Development of Indirect Competitive ELISA and Visualized Multicolor ELISA Based on Gold Nanorods Growth for the Determination of Zearalenone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Apparatus
2.2. Preparation of the Coating Antigen—The Conjugate of ZEN-OVA
2.3. Test Procedure of ic-ELISA
2.4. Specificity of ic-ELISA
2.5. AuNR Growth-Based Multicolor ELISA for ZEN
3. Results and Discussion
3.1. Preparation and Characterization of ZEN-CMO and ZEN-OVA Conjugate
3.2. Conditions Optimization of Traditional ELISA
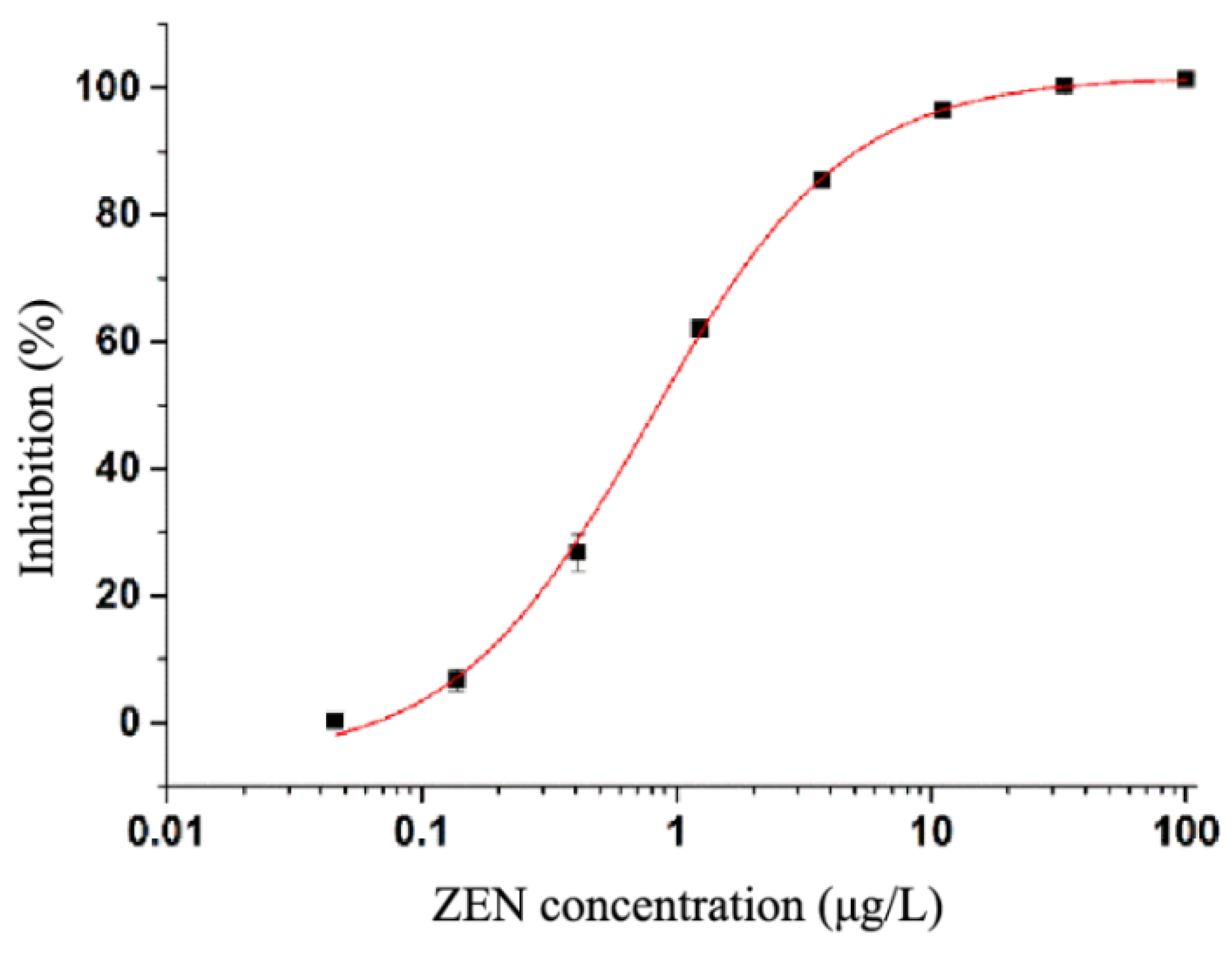
3.3. ic-ELISA Standard Curve
3.4. Specificity of Traditional ELISA
3.5. Multicolor ELISA Based on AuNRs Growth
3.6. Standard Curve of AuNPs Growth-Based Multicolor ELISA for ZEN Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Freire, L.; Sant’Ana, A.S. Modified mycotoxins: An updated review on their formation, detection, occurrence, and toxic effects. Food Chem. Toxicol. 2018, 111, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Mahato, D.K.; Devi, S.; Pandhi, S.; Sharma, B.; Maurya, K.K.; Mishra, S.; Dhawan, K.; Selvakumar, R.; Kamle, M.; Mishra, A.K.; et al. Occurrence, impact on agriculture, human health, and management strategies of zearalenone in food and feed: A review. Toxins 2021, 13, 92. [Google Scholar] [CrossRef]
- Mazaheri, M.; Maymand, M.M.; Gilasgar, A.; Akbarzadeh, A.; Manafi, M.H. Quantification of the zearalenone in maize oil with no clean-up. Food Control 2021, 127, 108166. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Molto, J.C.; Manes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, L.; Wu, D.; Liu, F. Anti-apoptotic action of zearalenone in MCF7 cells. Ecotox. Environ. Saf. 2005, 62, 441–446. [Google Scholar] [CrossRef]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. 2020, 60, 2710–2729. [Google Scholar] [CrossRef]
