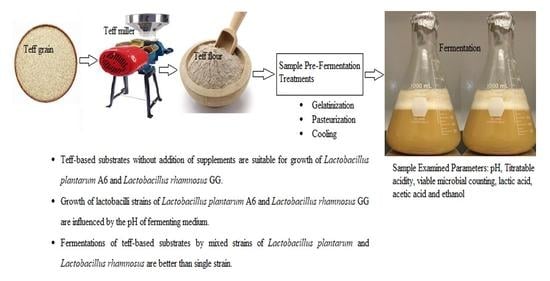
Teff-Based Probiotic Functional Beverage Fermented with Lactobacillus rhamnosus and Lactobacillus plantarum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Starter Culture, Storage and Substrate Preparation
2.3. Enumeration of the Viable Microbial Count
2.4. Substrate Fermentation with a Single Strain
2.5. Experimental Design
2.6. Fermentation of Substrate with Mixed Strains
2.7. Analytical Methods
2.7.1. The pH and TA
2.7.2. Organic Acid and Sugar
2.8. Statistical Analysis
3. Results and Discussion
3.1. Growth of Lactobacillus plantarum A6 (LA6) in Single-Strain Fermentation
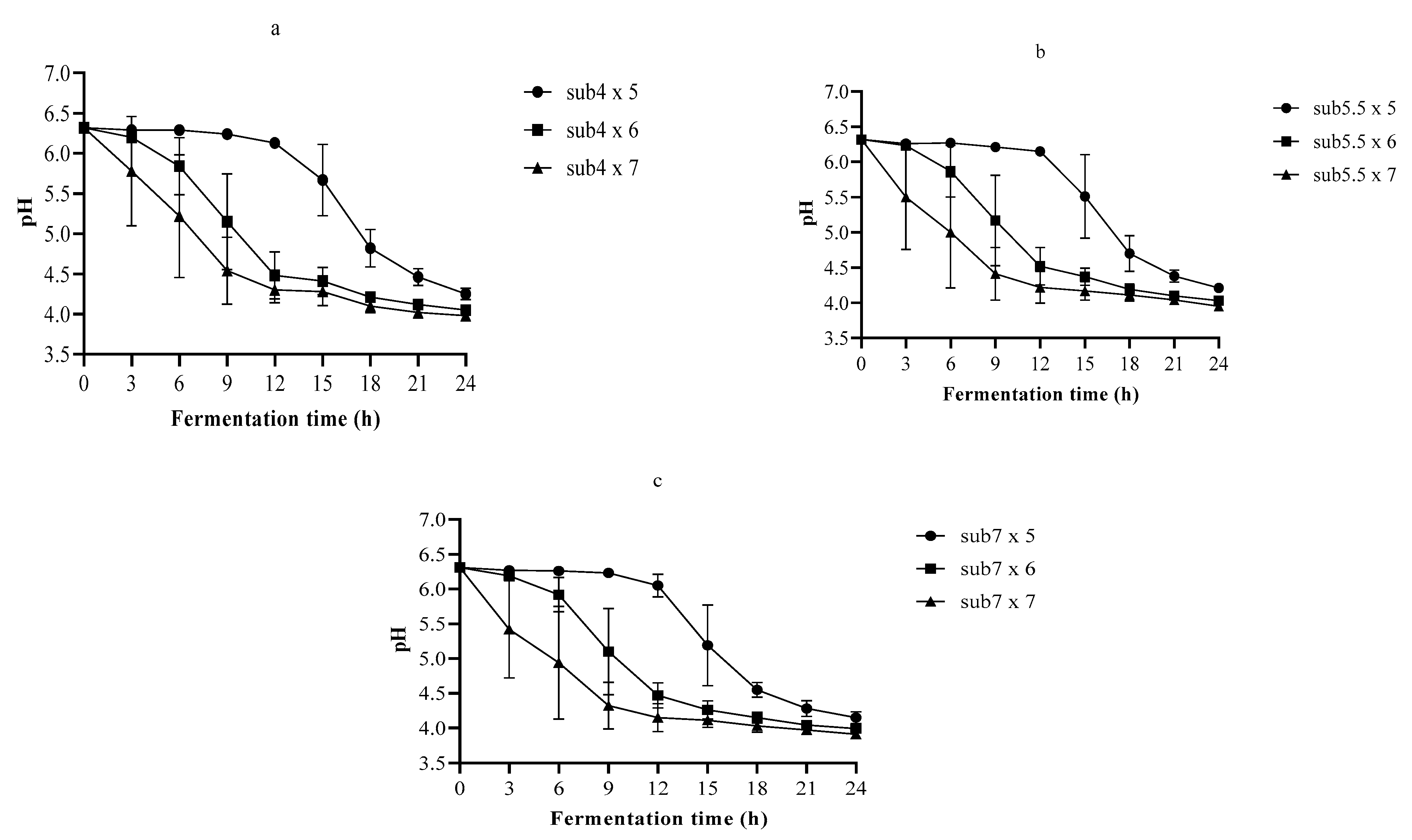
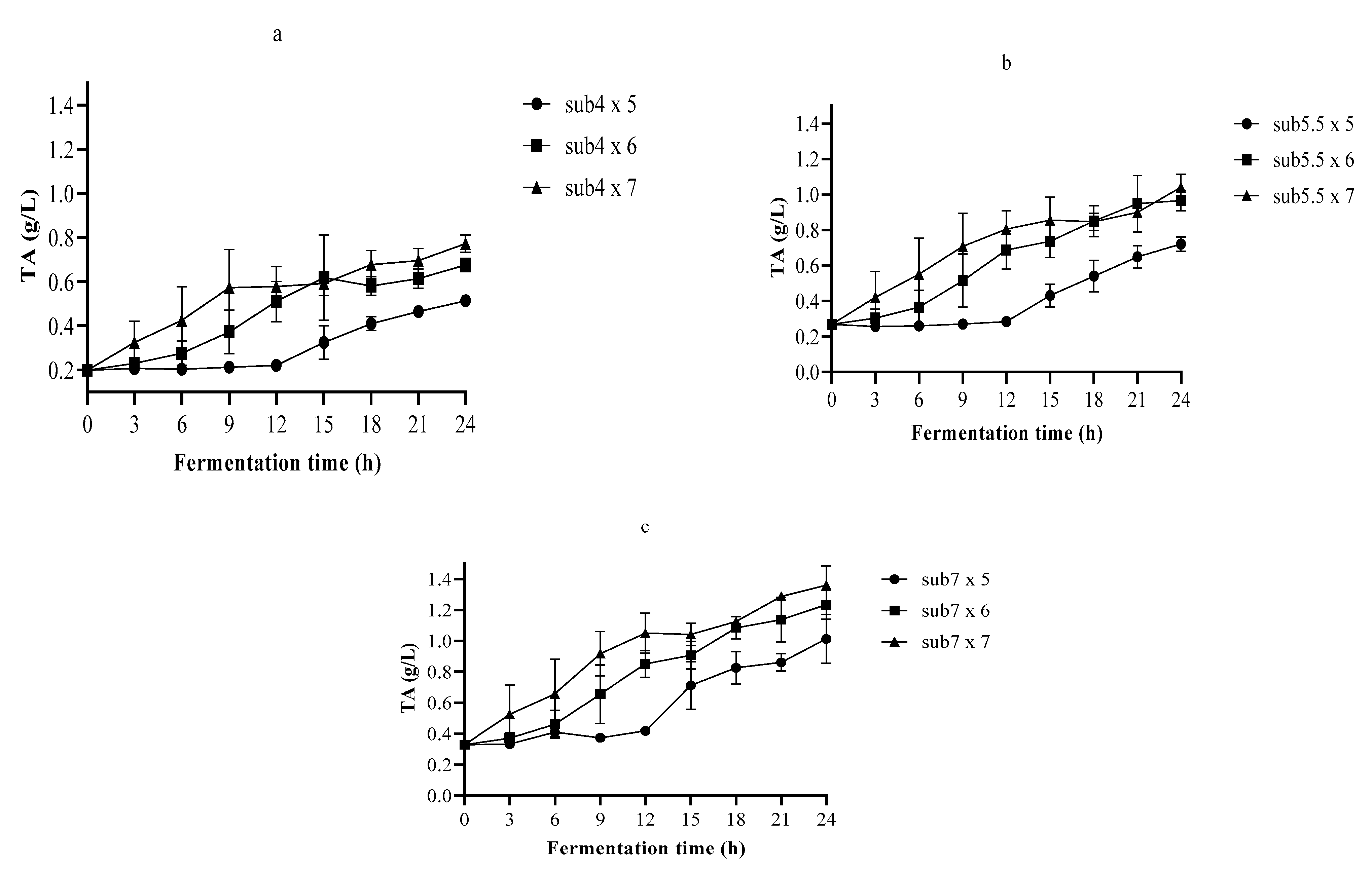
3.2. The pH and TA in Single-Strain Fermentation
3.3. Optimization of the Fermentation Process Using Nelder–Mead Simplex
3.4. Selective Enumeration of Tested Lactobacilli Strains
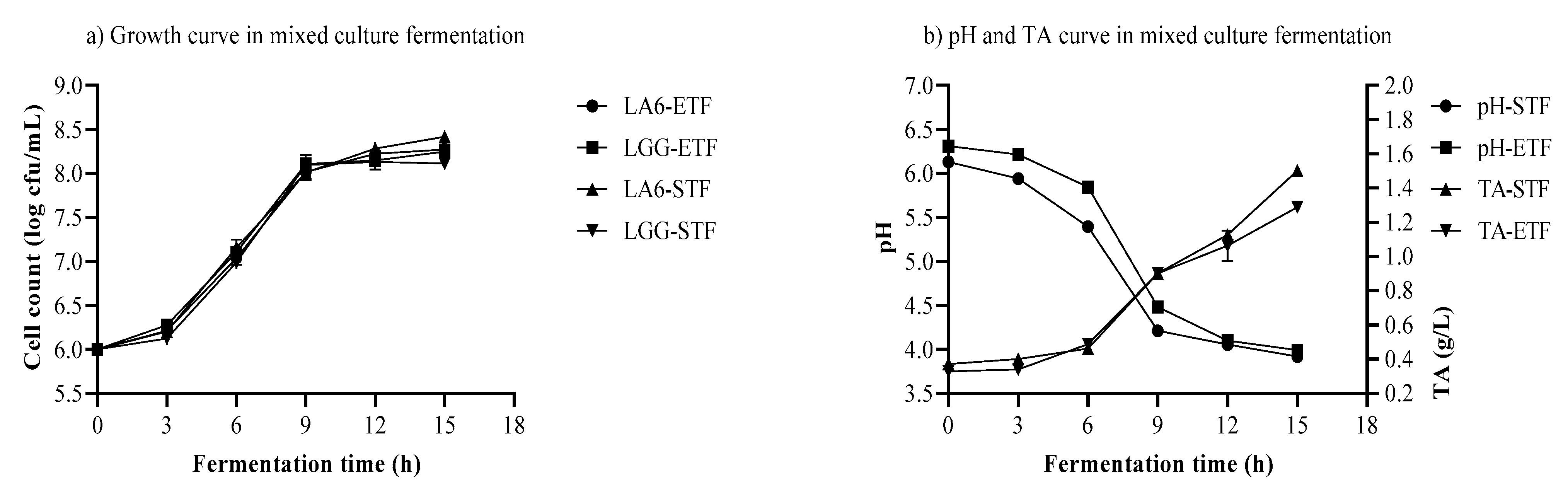
3.5. Growth of LA6 and LGG, pH, and TA in Mixed-Strain Fermentation
3.6. Organic Acid and Sugar in Mixed-Strain Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menezes, A.; Ramos, C.; Dias, D.R.; Schwan, R.F. Combination of probiotic yeast and lactic acid bacteria as starter culture to produce maize-based beverages. Food Res. Int. 2018, 111, 187–197. [Google Scholar] [CrossRef]
- Pimentel, T.C.; da Costa, W.K.A.; Barão, C.E.; Rosset, M.; Magnani, M. Vegan probiotic products: A modern tendency or the newest challenge in functional foods. Food Res. Int. 2020, 140, 110033. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Mridula, D.; Gupta, R.K. Development of sprouted wheat based probiotic beverage. J. Food Sci. Technol. 2013, 51, 3926–3933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Küster-Boluda, I.; Vidal-Capilla, I. Consumer attitudes in the election of functional foods. Span. J. Mark. ESIC 2017, 21, 65–79. [Google Scholar] [CrossRef]
- Valero-Cases, E.; Cerdá-Bernad, D.; Pastor, J.-J.; Frutos, M.-J. Non-Dairy Fermented Beverages as Potential Carriers to Ensure Probiotics, Prebiotics, and Bioactive Compounds Arrival to the Gut and Their Health Benefits. Nutrients 2020, 12, 1666. [Google Scholar] [CrossRef]
- Behera, S.S.; Panda, S.K. Ethnic and industrial probiotic foods and beverages: Efficacy and acceptance. Curr. Opin. Food Sci. 2020, 32, 29–36. [Google Scholar] [CrossRef]
