Effects of Improper Mechanical Force on the Production of Sonic Hedgehog, RANKL, and IL-6 in Human Periodontal Ligament Cells In Vitro
Abstract
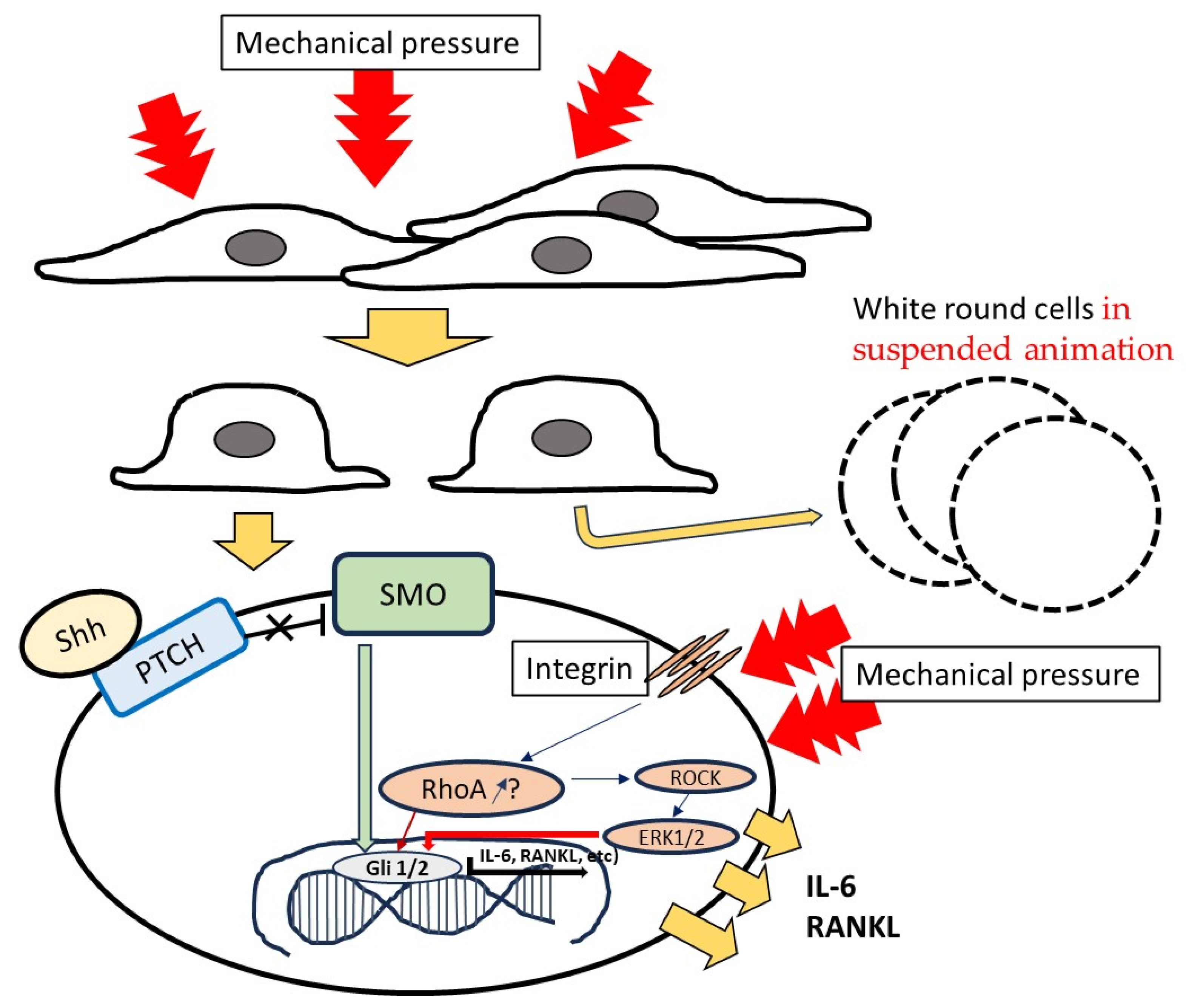
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Application of Compression Force
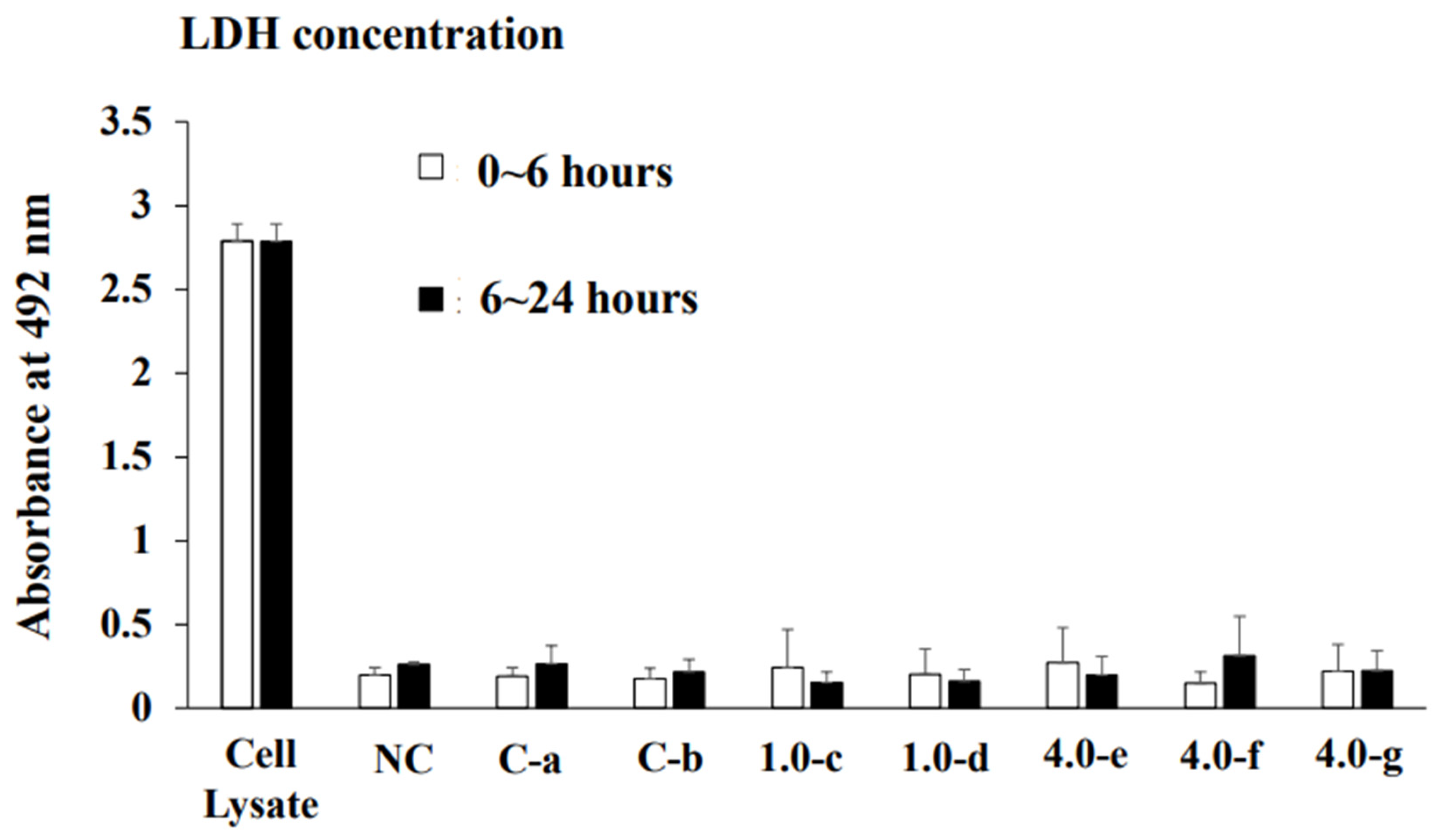
2.2. Measurement of Lactate Dehydrogenase (LDH)
2.3. Measurement of Cell Viability by MTT
2.4. SDS-PAGE and Western Blotting
2.5. Quantitative Measurement of RNAKL and IL-6
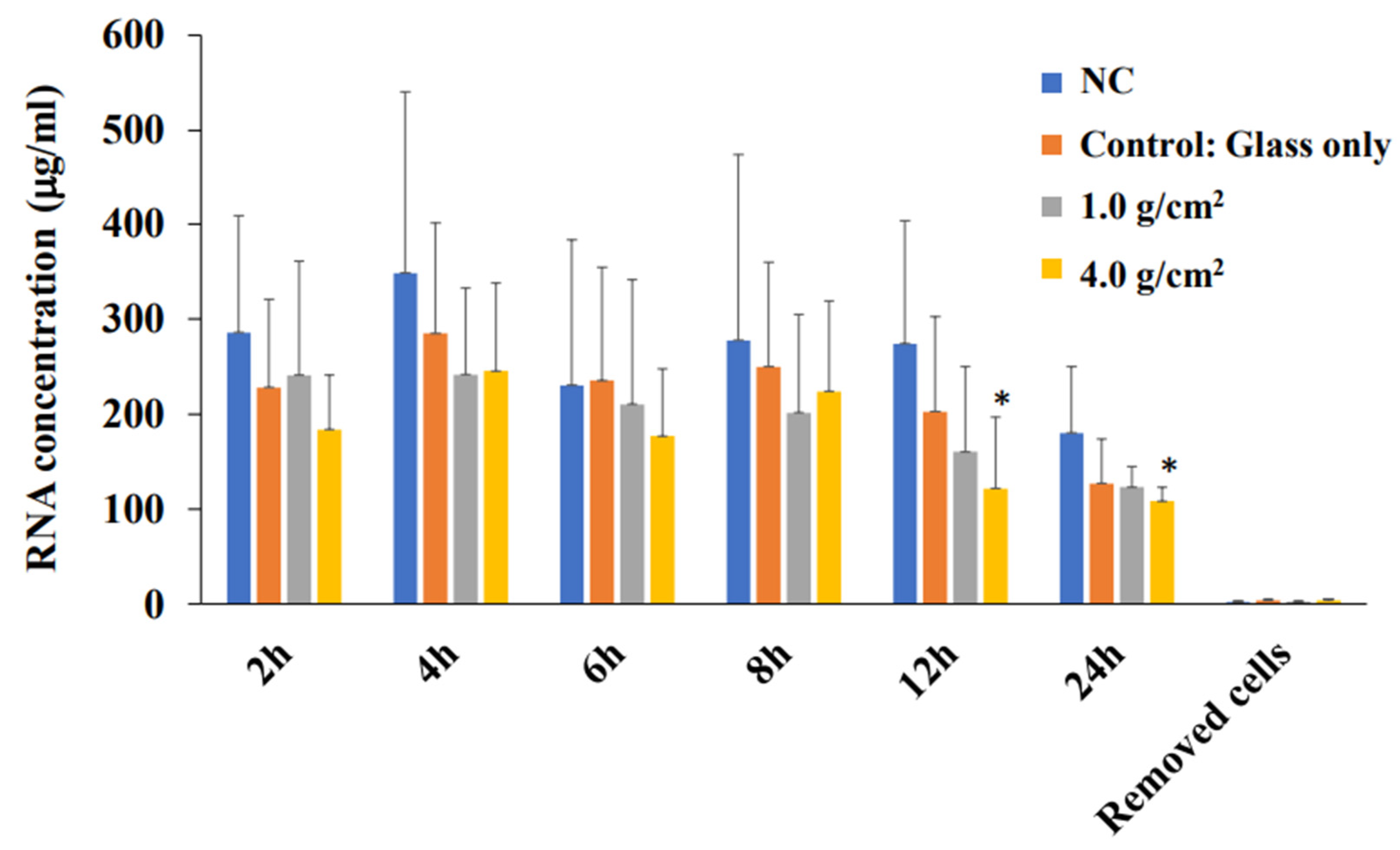
2.6. Total RNA Extraction and Real-Time PCR
2.7. Statistical Analysis
3. Results
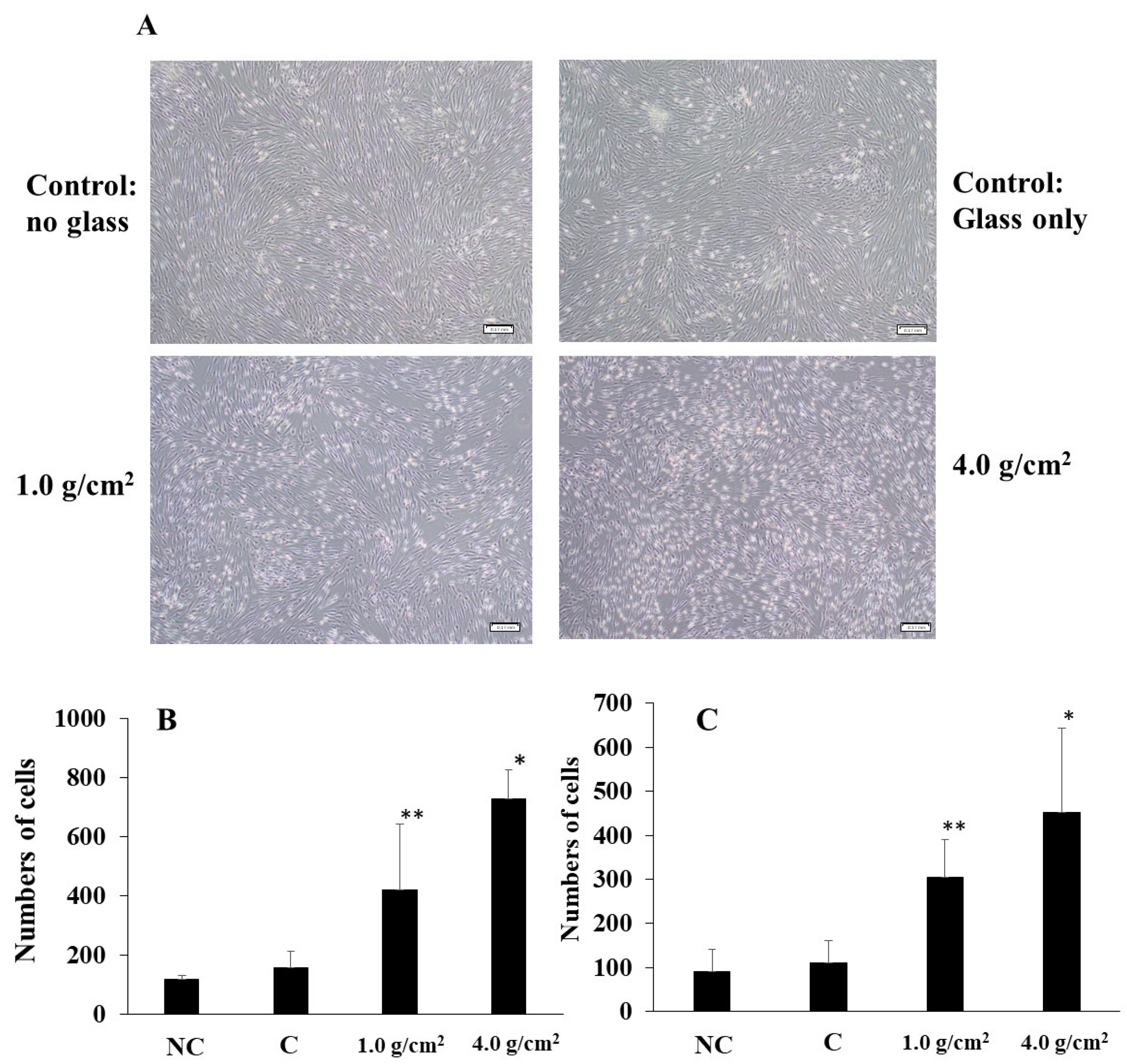
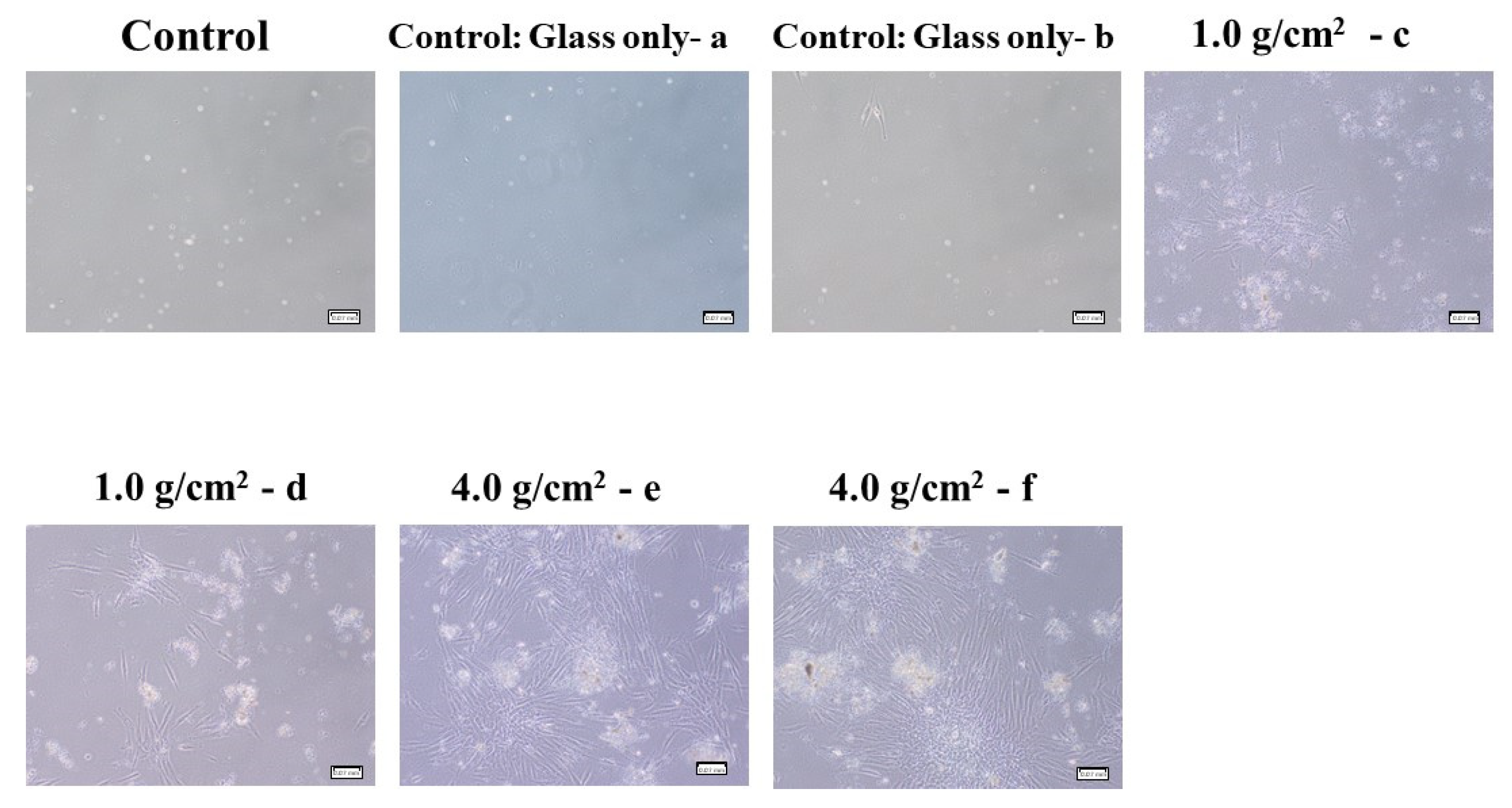
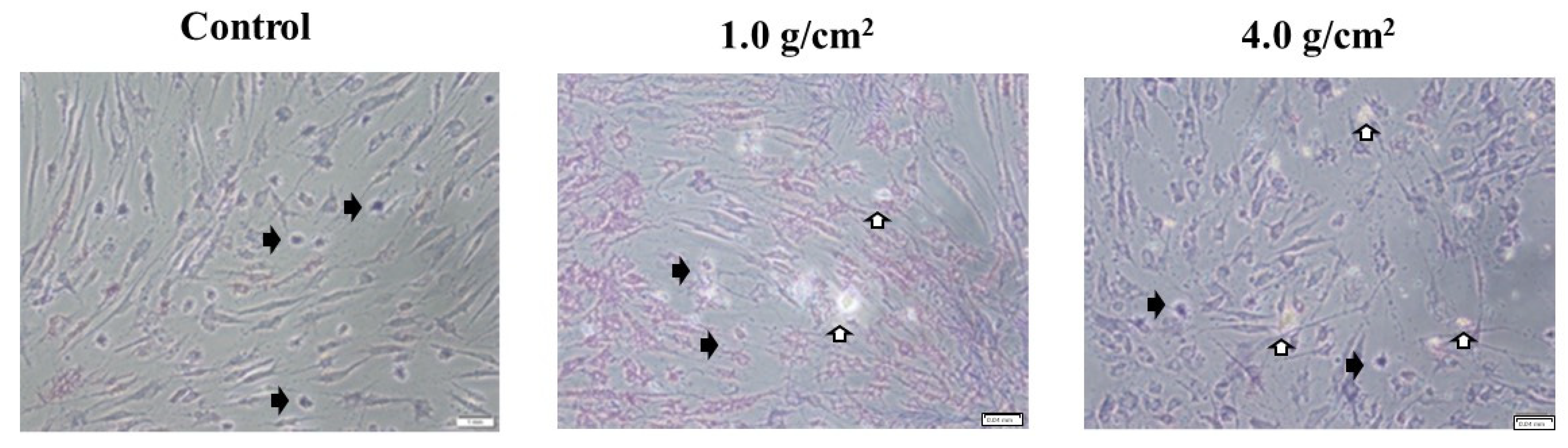
3.1. Effects of Orthodontic Force on the Cell Activities, Adherence, and Morphology
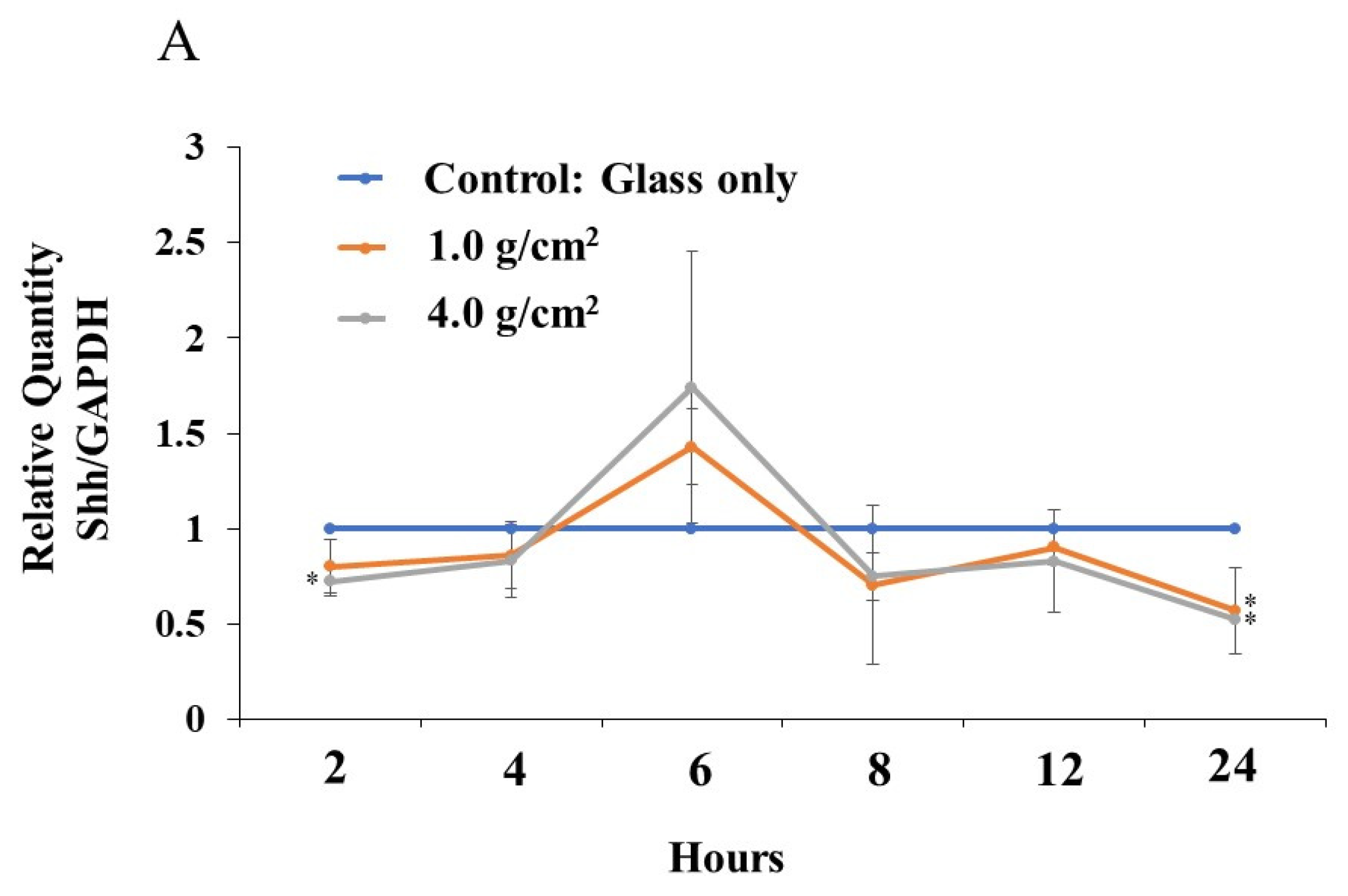
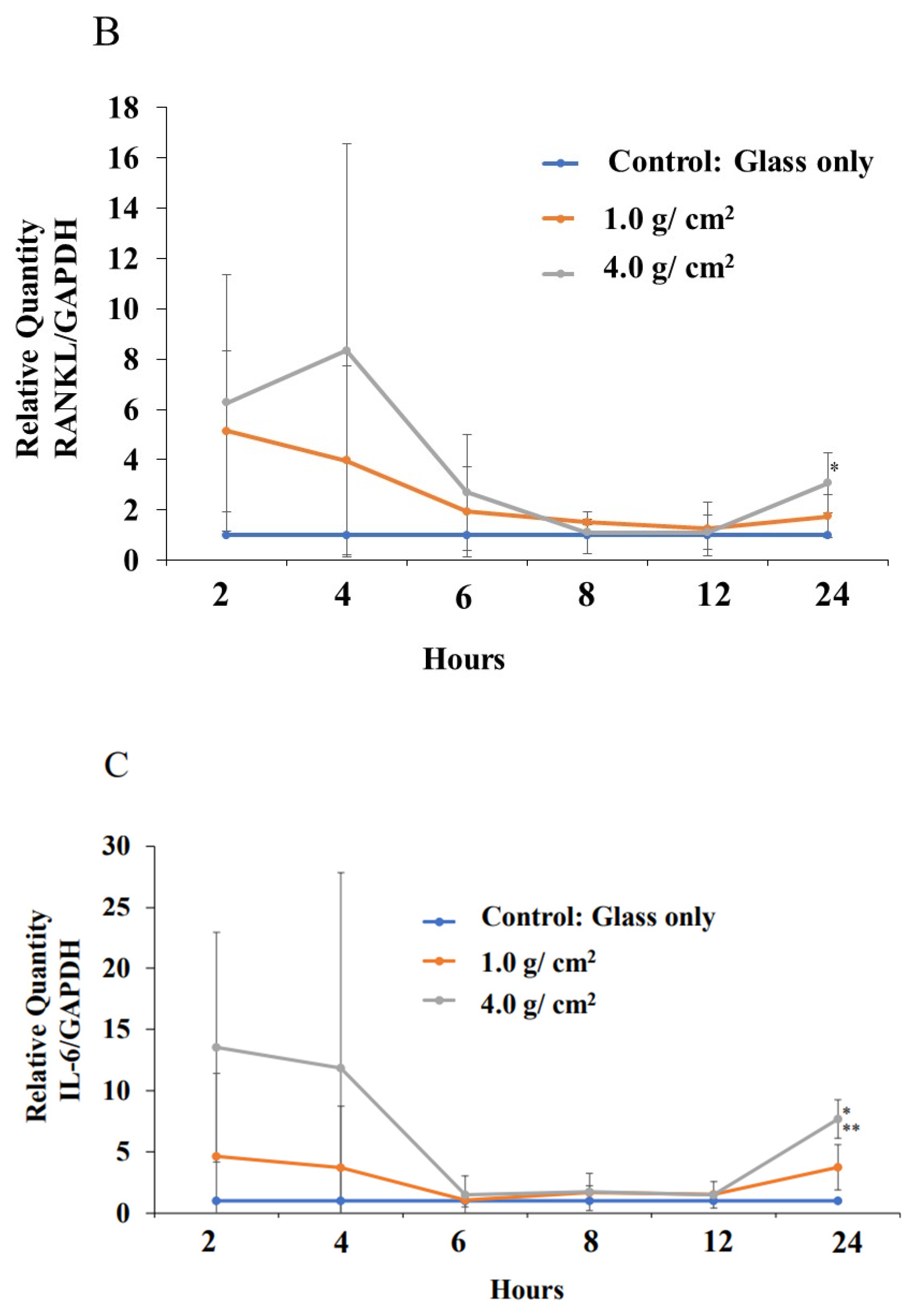
3.2. Effects of Orthodontic Force on the Expression of Shh, RANKL, and IL-6 from PDLF Cells
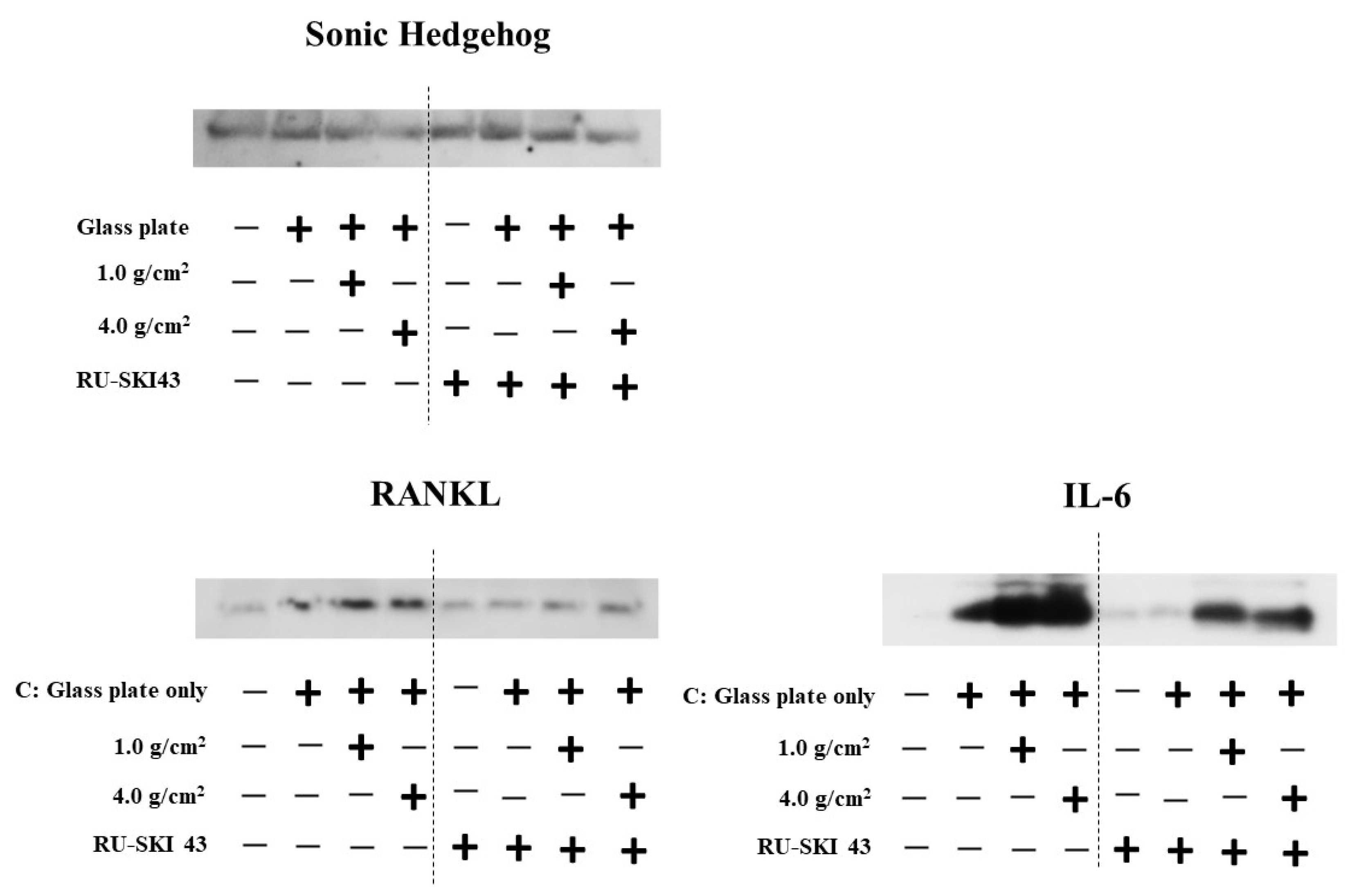
3.3. Effects of Shh on the Expression of RANKL and IL-6 from PDLF Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saffar, J.L.; Lasfargues, J.J.; Cherruau, M. Alveolar Bone and the Alveolar Process: The Socket that is Never Stable. Periodontol. 2000 1997, 13, 76–90. [Google Scholar] [CrossRef]
- Sodek, J.; McKee, M.D. Molecular and Cellular Biology of Alveolar Bone. Periodontol. 2000 2000, 24, 99–126. [Google Scholar] [CrossRef] [PubMed]
- Beertsen, W.; McCulloch, C.A.; Sodek, J. The Periodontal Ligament: A Unique, Multifunctional Connective Tissue. Periodontol. 2000 1997, 13, 20–40. [Google Scholar] [CrossRef]
- de Vries, T.J.; Schoenmaker, T.; Micha, D.; Hogervorst, J.; Bouskla, S.; Forouzanfar, T.; Pals, G.; Netelenbos, C.; Eekhoff, E.M.W.; Bravenboer, N. Periodontal Ligament Fibroblasts as a Cell Model to Study Osteogenesis and Osteoclastogenesis in Fibrodysplasia Ossificans Progressive. Bone 2018, 23, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Basdra, E.K.; Komposch, G. Osteoblast-like Properties of Human Periodontal Ligament Cells: An In Vitro Analysis. Eur. J. Orthod. 1997, 19, 615–621. [Google Scholar] [CrossRef]
