Effects of Cavitation Jet Treatment on the Structure and Emulsification Properties of Oxidized Soy Protein Isolate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Protein Oxidation
2.3. Cavitation Jet Treatment of Oxidized SPI
2.4. Determination of Carbonyl Content
2.5. Characterization of Particle Size Distribution
2.6. Evaluation of Emulsifying Properties
2.6.1. Preparation of Protein-Stabilized Emulsions
2.6.2. Determination of Mean Droplet Size and ζ-Potential of the Emulsion
2.6.3. Emulsification Activity Index and Emulsification Stability Index
2.7. Determination of Sulfhydryl Groups (SH) and Disulfide Groups (S–S)
2.8. Measurement of Surface Hydrophobicity (H0)
2.9. Intrinsic Fluorescence Emission Spectroscopy
2.10. Circular Dichroism Spectra Measurement
2.11. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis
2.12. Atomic Force Microscope
2.13. Statistical Analyses
3. Results and Discussion
3.1. Effect of Cavitation Jet Treatment on the Carbonyl Content of Oxidized SPI
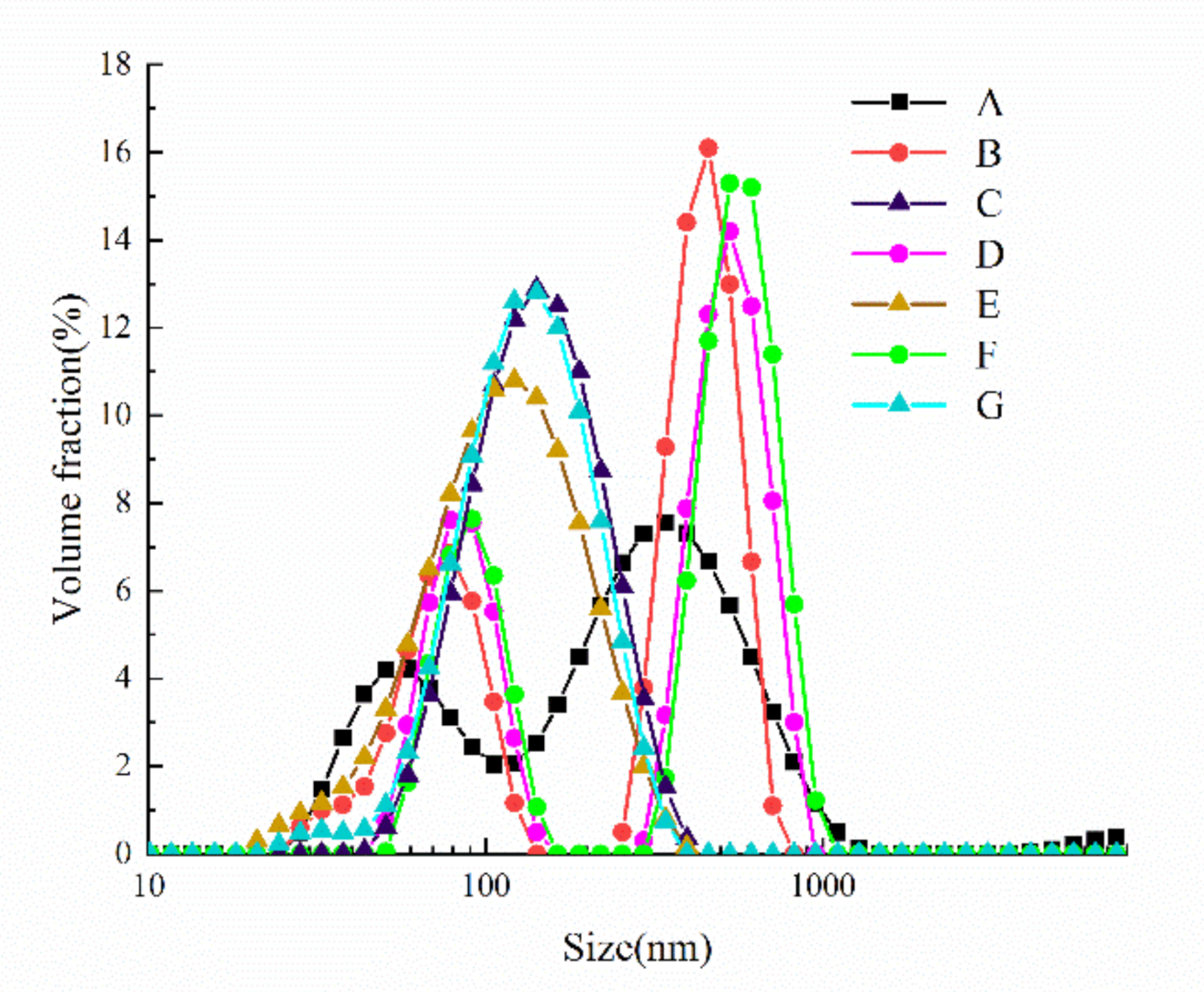
3.2. Effect of Cavitation Jet Treatment on the Particle Size Distribution of Oxidized SPI
3.3. Effect of Cavitation Jet Treatment on Hydrophobic Properties of Oxidized SPI
3.3.1. SH and S-S Bond Contents
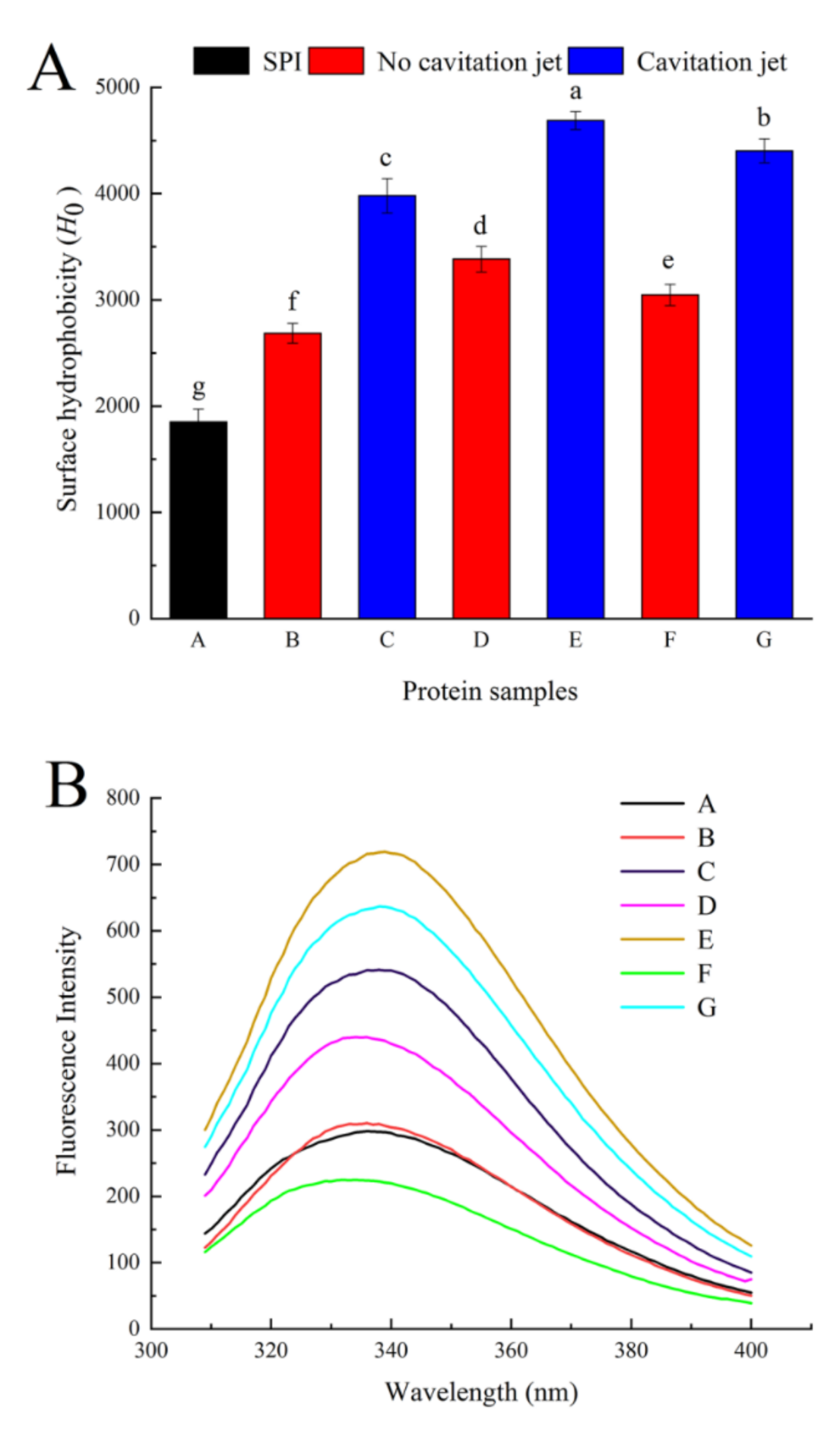
3.3.2. Surface Hydrophobicity (H0)
3.3.3. Intrinsic Fluorescence Emission Spectra
3.4. Effect of Cavitation Jet Treatment on the Emulsifying Properties of Oxidized SPI
3.4.1. Emulsifying Capabilities
3.4.2. Emulsification Stability
3.5. Effect of Cavitation Jet Treatment on the CD Spectrum of Oxidized SPI
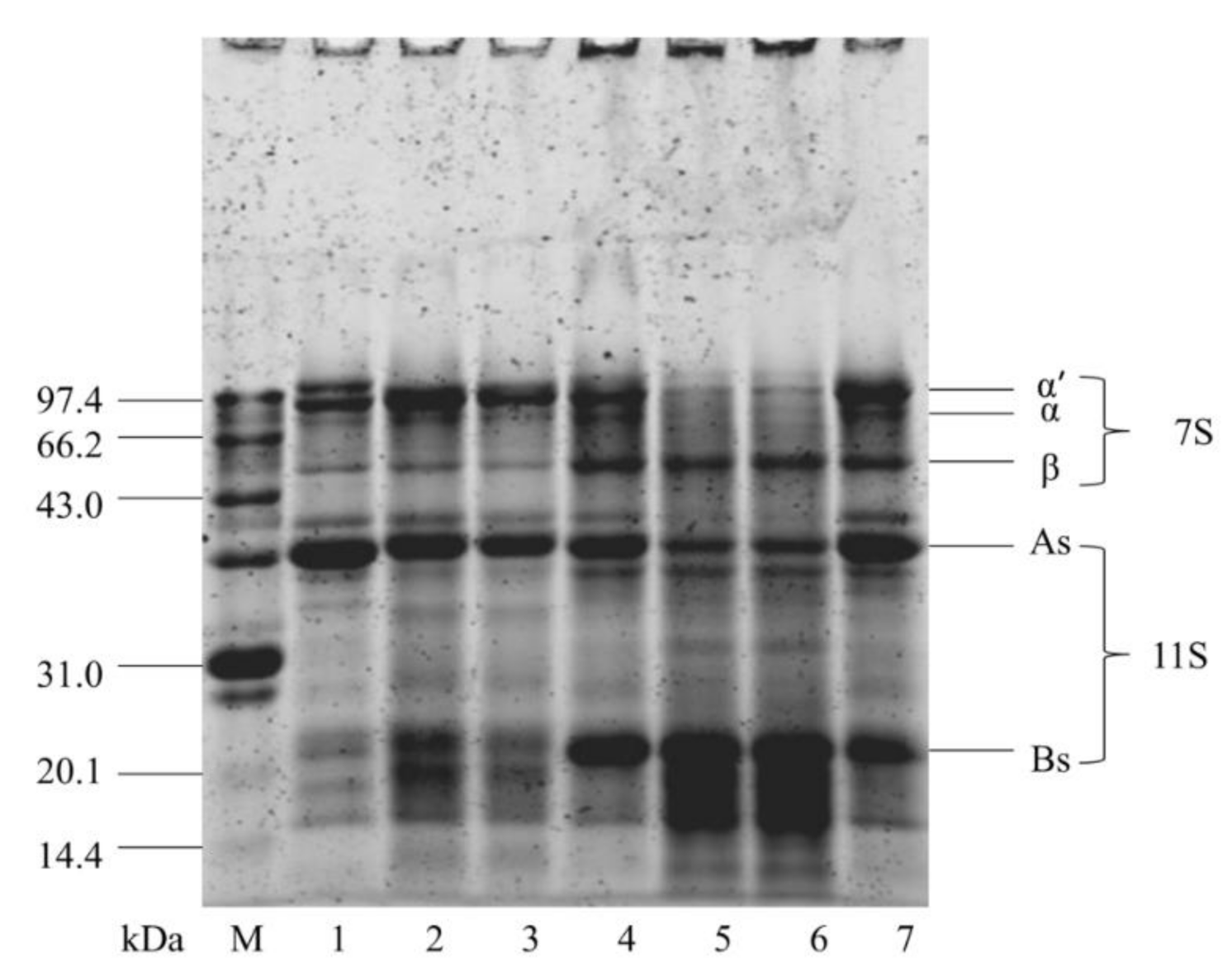
3.6. Effect of Cavitation Jet Treatment on SDS-PAGE of Oxidized SPI
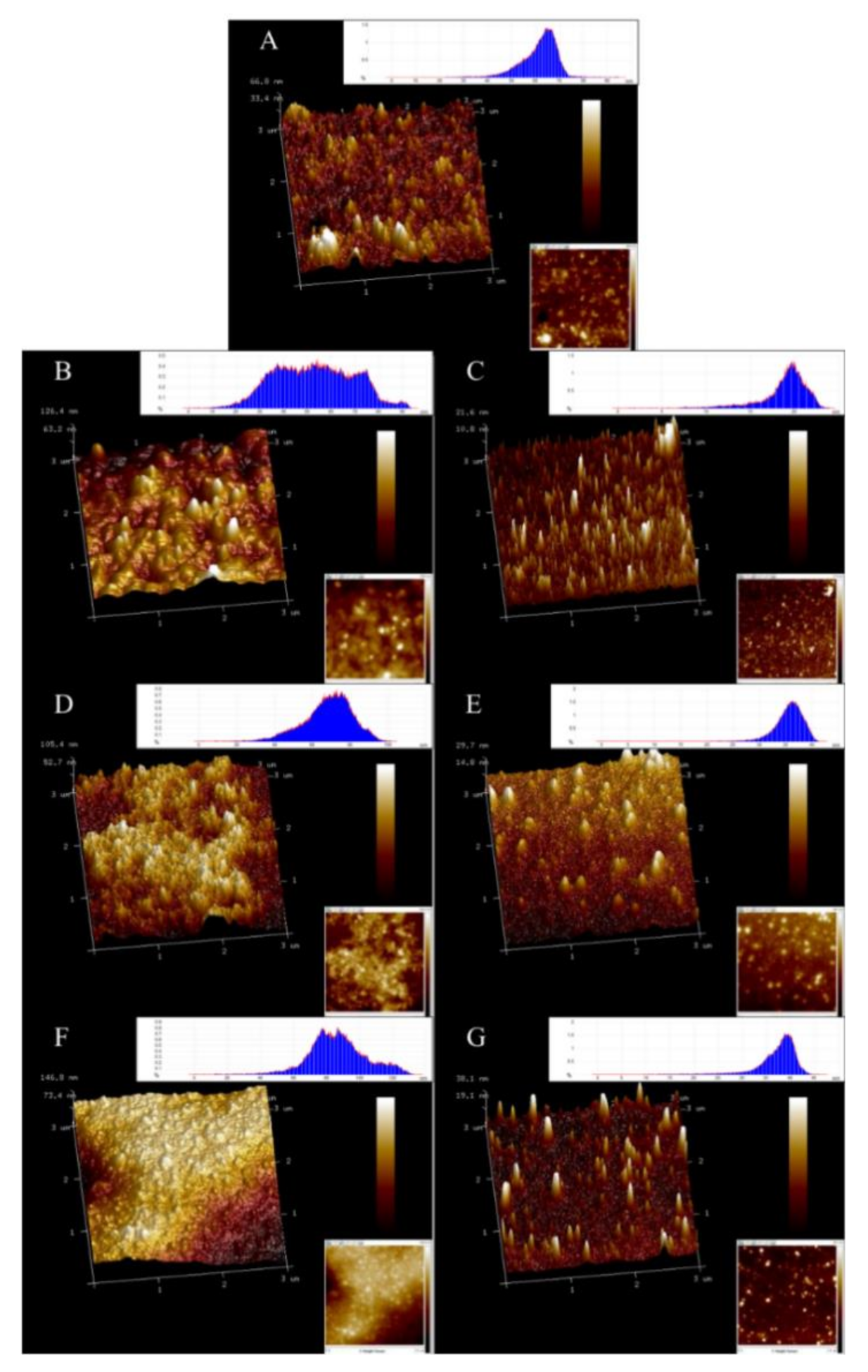
3.7. Effect of Cavitation Jet Treatment on the Surface Morphology of Oxidized SPI
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, C.-H. Nanostructured soy proteins: Fabrication and applications as delivery systems for bioactives (a review). Food Hydrocoll. 2019, 91, 92–116. [Google Scholar]
