Abstract
Asthma is a chronic, heterogeneous respiratory pathology characterized by reversible airway inflammation. Therapeutics focus on symptom reduction and control, aimed at preserving normal pulmonary function and inducing bronchodilatation. The objective of this review is to describe the adverse effects produced by anti-asthmatic drugs on dental health, according to the reported scientific evidence. A bibliographic review was carried out on databases, such as Web of science, Scopus, and ScienceDirect. Most anti-asthmatic medications are administered using inhalers or nebulizers, making it impossible to avoid contact of the drug with hard dental tissues and oral mucosa, and thus promoting a greater risk of oral alterations, mainly due to decreases in the salivary flow and pH. Such changes can cause diseases, such as dental caries, dental erosion, tooth loss, periodontal disease, bone resorption, as well as fungal infections, such as oral candidiasis.
1. Introduction
Asthma is a heterogeneous disease, usually characterized by a chronic inflammatory disorder of the airways, elevated hyper-reactivity of the tracheobronchial tree, increased mucus production, and by symptoms, such as wheezing, coughing, chest tightness, and dyspnea, that can vary over time and in intensity, affecting people of both sexes, of all ages, and all races [1,2,3,4].
In recent years, its prevalence has increased significantly in most countries, with high rates of morbidity in children and mortality in adults, affecting more than 300 million people worldwide [5,6,7]. Prior to adolescence, the prevalence of asthma is higher in men than in women, but this tends to reverse after adolescence, because hormone levels in many women of childbearing age and the use of contraceptives can directly interfere with the treatment of asthma [8,9]. Mortality has increased significantly in recent years. According to the World Health Organization (WHO), the world death toll was estimated at 416,000 in 2016, mainly affecting developing countries [10]. More than 80% of these deaths have occurred in low- and middle-income countries [11,12,13].
The cause of asthma is not known, but there are risk factors that have been identified and gene-environment interactions. Genetics play a fundamental role in asthma; large genetic studies have identified hundreds of genetic variants associated with an increased risk of asthma [14,15]. The heritability of asthma ranges from 35% to 95% [16]. Other risk factors include allergic stimuli, such as house dust mites, animals, mold, and pollen. Non-allergic stimuli include viral infections, tobacco smoke, and cold air [3,17,18].
Diagnosis is made by clinical evaluation of symptoms, such as dyspnea, wheezing, paroxysmal cough, and chest tightness, as well as the functional demonstration of reversible airway obstruction, either spontaneously or following medical treatment [19].
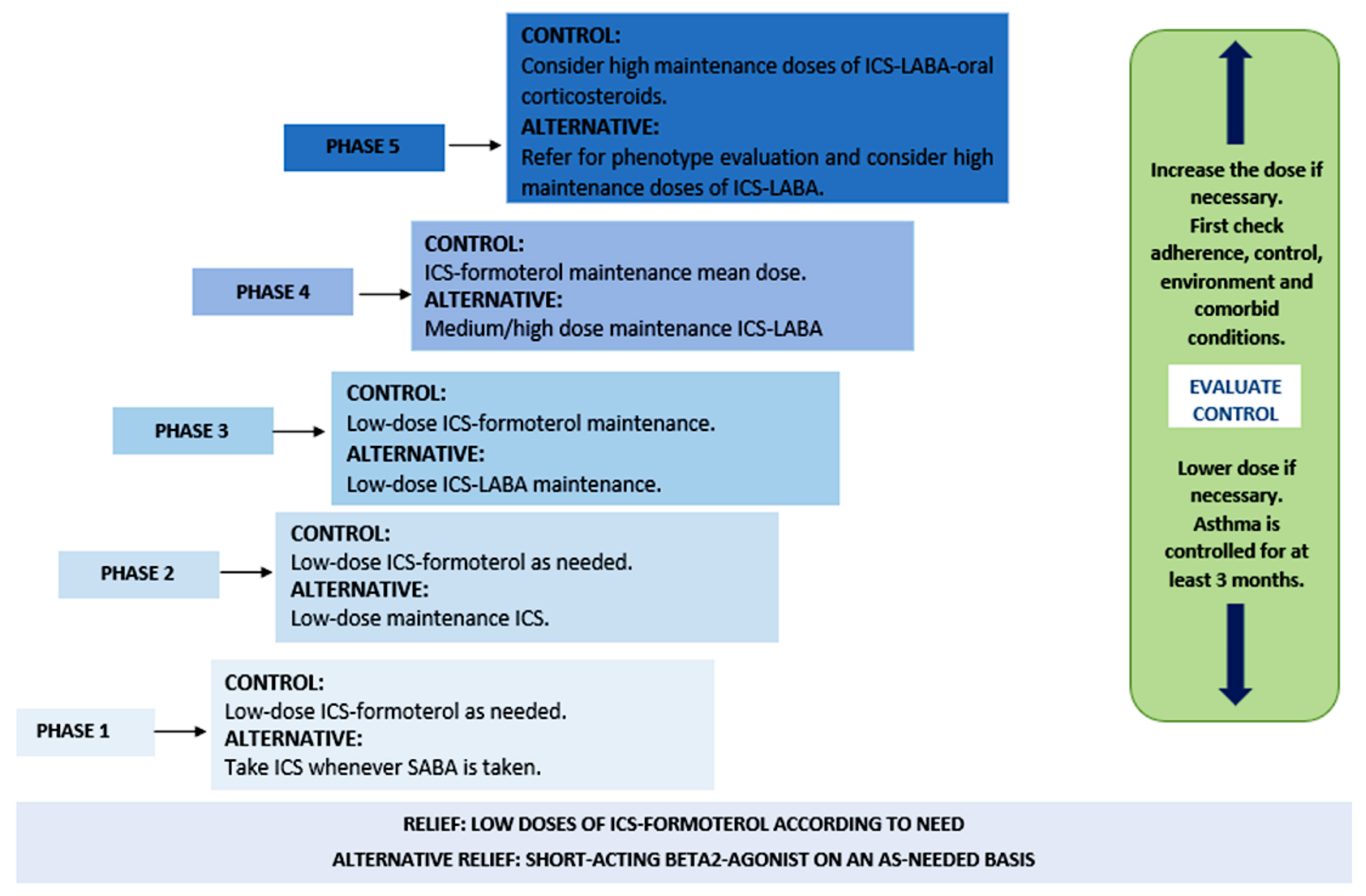
Considering the manifestations of this disease, patients with asthma can receive short-term treatment for constant exacerbations of the pathology, as well as long-term treatment for its maintenance and control, as described in Figure 1 [20,21].
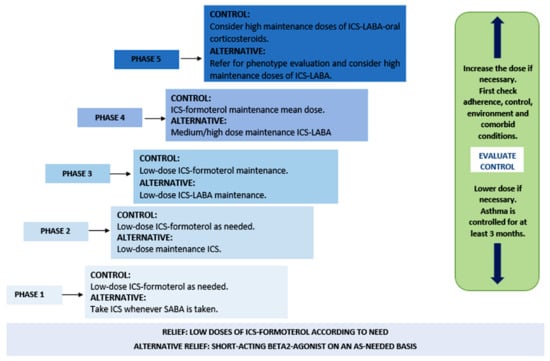
Figure 1.
Phases of asthma treatment. Abbreviations: ICS (inhaled corticosteroids), SABA (short-acting β2-adrenergic agonists), and LABA (long-acting β2-adrenergic agonists).
The prescribed anti-asthmatic medication is selected in relation to the severity of the disease and the type of asthma present [22]. There are national and international guidelines, such as those of the Global Initiative for Asthma (GINA), the National Asthma Education and Prevention Program (NAEPP), and the British Thoracic Society (BTS) [23,24], which provide recommendations on the diagnosis and treatment of asthmatic patients on an individualized basis by considering not only the severity of the disease, but also the phenotypic characteristics of the patient [2].
Drug therapy is mainly focused on two groups: bronchodilators and anti-inflammatory drugs. These are used to improve the patient’s symptoms by relaxing the muscles that surround the airways making it easier to breathe. The administration method used for these drugs is dependent on the state of severity present in each patient [25,26].
