Manifestations and Treatment of Hypovitaminosis in Oral Diseases: A Systematic Review
Abstract
:1. Introduction
1.1. Vitamin A
1.2. Vitamin B
1.3. Vitamin C
1.4. Vitamin D
1.5. Vitamin E
1.6. Vitamin K
2. Materials and Methods
2.1. Protocol and Registration
2.2. Literature Search Strategy
- -
- Vitamin A deficiency and oral disease;
- -
- Vitamin B deficiency and oral disease;
- -
- Vitamin C deficiency and oral disease;
- -
- Vitamin D deficiency and oral disease;
- -
- Vitamin E deficiency and oral disease;
- -
- Vitamin K deficiency and oral disease;
- -
- Vitamin deficiency and oral disease.
2.3. Eligibility Criteria
- -
- Articles published in English;
- -
- Research published between 2017 and 2023;
- -
- Original studies;
- -
- Studies conducted on human subjects.
- -
- Articles not written in English;
- -
- Papers published before 2017;
- -
- Case reports;
- -
- Studies conducted on animals.
2.4. Selection Process
2.5. Data Collection Process
2.6. Data Items
2.7. Risk of Bias Assessment
2.8. Effect Measures
2.9. Compliance with Ethical Guidelines
3. Results
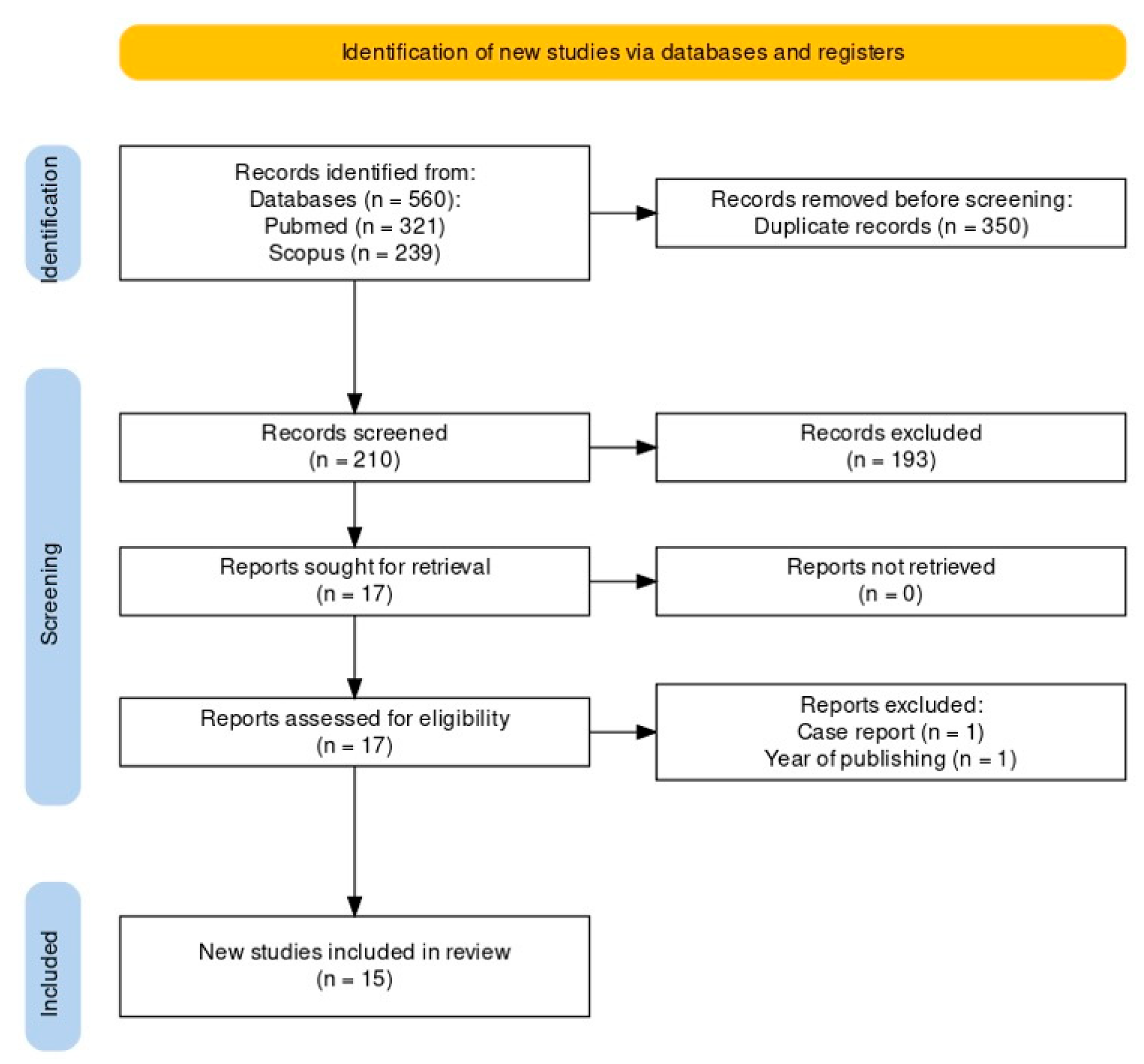
3.1. Study Selection
3.2. Risk-of-Bias Assessment
3.3. Nature of Included Studies
3.4. Diseases of the Oral Mucosa
3.5. Dental Caries
3.6. Gingival and Periodontal Disease
3.7. Malformations
3.8. Oral Carcinoma
3.9. Perioperative Bleeding
3.10. Burning Mouth Syndrome
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gutierrez Gossweiler, A.; Martinez-Mier, E.A. Chapter 6: Vitamins and Oral Health. Monogr. Oral Sci. 2019, 28, 59–67. [Google Scholar]
- Tolkachjov, S.N.; Bruce, A.J. Oral Manifestations of Nutritional Disorders. Clin. Dermatol. 2017, 35, 441–452. [Google Scholar] [CrossRef]
- Bolat, M.; Trandafir, L.; Ciubara, A.; Diaconescu, S. Oral manifestations of nuritional diseases in children. Rom. J. Oral Rehabil. 2016, 8, 56–60. [Google Scholar]
- Zinder, R.; Cooley, R.; Vlad, L.G.; Molnar, J.A. Vitamin A and Wound Healing. Nutr. Clin. Pract. 2019, 34, 839–849. [Google Scholar] [CrossRef]
- Wan, Z.; Zheng, J.; Zhu, Z.; Sang, L.; Zhu, J.; Luo, S.; Zhao, Y.; Wang, R.; Zhang, Y.; Hao, K.; et al. Intermediate Role of Gut Microbiota in Vitamin B Nutrition and Its Influences on Human Health. Front. Nutr. 2022, 9, 1031502. [Google Scholar] [CrossRef]
- Dreizen, S. Oral Indications of the Deficiency States. Postgrad. Med. 2016, 49, 97–102. [Google Scholar] [CrossRef]
- Freitas, J.; Bliven, P.; Case, R. Combined Zinc and Vitamin B6 Deficiency in a Patient with Diffuse Red Rash and Angular Cheilitis 6 Years after Roux-En-Y Gastric Bypass. BMJ Case Rep. 2019, 12, e230605. [Google Scholar] [CrossRef]
- Gondivkar, S.M.; Gadbail, A.R.; Gondivkar, R.S.; Sarode, S.C.; Sarode, G.S.; Patil, S.; Awan, K.H. Nutrition and Oral Health. Dis. Mon. 2019, 65, 147–154. [Google Scholar] [CrossRef]
- Hrubša, M.; Siatka, T.; Nejmanová, I.; Vopršalová, M.; Krčmová, L.K.; Matoušová, K.; Javorská, L.; Macáková, K.; Mercolini, L.; Remião, F.; et al. Biological Properties of Vitamins of the B-Complex, Part 1: Vitamins B1, B2, B3, and B5. Nutrients 2022, 14, 484. [Google Scholar] [CrossRef] [PubMed]