- Sneha, S.; Baker, S.C.; Green, A.; Storr, S.; Aiyappa, R.; Martin, S.; Pors, K. Intratumoural Cytochrome P450 expression in breast cancer: Impact on standard of care treatment and new efforts to develop tumour-selective therapies. Biomedicines 2021, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.L.; Hu, D.; Li, Y. Effects of zearalenone on mRNA expression and activity of cytochrorne P450 1A1 and 1B1 in MCF-7 cells. Ecotox. Environ. Safe. 2004, 58, 187–193. [Google Scholar] [CrossRef]
- Rogowska, A.; Pomastowski, P.; Sagandykova, G.; Buszewski, B. Zearalenone and its metabolites: Effect on human health, metabolism and neutralisation methods. Toxicon 2019, 162, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Gajęcka, M.; Janowski, T.; Jakimiuk, E.; Zielonka, Ł.; Podhalicz-Dziegielewska, M.; Jan Obremski, K.; Gajęcki, M. Influence of long-term zearalenone intoxication on the concentration of progesterone and 17 beta-oestradiol in blood plasma in bitches. Bull. Vet. Inst. Pulawy 2008, 52, 405–409. [Google Scholar]
- Ji, F.; He, D.; Olaniran, A.O.; Mokoena, M.P.; Xu, J.; Shi, J. Occurrence, toxicity, production and detection of Fusarium mycotoxin: A review. Food Prod. Process. Nutr. 2019, 1, 6. [Google Scholar] [CrossRef]
- Hong, X.; Mao, Y.H.; Yang, C.Q.; Liu, Z.J.; Li, M.; Du, D.L. Contamination of Zearalenone from China in 2019 by a Visual and Digitized Immunochromatographic Assay. Toxins 2020, 12, 521. [Google Scholar] [CrossRef]
- Pan, M.F.; Ma, T.Y.; Yang, J.Y.; Li, S.J.; Liu, S.M.; Wang, S. Development of lateral flow immunochromatographic assays using colloidal au sphere and nanorods as signal marker for the determination of zearalenone in cereals. Foods 2020, 9, 281. [Google Scholar] [CrossRef] [Green Version]
- GB2761-2017. National Standard of People’s Republic of China-Limitation of Mycotoxins in Food. Available online: http://down.foodmate.net/info/sort/2/7407.html (accessed on 17 September 2017).