However, most of the drugs are administered through inhalers or nebulizers [27]; therefore, it is impossible to avoid contact of the drug with hard dental tissues and the oral mucosa, inducing a higher risk of adverse effects, such as dental caries, dental erosion, periodontal disease, tooth loss, bone resorption, or oral candidiasis, following prolonged use [28,29]. These effects lead to decreases in the salivary pH and flow, thus reducing the protective effects of saliva, and increases the presence of Streptococcus mutans and Lactobacillus acidophilus, which increases an individual’s susceptibility to the development of oral pathologies [30,31,32].
The adverse effects generated by medications prescribed for asthma have been the subject of research for a long time, due to the appearance of oral and dental alterations [33]. Therefore, the objective of this review is to describe the adverse effects produced by anti-asthmatic medications on dental health, based on the reported scientific evidence.
2. Materials and Methods
This study was developed in response to the need to obtain information in the dental field, so a bibliographic search of scientific articles published between 2002 and 2022 was carried out in the Web of Science, Scopus, and ScienceDirect databases.
2.1. Bibliographic Search
We used search strategy terms indexed in the MeSH keyword health descriptor, such as “asthma”, “asthma; dentistry”, “asthma medication; dentistry”, and “asthma; oral health” to identify relevant studies in the databases indicated.
2.2. Inclusion Criteria
Studies reporting on the relationship between asthma and oral disorders and peer-reviewed articles written in English were included. No studies were excluded because of their design.
2.3. Exclusion Criteria
Studies that were not relevant to the review include articles written in Spanish, Chinese, or Russian, or gray literature. In addition, duplicate studies and studies with incomplete information, such as a lack of information on the drug used, were excluded. Likewise, information from letters to the editor, congress abstracts, and non-peer-reviewed trial registries were not included.
2.4. Data Analysis
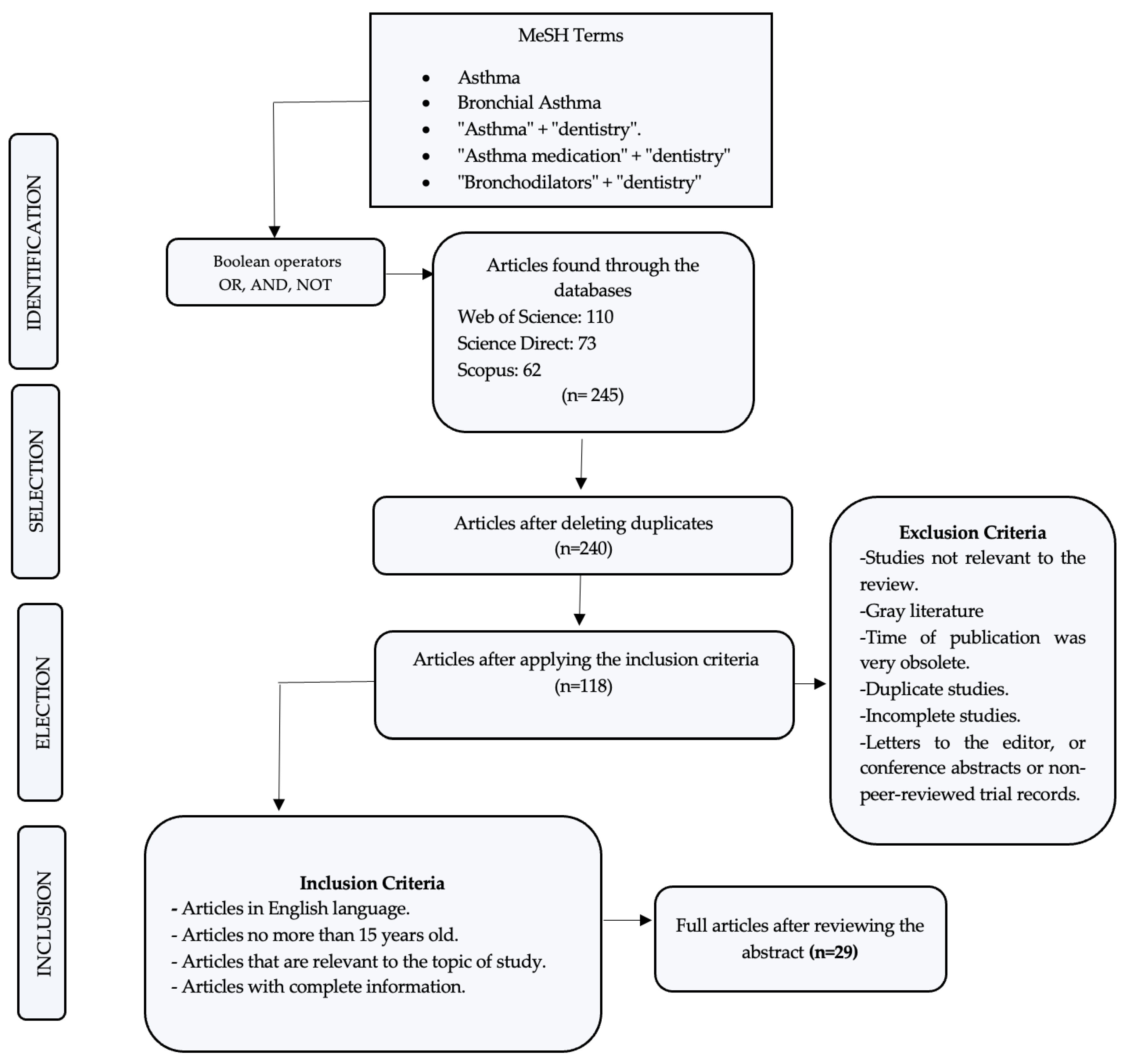
In the initial search, 245 articles related to this work were identified, and after a rigorous analysis and according to the inclusion criteria, only 29 were accepted to be part of the present review.
3. Results
Based on the described methodology and the scientific evidence, Figure 2 represents the PRISMA flow diagram, with the results obtained in this review.
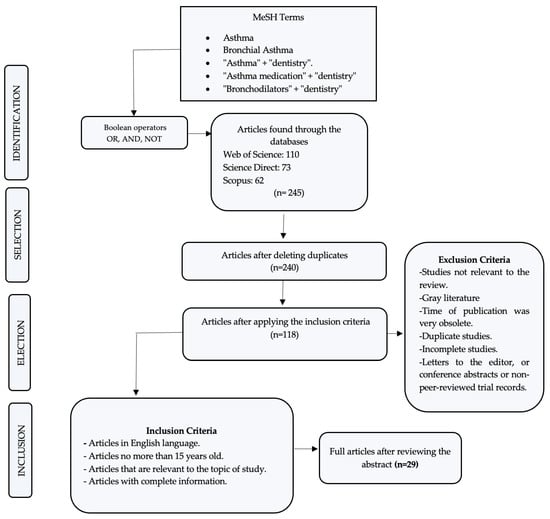
Figure 2.
Search flow diagram.
3.1. Effects on Dental Health
The association of asthma with adverse effects on the dental health, such as dental caries, periodontal disease, dental erosion, and alterations at the level of the oral mucosa, has been a subject of debate among dentists [34]. Anti-asthmatic medications, especially inhalers that maintain intimate contact with oral tissues, contain fermentable carbohydrates, such as lactose monohydrate, used with the purpose of improving their taste and thus increasing the patient’s tolerance to them. Their frequent use can cause, in most cases, a decrease in salivary flow. Studies indicate that prolonged use of β2-adrenergic agonists can reduce the salivary secretion rate of the parotid gland by 36%. Therefore, as the salivary flow decreases, the levels of salivary amylase, salivary peroxidase, lysozyme, and immunoglobulin A (IgA-S) decrease [2,30,35,36].
Saliva is composed of water and a variety of inorganic and organic components, which aid in the masticatory process and protect dental structures from existing pathogenic microorganisms. Therefore, the salivary composition plays an important role in oral health, as it acts as a defensive mechanism. In addition, anti-asthmatic medications are frequently administered at night before going to sleep without performing any routine method of oral hygiene, which could increase the risk of alterations at the level of the oral cavity [2,37,38,39].