- Tang, C.-H.; Ma, C.-Y. Effect of high pressure treatment on aggregation and structural properties of soy protein isolate. LWT Food Sci. Technol. 2009, 42, 606–611. [Google Scholar]
- Duque-Estrada, P.; Berton-Carabin, C.C.; Nieuwkoop, M.; Dekkers, B.L.; Janssen, A.E.M.; van der Goot, A.J. Protein Oxidation and In Vitro Gastric Digestion of Processed Soy-Based Matrices. J. Agric. Food Chem. 2019, 67, 9591–9600. [Google Scholar] [PubMed] [Green Version]
- Zhao, X.; Zhao, Q.; Chen, H.; Xiong, H. Distribution and effects of natural selenium in soybean proteins and its protective role in soybean beta-conglycinin (7S globulins) under AAPH-induced oxidative stress. Food Chem. 2019, 272, 201–209. [Google Scholar] [PubMed]
- Shacter, E. Quantification and significance of protein oxidation in biological samples. Drug Metab. Rev. 2000, 32, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Grune, T.; Jung, T.; Merker, K.; Davies, K.J. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and ‘aggresomes’ during oxidative stress, aging, and disease. Int. J. Biochem. Cell Biol. 2004, 36, 2519–2530. [Google Scholar] [PubMed]
- Grune, T. Oxidized protein aggregates: Formation and biological effects. Free Radic Biol. Med. 2020, 150, 120–124. [Google Scholar]
- Wu, W.; Hua, Y.; Lin, Q.; Xiao, H. Effects of oxidative modification on thermal aggregation and gel properties of soy protein by peroxyl radicals. Int. J. Food Sci. Technol. 2011, 46, 1891–1897. [Google Scholar]
- Ji, J.A.; Zhang, B.; Cheng, W.; Wang, Y.J. Methionine, tryptophan, and histidine oxidation in a model protein, PTH: Mechanisms and stabilization. J. Pharm. Sci. 2009, 98, 4485–4500. [Google Scholar]
- Zhou, F.; Zhao, M.; Zhao, H.; Sun, W.; Cui, C. Effects of oxidative modification on gel properties of isolated porcine myofibrillar protein by peroxyl radicals. Meat Sci. 2014, 96, 1432–1439. [Google Scholar]
- Liu, Z.Q.; Yu, W.; Liu, Z.L. Antioxidative and prooxidative effects of coumarin derivatives on free radical initiated and photosensitized peroxidation of human low-density lipoprotein. Chem. Phys. Lipids 1999, 103, 125–135. [Google Scholar] [CrossRef]
