Dent. Res. 2001, 80, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.; Carvalho, R.; Pegoraro, L.; Tay, F.; Thompson, V.P. Evaluation of a Self-limiting Concept in Dentinal Caries Removal. J. Dent. Res. 2006, 85, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Hesse, D.; Bonifácio, C.C.; Mendes, F.M.; Braga, M.M.; Imparato, J.C.P.; Raggio, D.P. Sealing versus partial caries removal in primary molars: A randomized clinical trial. BMC Oral Health 2014, 14, 58. [Google Scholar] [CrossRef]
- Spadaro, J.A.; Webster, D.A.; Becker, R.O. Silver polymethyl methacrylate antibacterial bone cement. Clin. Orthop. Relat. Res. 1979, 143, 266–270. [Google Scholar] [CrossRef]
- Barillo, D.J.; Marx, D.E. Silver in medicine: A brief history BC 335 to present. Burns 2014, 40 (Suppl. 1), S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Moyer, C.; Brentano, L.; Gravens, D.; Margraf, H.; Monafo, W. Treatment of large human burns with 0.5% silver nitrate solution. Arch. Surg. 1965, 90, 812–867. [Google Scholar] [CrossRef]
- Rosenblatt, A.; Stamford, T.; Niederman, R. Silver Diamine Fluoride: A Caries “Silver-Fluoride Bullet”. J. Dent. Res. 2009, 88, 116–125. [Google Scholar] [CrossRef]
- Sorkhdini, P.; Crystal, Y.O.; Tang, Q.; Lippert, F. The effect of silver diamine fluoride in preventing in vitro primary coronal caries under pH-cycling conditions. Arch. Oral Biol. 2020, 121, 104950. [Google Scholar] [CrossRef]
- Sorkhdini, P.; Crystal, Y.O.; Tang, Q.; Lippert, F. The effect of silver diamine fluoride on the remineralization of early enamel carious lesions under pH-cycling conditions. JADA Found. Sci. 2022, 1, 100006. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial Activity and Mechanism of Action of the Silver Ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef]
- Furr, J.; Russell, A.; Turner, T.; Andrews, A. Antibacterial activity of Actisorb Plus, Actisorb and silver nitrate. J. Hosp. Infect. 1994, 27, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.M.; Odelola, H.A.; Anderson, B. Effect of silver on whole cells and spheroplasts of a silver resistant Pseudomonas aeruginosa. Microbios 1984, 39, 151–157. [Google Scholar] [PubMed]
- Thurman, R.B.; Gerba, C.P.; Bitton, G. The molecular mechanisms of copper and silver ion disinfection of bacteria and viruses. Crit. Rev. Environ. Control 1989, 18, 295–315. [Google Scholar] [CrossRef]
- Seifo, N.; Robertson, M.; MacLean, J.; Blain, K.; Grosse, S.; Milne, R.; Seeballuck, C.; Innes, N. The use of silver diamine fluoride (SDF) in dental practice. Br. Dent. J. 2020, 228, 75–81. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Gotjamanos, T. Pulp response in primary teeth with deep residual caries treated with silver fluoride and glass ionomer cement (‘atraumatic’ technique). Aust. Dent. J. 1996, 41, 328–334. [Google Scholar] [CrossRef]
- Zander, H.; Burrill, D.Y. The Penetration of Silver Nitrate Solution into Dentin. J. Dent. Res. 1943, 22, 85–89. [Google Scholar] [CrossRef]
- Zander, H.; Smith, H. Penetration of Silver Nitrate Into Dentin II. J. Dent. Res. 1945, 24, 121–128. [Google Scholar] [CrossRef]
- Perreault, J.G.; Massler, M.; Schour, I. Reaction of odontoblasts to medicaments placed in cavity preparations in rat incisors. J. Am. Dent. Assoc. 1956, 52, 533–554. [Google Scholar] [CrossRef]
- Englander, H.R.; James, V.E.; Massler, M. Histologic effects of silver nitrate on human dentin and pulp. J. Am. Dent. Assoc. 1958, 57, 621–630. [Google Scholar] [CrossRef]