Table 1 summarizes the studies found on the adverse effects of anti-asthmatic drugs in the oral cavity. The studies are ordered by year of publication, type of study, parameters tested, drugs analyzed, study design, and most relevant results.

Table 1.
Characteristics of the included studies.
There are several international medical guidelines that establish the objectives for optimizing therapy in asthmatic patients, such as preventing and minimizing exacerbations of chronic and acute symptoms, and the requirement for emergency care. Several investigations have shown that many patients can achieve good asthma management through long-term control treatment, mainly with inhaled corticosteroids (ICS), long-acting β2-adrenergic agonists (LABA), and short-acting β2-adrenergic agonists (SABA) [4,42,60,61,62,63]. However, the increased presence of side effects that accompany the prolonged use of these medications should be considered [26,48,64]. Inhaled or nebulized SABAs resolve acute asthma symptoms, and their therapeutic effect occurs during the first 15 to 20 min of the first hour of acute asthma, inducing symptomatic relief, but they have no effect on airway inflammation and do not provide a sustained benefit [26,51,57,65]. The inhalation route is considered the most effective method of drug administration for asthmatic patients, because it avoids the effect of the first hepatic step with a minimal reduction in bioavailability and rapid absorption of the drug from the alveolar region to the bloodstream, thus improving the therapeutic efficacy. There are three pharmaceutical presentations that allow for the drug to be administered: through an inhalation aerosol, pressurized metered-dose inhaler (MDI), or a dry powder inhaler (DPI) or nebulizer [65,66,67,68]. The management of these formulations is complex at the pediatric level, and almost 30% of asthmatic adults have an inadequate inhalation technique, triggering 80% of the drug to be deposited in the oropharynx, which considerably changes the effectiveness of the treatment and can cause local or systemic side effects [55,69]. In addition, drugs have considerable effects on salivary components and properties, especially in terms of the buffering capacity, quantity, and viscosity. It is considered that any factor that reduces the quantity and quality of saliva can negatively affect oral health, because saliva plays a very important role in its preservation [37,43].
Based on the literature found, Table 2 of the drugs administered in asthma and the adverse effects that they produce was developed. Among the main adverse effects associated with anti-asthmatic medications are oropharyngeal candidiasis, laryngeal weakness, dental caries, decreased taste, burning tongue, tongue abrasion, and a decreased bone marrow density level, as well as the other adverse effects summarized in Table 2 [32,70].

Table 2.
Medications for asthma and adverse effects.
The main adverse effects that affect dental health produced by anti-asthmatic medications are described below.
3.1.1. Asthma and Dental Caries
Dental caries is one of the oral pathologies with the highest prevalence worldwide. Thus, it is considered a public health problem. It has a prevalence of 80% in children and 100% in adults [54,74,75,76]. The appearance of carious lesions results from an alteration in the balance between dietary-bacterial factors, the components of the host, and the use of various medications [77,78]. The main treatment for asthma is the inhalation of steroids, and this is an important causal factor in the development of dental caries, because of the direct changes in the salivary composition that occur [79,80,81]. Several factors contribute to the increased prevalence of dental caries in asthmatic patients, such as a reduced saliva flow caused especially by beta-2 adrenergic agonists, increased counts of Streptococcus mutans and Lactobacillus spp., and a decreased salivary pH below the critical value 30 min after treatment with beta-2 agonist inhalers [22,36,73,82].
In addition, most anti-asthmatic medications contain fermentable carbohydrates [83]. Therefore, frequent oral inhalation of these sugar-containing drugs, coupled with decreased salivary production, may contribute to an increased risk of dental caries. Previous research has shown that the duration of medication has a significant influence on the risk of developing dental caries in asthmatic patients, indicating that patients using salbutamol inhalers are at higher risk of developing carious lesions compared with non-asthmatic patients [84,85]. Similarly, the frequency of use and dosing times influence the prevalence of carious lesions. Studies have shown that children who use this type of medication more than twice a day experience more dental caries and that this risk increases in children whose dose is administered before bedtime [2].
Therefore, it is essential to prescribe fluoride supplements to asthmatic patients, especially those taking beta-2 adrenergic agonists. Patients should be educated to perform mouth rinses after using inhalers. Chewing sugarless gum for at least one minute after drug administration will help to neutralize salivary pH, stimulate salivary flow, and buffering oral acids [2,36,52,85,86].
3.1.2. Asthma and Oral Candidiasis
Oral candidiasis is the most prevalent opportunistic fungal infection at the level of the oral cavity. This fungal infection is caused by species of the genus Candida, with Candida albicans being the most common species, representing more than 80% of clinical isolates; however, there are other responsible species, such as C. tropicalis, C. glabrata, C. krusei, and C. parapsilosis, that enhance the resistance to antifungal therapy [52,87,88]. Pathological changes in the oral mucosal surface, immunological changes, and microenvironmental variations contribute to the development of this opportunistic infection [52,87]. Thus, local and systemic factors, such as decreased salivary flow, erosion of the oral mucosa, vitamin deficiency, and generalized immunosuppression play important roles in the etiology of this lesion [52].
There are certain population groups that are prone to the development of oral candidiasis, such as patients being treated with systemic immunosuppressants or topical steroids, those with a dry oral mucosa, and those with anemia, among other systemic conditions [52]. It has been highlighted that the regular use of inhaled corticosteroids in asthmatic patients is associated with the development of oral candidiasis, the prevalence of this infection as a possible adverse effect varies from 0 to 77% [89]. The relationship between the administration of inhaled corticosteroids and candidiasis is mainly due to the decreases in salivary IgA and histamine due to the actions of immunosuppressants, considering that only 20% of the inhaled dose actually reaches the lungs, so most of the inhaled drug remains in the oral cavity and oropharynx, affecting the physiology of the oral tissues, in addition to suppressing cellular immunity and phagocytosis. However, it has been reported that oral mucosal immunity normalizes upon discontinuation of corticosteroids [50,59,89,90,91,92].
To reduce the incidence of oral candidiasis in patients undergoing asthma treatment, it is recommended that mouth rinses are performed immediately after the administration of the drug and before going to sleep. For this purpose, rinses based on 0.05% neutral sodium fluoride or with an antimicrobial base can be used. It has even been shown that gargling with a diluted amphotericin solution considerably reduces the amount of Candida albicans in relation to gargling with water. In addition, regular dental check-ups are advised [89]. The administration of topical antifungals, such as nystatin, and medications that promote salivary flow, such as sugar-free chewing gum, are also recommended [93].
3.1.3. Asthma and Periodontal Disease
Periodontal diseases represent complex interactions between host defenses and bacterial pathogen aggression that result in inflammatory processes in the dental supporting tissues. Signs and symptoms may vary: from gingivitis, which refers to inflammation of the gingival tissues, to periodontitis, in which an inflammatory process of the supporting tissues occurs, involving clinical attachment loss and bone loss, possibly resulting in tooth loss [94,95]. Periodontal health appears to be strongly related to an adequate salivary flow. One of the most effective elements in the defense of the oral cavity is saliva, which has antibacterial, antiviral, and antifungal activity, as well as containing immunoglobulins and enzymes that play protective roles for the mucosa. Therefore, a decrease in the salivary flow directly affects periodontal tissues, and it has been reported that anti-asthmatic medications cause alterations to salivary secretion, negatively influencing periodontal health [96,97,98,99].
Therefore, the main cause of periodontal tissue deterioration in asthmatic patients is the reduction of the protective action of saliva. Another factor that contributes to the presence of these alterations is the tendency of these patients to breathe through the mouth, thus causing continuous dehydration of the alveolar mucosa, especially during an acute asthmatic attack [100,101,102]. They also have a higher prevalence of calculus due to elevated levels of calcium and phosphorus [85], and these patients may have high IgE levels in the gingival tissues, a factor that is also directly related to poor periodontal health. Previous research indicated that diseases other than asthma have direct relationships with periodontal disease [2,36], such as allergic rhinitis, obstructive sleep apnea, and adenoid and tonsil hypertrophy, among other respiratory diseases that favor mouth breathing [2].