- Duan, X.; Li, M.; Shao, J.; Chen, H.; Xu, X.; Jin, Z.; Liu, X. Effect of oxidative modification on structural and foaming properties of egg white protein. Food Hydrocoll. 2018, 75, 223–228. [Google Scholar] [CrossRef]
- Ye, L.; Liao, Y.; Zhao, M.; Sun, W. Effect of Protein Oxidation on the Conformational Properties of Peanut Protein Isolate. J. Chem. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Zhang, C.; Kong, X.; Hua, Y. Oxidative modification of soy protein by peroxyl radicals. Food Chem. 2009, 116, 295–301. [Google Scholar] [CrossRef]
- Fernandez-Avila, C.; Trujillo, A.J. Ultra-High Pressure Homogenization improves oxidative stability and interfacial properties of soy protein isolate-stabilized emulsions. Food Chem. 2016, 209, 104–113. [Google Scholar] [CrossRef]
- Song, X.; Zhou, C.; Fu, F.; Chen, Z.; Wu, Q. Effect of high-pressure homogenization on particle size and film properties of soy protein isolate. Ind. Crop. Prod. 2013, 43, 538–544. [Google Scholar] [CrossRef]
- Preece, K.E.; Hooshyar, N.; Krijgsman, A.; Fryer, P.J.; Zuidam, N.J. Intensified soy protein extraction by ultrasound. Chem. Eng. Process. Process Intensif. 2017, 113, 94–101. [Google Scholar] [CrossRef]
- Li, H.; Zhu, K.; Zhou, H.; Peng, W. Effects of high hydrostatic pressure treatment on allergenicity and structural properties of soybean protein isolate for infant formula. Food Chem. 2012, 132, 808–814. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Liu, C.; Zhong, Y.; Liu, W.; Wan, J. Activation and conformational changes of mushroom polyphenoloxidase by high pressure microfluidization treatment. Innov. Food Sci. Emerg. Technol. 2009, 10, 142–147. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Z.-Q.; Liu, C.-M.; Xie, M.-Y.; Tu, Z.-C.; Liu, J.-H.; Liang, R.-H. The effect of dynamic high-pressure microfluidization on the activity, stability and conformation of trypsin. Food Chem. 2010, 123, 616–621. [Google Scholar] [CrossRef]