- Arceo, N.; Sauk, J.J.; Moehring, J.; Foster, R.A.; Somerman, M.J. Human Periodontal Cells Initiate Mineral-like Nodules in vitro. J. Periodontol. 1991, 62, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Bloemen, V.; Schoenmaker, T.; de Vries, T.J.; Everts, V. Direct Cell-Cell Contact Between Periodontal Ligament Fibroblasts and Osteoclast Precursors Synergistically Increases the Expression of Genes Related to Osteoclastogenesis. J. Cell. Physiol. 2010, 222, 565–573. [Google Scholar] [CrossRef]
- Sringkarnboriboon, S.; Matsumoto, Y.; Soma, K. Root Resorption Related to Hypofunctional Periodontium in Experimental Tooth Movement. J. Dent. Res. 2003, 82, 486–490. [Google Scholar] [CrossRef]
- Hayashi, A.; Hayashi, H.; Kawata, T. Prevention of Root Resorption in Hypofunctional Teeth by Occlusal Function Recovery. Angle Orthod. 2016, 86, 214–220. [Google Scholar] [CrossRef]
- Kahane, N.; Kalcheim, C. From Bipotent Neuromesodermal Progenitors to Neural-Mesodermal Interactions During Embryonic Development. Int. J. Mol Sci. 2021, 22, 9141. [Google Scholar] [CrossRef]
- Ehlen, H.W.A.; Buelens, L.A.; Vortkamp, A. Hedgehog Signaling in Skeletal Development. Birth Defects Res. Part C Embryo Today 2006, 78, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Ayers, K.L.; Gallet, A.; Staccini-Lavenant, L.; Thérond, P.P. The Long-Range Activity of Hedgehog is Regulated in the Apical Extracellular Space by the Glypican Dally and the Hydrolase Notum. Dev. Cell 2010, 18, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.K.; Bi, Y.; Wan, C.; Chuang, P.-T.; Clemens, T.; Young, M.; Yang, Y. Hedgehog Signaling in Mature Osteoblasts Regulates Bone Formation and Resorption by Controlling PTHrP and RANKL expression. Dev. Cell 2008, 14, 674–688. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, Y.; Fu, Q.; Dong, Y. Osterix is Required for Sonic Hedgehog-Induced Osteoblastic MC3T3-E1 Cell Differentiation. Cell Biochem. Biophys. 