3.1.4. Asthma and Dental Erosion
Dental erosion is the progressive and irreversible erosion of mineralized dental tissues due to chemical processes without bacterial involvement [78,103], an example being anti-asthmatic medications that can contribute to the development of this non carious lesion by reducing salivary protection against extrinsic and intrinsic acids [104,105,106]. In addition, the main drugs used to treat asthma, especially those in powder form, can lead to a pH below 5.5 [40,41]. Similarly, beta-2 adrenergic agonists can cause the relaxation of other smooth muscles, such as the lower esophageal sphincter, and increase the amplitude of esophageal contraction, leading to symptoms, such as gastric reflux. Therefore, asthmatic patients tend to present with dental erosion due to constant acid attacks.
It is essential to instruct asthmatic patients to rinse their mouths with sodium bicarbonate or neutral sodium fluoride after using inhalers [104]. Similarly, these patients should be instructed that it is not advisable to brush their teeth immediately after exposure to acids, as this may further damage the already weakened enamel. Another measure that helps to reduce erosion rates in asthmatic subjects is the use of a spacer device during the administration of dry powder inhalers, these spacers consist of plastic devices that minimize the contact between the drug and the oropharynx, meaning that most of the drug is directed towards the lungs [2,17,36].
4. Discussion
Thomas et al. [36] pointed out that most asthma medications are significantly associated with alterations in oral health, and patients undergoing anti-asthma treatment should be instructed on the use of their prescribed inhalers, since constant and direct contact between the formulations and the oral cavity increases the risk of oral tissue affection and, therefore, the development of pathologies. Some dry powder inhalers are known to contain sugar to increase the tolerability of the taste of the drug when administered. Frequent oral inhalation of sugar combined with a reduced salivary flow and decreased saliva pH may contribute to an increase in dental caries [80]. Stensson et al. [77] explained that this association may be due to the presence of fermentable carbohydrates, such as lactose monohydrate, in anti-asthmatic medications. These excipients may increase the risk of oral conditions, such as dental caries, by acidifying the pH of the oral cavity due to acid formation from the fermentation of ingested carbohydrates. Shashikiran et al. [85] demonstrated that patients who use bronchodilators, such as salbutamol, are more prone to the development of dental caries in comparison with those taking other anti-asthmatic medications. Likewise, the authors stated that patients who consume beta-2 adrenergic agonists also present with a high incidence of dental caries due to a reduction of salivary flow and an increase in the proliferation of cariogenic microorganisms. Moreover, after investigating oral health indices in healthy children and children with mild to moderate asthma, Ehsani et al. [44], found that there were no significant differences between children with asthma and those without asthma with respect to the DMTF index (decayed, missing, filled teeth) with means of 3.34 in the asthmatic children and 3.0 in the control group. In the study, Brigic et al. [31] reported the cariogenic potential of inhaled anti-asthmatic medications, observing that non-asthmatic subjects presented with a significantly higher DMTF index than asthmatic subjects (p = 0.004). We also determined that there were no significant differences in the values of the plaque index between groups (p > 0.05).
Among the anti-asthmatic medications, it has been shown that high doses and prolonged duration of inhaled medications are closely related to adverse effects on oral tissues. Manuel et al. [104], reported that inhalers containing salbutamol and fluticasone propionate decrease salivary flow 30 min after inhalation of the drug, indicating that higher bronchodilator doses are associated with greater reductions in the levels of immunoglobulin A (IgA), calcium, lactoferrin, and total protein in the saliva, so that patients who do not benefit from the protective role of saliva are more vulnerable to the development of oral pathologies.
Another adverse effect caused by the consumption of anti-asthmatic medications is oral candidiasis. The growth of Candida in asthmatic patients is very high compared to healthy people. Anti-asthmatic medication makes the oral habitat prone to attack from opportunistic infections, such as oral candidiasis. [59]. Moreover, Lemmetyinen et al. [92] showed that one of the conditions related to asthma drugs is a viral infection. The authors found that the rate of herpes zoster was almost seven times higher in asthmatic subjects than in non-asthmatic subjects. The authors pointed out that this may be because asthmatic patients have an immune system deficiency, which may cause reactivation of the varicella zoster virus.
Gani et al. [2] pointed out that dental erosion is associated with the use of β2-adrenergic agonists, regardless of the type of inhaler used, but cofactors, such as lifestyle, a diet rich in acids, or diseases that cause abnormal relaxation of the lower esophageal sphincter muscle, can increase the risk of dental erosion. It has even been reported that this abnormal relaxation of the muscle can be associated with the use of theophylline. Shashikiran et al. [85] conducted a case-control study on patients from Southeast Queensland and found higher incidences of dental hypersensitivity, xerostomia, salivary gland abnormalities, gastric discomfort, and self-induced vomiting in asthmatic patients compared to non-asthmatic patients. They explained that asthmatics are at risk of dental erosion due to the extrinsic acid to which they are constantly exposed.
Mappangara et al. [102] stated that the use of corticosteroid anti-asthmatic medications, especially via inhaled methods, increases the risk of periodontal diseases, such as gingivitis and severe periodontitis. The immunosuppressive effect of corticosteroids may influence the periodontal tissue response. These agents inhibit the host response, resulting in the clinical expression of gingivitis, [2] thus demonstrating that bronchodilators administered by the inhalation route are the anti-asthmatic medications that cause the most adverse effects in the oral cavity.
4.1. Perspectives
Respiratory problems nowadays have reached a high percentage, especially in the pediatric population, hence the imperative need to study the anti-asthmatic medications used for their treatment; there are reports that the continuous use of these drugs is related to the generation of other pathologies. In the dental area, they are directly related to an increase in the percentage of dental caries, the presence of periodontal and gingival disease, tooth loss due to changes in the salivary composition, and pH. Therefore, this review seeks to highlight the importance of adverse effects on dental health to provide effective pharmacotherapeutic follow-up that largely prevents dental damage. It is necessary to mention that there are preventive measures to avoid adverse effects on dental health, such as adequate hygiene after the application of medications, and this is where the contribution of the patient is very significant, because it will allow to control and reduce the incidence of adverse effects in the oral cavity.
4.2. Strengths
Among the strengths found in this study is the mention of the importance of knowing the different adverse effects that occur after administering anti-asthmatics at the oral level, and we hope that through this collection of information, we can begin to investigate these consequences in depth, so that the health professional can see the issues and can solve them.
4.3. Limitations of the Study
Among the limitations, the lack of specific information on the relationship between the prescription of anti-asthmatics and the dental area is indicated, which promotes further research in this area. Added to this is the scarce information on the indication, dosage, and presentation of the drug in the different publications revised in this article.
5. Conclusions
The use of anti-asthmatic medications can lead to a series of oral alterations due to an imbalance in the protective factors associated with the oral cavity, which can cause diseases, such as dental caries, dental erosion, tooth loss, periodontal disease, bone resorption, as well as fungal infections, such as oral candidiasis. These alterations are mainly due to the decrease in salivary flow, which leads to a reduction in the protection of the oral cavity by saliva due to reduced levels of defense components, such as IgA, calcium, and lactoferrin, among others. Inhalation therapy is closely related to the production of these adverse effects due to the direct contact of the drug with the oral cavity and oropharynx, so it is essential to educate asthmatic patients about preventive measures, such as mouth rinses with sodium bicarbonate or neutral sodium fluoride after using inhalers; chewing sugarless gum for at least one minute after drug administration to help neutralize the salivary pH, stimulate salivary flow, and buffer oral acids; or even the use of a spacer device during the administration of dry powder inhalers to minimize contact between the drug and the oropharynx.