- Hugar, S.M.; Dhariwal, N.S.; Badakar, C.; Gokhale, N.; Mistry, L. Assessment of Vitamin B12 and Its Correlation with Dental Caries and Gingival Diseases in 10- to 14-Year-Old Children: A Cross-Sectional Study. Int. J. Clin. Pediatr. Dent. 2017, 10, 142–146. [Google Scholar]
- Stoopler, E.T.; Kuperstein, A.S. Glossitis Secondary to Vitamin B12 Deficiency Anemia. Can. Med. Assoc. J. 2013, 185, 582. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Munday, M.R.; Rodricks, R.; Fitzpatrick, M.; Flood, V.M.; Gunton, J.E. A Pilot Study Examining Vitamin C Levels in Periodontal Patients. Nutrients 2020, 12, 2255. [Google Scholar] [CrossRef] [PubMed]
- Murererehe, J.; Uwitonze, A.M.; Nikuze, P.; Patel, J.; Razzaque, M.S. Beneficial Effects of Vitamin C in Maintaining Optimal Oral Health. Front. Nutr. 2022, 8, 805809. [Google Scholar] [CrossRef] [PubMed]
- Van der Velden, U. Vitamin C and Its Role in Periodontal Diseases—The Past and the Present: A Narrative Review. Oral Health Prev. Dent. 2020, 18, 115–124. [Google Scholar]
- Botelho, J.; Machado, V.; Proença, L.; Delgado, A.S.; Mendes, J.J. Vitamin D Deficiency and Oral Health: A Comprehensive Review. Nutrients 2020, 12, 1471. [Google Scholar] [CrossRef] [PubMed]
- Diachkova, E.; Trifonova, D.; Morozova, E.; Runova, G.; Ashurko, I.; Ibadulaeva, M.; Fadeev, V.; Tarasenko, S. Vitamin d and Its Role in Oral Diseases Development. Scoping Rev. Dent. J. 2021, 9, 129. [Google Scholar]
- Traber, M.G.; Head, B. Vitamin E: How Much Is Enough, Too Much and Why! Free Radic. Biol. Med. 2021, 177, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Oghogho Omage, S.; Börmel, L.; Kluge, S.; Schubert, M.; Waller, M.; Lorkowski, S. Vitamin E and Metabolic Health: Relevance of Interactions with Other Micronutrients. Antioxidants 2022, 11, 1785. [Google Scholar] [CrossRef]
- Najeeb, S.N.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Almas, K. The Role of Nutrition in Periodontal Health: An Update. Nutrients 2016, 8, 530. [Google Scholar] [CrossRef]
- Mujer, M.T.P.; Rai, M.P.; Atti, V.; Dimaandal, I.L.; Chan, A.S.; Shrotriya, S.; Gundabolu, K.; Dhakal, P. An Update on the Reversal of Non-Vitamin K Antagonist Oral Anticoagulants. Adv. Hematol. 2020, 2020, 7636104. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Wells, G.; Shea, B.; Shea, B.; O’Connell, D.; Peterson, J.; Welch Losos, M.; Tugwell, P.; Ga, S.W.; Zello, G.A.; et al. The New Castle-Otawa Scale (NOS) for Assessing the Quality of Non Randomized Studies in Meta Analysis. Medicine 2014. [Google Scholar]
- Cui, J.; Lin, W.; Ma, X.; Wang, J. Clinical Evaluation and Therapeutic Effects of Combination Treatment with Mecobalamin + Vitamin E in Recurrent Oral Ulcer. Clin. Ther. 2022, 44, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Woelber, J.P.; Gärtner, M.; Breuninger, L.; Anderson, A.; König, D.; Hellwig, E.; Al-Ahmad, A.; Vach, K.; Dötsch, A.; Ratka-Krüger, P.; et al. The Influence of an Anti-Inflammatory Diet on Gingivitis. A Randomized Controlled Trial. J. Clin. Periodontol. 2019, 46, 481–490. [Google Scholar] [CrossRef]