2012, 64, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Kiuru, M.; Solomon, J.; Ghali, B.; van der Meulen, M.; Crystal, R.G.; Hidaka, C. Transient Overexpression of Sonic Hedgehog Alters the Architecture and Mechanical Properties of Trabecular Bone. J. Bone Miner. Res. 2009, 24, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, N.; Takahashi, N.; Katagiri, T.; Wada, S.; Findlay, D.M.; Martin, T.J.; Hirota, H.; Taga, T.; Kishimoto, T.; Suda, T. Interleukin (IL)-6 Induction of Osteoclast Differentiation Depends on IL-6 Receptors Expressed on Osteoblastic Cells but not on Osteoclast Progenitors. J. Exp. Med. 1995, 182, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, P.; Persson, E.; Conaway, H.H.; Lerner, U.H. IL-6, Leukemia Inhibitory Factor, and Oncostatin M Stimulate Bone Resorption and Regulate the Expression of Receptor Activator of NF-kappa B Ligand, Osteoprotegerin, and Receptor Activator of NF-kappa B in Mouse Calvariae. J. Immunol. 2002, 169, 3353–3362. [Google Scholar] [CrossRef] [PubMed]
- Wittrant, Y.; Théoleyre, S.; Chipoy, C.; Padrines, M.; Blanchard, F.; Heymann, D.; Rédini, F. RANKL/RANK/OPG: New Therapeutic Targets in Bone Tumours and Associated Osteolysis. Biochim. Biophys. Acta 2004, 1704, 49–57. [Google Scholar] [CrossRef]
- Shimo, T.; Matsumoto, K.; Takabatake, K.; Aoyama, E.; Takebe, Y.; Ibaragi, S.; Okui, T.; Kurio, K.; Takada, H.; Obata, K.; et al. The Role of Sonic Hedgehog Signaling in Osteoclastogenesis and Jaw Bone Destruction. PLoS ONE 2016, 11, e0151731. [Google Scholar] [CrossRef] [PubMed]
- Day, T.F.; Yang, Y. Wnt and hedgehog signaling pathways in bone development. J. Bone Jt. Surg. Am. 2008, 90 (Suppl. S1), S19–S24. [Google Scholar] [CrossRef]
- Cai, H.; Liu, A. Spop promotes skeletal development and homeostasis by positively regulating Ihh signaling. Proc. Natl. Acad. Sci. USA 2016, 113, 14751–14756. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Zhao, N.; Wang, L.; Yu, M.; Liu, H.; Song, A.; Huang, J.; Wang, G.; Yang, P. Bone Repair by Periodontal Ligament Stem Cell Seeded Nanohydroxyapatite-Chitosan Scaffold. Int. J. Nanomed. 2012, 7, 5405–5414. [Google Scholar] [CrossRef] [PubMed]