Author Contributions
Conceptualization, E.-M.P.-Q., J.J., J.S.-O. and K.C.-L.; methodology, E.-M.P.-Q., J.S.-O. and K.C.-L.; software, E.-M.P.-Q.; Validation, E.-M.P.-Q. and K.C.-L.; formal analysis, E.-M.P.-Q., J.S.-O. and K.C.-L.; investigation, E.-M.P.-Q., J.J., J.S.-O. and K.C.-L.; resources, E.-M.P.-Q.; data curation, E.-M.P.-Q., J.J., J.S.-O. and K.C.-L.; writing—original draft preparation, E.-M.P.-Q. and J.J.; writing—review and editing, E.-M.P.-Q., J.S.-O. and K.C.-L.; visualization, E.-M.P.-Q., J.S.-O. and K.C.-L.; supervision, E.-M.P.-Q.; funding acquisition, E.-M.P.-Q., J.S.-O. and K.C.-L. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Catholic University of Cuenca, Cuenca, Ecuador, it is anchored to the project called: “COMPARATIVE ANALYSIS OF PRECLINICAL PERFORMANCE INDICATORS BETWEEN TOPICAL SOLUTIONS OF COPPER FLUORIDES AND SILVER FLUORIDE”, approved under code: PICODS21-38.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| GINA | Global Initiative for Asthma |
| NAEPP | National Asthma Education and Prevention Program |
| BTS | British Thoracic Society |
| WHO | World Health Organization |
| ICS | Inhaled corticosteroids |
| LABA | Long-acting β2-adrenergic agonists |
| SABA | Short-acting β2-adrenergic agonists |
| MDI | Pressurized metered-dose inhaler |
| DPI | Dry power inhaler |
| SIg-A | Secreted immunoglobulin A |
| IgA | Immunoglobulin A |
| DMTF | Decayed, Missing, and Filled Teeth index |
| PFT | Pulmonary function tests |
| OH: SALSA | Health Oral San Antonio Longitudinal Study of Aging |
| LS | Lumbar spine |
| FN | Femoral neck |
| DEXA | Dual energy X-ray absorptiometry |
| BMD | Bone mineral density |
| PPD | Probing depth |
| CAL | Clinical attachment level. |
| START | Steroids as regular therapy. |
| GOHAI | General Oral Health Assessment Index. |
References
- Boonpiyathad, T.; Sözener, Z.C.; Satitsuksanoa, P.; Akdis, C.A. Immunologic Mechanisms in Asthma. Semin. Immunol. 2019, 46, 101333. [Google Scholar] [CrossRef]
- Gani, F.; Caminati, M.; Bellavia, F.; Baroso, A.; Faccioni, P.; Pancera, P.; Batani, V.; Senna, G. Oral Health in Asthmatic Patients: A Review: Asthma and Its Therapy May Impact on Oral Health. Clin. Mol. Allergy 2020, 18, 22. [Google Scholar] [CrossRef]
- Quirt, J.; Hildebrand, K.J.; Mazza, J.; Noya, F.; Kim, H. Asthma. Allergy Asthma Clin. Immunol. 2018, 14, 1–16. [Google Scholar] [CrossRef]
- O’Byrne, P.; Fabbri, L.M.; Pavord, I.D.; Papi, A.; Petruzzelli, S.; Lange, P. Asthma Progression and Mortality: The Role of Inhaled Corticosteroids. Eur. Respir. J. 2019, 54, 1900491. [Google Scholar] [CrossRef]
- Dharmage, S.C.; Perret, J.L.; Custovic, A. Epidemiology of Asthma in Children and Adults. Front. Pediatr. 2019, 7, 246. [Google Scholar] [CrossRef]
- Kudo, M.; Ishigatsubo, Y.; Aoki, I. Pathology of Asthma. Front. Microbiol. 2013, 4, 1–16. [Google Scholar] [CrossRef]
- Stern, J.; Pier, J.; Litonjua, A.A. Asthma Epidemiology and Risk Factors. Semin. Immunopathol. 2020, 42, 5–15. [Google Scholar] [CrossRef]
- Shah, R.; Newcomb, D.C. Sex Bias in Asthma Prevalence and Pathogenesis. Front. Immunol. 2018, 9, 2997. [Google Scholar] [CrossRef]
- Rehman, A.; Amin, F.; Sadeeqa, S. Prevalence of Asthma and Its Management: A Review. J. Pak. Med. Assoc. 2018, 68, 1823–1827. [Google Scholar]
- Global Strategy for Asthma Management and Prevention. 2021. Available online: https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf (accessed on 11 April 2023).
- Bostantzoglou, C.; Delimpoura, V.; Samitas, K.; Zervas, E.; Kanniess, F.; Gaga, M. Clinical Asthma Phenotypes in the Real World: Opportunities and Challenges. Breathe 2015, 11, 186–193. [Google Scholar] [CrossRef]
- Alahmadi, T.S.; Banjari, M.A.; Alharbi, A.S. The Prevalence of Childhood Asthma in Saudi Arabia. Int. J. Pediatr. Adolesc. Med. 2019, 6, 74–77. [Google Scholar] [CrossRef]
- Kamga, A.; Rochefort-Morel, C.; Le Guen, Y.; Ouksel, H.; Pipet, A.; Leroyer, C. Asthma and Smoking: A Review. Respir. Med. Res. 2022, 82, 100916. [Google Scholar] [CrossRef]
- Holloway, J.W.; Yang, I.A.; Holgate, S.T. Genetics of Allergic Disease. J. Allergy Clin. Immunol. 2010, 125, S81–S94. [Google Scholar] [CrossRef]
- Ranjbar, M.; Whetstone, C.E.; Omer, H.; Power, L.; Cusack, R.P.; Gauvreau, G.M. The Genetic Factors of the Airway Epithelium Associated with the Pathology of Asthma. Genes 2022, 13, 1870. [Google Scholar] [CrossRef]
- Ober, C.; Yao, T.C. The Genetics of Asthma and Allergic Disease: A 21st Century Perspective. Immunol. Rev. 2011, 242, 10–30. [Google Scholar] [CrossRef]
- Mims, J.W. Asthma: Definitions and Pathophysiology. Int. Forum Allergy Rhinol. 2015, 5, S2–S6. [Google Scholar] [CrossRef]
- Castillo, J.R.; Peters, S.P.; Busse, W.W. Asthma Exacerbations: Pathogenesis, Prevention, and Treatment. J. Allergy Clin. Immunol. Pract. 2017, 5, 918–927. [Google Scholar] [CrossRef]
- Alaki, S.M.; Ashiry, E.A.A.; Bakry, N.S.; Baghlaf, K.K.; Bagher, S.M. The Effects of Asthma and Asthma Medication on Dental Caries and Salivary Characteristics in Children. Oral Health Prev. Dent. 2013, 11, 113–120. [Google Scholar] [CrossRef]
- Nunes, C.; Pereira, A.M.; Morais-Almeida, M. Asthma Costs and Social Impact. Asthma Res. Pract. 2017, 3, 1. [Google Scholar] [CrossRef]
- dos Santos, C.C.O.; Bellini-Pereira, S.A.; Medina, M.C.G.; Normando, D. Allergies/Asthma and Root Resorption: A Systematic Review. Prog. Orthod. 2021, 22, 8. [Google Scholar] [CrossRef]
- Wu, F.; Liu, J. Asthma Medication Increases Dental Caries among Children in Taiwan: An Analysis Using the National Health Insurance Research Database. J. Dent. Sci. 2019, 14, 413–418. [Google Scholar] [CrossRef]
- GINA Report, Global Strategy for Asthma Management and Prevention. Available online: https://ginasthma.org/gina-reports/ (accessed on 22 May 2022).
- National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC)|NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/science/national-asthma-education-and-prevention-program-naepp (accessed on 23 May 2022).