- Yuce, H.B.; Gokturk, O.; Turkal, H.A.; Inanir, A.; Benli, I.; Demir, O. Assessment of Local and Systemic 25-Hydroxy-Vitamin D, RANKL, OPG, and TNF Levels in Patients with Rheumatoid Arthritis and Periodontitis. J. Oral Sci. 2017, 59, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Jankovskis, V.; Selga, G. Medicina Vitamin B and Zinc Supplements and Capsaicin Oral Rinse Treatment Options for Burning Mouth Syndrome. Medicina 2021, 57, 391. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.X.; Yang, X.W.; Fang, D.D. Lingual Linear Lesions: A Clinical Sign Strongly Suggestive of Severe Vitamin B12 Deficiency. Nutr. Clin. Pract. 2021, 36, 1041–1048. [Google Scholar] [CrossRef]
- Khayamzadeh, M.; Najafi, S.; Sadrolodabaei, P.; Vakili, F.; Kharrazi Fard, M.J. Determining Salivary and Serum Levels of Iron, Zinc and Vitamin B12 in Patients with Geographic Tongue. J. Dent. Res. Dent. Clin. Dent. Prospect. 2019, 13, 221–226. [Google Scholar] [CrossRef]
- Borsting, T.; Schuller, A.; van Dommelen, P.; Stafne, N.; Skeie, M.S.; Skaare, A.B.; Morkved, S.; Salvesen, K.A.; Stunes, A.K.; Mosti, M.P.; et al. Maternal Vitamin D Status in Pregnancy and Molar Incisor Hypomineralisation and Hypomineralised Second Primary Molars in the Offspring at 7–9 Years of Age: A Longitudinal Study. Eur. Arch. Paediatr. Dent. 2022, 23, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, J.S.; Rademacher, W.M.H.; Hazendonk, H.C.A.M.; Van Diermen, D.E.; Leebeek, F.W.G.; Rozema, F.R.; Kruip, M.J.H.A. Predictors of Oral Cavity Bleeding and Clinical Outcome after Dental Procedures in Patients on Vitamin K Antagonists: A Cohort Study. Thromb. Haemost. 2017, 117, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Chang, J.Y.F.; Wang, Y.P.; Wu, Y.C.; Chen, H.M.; Sun, A. Hemoglobin, Iron, Vitamin B12, and Folic Acid Deficiencies and Hyperhomocysteinemia in Behcet’s Disease Patients with Atrophic Glossitis. J. Formos. Med. Assoc. 2018, 117, 559–565. [Google Scholar] [CrossRef]
- Bahramian, A.; Falsafi, P.; Abbasi, T.; Ghanizadeh, M.; Abedini, M.; Kavoosi, F.; Kouhsoltani, M.; Noorbakhsh, F.; Dabbaghi Tabriz, F.; Rajaeih, S.; et al. Comparing Serum and Salivary Levels of Vitamin D in Patients with Recurrent Aphthous Stomatitis and Healthy Individuals. J. Dent. Shiraz Univ. Med. Sci. 2018, 19, 295–300. [Google Scholar]
- Kim, I.J.; Lee, H.S.; Ju, H.J.; Na, J.Y.; Oh, H.W. A Cross-Sectional Study on the Association between Vitamin D Levels and Caries in the Permanent Dentition of Korean Children. BMC Oral Health 2018, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Gyll, J.; Ridell, K.; Öhlund, I.; Karlsland Åkeson, P.; Johansson, I.; Lif Holgerson, P. Vitamin D Status and Dental Caries in Healthy Swedish Children. Nutr. J. 2018, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Nuszkiewicz, J.; Czuczejko, J.; Maruszak, M.; Pawłowska, M.; Woźniak, A.; Małkowski, B.; Szewczyk-Golec, K. Parameters of Oxidative Stress, Vitamin D, Osteopontin, and Melatonin in Patients with Lip, Oral Cavity, and Pharyngeal Cancer. Oxid. Med. Cell Longev. 2021, 2021, 2364931. [Google Scholar] [CrossRef] [PubMed]