- Tanaka, T.; Yoneda, A.; Inoue, S.; Sugiura, Y.; Ueno, Y. Simultaneous determination of trichothecene mycotoxins and zearalenone in cereals by gas chromatography-mass spectrometry. J. Chromatogr. A 2000, 882, 23–28. [Google Scholar] [CrossRef]
- Amelin, V.G.; Karaseva, N.M.; Tretyakov, A.V. Simultaneous determination of trichothecene micotoxins, ochratoxin A, and zearalenone in grain and products of its processing, feed premixes, and meat by gas chromatography. J. Anal. Chem. 2013, 68, 61–67. [Google Scholar] [CrossRef]
- Qian, M.R.; Zhang, H.; Wu, L.Q.; Jin, N.; Wang, J.M.; Jiang, K.Z. Simultaneous determination of zearalenone and its derivatives in edible vegetable oil by gel permeation chromatography and gas chromatography-triple quadrupole mass spectrometry. Food Chem. 2015, 166, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Deng, C.L.; Zhou, S.; Zhao, Y.F.; Wang, D.; Wang, X.D.; Gong, Y.Y.; Wu, Y.N. High-throughput and sensitive determination of urinary zearalenone and metabolites by UPLC-MS/MS and its application to a human exposure study. Anal. Bioanal. Chem. 2018, 410, 5301–5312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, D.L.; Li, C.L.; Zhou, S.; Zhao, Y.F.; Gong, Y.Y.; Gong, Z.Y.; Wu, Y.N. Determination of trace zearalenone and its metabolites in human serum by a high-throughput UPLC-MS/MS analysis. Appl. Sci. 2019, 9, 741. [Google Scholar] [CrossRef] [Green Version]
- Tahoun, I.F.; Gab-Allah, M.A.; Yamani, R.N.; Shehata, A.B. Development and validation of a reliable LC-MS/MS method for simultaneous determination of deoxynivalenol and T-2 toxin in maize and oats. Microchem. J. 2021, 169, 106599. [Google Scholar] [CrossRef]
- Engvall, E. Enzyme-linked immunosorbent assay (ELISA), quantitative assay of immunoglobulin G. Immunochemistry 1971, 8, 871–874. [Google Scholar] [CrossRef]
- Li, S.J.; Sheng, W.; Wen, W.J.; Gu, Y.; Wang, J.P.; Wang, S. Three kinds of lateral flow immunochromatographic assays based on the use of nanoparticle labels for fluorometric determination of zearalenone. Microchim. Acta 2018, 185, 238. [Google Scholar] [CrossRef]
- Gemes, B.; Takacs, E.; Gadoros, P.; Barocsi, A.; Kocsanyi, L.; Lenk, S.; Csakanyi, A.; Kautny, S.; Domjan, L.; Szarvas, G.; et al. Development of an immunofluorescence assay module for determination of the mycotoxin zearalenone in water. Toxins 2021, 13, 182. [Google Scholar] [CrossRef]
- Li, R.X.; Wen, Y.; Yang, L.Q.; Liu, A.G.; Wang, F.L.; He, P.L. Dual quantum dot nanobeads-based fluorescence-linked immunosorbent assay for simultaneous detection of aflatoxin B1 and zearalenone in feedstuffs. Food Chem. 2022, 366, 130527. [Google Scholar] [CrossRef]
- Liu, R.X.; Shi, R.R.; Zou, W.T.; Chen, W.H.; Yin, X.C.; Zhao, F.C.; Yang, Z.Y. Highly sensitive phage-magnetic-chemiluminescent enzyme immunoassay for determination of zearalenone. Food Chem. 2020, 325, 126905. [Google Scholar] [CrossRef]