- Sin, D.D.; Man, J.; Sharpe, H.; Gan, W.Q.; Man, S.F.P. Pharmacological Management to Reduce Exacerbations in Adults with Asthma: A Systematic Review and Meta-Analysis. J. Am. Med. Assoc. 2004, 292, 367–376. [Google Scholar] [CrossRef]
- Lommatzsch, M.; Virchow, J.C. Severe Asthma. Dtsch. Arztebl. Int. 2014, 111, 847–855. [Google Scholar] [CrossRef]
- Arafa, A.; Aldahlawi, S.; Fathi, A. Assessment of the Oral Health Status of Asthmatic Children. Eur. J. Dent. 2017, 11, 357–363. [Google Scholar] [CrossRef]
- Orellana-Alpala, C.; Pacheco-Quito, E.-M.; Calle-Prado, D.; Cuenca-León, K. Knowledge Level Regarding the Prescription of Antibiotics within the Dentistry Field. Arch. Venez. Farmacol. Ter. 2022, 41, 665–670. [Google Scholar] [CrossRef]
- Velez-León, E.M.; Vargas, K.L.; Cuenca-León, K.; Acurio-Vargas, C.; Zumba, A.; Pacheco-Quito, E.M. Ambulatory Sedation for Dental Procedures-Case of Cuenca, Ecuador. Children 2022, 9, 1618. [Google Scholar] [CrossRef]
- Brigic, A.; Kobaslija, S.; Zukanovic, A. Cariogenic Potential of Inhaled Antiasthmatic Drugs. Med. Arch. 2015, 69, 247–250. [Google Scholar] [CrossRef]
- Brigic, A.; Kobaslija, S.; Zukanovic, A. Antiasthmatic Inhaled Medications as Favoring Factors for Increased Concentration of Streptococcus Mutans. Mater. Socio Med. 2015, 27, 237. [Google Scholar] [CrossRef]
- Botelho, M.P.J.; MacIel, S.M.; Cerci Neto, A.; Dezan, C.C.; Fernandes, K.B.P.; De Andrade, F.B. Cariogenic Microorganisms and Oral Conditions in Asthmatic Children. Caries Res. 2011, 45, 386–392. [Google Scholar] [CrossRef]
- Ersin, N.K.; Gülen, F.; Eronat, N.; Cogulu, D.; Demir, E.; Tanaç, R.; Aydemir, Ş. Oral and Dental Manifestations of Young Asthmatics Related to Medication, Severity and Duration of Condition. Pediatr. Int. 2006, 48, 549–554. [Google Scholar] [CrossRef]
- Stensson, M.; Wendt, L.K.; Koch, G.; Oldaeus, G.; Ramberg, P.; Birkhed, D. Oral Health in Young Adults with Long-Term, Controlled Asthma. Acta Odontol. Scand. 2011, 69, 158–164. [Google Scholar] [CrossRef]
- Doğan, M.; Şahiner, Ü.M.; Ataç, A.S.; Ballıkaya, E.; Soyer, Ö.U.; Şekerel, B.E. Oral Health Status of Asthmatic Children Using Inhaled Corticosteroids. Turk. J. Pediatr. 2021, 63, 77–85. [Google Scholar] [CrossRef]
- Thomas, M.S.; Parolia, A.; Kundabala, M.; Vikram, M. Asthma and Oral Health: A Review. Aust. Dent. J. 2010, 55, 128–133. [Google Scholar] [CrossRef]
- Świątkowska-Bury, M.; Zawadzka-Krajewska, A.; Kulus, M.; Olczak-Kowalczyk, D. The Effect of the Type of Inhaled Anti-Asthmatic Therapy on the Properties of Saliva in Children—A Phantom Study. J. Stomatol. 2021, 74, 22–27. [Google Scholar] [CrossRef]
- Dawes, C.; Wong, D.T.W. Role of Saliva and Salivary Diagnostics in the Advancement of Oral Health. J. Dent. Res. 2019, 98, 133–141. [Google Scholar] [CrossRef]
- Rezende, G.; dos Santos, N.M.L.; Stein, C.; Hilgert, J.B.; Faustino-Silva, D.D. Asthma and Oral Changes in Children: Associated Factors in a Community of Southern Brazil. Int. J. Paediatr. Dent. 2019, 29, 456–463. [Google Scholar] [CrossRef]
- Sivasithamparam, K.; Young, W.G.; Jirattanasopa, V.; Priest, J.; Khan, F.; Harbrow, D.; Daley, T.J. Dental Erosion in Asthma: A Case-Control Study from South East Queensland. Aust. Dent. J. 2002, 47, 298–303. [Google Scholar] [CrossRef]
- Dugmore, C.R.; Rock, W.P. A Multifactorial Analysis of Factors Associated with Dental Erosion. Br. Dent. J. 2004, 196, 283–286. [Google Scholar] [CrossRef]
- Huchon, G.; Magnussen, H.; Chuchalin, A.; Dymek, L.; Gonod, F.B.; Bousquet, J. Lung Function and Asthma Control with Beclomethasone and Formoterol in a Single Inhaler. Respir. Med. 2009, 103, 41–49. [Google Scholar] [CrossRef]
- Boskabady, M.; Nematollahi, H.; Boskabady, M.H. Effect of Inhaled Medication and Inhalation Technique on Dental Caries in Asthmatic Patients. Iran. Red Crescent Med. J. 2012, 14, 816–821. [Google Scholar] [CrossRef]
- Ehsani, S.; Moin, M.; Meighani, G.; Pourhashemi, S.J.; Khayatpisheh, H.; Yarahmadi, N. Oral Health Status in Preschool Asthmatic Children in Iran. Iran J. Allergy Asthma Immunol. 2013, 12, 254–261. [Google Scholar] [PubMed]
- Godara, N.; Khullar, M.; Godara, R.; Singh, V. Evaluation of Cariogenic Potential of Dry Powder Inhalers: A Case-Control Study. Lung India 2013, 30, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Prasanthi, B.; Kannan, N.; Patil, R. Effect of Diuretics on Salivary Flow, Composition and Oral Health Status: A Clinico-Biochemical Study. Ann. Med. Health Sci. Res. 2014, 4, 549. [Google Scholar] [CrossRef]
- Monadi, M.; Javadian, Y.; Cheraghi, M.; Heidari, B.; Amiri, M. Impact of Treatment with Inhaled Corticosteroids on Bone Mineral Density of Patients with Asthma: Related with Age. Osteoporos. Int. 2015, 26, 2013–2018. [Google Scholar] [CrossRef] [PubMed]
- Reddel, H.K.; Busse, W.W.; Pedersen, S.; Tan, W.C.; Chen, Y.Z.; Jorup, C.; Lythgoe, D.; O’Byrne, P.M. Should Recommendations about Starting Inhaled Corticosteroid Treatment for Mild Asthma Be Based on Symptom Frequency: A Post-Hoc Efficacy Analysis of the START Study. Lancet 2017, 389, 157–166. [Google Scholar] [CrossRef]
- Rodríguez, F.; Duran, A.; Muñoz, Z.; Palomera, E.; Serra-Prat, M.; Boixeda, R.; Vicente, V.; Almirall, J. Oral Health and Risk of Pneumonia in Asthmatic Pacients with Inhaled Treatment. Med. Clin. 2018, 150, 455–459. [Google Scholar] [CrossRef]
- Ashuja, R.B.; Nandini, D.B.; Vidyasagar, B.; Ashwini, R.; Donoghue, M.; Madhushankari, G.S. Oral Carriage of Cariogenic Bacteria and Candida Albicans in Asthmatic Adults before and after Anti-Asthma Medication: A Longitudinal Study. J. Oral Maxillofac. Pathol. 2018, 22, 144–145. [Google Scholar] [CrossRef]
- Hu, L.; He, C.; Zhao, C.; Chen, X.; Hua, H.; Yan, Z. Characterization of Oral Candidiasis and the Candida Species Profile in Patients with Oral Mucosal Diseases. Microb. Pathog. 2019, 134, 103575. [Google Scholar] [CrossRef]
- Hassanpour, K.; Tehrani, H.; Goudarzian, M.; Beihaghi, S.; Ebrahimi, M.; Amiri, P. Comparison of the Frequency of Dental Caries in Asthmatic Children under Treatment with Inhaled Corticosteroids and Healthy Children in Sabzevar in 2017–2018. Electron. J. Gen. Med. 2019, 16, em119. [Google Scholar] [CrossRef]