- Rigassio Radler, D.; Lister, T. Nutrient Deficiencies Associated with Nutrition-focused Physical Findings of the Oral Cavity. Nutr. Clin. Pract. 2013, 28, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Shipton, M.J.; Thachil, J. Vitamin B 12 Deficiency—A 21st Century Perspective. Clin. Med. 2015, 15, 145. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D Supplementation Guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef]
- Singh, N.; Chander Narula, S.; Kumar Sharma, R.; Tewari, S.; Kumar Sehgal, P. Vitamin E Supplementation, Superoxide Dismutase Status, and Outcome of Scaling and Root Planing in Patients With Chronic Periodontitis: A Randomized Clinical Trial. J. Periodontol. 2014, 85, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, T. Malnutrition and Diet Role in Prevention of Oral Disease. EC Dent. Sci. 2019, 18, 2206–2213. [Google Scholar]
| Patient and population (P) | Adults |
| Intervention (I) | Increase in vitamin intake |
| Comparator or control group | Typical intake of vitamins |
| Outcomes (O) | Relief from hypovitaminosis symptoms |
| Patient and population (P) | Adults |
| Intervention (I) | Vitamin treatment of oral diseases |
| Comparator or control group | Typical intake of vitamins |
| Outcomes (O) | Regression of oral diseases |
| Study | Randomization Process | Deviations from Intended Interventions | Missing Outcome Data | Measurement of Outcome Data | Selection of the Reported Result | Overall Risk-of-Bias Judgment |
|---|---|---|---|---|---|---|
| Cui, J. et al. [25] | Some concerns | Some concerns | Low risk | Some concerns | Some concerns | Some concerns |
| Woelber, J.P. et al. [26] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Balci Yuce, H. et al. [27,28] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Jankovskis, V. et al. [29] | Low risk | Low risk | Low risk | Low risk | Some concerns | Low risk |
| Study | Bias due to Confounding | Bias in Selection of Participants for the Study | Bias in Classification of Interventions | Bias due to Deviations from Intended Interventions | Bias due to Missing Data | Bias in Measurement of Outcomes | Bias in Selection of the Reported Result | Overall Risk-of-Bias Judgment |
|---|---|---|---|---|---|---|---|---|
| Bao, Z. et al. [29] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Khayamzadeh, M. et al. [30] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Borsting, T. et al. [31] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Biedermann, J.S. et al. and Wu, Y.H. et al. [32,33] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Study | Selection | Comparability | Outcome | Overall |
|---|---|---|---|---|
| Wu, Y.-H. et al. [33] | 5 | 1 | 3 | 9 (High Quality) |
| Bahramian, A. et al. [34] | 4 | 1 | 3 | 8 (High Quality) |
| Hugar, S. et al. [13] | 3 | 1 | 3 | 7 (High Quality) |
| Kim, I.-J. et al. [35] | 5 | 1 | 2 | 8 (High Quality) |
| Gyll, J. et al. [36] | 5 | 1 | 3 | 9 (High Quality) |
| Munday, M.-R. et al. [13] | 5 | 1 | 2 | 8 (High Quality) |