- Kunii, R.; Yamaguchi, M.; Tanimoto, Y.; Asano, M.; Yamada, K.; Goseki, T.; Kasai, T. Role of Interleukin-6 in Orthodontically Induced Inflammatory Root Resorption in Humans. Korean J. Orthod. 2013, 43, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Iwane, T.; Kikuta, J. Notch Signaling Response to Heavy Compression Force Induces Orthodontic Root Resorption via RNAKL and IL-6 from Cementoblasts. Int. J. Oral-Med. Sci. 2020, 18, 287–295. [Google Scholar] [CrossRef]
- Petrova, E.; Rios-Esteves, J.; Ouerfelli, O.; Glickman, J.F.; Resh, M.D. Inhibitors of Hedgehog Acyltransferase Block Sonic Hedgehog Signaling. Nat. Chem. Biol. 2013, 9, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Honselmann, K.C.; Pross, M.; Jung, C.M.F.; Wellner, U.F.; Deichmann, S.; Keck, T.; Bausch, D. Regulation Mechanisms of the Hedgehog Pathway in Pancreatic Cancer: A Review. J. Pancreas 2015, 16, 25–32. [Google Scholar]
- Ponchel, F.; Toomes, C.; Bransfield, K.; Leong, F.T.; Douglas, S.H.; Sarah, L.; Field, S.L.; Bell, S.M.; Combaret, V.; Puisieux, A.; et al. Real-time PCR Based on SYBR-Green I Fluorescence: An Alternative to the TaqMan Assay for a Relative Quantification of Gene Rearrangements, Gene Amplifications and Micro Gene Deletions. BMC Biotechnol. 2003, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, Q.; Huang, Y.; Li, X.; Zhu, Y.; Jia, L.; Zheng, Y.; Li, W. Mechanical Force Inhibited hPDLSCs Proliferation with the Downregulation of MIR31HG via DNA Methylation. Oral Dis. 2021, 27, 1268–1282. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.L.; Xian, C.Y.; Wang, Y.L. The mgf Expression of Osteoblasts in Response to Mechanical Overload. Arch. Oral Biol. 2006, 51, 1080–1085. [Google Scholar] [CrossRef]
- Marchesan, J.T.; Scanlon, C.S.; Soehren, S.; Matsuo, M.; Kapila, Y.L. Implications of Cultured Periodontal Ligament Cells for the Clinical and Experimental Setting: A Review. Arch. Oral Biol. 2011, 56, 933–943. [Google Scholar] [CrossRef]