- Seltzer, S.; Bender, I.; Kaufman, I.J. Histologic changes in dental pulps of dogs and monkeys following application of pressure, drugs, and microorganisms on prepared cavities. Oral Surg. Oral Med. Oral Pathol. 1961, 14, 327–346. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, S.; Bender, I.; Kaufman, I.J. Histologic changes in dental pulps of dogs and monkeys following application of pressure, drugs, and microorganisms on prepared cavities: Part II. Changes observable more than one month after Application of Traumatic Agents. Oral Surg. Oral Med. Oral Pathol. 1961, 14, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Srisomboon, S.; Kettratad, M.; Stray, A.; Pakawanit, P.; Rojviriya, C.; Patntirapong, S.; Panpisut, P. Effects of Silver Diamine Nitrate and Silver Diamine Fluoride on Dentin Remineralization and Cytotoxicity to Dental Pulp Cells: An In Vitro Study. J. Funct. Biomater. 2022, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, Y.; Aritomi, K.; Goto, G. Pulpal response to diammine silver fluoride. (2). Application on exposed pulps. Shoni Shikagaku Zasshi 1990, 28, 327–337. [Google Scholar] [PubMed]
- Shah, N.; Korwar, A.; Sharma, S.; Logani, A. Pulp response to high fluoride releasing glass ionomer, silver diamine fluoride, and calcium hydroxide used for indirect pulp treatment: An in-vivo comparative study. Contemp. Clin. Dent. 2015, 6, 288–292. [Google Scholar] [CrossRef]
- Rossi, G.; Squassi, A.; Mandalunis, P.; Kaplan, A. Effect of silver diamine fluoride (SDF) on the dentin-pulp complex: Ex vivo histological analysis on human primary teeth and rat molars. Acta Odontol. Latinoam. 2017, 30, 5–12. [Google Scholar]
- Bimstein, E.; Damm, D. Human Primary Tooth Histology Six Months after Treatment with Silver Diamine Fluoride. J. Clin. Pediatr. Dent. 2018, 42, 442–444. [Google Scholar] [CrossRef]
- Hu, S.; Muniraj, G.; Mishra, A.; Hong, K.; Lum, J.L.; Hong, C.H.L.; Rosa, V.; Sriram, G. Characterization of silver diamine fluoride cytotoxicity using microfluidic tooth-on-a-chip and gingival equivalents. Dent. Mater. 2022, 38, 1385–1394. [Google Scholar] [CrossRef]
- Targino, A.G.R.; Flores, M.A.P.; Junior, V.E.D.S.; Bezerra, F.D.G.B.; Freire, H.D.L.; Galembeck, A.; Rosenblatt, A. An innovative approach to treating dental decay in children. A new anti-caries agent. J. Mater. Sci. Mater. Med. 2014, 25, 2041–2047. [Google Scholar] [CrossRef]
- Gotjamanos, T.; Afonso, F. Unacceptably high levels of fluoride in commercial preparations of silver fluoride. Aust. Dent. J. 1997, 42, 52–53. [Google Scholar] [CrossRef]
- Gardner, A.F.; Higel, R.W. An evaluation of agents used in cavity sterilization. Aust. Dent. J. 1962, 7, 53–61. [Google Scholar] [CrossRef]
- Gotjamanos, T.; Orton, V. Fluorideion concentration in 40 per cent silver fluoride solutions determined by ion selective electrode and ion chromatography techniques. Aust. Dent. J. 1998, 43, 55–56. [Google Scholar] [CrossRef] [PubMed]
- Stebbins, E. What value has argenti nitras as a therapeutic agent in dentistry? Int. Dent. J. 1891, 12, 661–670. [Google Scholar]
- Bauer, M.; Balogh, D.; Kompatscher, P. 10% Silver nitrate solution for topical treatment in scalding. Chir. Plast. 1984, 8, 25–36. [Google Scholar] [CrossRef]
- Howe, P.R. A method of sterilizing and at the same time impregnating with a metal affected dentinal tissue. Dent. Cosm. 1917, 59, 891–904. [Google Scholar]
- Klein, H.; Knutson, J.W. XIII. Effect of Ammoniacal Silver Nitrate on Caries in the First Permanent Molar. J. Am. Dent. Assoc. 1942, 29, 1420–1426. [Google Scholar] [CrossRef]
- James, P.; Parfitt, G. A clinical note on the use of silver nitrate in the prevention of fissure caries in newly erupted first permanant molars. Br. Dent. J. 1954, 96, 35–36. [Google Scholar]
- Schultz-Haudt, S.; Taylor, R.; Brudevold, F. Silver nitrate treatment of proximal caries in primary molars. J. Dent. Child. 1956, 23, 184–186. [Google Scholar]
- Hyde, E.J. Caries-inhibiting action of three different topically-applied agents on incipient lesions in newly erupted teeth: Results after 24 months. J. Can. Dent. Assoc. 1973, 39, 189–193. [Google Scholar]