- Huang, L.; Shen, M.; Zhang, X.; Jiang, L.; Song, Q.; Xie, J. Effect of high-pressure microfluidization treatment on the physicochemical properties and antioxidant activities of polysaccharide from Mesona chinensis Benth. Carbohydr. Polym. 2018, 200, 191–199. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, M.; Sun, W.; Zhao, G.; Ren, J. Effects of microfluidization treatment and transglutaminase cross-linking on physicochemical, functional, and conformational properties of peanut protein isolate. J. Agric. Food Chem. 2011, 59, 8886–8894. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, J.; Yu, L.; Wu, K. Improved emulsifying capabilities of hydrolysates of soy protein isolate pretreated with high pressure microfluidization. LWT Food Sci. Technol. 2016, 69, 1–8. [Google Scholar] [CrossRef]
- Wu, C.; Teng, F.; McClements, D.J.; Zhang, S.; Li, Y.; Wang, Z. Effect of cavitation jet processing on the physicochemical properties and structural characteristics of okara dietary fiber. Food Res. Int. 2020, 134, 109251. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.E.; Li, C.; Yang, F.; Huang, Y.; Huang, C.; Zhang, K.; Yan, L. Comparison of hydrodynamic and ultrasonic cavitation effects on soy protein isolate functionality. J. Food Eng. 2020, 265, 109697. [Google Scholar] [CrossRef]
- Huang, Y.; Hua, Y.; Qiu, A. Soybean protein aggregation induced by lipoxygenase catalyzed linoleic acid oxidation. Food Res. Int. 2006, 39, 240–249. [Google Scholar] [CrossRef]
- Li, Y.; Wu, C.L.; Liu, J.; Zhu, Y.; Zhang, X.Y.; Jiang, L.Z.; Qi, B.K.; Zhang, X.N.; Wang, Z.J.; Teng, F. Soy Protein Isolate-Phosphatidylcholine Nanoemulsions Prepared Using High-Pressure Homogenization. Nanomaterials 2018, 8, 307. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Ding, X.; Li, Y.; Ma, H. The aggregation, structures and emulsifying properties of soybean protein isolate induced by ultrasound and acid. Food Chem 2019, 279, 114–119. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Shen, L.; Tang, C.-H. Microfluidization as a potential technique to modify surface properties of soy protein isolate. Food Res. Int. 2012, 48, 108–118. [Google Scholar] [CrossRef]