| Nuszkiewicz, J. et al. [37] | 4 | 1 | 2 | 7 (High Quality) |
| Author | Title | Year | Type of Study | Oral Manifestation/s | Number of Participants | Age Range of Participants | Intervention/Exposure | Comparator/Control | Vitamin | Outcomes of Interest | Main Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wu, Y.-H. et al. [33] | Hemoglobin, iron, vitamin B12, and folic acid deficiencies and hyperhomocysteinemia in Behcet’s disease patients with atrophic glossitis | 2018 | Cross-sectional study | Recurrent aphthous stomatitis (RAS), atrophic glossitis (AG) | 30 AG + RAS/BD patients, 33 AG־RAS/BD patients, and 126 healthy control subjects | 18–82 | Measurement of blood hemoglobin, iron, vitamin B12, folic acid, and homocysteine concentrations | Healthy control subjects | B9, B12 | Hemoglobin, iron, vitamin B12, and folic acid deficiencies | AG + RAS/BD patients had significantly higher frequencies of hemoglobin, iron, vitamin B12, and folic acid deficiencies than healthy control subjects. They also had significantly higher frequencies of hemoglobin and vitamin B12 deficiencies than AG־RAS/BD patients. AG־RAS/BD patients had significantly higher frequencies of hemoglobin and iron deficiencies than healthy control subjects |
| Bao, Z. et al. [29] | Lingual Linear Lesions: A Clinical Sign Strongly Suggestive of Severe Vitamin B12 Deficiency | 2020 | Retrospective study | Lingual linear lesion (LLL) | 57 patients presenting with lingual linear lesions (LLLs) | 18–80 | Measurement of serum levels of vitamin B12, folate, ferritin, hemoglobin, and zinc | / | B12 | Serum levels of vitamin B12, folate, ferritin, hemoglobin, and zinc | 98.25% of patients had severe serum vitamin B12 deficiency. LLLs were associated with significantly lower mean levels of vitamin B12 and significantly higher mean MCV, rapid improvement after replacement therapy |
| Khayamzadeh, M. et al. [30] | Determining salivary and serum levels of iron, zinc and vitamin B12 in patients with geographic tongue | 2019 | Case–control study | Geographic tongue | 20 patients with geographic tongue, 20 healthy individuals | 19–49 | Evaluation of iron, zinc, and vitamin B12 levels in blood and saliva | Healthy subjects without geographic tongue | B12 | Salivary and serum levels of iron, zinc, and vitamin B12 | Patients with geographic tongue had lower salivary zinc levels compared to the control group. No significant differences were found in serum levels of iron, zinc, and vitamin B12 |
| Cui, J. et al. [25] | Clinical Evaluation and Therapeutic Effects of Combination Treatment withMecobalamin + Vitamin E in Recurrent Oral Ulcer | 2022 | Randomized controlled trial | Recurrent oral ulcer | 58 patients with aphthous oral ulcers (29 in the experimental group, 29 in the control group) | 45–50 | Mecobalamin tablets + vitamin E | Fe complex enzyme gargle | B12 | Pain level (VAS score), ulcer status and number, proinflammatory cytokine levels, quality of life (SF-36), mean intermission time, mean ulcer healing time, and total effectiveness rate | Longer total intermission time, shorter mean ulcer healing time, fewer total ulcers, lower pain scores in test group, improved QoL in test group, and lower proinflammatory cytokine levels in test group |