- Chu, C.-H.; Gao, S.S.; Li, S.K.; Wong, M.C.; Lo, E.C. The effectiveness of the biannual application of silver nitrate solution followed by sodium fluoride varnish in arresting early childhood caries in preschool children: Study protocol for a randomised controlled trial. Trials 2015, 16, 426. [Google Scholar] [CrossRef]
- Saito, T.; Toyooka, H.; Ito, S.; Crenshaw, M.A. In vitro Study of Remineralization of Dentin: Effects of Ions on Mineral Induction by Decalcified Dentin Matrix. Caries Res. 2003, 37, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.L.; Nudelman, F.; Marzec, B.; Walker, J.; Lo, E.; Walls, A.; Chu, C. Formation of Fluorohydroxyapatite with Silver Diamine Fluoride. J. Dent. Res. 2017, 96, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.L.; Ito, L.; Cao, Y.; Li, Q.; Lo, E.C.; Chu, C. Inhibitory effect of silver diamine fluoride on dentine demineralisation and collagen degradation. J. Dent. 2013, 41, 809–817. [Google Scholar] [CrossRef]
- Nishino, M.; Yoshida, S.; Sobue, S.; Kato, J.; Nishida, M. Effect of topically applied ammoniacal silver fluoride on dental caries in children. J. Osaka Univ. Dent. Sch. 1969, 9, 149–155. [Google Scholar] [PubMed]
- Mitwalli, H.; Mourao, M.D.; Dennison, J.; Yaman, P.; Paster, B.J.; Fontana, M. Effect of Silver Diamine Fluoride Treatment on Microbial Profiles of Plaque Biofilms from Root/Cervical Caries Lesions. Caries Res. 2019, 53, 555–566. [Google Scholar] [CrossRef]
- Chopra, I. The increasing use of silver-based products as antimicrobial agents: A useful development or a cause for concern? J. Antimicrob. Chemother. 2007, 59, 587–590. [Google Scholar] [CrossRef]
- Natarajan, D. Silver Modified Atraumatic Restorative Technique: A Way Towards “SMART” Pediatric Dentistry During the COVID-19 Pandemic. Front. Dent. 2022, 19, 12. [Google Scholar] [CrossRef]
- Benzian, H.; Niederman, R. A Dental Response to the COVID-19 Pandemic—Safer Aerosol-Free Emergent (SAFER) Dentistry. Front. Med. 2020, 7, 520. [Google Scholar] [CrossRef]
- Oliveira, B.H.; Rajendra, A.; Veitz-Keenan, A.; Niederman, R. The Effect of Silver Diamine Fluoride in Preventing Caries in the Primary Dentition: A Systematic Review and Meta-Analysis. Caries Res. 2018, 53, 24–32. [Google Scholar] [CrossRef]
- Tirupathi, S.P.; Svsg, N.; Rajasekhar, S.; Nuvvula, S. Comparative cariostatic efficacy of a novel Nano-silver fluoride varnish with 38% silver diamine fluoride varnish a double-blind randomized clinical trial. J. Clin. Exp. Dent. 2019, 11, e105–e112. [Google Scholar] [CrossRef]
- Seifo, N.; Cassie, H.; Radford, J.R.; Innes, N.P.T. Silver diamine fluoride for managing carious lesions: An umbrella review. BMC Oral Health 2019, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Noronha, V.T.; Paula, A.J.; Durán, G.; Galembeck, A.; Cogo-Müller, K.; Franz-Montan, M.; Durán, N. Silver nanoparticles in dentistry. Dent. Mater. 2017, 33, 1110–1126. [Google Scholar] [CrossRef]
- Lung, C.Y.K.; Abdalla, M.M.; Chu, C.H.; Yin, I.; Got, S.-R.; Matinlinna, J.P. A Multi-Element-Doped Porous Bioactive Glass Coating for Implant Applications. Materials 2021, 14, 961. [Google Scholar] [CrossRef] [PubMed]
- Kittler, S.; Greulich, C.; Diendorf, J.; Köller, M.; Epple, M. Toxicity of Silver Nanoparticles Increases during Storage Because of Slow Dissolution under Release of Silver Ions. Chem. Mater. 2010, 22, 4548–4554. [Google Scholar] [CrossRef]
- Khorrami, S.; Zarrabi, A.; Khaleghi, M.; Danaei, M.; Mozafari, M.R. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed. 2018, 13, 8013–8024. [Google Scholar] [CrossRef] [PubMed]