- Laemmli, U. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Berlett, B.S. Reactive oxygen-mediated protein oxidation in aging and disease. Drug Metab. Rev. 1998, 30, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lu, Y.; Han, J.; Chen, Q.; Kong, B. Structure-modification by moderate oxidation in hydroxyl radical-generating systems promote the emulsifying properties of soy protein isolate. Food Struct. 2015, 6, 21–28. [Google Scholar] [CrossRef]
- Headlam, H.A.; Davies, M.J. Markers of protein oxidation: Different oxidants give rise to variable yields of bound and released carbonyl products. Free Radic Biol. Med. 2004, 36, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhao, M.; Sun, W.; Ren, J.; Cui, C. Effect of oxidation on the emulsifying properties of soy protein isolate. Food Res. Int. 2013, 52, 26–32. [Google Scholar] [CrossRef]
- Jung, S.; Nam, K.C.; Ahn, D.U.; Kim, H.J.; Jo, C. Effect of phosvitin on lipid and protein oxidation in ground beef treated with high hydrostatic pressure. Meat Sci. 2013, 95, 8–13. [Google Scholar] [CrossRef]
- Zheng, J.; Bizzozero, O.A. Traditional reactive carbonyl scavengers do not prevent the carbonylation of brain proteins induced by acute glutathione depletion. Free Radic Res. 2010, 44, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Liu, X.; Ren, X.; Huang, Y.; Huang, C.; Zhang, K. Swirling cavitation improves the emulsifying properties of commercial soy protein isolate. Ultrason. Sonochem. 2018, 42, 471–481. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, F.; Zhao, M.; Yang, B.; Cui, C. Physicochemical changes of myofibrillar proteins during processing of Cantonese sausage in relation to their aggregation behaviour and in vitro digestibility. Food Chem. 2011, 129, 472–478. [Google Scholar] [CrossRef]