- Fu, J.M.; Zhou, Y.F.; Huang, X.L.; Zhang, W.J.; Wu, Y.H.; Fang, H.; Zhang, C.Z.; Xiong, Y.H. Dramatically enhanced immunochromatographic assay using cascade signal amplification for ultrasensitive detection of Escherichia coli O157: H7 in milk. J. Agric. Food Chem. 2020, 68, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.Y.; Liu, B.; Sheng, W.; Zhang, Y.; Liu, Q.; Li, S.J.; Wang, S. Fluoroimmunoassays for the detection of zearalenone in maize using CdTe/CdS/ZnS quantum dots. Food Chem. 2018, 255, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Pei, K.; Wu, Y.Q.; Duan, H.; Lai, W.H.; Xiong, Y.H. Plasmonic ELISA based on enzyme-assisted etching of Au nanorods for the highly sensitive detection of aflatoxin B-1 in corn samples. Sens. Actuators B Chem. 2018, 267, 320–327. [Google Scholar] [CrossRef]
- Liu, Z.J.; Wang, X.Y.; Ren, X.X.; Li, W.B.; Sun, J.F.; Wang, X.W.; Huang, Y.Q.; Guo, Y.G.; Zeng, H.W. Novel fluorescence immunoassay for the detection of zearalenone using HRP-mediated fluorescence quenching of gold-silver bimetallic nanoclusters. Food Chem. 2021, 355, 129633. [Google Scholar] [CrossRef]
- Thouvenot, D.; Morfin, R.F. Radioimmunoassay for zearalenone and zearalanol in human serum: Production, properties, and use of porcine antibodies. Appl. Environ. Microb. 1983, 45, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, H.; Li, Y.; Shao, Y.N.; Huang, X.L.; Xiong, Y.H. Multicolor quantum dot nanobeads for simultaneous multiplex immunochromatographic detection of mycotoxins in maize. Sens. Actuators B Chem. 2019, 291, 411–417. [Google Scholar] [CrossRef]






| Determinand | Structure | IC50 (μg/L) | Cross-Reaction Rate |
|---|---|---|---|
| Zearalenone |  | 0.85 | 100 |
| α-Zearalanol |  | 2.41 | 35.27 |
| β-Zearalanol |  | 1.86 | 45.70 |
| α-Zearalenol |  | 2.86 | 29.72 |
| β-Zearalenol |  | 4.74 | 17.93 |
| Zearalanone |  | 53.79 | 1.58 |
| AFB1 |  | >1000 | <0.01 |
| OTA |  | >1000 | <0.01 |
| FB2 |  | >1000 | <0.01 |
| T-2 toxin |  | >1000 | <0.01 |
| Method Applied | Materials | Limit of Detection | References |
|---|---|---|---|
| GC-MS | - | 5 ng/g | [15] |
| LC-MS/MS | - | 0.02 ng/mL | [18] |
| Lateral flow immunochromatographic assays | Colloidal gold, quantum dots, polystyrene microspheres | 10, 1, 1 μg/L | [22] |
| Fluorescence-linked immunosorbent assay | CdTe/CdS/ZnS quantum dots | 0.012 ng/mL | [27] |
| Multiplexed immunochromatographic assay | Dual CdSe/ZnS quantum dot nanobeads | 10 ng/mL | [31] |
| ic-ELISA | - | 0.22 ± 0.08 μg/L (IC15) | This work |
| Visualized multicolor ELISA | AuNRs | 0.1 μg/L | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Liu, K.; Yang, X.; Yang, J.; Pan, M.; Wang, S. Development of Indirect Competitive ELISA and Visualized Multicolor ELISA Based on Gold Nanorods Growth for the Determination of Zearalenone. Foods 2021, 10, 2654. https://doi.org/10.3390/foods10112654
Ma T, Liu K, Yang X, Yang J, Pan M, Wang S. Development of Indirect Competitive ELISA and Visualized Multicolor ELISA Based on Gold Nanorods Growth for the Determination of Zearalenone. Foods. 2021; 10(11):2654. https://doi.org/10.3390/foods10112654
Chicago/Turabian StyleMa, Tianyu, Kaixin Liu, Xiao Yang, Jingying Yang, Mingfei Pan, and Shuo Wang. 2021. "Development of Indirect Competitive ELISA and Visualized Multicolor ELISA Based on Gold Nanorods Growth for the Determination of Zearalenone" Foods 10, no. 11: 2654. https://doi.org/10.3390/foods10112654