- Angarita-Díaz, M.P.; Forero-Escobar, D.; Cerón-Bastidas, X.A.; Cisneros-Hidalgo, C.A.; Dávila-Narvaez, F.; Bedoya-Correa, C.M.; Freitas, S.C.; Cabrera-Arango, C.L.; Melo-Colina, R. Effects of a functional food supplemented with probiotics on biological factors related to dental caries in children: A pilot study. Eur. Arch. Paediatr. Dent. 2019, 21, 161–169. [Google Scholar] [CrossRef]
- Setta, M.C.; Matemu, A.; Mbega, E.R. Potential of probiotics from fermented cereal-based beverages in improving health of poor people in Africa. J. Food Sci. Technol. 2020, 57, 3935–3946. [Google Scholar] [CrossRef]
- Freire, A.L.; Ramos, C.; Schwan, R.F. Effect of symbiotic interaction between a fructooligosaccharide and probiotic on the kinetic fermentation and chemical profile of maize blended rice beverages. Food Res. Int. 2017, 100, 698–707. [Google Scholar] [CrossRef]
- Das, A.; Raychaudhuri, U.; Chakraborty, R. Cereal based functional food of Indian subcontinent: A review. J. Food Sci. Technol. 2011, 49, 665–672. [Google Scholar] [CrossRef]
- Cante, R.C.; Gallo, M.; Nigro, F.; Passannanti, F.; Salameh, D.; Budelli, A.; Nigro, R. Lactic fermentation of cooked navy beans by Lactobacillus paracasei CBA L74 aimed at a potential production of functional legume-based foods. Can. J. Chem. Eng. 2020, 98, 1955–1961. [Google Scholar] [CrossRef]
- Zhu, F. Chemical composition and food uses of teff (Eragrostis tef). Food Chem. 2018, 239, 402–415. [Google Scholar] [CrossRef]
- Gebru, Y.A.; Hyun-Ii, J.; Young-Soo, K.; Myung-Kon, K.; Kwang-Pyo, K. Variations in Amino Acid and Protein Profiles in White versus Brown Teff (Eragrostis Tef) Seeds, and Effect of Extraction Methods on Protein Yields. Foods 2019, 8, 202. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, K.D.O.D.; Paes, S.D.N.D.; De Oliveira, I.R.; Reis, I.P.; Augusta, I.M. Teff: Suitability for Different Food Applications and as a Raw Material of Gluten-free, a Literature Review. J. Food Nutr. Res. 2018, 6, 74–81. [Google Scholar] [CrossRef]