| Hugar, S. et al. [10] | Assessment of Vitamin B12 and Its Correlation with Dental Caries and Gingival Diseases in 10- to 14-year-old Children: A Cross-sectional Study | 2017 | Cross-sectional study | Dental caries and gingival disease | 42 healthy children (21 boys, 21 girls) | 10–14 | Serum vitamin B12 levels | / | B12, E | Dental caries (DMFT score) and gingival diseases (PI, GI scores) | 64% of children had vitamin B12 deficiency, higher DMFT scores in deficient children, negative correlation between vitamin B12 levels and dental caries, and gingival indices |
| Jankovskis, V. et al. [28] | Vitamin B and Zinc Supplements and Capsaicin Oral Rinse Treatment Options for Burning Mouth Syndrome | 2021 | Randomized controlled study | Burning mouth syndrome (BMS) | 89 patients (BMS), 20 patients (capsaicin rinse) | Not provided Mean age: 59 ± 19 years | Vitamin B complex and zinc supplements; 0.02% topical capsaicin rinse | / | B12 | Pain/burning levels (assessed via visual analog scale) and salivary flow | Both treatment methods showed statistically significant reductions in pain/burning levels and no significant changes in salivary flow |
| Munday, M.-R. et al. [13] | A Pilot Study Examining Vitamin C Levels in Periodontal Patients | 2020 | Cross-sectional study | Periodontal disease | 20 patients with periodontitis | Not provided Mean age: 65 ± 9 | Serum vitamin C and C-reactive protein (CRP) | / | C | Serum vitamin C levels, C-reactive protein (CRP) levels, periodontal disease stage, and dietary intake | 6 out of 20 patients had vitamin C levels below the institutional normal range, low vitamin C was associated with higher periodontal disease stage, elevated CRP was found in 2/3 of individuals with low vitamin C, with a significant negative correlation between vitamin C and CRP, and vitamin C levels did not correlate with patient-reported fruit or vegetable consumption but were associated with high processed meat intake |
| Woelber, J. P. et al. [26] | The influence of an anti-inflammatory diet on gingivitis. A randomized controlled trial | 2019 | Randomized controlled trial | Gingivitis | 30 participants (15 in the experimental group, 15 in the control group) | Not provided Mean age of experimental group: 27,3 ± 4,7 years Mean age of control group: 33,7 ± 1 | Diet low in processed carbohydrates and animal proteins, rich in omega-3 fatty acids, vitamin C, vitamin D, antioxidants, plant nitrates, and fibers for 4 weeks. Both groups suspended inter-dental cleaning | No change in diet. Both groups suspended inter-dental cleaning | C, D | Gingival bleeding (GI), plaque index (PI), probing pocket depths, bleeding on pocket probing, periodontal inflamed surface areas (PISA), body mass index, weight, and subgingival microbiome composition | Experimental group showed significant reduction in gingival bleeding (GI), significant increase in vitamin D values, and significant weight loss. No significant differences in plaque values between groups. No significant differences in inflammatory serological parameters or subgingival microbiome composition between groups |