- Eaton, P. Protein thiol oxidation in health and disease: Techniques for measuring disulfides and related modifications in complex protein mixtures. Free Radic Biol. Med. 2006, 40, 1889–1899. [Google Scholar] [CrossRef]
- Visschers, R.W.; de Jongh, H.H. Disulphide bond formation in food protein aggregation and gelation. Biotechnol. Adv. 2005, 23, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Vivian, J.T.; Callis, P.R. Mechanisms of Tryptophan Fluorescence Shifts in Proteins. Biophys. J. 2001, 80, 2093–2109. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Zhao, M.; Yang, B.; Zhao, H.; Cui, C. Oxidation of sarcoplasmic proteins during processing of Cantonese sausage in relation to their aggregation behaviour and in vitro digestibility. Meat Sci. 2011, 88, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.Z.; Liu, W.; Liu, C.M.; Wang, Q.H.; Li, T.; Tu, Z.C.; Luo, S.J.; Cai, X.F.; Xu, Y.J. Aggregation and conformational changes of bovine beta-lactoglobulin subjected to dynamic high-pressure microfluidization in relation to antigenicity. J. Dairy Sci. 2012, 95, 4237–4245. [Google Scholar] [CrossRef]
- Shen, X.; Fang, T.; Gao, F.; Guo, M. Effects of ultrasound treatment on physicochemical and emulsifying properties of whey proteins pre- and post-thermal aggregation. Food Hydrocoll. 2017, 63, 668–676. [Google Scholar] [CrossRef]
- Oliete, B.; Potin, F.; Cases, E.; Saurel, R. Modulation of the emulsifying properties of pea globulin soluble aggregates by dynamic high-pressure fluidization. Innov. Food Sci. Emerg. Technol. 2018, 47, 292–300. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Gan, X.; Li, H. Effect of peroxyl radicals on the structure and gel properties of isolated rabbit meat myofibrillar proteins. Int. J. Food Sci. Technol. 2018, 53, 2687–2696. [Google Scholar] [CrossRef]
- Chandrapala, J.; Zisu, B.; Palmer, M.; Kentish, S.; Ashokkumar, M. Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrason. Sonochem. 2011, 18, 951–957. [Google Scholar] [CrossRef]
- Hu, H.; Wu, J.; Li-Chan, E.C.Y.; Zhu, L.; Zhang, F.; Xu, X.; Fan, G.; Wang, L.; Huang, X.; Pan, S. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocoll. 2013, 30, 647–655. [Google Scholar] [CrossRef]