- Bationo, F.; Songré-Ouattara, L.T.; Hemery, Y.M.; Hama-Ba, F.; Parkouda, C.; Chapron, M.; Le Merrer, M.; Leconte, N.; Sawadogo-Lingani, H.; Diawara, B. Improved processing for the production of cereal-based fermented porridge enriched in folate using selected lactic acid bacteria and a back slopping process. LWT 2019, 106, 172–178. [Google Scholar] [CrossRef]
- Matejčeková, Z.; Liptáková, D.; Valík, Ľ. Functional probiotic products based on fermented buckwheat with Lactobacillus rhamnosus. LWT 2017, 81, 35–41. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, F.; Chai, Z.; Liu, M.; Battino, M.; Meng, X. Mixed fermentation of blueberry pomace with L. rhamnosus GG and L. plantarum-1: Enhance the active ingredient, antioxidant activity and health-promoting benefits. Food Chem. Toxicol. 2019, 131, 110541. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Guyot, J.-P.; Icard-Vernière, C.; Rochette, I.; Loiseau, G. Effect of high pressure homogenisation on the capacity of Lactobacillus plantarum A6 to ferment rice/soybean slurries to prepare high energy density complementary food. Food Chem. 2007, 102, 1288–1295. [Google Scholar] [CrossRef]
- AACC. American Association of Cereal Chemists Approved Methods of Analysis, 11th ed.; Cereals & Grains Association: St. Paul, MN, USA, 1999. [Google Scholar]
- Nielsen, S.S. Correction to: Food Analysis Fifth Edition. In Food Analysis; Springer: Cham, Switzerland, 2019; pp. C1–C2. [Google Scholar] [CrossRef]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Rathore, S.; Salmerón, I.; Pandiella, S.S. Production of potentially probiotic beverages using single and mixed cereal substrates fermented with lactic acid bacteria cultures. Food Microbiol. 2012, 30, 239–244. [Google Scholar] [CrossRef]
- Rasika, D.M.; Vidanarachchi, J.K.; Rocha, R.S.; Balthazar, C.F.; Cruz, A.G.; Sant’Ana, A.S.; Ranadheera, C.S. Plant-based milk substitutes as emerging probiotic carriers. Curr. Opin. Food Sci. 2020, 38, 8–20. [Google Scholar] [CrossRef]
- Espirito-Santo, A.P.D.; Mouquet-Rivier, C.; Humblot, C.; Cazevieille, C.; Verniere, C.I.; Soccol, C.R.; Guyot, J.-P. Influence of cofermentation by amylolytic Lactobacillus strains and probiotic bacteria on the fermentation process, viscosity and microstructure of gruels made of rice, soy milk and passion fruit fiber. Food Res. Int. 2014, 57, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Humblot, C.; Turpin, W.; Chevalier, F.; Picq, C.; Rochette, I.; Guyot, J.