- Khassawneh, B.; Alhabashneh, R.; Ibrahim, F. The Association between Bronchial Asthma and Periodontitis: A Case-Control Study in Jordan. J. Asthma. 2019, 56, 404–410. [Google Scholar] [CrossRef]
- Chumpitaz-Cerrate, V.; A Bellido-Meza, J.; Chávez-Rimache, L.; Rodríguez-Vargas, C. Impact of Inhaler Use on Dental Caries in Asthma Pediatrics Patients: A Case-Control Study. Arch. Argent. Pediatr. 2020, 118, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Bairappan, S.; Puranik, M.P.; Sowmya, K.R. Impact of Asthma and Its Medication on Salivary Characteristics and Oral Health in Adolescents: A Cross-Sectional Comparative Study. Spec. Care Dent. 2020, 40, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Brasil-Oliveira, R.; Cruz, Á.A.; Souza-Machado, A.; Pinheiro, G.P.; Inácio, D.D.S.; Sarmento, V.A.; Lins-Kusterer, L. Oral Health-Related Quality of Life in Individuals with Severe Asthma. J. Bras. Pneumol. 2021, 47, e20200117. [Google Scholar] [CrossRef] [PubMed]
- Akiki, Z.; Saadeh, D.; Farah, R.; Hallit, S.; Sacre, H.; Hosseini, H.; Salameh, P. Asthma Prevalence and Associated Factors among Lebanese Adults: The First National Survey. BMC Pulm. Med. 2021, 21, 162. [Google Scholar] [CrossRef] [PubMed]
- Slob, E.M.A.; Richards, L.B.; Vijverberg, S.J.H.; Longo, C.; Koppelman, G.H.; Pijnenburg, M.W.H.; Bel, E.H.D.; Neerincx, A.H.; Herrera Luis, E.; Perez-Garcia, J.; et al. Genome-Wide Association Studies of Exacerbations in Children Using Long-Acting Beta2-Agonists. Pediatr. Allergy Immunol. 2021, 32, 1197–1207. [Google Scholar] [CrossRef]
- Abidullah, M.; Sanober, A.; Kumar, S.; Gaddikeri, K.; Soorneedi, N.; Fatema, R.; Ahmed, S.M. Salivary Candida Albicans in Asthmatic Patients Taking Anti-Asthmatic Medication. J. Med. Life 2022, 15, 1110. [Google Scholar] [CrossRef]
- Postma, D.S.; Kerstjens, H.A.M.; Ten Hacken, N.H.T. Inhaled Corticosteroids and Long-Acting Beta-Agonists in Adult Asthma: A Winning Combination in All? Naunyn Schmiedebergs Arch. Pharmacol. 2008, 378, 203215, Erratum in Naunyn Schmiedebergs Arch. Pharmacol. 2009, 380, 97. [Google Scholar] [CrossRef]
- Jeminiwa, R.; Hohmann, L.; Qian, J.; Garza, K.; Hansen, R.; Fox, B.I. Impact of EHealth on Medication Adherence among Patients with Asthma: A Systematic Review and Meta-Analysis. Respir. Med. 2019, 149, 59–68. [Google Scholar] [CrossRef]
- Adams, N.P.; Jones, P.W. The Dose-Response Characteristics of Inhaled Corticosteroids When Used to Treat Asthma: An Overview of Cochrane Systematic Reviews. Respir. Med. 2006, 100, 1297–1306. [Google Scholar] [CrossRef]
- Pandya, D.; Puttanna, A.; Balagopal, V. Systemic Effects of Inhaled Corticosteroids: An Overview. Open Respir. Med. J. 2015, 8, 59–65. [Google Scholar] [CrossRef]
- Reddel, H.K.; Taylor, D.R.; Bateman, E.D.; Boulet, L.P.; Boushey, H.A.; Busse, W.W.; Casale, T.B.; Chanez, P.; Enright, P.L.; Gibson, P.G.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Asthma Control and Exacerbations—Standardizing Endpoints for Clinical Asthma Trials and Clinical Practice. Am. J. Respir. Crit. Care Med. 2009, 180, 59–99. [Google Scholar] [CrossRef] [PubMed]
- Laube, B.L.; Janssens, H.M.; De Jongh, F.H.C.; Devadason, S.G.; Dhand, R.; Diot, P.; Everard, M.L.; Horvath, I.; Navalesi, P.; Voshaar, T.; et al. What the Pulmonary Specialist Should Know about the New Inhalation Therapies. Eur. Respir. J. 2011, 37, 1308–1331. [Google Scholar] [CrossRef] [PubMed]
- Muchão, F.P.; Da Silva Filho, L.V.R.F. Advances in Inhalation Therapy in Pediatrics. J. Pediatr. 2010, 86, 367–376. [Google Scholar] [CrossRef]
- Cheng, Y.S. Mechanisms of Pharmaceutical Aerosol Deposition in the Respiratory Tract. AAPS PharmSciTech 2014, 15, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Dal Negro, R.W. Dry Powder Inhalers and the Right Things to Remember: A Concept Review. Multidiscip. Respir. Med. 2015, 10, 13. [Google Scholar] [CrossRef]
- Haughney, J.; Price, D.; Barnes, N.C.; Virchow, J.C.; Roche, N.; Chrystyn, H. Choosing Inhaler Devices for People with Asthma: Current Knowledge and Outstanding Research Needs. Respir. Med. 2010, 104, 1237–1245. [Google Scholar] [CrossRef]
- Choi, H.; Bae, K.H.; Lee, J.W. Association between Age at Asthma Diagnosis and Tooth Loss. Acta Odontol. Scand. 2018, 76, 466–472. [Google Scholar] [CrossRef]
- Patel, S.J.; Teach, S.J. Asthma. Pediatr. Rev. 2019, 40, 549–565. [Google Scholar] [CrossRef]
- Zahran, H.S.; Bailey, C.M.; Qin, X.; Johnson, C. Long-Term Control Medication Use and Asthma Control Status among Children and Adults with Asthma. J. Asthma 2017, 54, 1065–1072. [Google Scholar] [CrossRef]
- Elyassi Gorji, N.; Nasiri, P.; Malekzadeh Shafaroudi, A.; Moosazadeh, M. Comparison of Dental Caries (DMFT and DMFS Indices) between Asthmatic Patients and Control Group in Iran: A Meta-Analysis. Asthma Res. Pract. 2021, 7, 2. [Google Scholar] [CrossRef]
- Alshammari, F.R.; Alamri, H.; Aljohani, M.; Sabbah, W.; O’Malley, L.; Glenny, A.M. Dental Caries in Saudi Arabia: A Systematic Review. J. Taibah. Univ. Med. Sci. 2021, 16, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Vélez-León, E.M.; Albaladejo-Martínez, A.; Cuenca-León, K.; Encalada-Verdugo, L.; Armas-Vega, A.; Melo, M. Caries Experience and Treatment Needs in Urban and Rural Environments in School-Age Children from Three Provinces of Ecuador: A Cross-Sectional Study. Dent. J. 2022, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Vélez-León, E.; Albaladejo, A.; Cuenca-León, K.; Jiménez-Romero, M.; Armas-Vega, A.; Melo, M. Prevalence of Caries According to the ICDAS II in Children from 6 and 12 Years of Age from Southern Ecuadorian Regions. Int. J. Environ. Res. Public Health 2022, 19, 7266. [Google Scholar] [CrossRef]
- Stensson, M.; Wendt, L.K.; Koch, G.; Oldaeus, G.; Lingström, P.; Birkhed, D. Caries Prevalence, Caries-Related Factors and Plaque PH in Adolescents with Long-Term Asthma. Caries Res. 2011, 44, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Vélez-León, E.; Albaladejo-Martínez, A.; Pacheco-Quito, E.-M.; Armas-Vega, A.; Delgado-Gaete, A.; Pesántez-Ochoa, D.; Melo, M. Developmental Enamel Defects in Children from the Southern Region of Ecuador. Children 2022, 9, 1755. [Google Scholar] [CrossRef] [PubMed]