- Li, M.; Zhang, C.; Yang, Y. Effects of Mechanical Forces on Osteogenesis and Osteoclastogenesis in Human Periodontal Ligament Fibroblasts. Bone Jt. Res. 2019, 8, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, N.; Suzuki, N.; Maeno, M.; Mayahara, K.; Yanagisawa, M.; Otsuka, K.; Shimizu, N. Optimal Compressive Force Induces Bone Formation via Increasing Bone Sialoprotein and Prostaglandin E2 Production Appropriately. Life Sci. 2005, 77, 3168–3182. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, N.; Suzuki, N.; Maeno, M.; Yanagisawa, M.; Koyama, Y.; Otsuka, K.; Shimizu, N. Optimal Compressive Force Induces Bone Formation via Increasing Bone Morphogenetic Proteins Production and Decreasing Their Antagonists Production by Saos-2 Cells. Life Sci. 2006, 78, 2697–2706. [Google Scholar] [CrossRef] [PubMed]
- Blawat, K.; Mayr, A.; Hardt, M.; Kirschneck, C.; Nokhbehsaim, M.; Behl, C.; Deschner, J.; Jäger, A.; Memmert, S. Regulation of Autophagic Signaling by Mechanical Loading and Inflammation in Human PDL Fibroblasts. Int. J. Mol. Sci. 2020, 21, 9446. [Google Scholar] [CrossRef] [PubMed]
- Kikuta, J.; Yamaguchi, M.; Shimizu, M.; Yoshino, T.; Kasai, K. Notch Signaling Induces Root Resorption via RANKL and IL-6 from hPDL Cells. J. Dent. Res. 2015, 94, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Schroder, A.; Bauer, K.; Apanier, G.; Proff, P.; Wolf, M.; Kirschneck, C. Expression Kinetics of Human Periodontal Ligament Fibroblasts in the Early Phases of Orthodontic Tooth Movement. J. Orofac. Orthop. 2018, 79, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Kim, J.Y.; Bhattarai, G.; So, H.S.; Kook, S.H.; Lee, J.C. Periodontal Ligament-Mimetic Fibrous Scaffolds Regulate YAP-Associated Fibroblast Behaviors and Promote Regeneration of Periodontal Defect in Relation to the Scaffold Topography. ACS Appl. Mater. Interfaces 2023, 15, 599–616. [Google Scholar] [CrossRef]