-P. Determination of expression and activity of genes involved in starch metabolism in Lactobacillus plantarum A6 during fermentation of a cereal-based gruel. Int. J. Food Microbiol. 2014, 185, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Kedia, G.; Vázquez, J.A.; Pandiella, S.S. Fermentability of whole oat flour, PeriTec flour and bran by Lactobacillus plantarum. J. Food Eng. 2008, 89, 246–249. [Google Scholar] [CrossRef] [Green Version]
- Salmerón, I.; Thomas, K.; Pandiella, S.S. Effect of substrate composition and inoculum on the fermentation kinetics and flavour compound profiles of potentially non-dairy probiotic formulations. LWT 2013, 55, 240–247. [Google Scholar] [CrossRef]
- Salmerón, I.; Thomas, K.; Pandiella, S.S. Effect of potentially probiotic lactic acid bacteria on the physicochemical composition and acceptance of fermented cereal beverages. J. Funct. Foods 2015, 15, 106–115. [Google Scholar] [CrossRef]
- Urquizo, F.L.; Torres, S.M.G.; Tolonen, T.; Jaakkola, M.; Pena-Niebuhr, M.G.; Von Wright, A.; Repo-Carrasco-Valencia, R.; Korhonen, H.; Plumed-Ferrer, C. Development of a fermented quinoa-based beverage. Food Sci. Nutr. 2016, 5, 602–608. [Google Scholar] [CrossRef] [Green Version]
- Kantachote, D.; Ratanaburee, A.; Hayisama-Ae, W.; Sukhoom, A.; Nunkaew, T. The use of potential probiotic Lactobacillus plantarum DW12 for producing a novel functional beverage from mature coconut water. J. Funct. Foods 2017, 32, 401–408. [Google Scholar] [CrossRef]
- Herrera-Ponce, A.; Nevárez-Morillón, G.; Ortega-Rívas, E.; Perez-Vega, S.; Salmerón, I. Fermentation adaptability of three probiotic Lactobacillus strains to oat, germinated oat and malted oat substrates. Lett. Appl. Microbiol. 2014, 59, 449–456. [Google Scholar] [CrossRef]
- Tobil, M.; Deh, C.Y.; Agbenorhevi, J.K.; Sampson, G.O.; Kpodo, F. Effect of Okra Pectin on the Sensory, Physicochemical and Microbial Quality of Yoghurt. Food Nutr. Sci. 2020, 11, 442–456. [Google Scholar] [CrossRef]
- Byakika, S.; Mukisa, I.M.; Byaruhanga, Y.B.; Male, D.; Muyanja, C. Influence of food safety knowledge, attitudes and practices of processors on microbiological quality of commercially produced traditional fermented cereal beverages, a case of Obushera in Kampala. Food Control. 2019, 100, 212–219. [Google Scholar] [CrossRef]