| Bahramian, A. et al. [34] | Comparing Serum and Salivary Levels of Vitamin D in Patients with Recurrent Aphthous Stomatitis and Healthy Individuals | 2018 | Cross-sectional study | Recurrent aphthous stomatitis (RAS) | 26 RAS patients, 26 healthy individuals | 18–60 | Evaluation of serum and salivary vitamin D levels | Healthy Individuals | D | Serum and salivary levels of vitamin D | Serum vitamin D levels were significantly lower in patients with RAS compared to healthy individuals. Salivary vitamin D levels did not show a significant difference between patients with RAS and healthy individuals. A significant positive correlation was observed between serum and salivary levels of vitamin D in all participants |
| Kim, I.-J. et al. [35] | A cross-sectional study on the association between vitamin D levels and caries in the permanent dentition of Korean children | 2018 | Cross-sectional study | Dental caries | 1688 children | 10–12 | Blood vitamin D [25(OH)D] concentrations | / | D | Association between 25(OH)D levels and dental caries | The group with 25(OH)D levels < 50 nmol/L had higher proportion of caries in permanent dentition and first molar, no significant correlation between 25(OH)D levels and caries when controlling for external factors, but significant correlation with first molar caries. Children with 25(OH)D levels < 50 nmol/L 1.295 times more likely to have first molar caries and negative correlation between 25(OH)D levels and DMFT |
| Gyll, J. et al. [36] | Vitamin D status and dental caries in healthy Swedish children | 2018 | Cross-sectional study | Dental caries | 85 children | 8 | Vitamin D supplementation | / | D | Dental caries, enamel defects, saliva LL37 levels | Weak inverse association between vitamin D status at 6 years and caries 2 years later. Higher vitamin D levels correlated with being caries-free. Vitamin D status unrelated to enamel defects but positively associated with saliva LL37 levels |
| Balci Yuce, H. et al. [27] | Assessment of local and systemic 25-hydroxy-vitamin D, RANKL, OPG, and TNF levels in patients with rheumatoid arthritis and periodontitis | 2017 | Randomized controlled trial | Periodontal disease | 17 RA + CP patients, 18 CP patients, 18 healthy controls | RA + CP patient: 37–61 CP patients: 36–65. Control group: 36–65 | Nonsurgical periodontal treatment | Healthy individuals | D | Clinical periodontal parameters, GCF, and serum levels of vitamin D | GCF vitamin D levels were higher in RA +CP and CP groups than in healthy controls but decreased in the RA + CP group after periodontal treatment. Local vitamin D levels might be an important indicator of periodontal bone loss |