- Chao, C.C.; Ma, Y.S.; Stadtman, E.R. Modification of protein surface hydrophobicity and methionine oxidation by oxidative systems. Proc. Natl. Acad. Sci. USA 1997, 94, 2969–2974. [Google Scholar] [CrossRef] [Green Version]
- Park, D.; Xiong, Y.L.; Alderton, A.L.; Ooizumi, T. Biochemical changes in myofibrillar protein isolates exposed to three oxidizing systems. J. Agric. Food Chem. 2006, 54, 4445–4451. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Sun, F.; Li, Y.; Liu, Q.; Kong, B. Modification of gel properties of soy protein isolate by freeze-thaw cycles are associated with changes of molecular force involved in the gelation. Process Biochem. 2017, 52, 200–208. [Google Scholar] [CrossRef]
- Huang, L.; Ding, X.; Dai, C.; Ma, H. Changes in the structure and dissociation of soybean protein isolate induced by ultrasound-assisted acid pretreatment. Food Chem. 2017, 232, 727–732. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Sample Serial Number | ||||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | |
| AAPH concentration (mmol/L) | 0 | 0.2 | 0.2 | 1 | 1 | 5 | 5 |
| Cavitation jet pressure (MPa) | 0 | 0 | 90 | 0 | 90 | 0 | 90 |
| Protein Samples | Carbonyl (nmol/mg) | Free SH (μmol/mg) | Total SH (μmol/mg) | S-S (μmol/mg) |
|---|---|---|---|---|
| A | 5.67 ± 0.03 f | 7.07 ± 0.03 a | 9.73 ± 0.03 a | 3.80 ± 0.02 b |
| B | 5.86 ± 0.02 c | 6.39 ± 0.03 c | 9.70 ± 0.03 ab | 3.89 ± 0.03 ab |
| C | 5.77 ± 0.02 d | 6.89 ± 0.01 b | 9.71 ± 0.04 a | 3.83 ± 0.02 ab |
| D | 5.95 ± 0.03 b | 5.88 ± 0.02 f | 9.67 ± 0.02 ab | 3.93 ± 0.03 ab |
| E | 5.71 ± 0.02 e | 6.01 ± 0.02 d | 9.69 ± 0.01 ab | 3.88 ± 0.03 ab |
| F | 6.03 ± 0.01 a | 5.22 ± 0.02 g | 9.64 ± 0.03 b | 3.97 ± 0.03 a |
| G | 5.72 ± 0.01 e | 5.95 ± 0.02 e | 9.67 ± 0.05 ab | 3.90 ± 0.03 ab |
| Protein Samples | Dispersion | Emulsion Droplet | |
|---|---|---|---|
| d4,3 (nm) | d4,3 (μm) | ζ-Potential (mV) | |
| A | 266.21 ± 7.94 f | 4.32 ± 0.04 f | −22.53 ± 0.81 b |