- Ding, W.; Shah, N. Acid, Bile, and Heat Tolerance of Free and Microencapsulated Probiotic Bacteria. J. Food Sci. 2007, 72, M446–M450. [Google Scholar] [CrossRef]
- Peres, C.; Peres, C.; Hernandez-Mendoza, A.; Malcata, F. Review on fermented plant materials as carriers and sources of potentially probiotic lactic acid bacteria—With an emphasis on table olives. Trends Food Sci. Technol. 2012, 26, 31–42. [Google Scholar] [CrossRef]
- Graves, T.; Narendranath, N.V.; Dawson, K.; Power, R. Interaction effects of lactic acid and acetic acid at different temperatures on ethanol production by Saccharomyces cerevisiae in corn mash. Appl. Microbiol. Biotechnol. 2006, 73, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Kocková, M.; Dilongová, M.; Hybenová, E.; Valík, Ľ. Evaluation of Cereals and Pseudocereals Suitability for the Development of New Probiotic Foods. J. Chem. 2013, 2013, 414303. [Google Scholar] [CrossRef] [Green Version]
- Pires, B.D.A.; Campos, R.C.D.A.B.; Canuto, J.W.; Dias, T.D.M.C.; Martins, E.M.F.; Licursi, L.; Júnior, B.R.D.C.L.; Martins, M.L. Lactobacillus rhamnosus GG in a mixed pineapple (Ananas comosus L. Merril) and jussara (Euterpe edulis Martius) beverage and its survival in the human gastrointestinal tract. LWT 2020, 134, 110028. [Google Scholar] [CrossRef]
- Santos, C.; Libeck, B.D.S.; Schwan, R.F. Co-culture fermentation of peanut-soy milk for the development of a novel functional beverage. Int. J. Food Microbiol. 2014, 186, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.S.; Sen, S.S.; Saha, S.; Sukumaran, V.; Park, S.C. Use of a Potential Probiotic, Lactobacillus plantarum L7, for the Preparation of a Rice-Based Fermented Beverage. Front. Microbiol. 2018, 9, 473. [Google Scholar] [CrossRef]
- Li, D.; Duan, F.; Tian, Q.; Zhong, D.; Wang, X.; Jia, L. Physiochemical, microbiological and flavor characteristics of traditional Chinese fermented food Kaili Red Sour Soup. LWT 2021, 142, 110933. [Google Scholar] [CrossRef]

| Experiment No. | Substrate (% w/v) | Inoculum (log cfu/mL) | Experiment No. | Substrate (% w/v) | Inoculum (log cfu/mL) |
|---|---|---|---|---|---|
| 1 | 5.5 | 7 | 15 | 4 | 6 |
