Microbiological Food Safety of Seaweeds
Abstract
:1. Introduction
2. Pathogenic Microorganisms in Seaweed
2.1. Bacillus sp.
2.2. Pathogenic Vibrios
2.3. Aeromonas sp.
2.4. Escherichia coli, Salmonella spp., Listeria monocytogenes, Staphylococcus aureus, and Other Microorganisms Associated with Health Hazard in Seaweed
2.5. Viruses
2.6. Antimicrobial Resistance
3. Processing and Factors That Control Microbial Growth in Seaweed
3.1. Drying
3.2. Thermal Processing
3.3. Fermentation
3.4. Freezing
3.5. Salting
3.6. Gamma Radiation
3.7. Emerging Trends (Other Novel Technologies)
4. Guidelines and Legislation
5. Data Gaps
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. The global status of seaweed production, trade and utilization. Globefish Research Programme 2018, 124, 120. [Google Scholar]
- Hollants, J.; Leliaert, F.; De Clerck, O.; Willems, A. What we can learn from sushi: A review on seaweed-bacterial associations. FEMS Microbiol. Ecol. 2013, 83, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goecke, F.; Thiel, V.; Wiese, J.; Labes, A.; Imhoff, J.F. Algae as an important environment for bacteria—Phylogenetic relationships among new bacterial species isolated from algae. Phycologia 2013, 52, 14–24. [Google Scholar] [CrossRef]
- Singh, R.P.; Reddy, C.R.K. Seaweed-microbial interactions: Key functions of seaweed-associated bacteria. FEMS Microbiol. Ecol. 2014, 88, 213–230. [Google Scholar] [CrossRef]
- Selvarajan, R.; Sibanda, T.; Venkatachalam, S.; Ogola, H.J.O.; Obieze, C.C.; Msagati, T.A. Distribution, Interaction and Functional Profiles of Epiphytic Bacterial Communities from the Rocky Intertidal Seaweeds, South Africa. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, N.B.; Lundsteen, S. Forekomst af Mikroorganismer på Tang—Specielt på Spiseligt Tang, der Forekommer i de Danske Farvande; DCA Report No. 048; Aarhus Universitet: Aarhus, Denmark, 2014. [Google Scholar]
- Duinker, A.; Roiha, I.S.; Amlund, H.; Dahl, L.; Lock, E.-J.; Kögel, T.; Måge, A.; Lunestad, B.T. Potential Risks Posed by Macroalgae for Applications as Feed and Food—A Norwegian Perspective; National Institute of Nutrition and Seafood Research (NIFES): Bergen, Norway, 2016. [Google Scholar]
- Lytou, A.E.; Schoina, E.; Liu, Y.; Michalek, K.; Stanley, M.S.; Panagou, E.Z.; Nychas, G.J.E. Quality and safety assessment of edible seaweeds Alaria esculenta and Saccharina latissima cultivated in Scotland. Foods 2021, 10, 2210. [Google Scholar] [CrossRef]
- Mazure, H.G.F.; Field, J.G. Density and ecological importance of bacteria on kelp fronds in an upwelling region. J. Exp. Mar. Biol. Ecol. 1980, 43, 173–182. [Google Scholar] [CrossRef]
- Bengtsson, M.M.; Sjøtun, K.; Øvreås, L. Seasonal dynamics of bacterial biofilms on the kelp Laminaria hyperborea. Aquat. Microb. Ecol. 2010, 60, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Lakshmanaperumalsamy, P.; Purushothaman, A. Heterotrophic bacteria associated with seaweed. Proc. Plant Sci. 1982, 91, 487–493. [Google Scholar] [CrossRef]
- Chan, E.C.S.; McManus, E.A. Distribution, characterization, and nutrition of marine microorganisms from algae Polysiphonia lanosa and Ascophyllum nodosum. Can. J. Microbiol. 1969, 15, 409–420. [Google Scholar] [CrossRef]
- Kong, M.K.; Chan, K. Study on the bacterial flora isolated from marine algae. Bot. Mar. 1979, 22, 83–97. [Google Scholar] [CrossRef]
- Cundell, A.M.; Sleeter, T.D.; Mitchell, R. Microbial populations associated with surface of brown algae Ascophyllum nodosum. Microb. Ecol. 1977, 4, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Nayyar, D.; Skonberg, D.I. Contrasting effects of two storage temperatures on the microbial, physicochemical, and sensory properties of two fresh red seaweeds, Palmaria palmata and Gracilaria tikvahiae. J. Appl. Phycol. 2019, 31, 731–739. [Google Scholar] [CrossRef]
- Largo, D.B.; Fukami, K.; Adachi, M.; Nishijima, T. Direct enumeration of bacteria from macroalgae by epifluorescence microscopy as applied to the fleshy red algae Kappaphycus alvarezii and Gracilaria spp. (Rhodophyta). J. Phycol. 1997, 33, 554–557. [Google Scholar] [CrossRef]
- Karacalar, U.; Turan, G. Microbiological assays on edible seaweed Ulva lactuca (L.) cultured in outdoor tanks. J. Appl. Biol. Sci. 2008, 2, 27–30. [Google Scholar]
- Moore, J.E.; Xu, J.; Millar, B.C. Diversity of the microflora of edible macroalga (Palmaria palmata). Food Microbiol. 2002, 19, 249–257. [Google Scholar] [CrossRef]