| B | 355.03 ± 9.16 d | 4.69 ± 0.08 e | −23.87 ± 1.03b c |
| C | 306.09 ± 3.95 e | 4.14 ± 0.08 g | −23.97 ± 1.21b c |
| D | 439.38 ± 7.59 b | 5.62 ± 0.15 c | −25.03 ± 0.58 c |
| E | 389.05 ± 4.52 c | 5.02 ± 0.08 d | −27.69 ± 0.98 d |
| F | 503.84 ± 5.73 a | 6.59 ± 0.09 a | −20.80 ± 0.61 a |
| G | 434.26 ± 4.58 b | 5.87 ± 0.08 b | −20.73 ± 1.12 a |
| Protein Samples | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random Coil (%) |
|---|---|---|---|---|
| A | 19.07 ± 0.04 b | 22.82 ± 0.09 c | 16.04 ± 0.08 d | 42.93 ± 0.09 f |
| B | 17.20 ± 0.11 c | 21.81 ± 0.06 d | 16.79 ± 0.09 c | 44.17 ± 0.07 e |
| C | 19.58 ± 0.06 a | 20.12 ± 0.09 f | 17.90 ± 0.10 b | 42.35 ± 0.06 g |
| D | 13.78 ± 0.06 e | 23.63 ± 0.07 a | 12.58 ± 0.07 g | 50.03 ± 0.11 a |
| E | 17.27 ± 0.07 c | 22.97 ± 0.07 b | 14.46 ± 0.08 f | 45.25 ± 0.05 d |
| F | 16.14 ± 0.10 d | 21.15 ± 0.05 e | 15.07 ± 0.09 e | 48.66 ± 0.08 b |
| G | 19.71 ± 0.09 a | 15.66 ± 0.07 g | 18.66 ± 0.08 a | 45.95 ± 0.05 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Wu, C.; Li, L.; Zheng, L.; Tian, T.; Jiang, L.; Li, Y.; Teng, F. Effects of Cavitation Jet Treatment on the Structure and Emulsification Properties of Oxidized Soy Protein Isolate. Foods 2021, 10, 2. https://doi.org/10.3390/foods10010002
He M, Wu C, Li L, Zheng L, Tian T, Jiang L, Li Y, Teng F. Effects of Cavitation Jet Treatment on the Structure and Emulsification Properties of Oxidized Soy Protein Isolate. Foods. 2021; 10(1):2. https://doi.org/10.3390/foods10010002
Chicago/Turabian StyleHe, Mingyu, Changling Wu, Lijia Li, Li Zheng, Tian Tian, Lianzhou Jiang, Yang Li, and Fei Teng. 2021. "Effects of Cavitation Jet Treatment on the Structure and Emulsification Properties of Oxidized Soy Protein Isolate" Foods 10, no. 1: 2. https://doi.org/10.3390/foods10010002
APA StyleHe, M., Wu, C., Li, L., Zheng, L., Tian, T., Jiang, L., Li, Y., & Teng, F. (2021). Effects of Cavitation Jet Treatment on the Structure and Emulsification Properties of Oxidized Soy Protein Isolate. Foods, 10(1), 2. https://doi.org/10.3390/foods10010002