| 2 | 7 | 7 | 16 | 7 | 6 |
| 3 | 7 | 7 | 17 | 7 | 5 |
| 4 | 4 | 7 | 18 | 5.5 | 5 |
| 5 | 5.5 | 5 | 19 | 4 | 5 |
| 6 | 5.5 | 6 | 20 | 5.5 | 6 |
| 7 | 4 | 6 | 21 | 4 | 7 |
| 8 | 5.5 | 7 | 22 | 5.5 | 6 |
| 9 | 7 | 5 | 23 | 5.5 | 7 |
| 10 | 5.5 | 5 | 24 | 4 | 6 |
| 11 | 7 | 6 | 25 | 7 | 7 |
| 12 | 7 | 6 | 26 | 7 | 5 |
| 13 | 4 | 5 | 27 | 4 | 7 |
| 14 | 4 | 5 |
| Inoculum (log cfu/mL) | Substrate (% w/v) | Estimated Growth Parameters | Goodness of Fit | ||
|---|---|---|---|---|---|
| Growth Rate (h−1) | Lag Phase (h) | R2 | SE of Fit | ||
| 5 | 4 | 0.215 | 3.490 | 0.998 | 0.063 |
| 5.5 | 0.262 | 4.476 | 0.996 | 0.086 | |
| 7 | 0.277 | 4.580 | 0.998 | 0.067 | |
| 6 | 4 | 0.273 | 4.022 | 0.993 | 0.079 |
| 5.5 | 0.274 | 4.257 | 0.994 | 0.076 | |
| 7 | 0.294 | 4.997 | 0.988 | 0.114 | |
| 7 | 4 | 0.221 | 3.780 | 0.968 | 0.095 |
| 5.5 | 0.313 | 5.173 | 0.960 | 0.118 | |
| 7 | 0.249 | 3.606 | 0.979 | 0.088 | |
| Inoculum (Log cfu/mL) | Substrate (% w/v) | Estimated Parameters | Goodness of Fit | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rate (h−1) | Lag Phase (h) | R2 | SE of Fit | ||||||
| pH | TA | pH | TA | pH | TA | pH | TA | ||
| 5 | 4 | −0.166 | 0.024 | 10.622 | 10.530 | 0.968 | 0.976 | 0.152 | 0.019 |
| 5.5 | −0.166 | 0.036 | 10.24 | 10.685 | 0.958 | 0.986 | 0.180 | 0.022 | |
| 7 | −0.267 | 0.045 | 11.243 | 9.106 | 0.996 | 0.937 | 0.060 | 0.065 | |
| 6 | 4 | −0.227 | 0.046 | 4.135 | 5.713 | 0.984 | 0.970 | 0.114 | 0.031 |
| 5.5 | −0.218 | 0.033 | 3.997 | 0.000 | 0.987 | 0.972 | 0.104 | 0.045 | |
| 7 | −0.244 | 0.040 | 4.464 | 0.000 | 0.990 | 0.964 | 0.094 | 0.063 | |
| 7 | 4 | −0.185 | 0.021 | 0.000 | 0.000 | 0.984 | 0.929 | 0.106 | 0.048 |
| 5.5 | −0.203 | 0.029 | 0.000 | 0.000 | 0.988 | 0.918 | 0.089 | 0.072 | |
| 7 | −0.217 | 0.040 | 0.000 | 0.000 | 0.985 | 0.954 | 0.099 | 0.071 | |
| Exp. | Time (h) | Inoculum of LA6 (log cfu/mL) | Growth of LA6 (log cfu/mL) | pH | TA (g/L) | QF |
|---|---|---|---|---|---|---|
| 1 | 12.000 | 6.000 | 8.078 ± 0.250 a,b | 4.473 ± 0.182 b | 0.851 ± 0.087 b | 1.905 ± 0.048 b |
| 2 | 12.000 | 5.500 | 7.781 ± 0.020 b | 4.840 ± 0.014 a | 0.622 ± 0.000 c | 1.799 ± 0.005 c |
| 3 | 13.500 | 6.000 | 8.265 ± 0.042 a | 4.245 ± 0.035 b,c | 0.901 ± 0.000 a,b | 1.975 ± 0.003 a,b |