| Borsting, T. et al. [31] | Maternal vitamin D status in pregnancy and molar incisor hypomineralisation and hypomineralised second primary molars in the offspring at 7–9 years of age: a longitudinal study | 2022 | Longitudinal study | Molar incisor hypomineralization (MIH) and hypomineralized second primary molars (HSPM) | 176 mother and child pairs | 7–9 | Maternal serum 25-hydroxyvitamin D levels | / | D | Molar incisor hypomineralization (MIH) and hypomineralized second primary molars (HSPM) | Insufficient maternal serum vitamin D at mid-pregnancy was associated with a higher number of affected teeth among the offspring with MIH at 7–9 years of age. Further prospective studies are needed to investigate whether this finding is replicable and to clarify the role of maternal vitamin D status during pregnancy and MIH, as well as HSPM, in children |
| Nuszkiewicz, J. et al. [37] | Parameters of Oxidative Stress, Vitamin D, Osteopontin, and Melatonin in Patients with Lip, Oral Cavity, and Pharyngeal Cancer | 2021 | Cross-sectional study | Lip, oral cavity, or pharyngeal cancer in situ | 25 LOCP patients (YCG), 20 LOCP elderly patients (OCG), 25 healthy volunteers | Not provided Mean age of YCG: 58:24 ± 1:29. Mean age of OCG: 69:7 ± 1:49. Mean age of control group: 55:36 ± 1:17 | Concentrations of vitamin D, osteopontin, melatonin, and malondialdehyde; activities of antioxidant enzymes | Healthy volunteers | D | Vitamin D deficiency and disturbed oxidant–antioxidant homeostasis observed in LOCP patients; association of osteopontin with LOCP carcinogenesis | Disruption of oxidant–antioxidant homeostasis in the lip, oral cavity, and pharyngeal cancer patients impaired antioxidant enzymatic defense, and increased lipid peroxidation, correlated with high levels of osteopontin, was determined in this type of cancer. However, vitamin D deficiency in the LOCP patients was found |
| Biedermann, J.S. et al. [32] | Predictors of oral cavity bleeding and clinical outcome after dental procedures in patients on vitamin K antagonists | 2017 | Cohort study | Periprocedural bleeding | 1845 patients | 64–81 | VKA management strategies (continuation with tranexamic acid mouthwash, interruption with or without bridging) | / | K | Clinically relevant oral cavity bleeding, non-oral cavity bleeding, thromboembolic events, hospitalization, all-cause mortality within 30 days | Bridging therapy, antiplatelet therapy, and a supratherapeutic or unobjectified INR before the procedure were identified as strongest predictors of oral cavity bleeding. Different management strategies showed varying risks of oral cavity bleeding |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bačun, B.; Galić, D.; Pul, L.; Tomas, M.; Kuiš, D. Manifestations and Treatment of Hypovitaminosis in Oral Diseases: A Systematic Review. Dent. J. 2024, 12, 152. https://doi.org/10.3390/dj12060152
Bačun B, Galić D, Pul L, Tomas M, Kuiš D. Manifestations and Treatment of Hypovitaminosis in Oral Diseases: A Systematic Review. Dentistry Journal. 2024; 12(6):152. https://doi.org/10.3390/dj12060152
Chicago/Turabian StyleBačun, Barbara, Dora Galić, Luka Pul, Matej Tomas, and Davor Kuiš. 2024. "Manifestations and Treatment of Hypovitaminosis in Oral Diseases: A Systematic Review" Dentistry Journal 12, no. 6: 152. https://doi.org/10.3390/dj12060152