- Bapat, R.A.; Chaubal, T.V.; Joshi, C.P.; Bapat, P.R.; Choudhury, H.; Pandey, M.; Gorain, B.; Kesharwani, P. An overview of application of silver nanoparticles for biomaterials in dentistry. Mater. Sci. Eng. C 2018, 91, 881–898. [Google Scholar] [CrossRef]
- Ramkumar, V.S.; Pugazhendhi, A.; Gopalakrishnan, K.; Sivagurunathan, P.; Saratale, G.D.; Dung, T.N.B.; Kannapiran, E. Biofabrication and characterization of silver nanoparticles using aqueous extract of seaweed Enteromorpha compressa and its biomedical properties. Biotechnol. Rep. 2017, 14, 1–7. [Google Scholar] [CrossRef]
- Hackenberg, S.; Scherzed, A.; Kessler, M.; Hummel, S.; Technau, A.; Froelich, K.; Ginzkey, C.; Koehler, C.; Hagen, R.; Kleinsasser, N. Silver nanoparticles: Evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol. Lett. 2011, 201, 27–33. [Google Scholar] [CrossRef]
- Quinzii, C.M.; Lopez, L.C.; Gilkerson, R.W.; Dorado, B.; Coku, J.; Naini, A.B.; Lagier-Tourenne, C.; Schuelke, M.; Salviati, L.; Carrozzo, R.; et al. Reactive oxygen species, oxidative stress, and cell death correlate with level of CoQ10 deficiency. FASEB J. 2010, 24, 3733–3743. [Google Scholar] [CrossRef]
- Durán, N.; Nakazato, G.; Seabra, A.B. Antimicrobial activity of biogenic silver nanoparticles, and silver chloride nanoparticles: An overview and comments. Appl. Microbiol. Biotechnol. 2016, 100, 6555–6570. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, L.; Zhou, X.; Yu, Y.; Li, Z.; Zuo, D.; Wu, Y. Silver nanoparticles induce protective autophagy via Ca2+/CaMKKβ/AMPK/mTOR pathway in SH-SY5Y cells and rat brains. Nanotoxicology 2019, 13, 369–391. [Google Scholar] [CrossRef] [PubMed]
- García-Contreras, R.; Argueta-Figueroa, L.; Mejía-Rubalcava, C.; Jiménez-Martínez, R.; Cuevas-Guajardo, S.; Sánchez-Reyna, P.A.; Zeron, H.M. Perspectives for the use of silver nanoparticles in dental practice. Int. Dent. J. 2011, 61, 297–301. [Google Scholar] [CrossRef]
- E Silva, A.V.C.; Teixeira, J.A.; Mota, C.C.; Lins, E.C.C.C.; Correia de Melo Júnior, P.; de Souza Lima, M.G.; Arnaud, M.; Galembeck, A.; Gadelha, A.T.; Pereira, J.R.D.; et al. In Vitro morphological, optical and microbiological evaluation of nanosilver fluoride in the remineralization of deciduous teeth enamel. Nanotechnol. Rev. 2018, 7, 509–520. [Google Scholar] [CrossRef]
- Dos Santos, V., Jr.; Vasconcelos Filho, A.; Targino, A.; Flores, M.; Galembeck, A.; Caldas, A.F., Jr.; Rosenblatt, A. A new “silver-bullet” to treat caries in children–nano silver fluoride: A randomised clinical trial. J. Dent. 2014, 42, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Puppala, N.; Nagireddy, V.R.; Reddy, D.; Kondamadugu, S.; Mareddy, A.; Chris, A. Nanosilver Fluoride—A Paradigm Shift for Arrest in Dental Caries in Primary Teeth of Schoolchildren: A Randomized Controlled Clinical Trial. Int. J. Clin. Pediatr. Dent. 2019, 12, 484–490. [Google Scholar] [CrossRef]
- Zhao, I.S.; Mei, M.L.; Li, Q.-L.; Lo, E.C.M.; Chu, C.-H. Arresting simulated dentine caries with adjunctive application of silver nitrate solution and sodium fluoride varnish: An In Vitro study. Int. Dent. J. 2017, 67, 206–214. [Google Scholar] [CrossRef]
- Mei, M.L.; Chu, C.H.; Lo, E.C.M.; Samaranayake, L.P. Fluoride and silver concentrations of silver diammine fluoride solutions for dental use. Int. J. Paediatr. Dent. 2012, 23, 279–285. [Google Scholar] [CrossRef]
- Seltzer, S.; Werther, L. Conservative Silver Nitrate Treatment of Borderline Cases of Deep Dental Caries. J. Am. Dent. Assoc. 1941, 28, 1586–1594. [Google Scholar] [CrossRef]
- ISO 10993-5: 2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Luong, M.; Sadr, A.; Chan, D.C.N. Dentin Discoloration and Pulpal Ion Concentrations Following Silver Diamine Fluoride and Potassium Iodide Treatment. Oper. Dent. 2022, 47, 640–647. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).