| 4 | 13.500 | 6.226 | 8.304 ± 0.011 a | 4.330 ± 0.000 b,c | 0.901 ± 0.000 a,b | 1.962 ± 0.001 a,b |
| 5 | 15.000 | 6.226 | 8.383 ± 0.017 a | 4.140 ± 0.014 c | 1.001 ± 0.000 a | 2.013 ± 0.006 a |
| 6 | 15.000 | 6.000 | 8.400 ± 0.043 a | 4.2 ± 0.014 b,c | 1.001 ± 0.000 a | 2.003 ± 0.002 a |
| 7 | 14.250 | 6.068 | 8.346 ± 0.022 a | 4.335 ± 0.021 b,c | 1.001 ± 0.000 a | 1.966 ± 0.007 a,b |
| 8 | 14.625 | 6.100 | 8.393 ± 0.024 a | 4.385 ± 0.007 b,c | 0.975 ± 0.037 a,b | 1.961 ± 0.001 a,b |
| Exp. | Time (h) | Inoculum (log cfu/mL) | Maltose (mg/L) | Glucose (mg/L) | Lactic Acid (mg/L) | Acetic Acid (mg/L) | Ethanol (mg/L) |
|---|---|---|---|---|---|---|---|
| 2 | 12 | 5.500 | 294.5 ± 3.536 c | 1070.5 ± 16.263 a | 522.0 ± 8.485 f | 37.5 ± 0.707 c | 6.0 ± 0.000 e |
| 3 | 13.5 | 6.000 | 318.0 ± 1.414 b | 557.0 ± 2.828 b | 820.0 ± 2.828 d | 43.0 ± 1.414 c | 19.5 ± 0.707 d |
| 4 | 13.5 | 6.226 | 204.5 ± 0.707 f | 241.0 ± 1.414 c | 584.0 ± 1.414 e | 39.0 ± 4.243 c | 21.5 ± 0.707 d |
| 5 | 15 | 6.226 | 242.0 ± 2.828 e | 0.0 ± 0.000 e | 1051.0 ± 9.899 b | 53.0 ± 0.000 b | 54.5 ± 0.707 a,b |
| 6 | 15 | 6.000 | 289.0 ± 5.657 c,d | 0.0 ± 0.000 e | 1140.5 ± 23.335 a | 82.5 ± 0.707 a | 48.5 ± 0.707 c |
| 7 | 14.25 | 6.068 | 274.5 ± 3.536 d | 0.0 ± 0.000 e | 1049.5 ± 16.263 b | 62.5 ± 2.121 b | 52.5 ± 0.707 b |
| 8 | 14.625 | 6.100 | 354.0 ± 7.071 a | 110.5 ± 2.121 d | 895.0 ± 14.142 c | 58.0 ± 4.243 b | 57.5 ± 2.121 a |
| Culture Media | Incubation Time (h) | LA6 | LGG | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Anaerobiosis | Aerobiosis | Anaerobiosis | Aerobiosis | ||||||
| 30 °C | 37 °C | 30 °C | 37 °C | 30 °C | 37 °C | 30 °C | 37 °C | ||
| MRS agar | 24 | + | + | + | + | +/− | + | − | +/− |
| 36 | + | + | + | + | + | + | +/− | + | |
| 48 | + | + | + | + | +/− | + | + | + | |
| Microbes and Acidity | Estimated Parameters | Goodness of Fit | ||||||
|---|---|---|---|---|---|---|---|---|
| Rate (h−1) | Lag Phase (h) | R2 | SE of Fit | |||||
| ETF | STF | ETF | STF | ETF | STF | ETF | STF | |
| LA6 | 0.350 | 0.326 | 3.044 | 2.49 | 0.999 | 0.995 | 0.034 | 0.078 |
| LGG | 0.357 | 0.482 | 2.860 | 4.04 | 0.994 | 0.998 | 0.079 | 0.050 |
| pH | −0.484 | −0.427 | 5.245 | 4.505 | 0.996 | 0.988 | 0.071 | 0.109 |