- Kaku, M.; Yamauchi, M. Mechano-regulation of Collagen Biosynthesis in Periodontal Ligament. J. Prosthodont. Res. 2014, 58, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Liang, M. Periodontal Ligament Stem Cells: Current Status, Concerns, and Future Prospects. Stem Cells Int. 2015, 2015, 972313. [Google Scholar] [CrossRef]
- Lim, S.-S.; Kook, S.-H.; Lee, J.-C. COMP-Ang1 Enhances DNA Synthesis and Cell Cycle Progression in Human Periodontal Ligament Cells via Tie2-Mediated Phosphorylation of PI3K/Akt and MAPKs. Mol. Cell. Biochem. 2016, 416, 157–168. [Google Scholar] [CrossRef]
- Nan, D.N.; Everts, V.; Ferreira, J.N.; Trachoo, V.; Osathanon, T.; Klincumhom, N.; Pavasant, P. Alteration of Extracellular Matrix Proteins in Atrophic Periodontal Ligament of Hypofunctional Rat Molars. BDJ Open 2023, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Andre, P.; Ye, L.; Yang, Y.-Z. The Hedgehog Signaling Pathway in Bone Formation. Int. J. Oral Sci. 2015, 7, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, Y.; Liao, Z.; Liu, Q.; Lei, X.; Li, M.; Zhang, S.; Hong, D.; Zhu, M.; Li, B.; et al. Genetic and Pharmacological Activation of Hedgehog Signaling Inhibits Osteoclastogenesis and Attenuates Titanium Particle-Induced Osteolysis Partly Through Suppressing the JNK/c-Fos-NFATc1 Cascade. Theranostics 2020, 10, 6638–6660. [Google Scholar] [CrossRef] [PubMed]
- Kawahira, N.; Ohtsuka, D.; Kida, N.; Ken-Ichi Hironaka, K.-I.; Morishita, Y. Quantitative Analysis of 3D Tissue Deformation Reveals Key Cellular Mechanism Associated with Initial Heart Looping. Cell Rep. 2020, 30, 3889–3903. [Google Scholar] [CrossRef] [PubMed]
- Morishita, Y.; Kuroiwa, A.; Suzuki, T. Quantitative Analysis of Tissue Deformation Dynamics Reveals Three Characteristic Growth Modes and Globally Aligned Anisotropic Tissue Deformation During Chick Limb Development. Development 2015, 142, 1672–1683. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, S.; Yeliz Boglev, Y.; Owens, H.; Goldie, S.J. The Role of Sonic Hedgehog in Craniofacial Patterning, Morphogenesis and Cranial Neural Crest Survival. J. Dev. Biol. 2016, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Morita, R.; Miho Kihira, M.; Nakatsu, Y.; Nomoto, Y.; Ogawa, M.; Ohashi, K.; Mizuno, K.; Tachikawa, T.; Ishimoto, Y.; Morishita, Y.; et al. Coordination of Cellular Dynamics Contributes to Tooth Epithelium Deformations. PLoS ONE 2016, 11, e0161336. [Google Scholar] [CrossRef] [PubMed]
- Corrigall, D.; Walther, R.F.; Rodriguez, L.; Pierre Fichelson, P.; Pichaud, F. Hedgehog Signaling is a Principal Inducer of Myosin-II-driven Cell Ingression in Drosophila Epithelia. Dev. Cell 2007, 13, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Polizio, A.H.; Chinchilla, P.; Chen, X.; Manning, D.R.; Riobo, N.A. Sonic Hedgehog Activates the GTPases Rac1 and RhoA in a Gli-Independent Manner Through Coupling of Smoothened to GI proteins. Sci. Signal 2011, 4, pt7. [Google Scholar]
- Katsumi, A.; Milanini, J.; Kiosses, W.B.; Miguel, A.; del Pozo, M.A.; Kaunas, R.; Chien, S.; Hahn, K.M.; Schwartz, M.A. Effects of Cell Tension on the Small GTPase Rac. J. Cell Biol. 2002, 158, 153–164. [Google Scholar] [CrossRef]
- Shyy, J.Y.; Chien, S. Role of integrins in cellular responses to mechanical stress and adhesion. Curr. Opin. Cell Biol. 1997, 9, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Lessey, E.C.; Guilluy, C.; Burridge, K. From Mechanical Force to RhoA Activation. Biochemistry 2012, 51, 7420–7432. [Google Scholar] [CrossRef] [PubMed]
- Khatiwala, C.B.; Kim, P.D.; Peyton, S.R.; Putnam, A.J. ECM Compliance Regulates Osteogenesis by Influencing MAPK Signaling Downstream of RhoA and ROCK. J. Bone Min. Res. 2009, 24, 886–898. [Google Scholar] [CrossRef]
- Hamamura, K.; Swarnkar, G.; Tanjung, N.; Cho, E.; Li, J.; Na, S.; Yokota, H. RhoA-mediated signaling in mechanotransduction of osteoblasts. Connect. Tissue Res. 2012, 53, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, C.; Zhang, J.; Bao, Y.; Tang, Y.; Lv, X.; Ma, B.; Wu, X.; Mao, G. RhoA promotes osteoclastogenesis and regulates bone remodeling through mTOR-NFATc1 signaling. Mol. Med. 2023, 29, 102. [Google Scholar] [CrossRef] [PubMed]