- Bozejac, B.V.; Stojšin, I.; Đurić, M.; Zvezdin, B.; Brkanić, T.; Budišin, E.; Vukoje, K.; Sečen, N. Impact of Inhalation Therapy on the Incidence of Carious Lesions in Patients with Asthma and COPD. J. Appl. Oral Sci. 2017, 25, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Hatipoğlu, Ö.; Pertek Hatipoğlu, F. Association between Asthma and Caries-Related Salivary Factors: A Meta-Analysis. J. Asthma 2022, 59, 38–53. [Google Scholar] [CrossRef]
- Cherkasov, S.V.; Popova, L.Y.; Vivtanenko, T.V.; Demina, R.R.; Khlopko, Y.A.; Balkin, A.S.; Plotnikov, A.O. Oral Microbiomes in Children with Asthma and Dental Caries. Oral Dis. 2019, 25, 898–910. [Google Scholar] [CrossRef]
- Azarpazhooh, A.; Leake, J.L. Systematic Review of the Association Between Respiratory Diseases and Oral Health. J. Periodontol. 2006, 77, 1465–1482. [Google Scholar] [CrossRef]
- Maupomé, G.; Shulman, J.D.; Medina-Soils, C.E.; Ladeinde, O. Is There a Relationship between Asthma and Dental Caries?: A Critical Review of the Literature. J. Am. Dent. Assoc. 2010, 141, 1061–1074. [Google Scholar] [CrossRef]
- Shah, P.D.; Badner, V.M.; Rastogi, D.; Moss, K.L. Association between Asthma and Dental Caries in US (United States) Adult Population. J. Asthma 2021, 58, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Shashikiran, N.D.; Reddy, V.V.S.; Krishnam Raju, P. Effect of Antiasthmatic Medication on Dental Disease: Dental Caries and Periodontal Disease. J. Indian Soc. Pedod. Prev. Dent. 2007, 25, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Steinbacher, D.M.; Glick, M. The Dental Patient with Asthma: An Update and Oral Health Considerations. J. Am. Dent. Assoc. 2001, 132, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Al-Haddad, K.A.; Al-dossary, O.A.E.; Al-Shamahy, H.A. Prevalence and Associated Factors of Oral Non-Candida Albicans Candida Carriage in Denture Wearers in Sana’a City—Yemen. Univers. J. Pharm. Res. 2018, 3, 69–73. [Google Scholar] [CrossRef]
- Pour, A.H.; Salari, S.; Nejad Almani, P.G. Oropharyngeal Candidiasis in HIV/AIDS Patients and Non-HIV Subjects in the Southeast of Iran. Curr. Med. Mycol. 2018, 4, 1–6. [Google Scholar] [CrossRef]
- GÜMRÜ, B.; AKKİTAP, M.P. Oral Candidiasis as a Local Adverse Effect of Inhaled Corticosteroids: What the Dental Practitioner Should Know. Black Sea J. Health Sci. 2021, 5, 107–115. [Google Scholar] [CrossRef]
- Vila, T.; Sultan, A.S.; Montelongo-Jauregui, D.; Jabra-Rizk, M.A. Oral Candidiasis: A Disease of Opportunity. J. Fungi 2020, 6, 15. [Google Scholar] [CrossRef]
- Sharma, S.; Gaur, P.; Gupta, S.; Kant, S. Impact of Asthma on Oral Health: A Review. Int. J. Recent. Sci. Res. 2018, 9, 26512–26514. [Google Scholar]
- Lemmetyinen, R.; Karjalainen, J.; But, A.; Renkonen, R.; Pekkanen, J.; Haukka, J.; Toppila-Salmi, S. Diseases with Oral Manifestations among Adult Asthmatics in Finland: A Population-Based Matched Cohort Study. BMJ Open 2021, 11, e053133. [Google Scholar] [CrossRef]
- Cuenca-León, K.; Pacheco-Quito, E.M.; Granda-Granda, Y.; Vélez-León, E.; Zarzuelo-Castañeda, A. Phytotherapy: A Solution to Decrease Antifungal Resistance in the Dental Field. Biomolecules 2022, 12, 789. [Google Scholar] [CrossRef]
- Ferreira, M.K.M.; Ferreira, R.d.O.; Castro, M.M.L.; Magno, M.B.; Almeida, A.P.C.P.S.C.; Fagundes, N.C.F.; Maia, L.C.; Lima, R.R. Is There an Association between Asthma and Periodontal Disease among Adults? Systematic Review and Meta-Analysis. Life Sci. 2019, 223, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Filho, I.S.; da Cruz, S.S.; Trindade, S.C.; Passos-Soares, J.d.S.; Carvalho-Filho, P.C.; Figueiredo, A.C.M.G.; Lyrio, A.O.; Hintz, A.M.; Pereira, M.G.; Scannapieco, F. Periodontitis and Respiratory Diseases: A Systematic Review with Meta-Analysis. Oral Dis. 2020, 26, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Yaghobee, S.; Paknejad, M.; Khorsand, A. Association between Asthma and Periodontal Disease. Front. Dent. (J. Dent. Tehran Univ. Med. Sci.) 2018, 5, 47–51. [Google Scholar]
- Moraschini, V.; de Albuquerque Calasans-Maia, J.; Calasans-Maia, M.D. Association Between Asthma and Periodontal Disease: A Systematic Review and Meta-Analysis. J. Periodontol. 2017, 89, 440–455. [Google Scholar] [CrossRef]
- Rivera, R.; Andriankaja, O.M.; Perez, C.M.; Joshipura, K. Relationship between Periodontal Disease and Asthma among Overweight/Obese Adults. J. Clin. Periodontol. 2016, 43, 566–571. [Google Scholar] [CrossRef]
- Gomes-Filho, I.S.; Soledade-Marques, K.R.; Seixas da Cruz, S.; de Santana Passos-Soares, J.; Trindade, S.C.; Souza-Machado, A.; Fischer Rubira-Bullen, I.R.; de Moraes Marcílio Cerqueira, E.; Barreto, M.L.; Costa de Santana, T.; et al. Does Periodontal Infection Have an Effect on Severe Asthma in Adults? J. Periodontol. 2014, 85, e179–e187. [Google Scholar] [CrossRef] [PubMed]
- Candeo, L.C.; Rigonato-Oliveira, N.C.; Brito, A.A.; Marcos, R.L.; França, C.M.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A.; Bussadori, S.K.; Vieira, R.P.; Lino-Dos-Santos-Franco, A.; et al. Effects of Periodontitis on the Development of Asthma: The Role of Photodynamic Therapy. PLoS ONE 2017, 12, e0187945. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.-C.; Chang, P.-Y.; Lin, C.-L.; Wei, C.-C.; Tu, C.-Y.; Hsia, T.-C.; Shih, C.-M.; Hsu, W.-H.; Sung, F.-C.; Kao, C.-H. Risk of Periodontal Disease in Patients with Asthma: A Nationwide Population-Based Retrospective Cohort Study. J. Periodontol. 2017, 88, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Mappangara, S.; Basir, I.; Oktawati, S.; Adam, M.; Achmad, H. Periodontal Disease Associated with Corticosteroid in Asthma Patients-A Systematic Review. Int. J. Appl. Pharm. 2019, 11, 68–70. [Google Scholar] [CrossRef]
- Taji, S.; Seow, W.K. A Literature Review of Dental Erosion in Children. Aust. Dent. J. 2010, 55, 358–367. [Google Scholar] [CrossRef]
- Manuel, S.T.; Kundabaka, M.; Shetty, N.; Parolia, A. Asthma and Dental Erosion. Kathmandu Univ. Med. J. 2008, 6, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Pandit, I.K.; Srivastava, N.; Gugnani, N. Dental Erosion in Children. J. Oral Health Comm. Dent. 2009, 3, 56–61. [Google Scholar]
- Lussi, A.; Jaeggi, T. Dental Erosion in Children. Monogr. Oral Sci. 2006, 20, 140–151. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).