| TA | 0.070 | 0.112 | - | 4.903 | 0.931 | 0.983 | 0.107 | 0.061 |
| Time (h) | Maltose (mg/L) | Glucose (mg/L) | Lactic Acid (mg/L) | Acetic Acid (mg/L) | Ethanol (mg/L) |
|---|---|---|---|---|---|
| 0 | 244.5 ± 0.707 b | 1250.0 ± 12.728 c | 0.0 ± 0.000 d | 20.0 ± 1.414 d | ND |
| 3 | 244.0 ± 8.485 b | 1483.0 ± 33.941 b | 58.0 ± 4.243 d | 29.0 ± 1.414 d | ND |
| 6 | 301.5 ± 0.707 a | 1698.5 ± 0.707 a | 292.0 ± 0.000 c | 32.0 ± 2.828 c,d | ND |
| 9 | 243.5 ± 12.021 b | 743.5 ± 31.820 d | 1038.5 ± 44.548 b | 49.5 ± 4.950 b,c | 44.0 ± 2.828 a |
| 12 | 206.5 ± 16.263 c | 0.0 ± 0.000 e | 1180.5 ± 67.175 a,b | 61.5 ± 4.950 b | 37.5 ± 2.121 a |
| 15 | 124.0 ± 4.243 d | 0.0 ± 0.000 e | 1320.0 ± 48.083 a | 146.5 ± 9.192 a | ND |
| Time (h) | Maltose (mg/L) | Glucose (mg/L) | Lactic Acid (mg/L) | Acetic Acid (mg/L) |
|---|---|---|---|---|
| 0 | 252.5 ± 7.778 a,b | 1544.0 ± 35.355 a | 0.0 ± 0.000 e | 40.0 ± 2.828 d |
| 3 | 232.5 ± 6.364 b | 1458.5 ± 45.962 a,b | 69.5 ± 2.121 e | 35.5 ± 2.121 d |
| 6 | 257.5 ± 0.707 a,b | 1435.5 ± 10.607 b | 373.5 ± 2.121 d | 52.0 ± 5.657 d |
| 9 | 279.5 ± 7.778 a | 575.5 ± 16.263 c | 1339.0 ± 33.941 b | 81.0 ± 2.828 c |
| 12 | 183.0 ± 0.000 c | 157.0 ± 0.000 d | 1144.5 ± 0.707 c | 120.0 ± 0.000 b |
| 15 | 236.5 ± 12.021 b | 0.0 ± 0.000 e | 1672.5 ± 36.062 a | 231.5 ± 12.021 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alemneh, S.T.; Emire, S.A.; Hitzmann, B. Teff-Based Probiotic Functional Beverage Fermented with Lactobacillus rhamnosus and Lactobacillus plantarum. Foods 2021, 10, 2333. https://doi.org/10.3390/foods10102333
Alemneh ST, Emire SA, Hitzmann B. Teff-Based Probiotic Functional Beverage Fermented with Lactobacillus rhamnosus and Lactobacillus plantarum. Foods. 2021; 10(10):2333. https://doi.org/10.3390/foods10102333
Chicago/Turabian StyleAlemneh, Sendeku Takele, Shimelis Admassu Emire, and Bernd Hitzmann. 2021. "Teff-Based Probiotic Functional Beverage Fermented with Lactobacillus rhamnosus and Lactobacillus plantarum" Foods 10, no. 10: 2333. https://doi.org/10.3390/foods10102333
APA StyleAlemneh, S. T., Emire, S. A., & Hitzmann, B. (2021). Teff-Based Probiotic Functional Beverage Fermented with Lactobacillus rhamnosus and Lactobacillus plantarum. Foods, 10(10), 2333. https://doi.org/10.3390/foods10102333