- Dutzmann, J.; Koch, A.; Weisheit, S.; Sonnenschein, K.; Korte, L.; Haertlé, M.; Thum, T.; Bauersachs, J.; Sedding, D.G.; Daniel, J.-M. Sonic Hedgehog-Dependent Activation of Adventitial Fibroblasts Promotes Neointima Formation. Cardiovasc. Res. 2017, 113, 1653–1663. [Google Scholar] [CrossRef]
- Su, P.H.; Huang, R.L.; Lai, H.C.; Chen, L.Y.; Weng, Y.C.; Wang, C.C.; Wu, C.C. NKX6-1 Mediates Cancer Stem-Like Properties and Regulates Sonic Hedgehog Signaling in Leiomyosarcoma. J. Biomed. Sci. 2021, 28, 32. [Google Scholar] [CrossRef]




| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Shh | GGACAGGCTGATGACTCAGA | GCCCTCGTAGTGCAGAGACT |
| RANKL | GCCTTTCAAGGAGCTGTGCAAAA | GAGCAAAAGGCTGAGCTTCAAGC |
| IL-6 | AGACAGCCACTCACCTCTTCAG | TTCTGCCAGTGCCTCTTTGC |
| GAPDH | GTCAGTGGTGGACCTGACCT | TGCTGTAGCCAAATTCGTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, E.; Negishi, S.; Kikuta, J.; Shimizu, M.; Senpuku, H. Effects of Improper Mechanical Force on the Production of Sonic Hedgehog, RANKL, and IL-6 in Human Periodontal Ligament Cells In Vitro. Dent. J. 2024, 12, 108. https://doi.org/10.3390/dj12040108
Yamashita E, Negishi S, Kikuta J, Shimizu M, Senpuku H. Effects of Improper Mechanical Force on the Production of Sonic Hedgehog, RANKL, and IL-6 in Human Periodontal Ligament Cells In Vitro. Dentistry Journal. 2024; 12(4):108. https://doi.org/10.3390/dj12040108
Chicago/Turabian StyleYamashita, Erika, Shinichi Negishi, Jun Kikuta, Mami Shimizu, and Hidenobu Senpuku. 2024. "Effects of Improper Mechanical Force on the Production of Sonic Hedgehog, RANKL, and IL-6 in Human Periodontal Ligament Cells In Vitro" Dentistry Journal 12, no. 4: 108. https://doi.org/10.3390/dj12040108
APA StyleYamashita, E., Negishi, S., Kikuta, J., Shimizu, M., & Senpuku, H. (2024). Effects of Improper Mechanical Force on the Production of Sonic Hedgehog, RANKL, and IL-6 in Human Periodontal Ligament Cells In Vitro. Dentistry Journal, 12(4), 108. https://doi.org/10.3390/dj12040108