- Kudaka, J.; Itokazu, K.; Taira, K.; Nidaira, M.; Okan, S.; Nakamura, M.; Iwanaga, S.; Tominagai, M.; Ohno, A. Investigation and culture of microbial contaminants of Caulerpa lentillifera (sea grape). J. Food Hyg. Soc. Jpn. 2008, 49, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Musa, N.; Wei, L.S. Bacteria attached on cultured seaweed Gracilaria changii at Mengabang Telipot, Terengganu. Acad. J. Plant Sci. 2008, 1, 1–4. [Google Scholar]
- Blikra, M.J.; Løvdal, T.; Vaka, M.R.; Roiha, I.S.; Lunestad, B.T.; Lindseth, C.; Skipnes, D. Assessment of food quality and microbial safety of brown macroalgae (Alaria esculenta and Saccharina latissima). J. Sci. Food Agric. 2019, 99, 1198–1206. [Google Scholar] [CrossRef]
- Rogerson, A. On the abundance of marine naked amebas on the surfaces of 5 species of macroalgae. FEMS Microbiol. Ecol. 1991, 85, 301–312. [Google Scholar] [CrossRef]
- Del Olmo, A.; Picon, A.; Nunez, M. The microbiota of eight species of dehydrated edible seaweeds from North West Spain. Food Microbiol. 2018, 70, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Martelli, F.; Marrella, M.; Lazzi, C.; Neviani, E.; Bernini, V. Microbiological contamination of ready to eat algae and evaluation of Bacillus cereus behavior by microbiological challenge test. J. Food Prot. 2021, 84, 1275–1280. [Google Scholar] [CrossRef]
- Ziino, G.; Nibali, V.; Panebianco, A. Bacteriological investigation on “Mauro” sold in Catania. Vet. Res. Commun. 2010, 34, S157–S161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fødevarestyrelsen. Prøveresultater—Hygiejnisk Kvalitet af Spiselig Tang. Available online: https://www.foedevarestyrelsen.dk/Kontrol/Kontrolresultater/Proeveresultater/Sider/Proeveresultater_fisk_og_fiskeprodukter_spiselig_tang.aspx (accessed on 5 June 2021).
- Banach, J.L.; Hoek-van den Hil, E.F.; van der Fels-Klercx, H.J. Food safety hazards in the European seaweed chain. Compr. Rev. Food Sci. Food Saf. 2020, 19, 332–364. [Google Scholar] [CrossRef]
- Sakon, N.; Sadamasu, K.; Shinkai, T.; Hamajima, Y.; Yoshitomi, H.; Matsushima, Y.; Takada, R.; Terasoma, F.; Nakamura, A.; Komano, J.; et al. Foodborne Outbreaks Caused by Human Norovirus GII.P17-GII.17-Contaminated Nori, Japan, 2017. Emerg. Infect. Dis. 2018, 24, 920–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.G.; Oh, M.H.; Lee, G.Y.; Hwang, I.G.; Kwak, H.S.; Kang, Y.S.; Koh, Y.H.; Jun, H.K.; Kwon, K.S. Analysis of major foodborne pathogens in various foods in Korea. Food Sci. Biotechnol. 2008, 17, 483–488. [Google Scholar]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef]
- Bennani, H.; Mateus, A.; Mays, N.; Eastmure, E.; Stark, K.D.C.; Hasler, B. Overview of Evidence of Antimicrobial Use and Antimicrobial Resistance in the Food Chain. Antibiotics-Basel 2020, 9, 49. [Google Scholar] [CrossRef] [Green Version]
- Logan, N.A.; De Vos, P.; Genus, I. Bacillus . In Bergey’s Manual of Systematic Bacteriology; De Vos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A., Schleifer, K., Whitman, W.B., Eds.; Springer: New York, NY, USA, 2009; Volume 3, pp. 21–128. [Google Scholar]
- Kramer, J.M.; Gilbert, R.J. Bacillus cereus and other Bacillus species. In Foodborne Bacterial Pathogens; Doyle, M.P., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1989; pp. 21–70. [Google Scholar]
- Salkinoja-Salonen, M.S.; Vuorio, R.; Andersson, M.A.; Kampfer, P.; Andersson, M.C.; Honkanen-Buzalski, T.; Scoging, A.C. Toxigenic strains of Bacillus licheniformis related to food poisoning. Appl. Environ. Microbiol. 1999, 65, 4637–4645. [Google Scholar] [CrossRef] [Green Version]
- From, C.; Hormazabal, V.; Granum, P.E. Food poisoning associated with pumilacidin-producing Bacillus pumilus in rice. Int. J. Food Microbiol. 2007, 115, 319–324. [Google Scholar] [CrossRef]
- Madslien, E.H.; Ronning, H.T.; Lindback, T.; Hassel, B.; Andersson, M.A.; Granum, P.E. Lichenysin is produced by most Bacillus licheniformis strains. J. Appl. Microbiol. 2013, 115, 1068–1080. [Google Scholar] [CrossRef]
- Jamal, M.T.; Morris, P.C.; Hansen, R.; Jamieson, D.J.; Burgess, J.G.; Austin, B. Recovery and characterization of a 30.7-kDa protein from Bacillus licheniformis associated with inhibitory activity against methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, and Listeria monocytogenes. Mar. Biotechnol. 2006, 8, 587–592. [Google Scholar] [CrossRef]
- Kanagasabhapathy, M.; Yamazaki, G.; Ishida, A.; Sasaki, H.; Nagata, S. Presence of quorum-sensing inhibitor-like compounds from bacteria isolated from the brown alga Colpomenia sinuosa. Lett. Appl. Microbiol. 2009, 49, 573–579. [Google Scholar] [CrossRef]
- Singh, R.P.; Bijo, A.J.; Baghel, R.S.; Reddy, C.R.K.; Jha, B. Role of bacterial isolates in enhancing the bud induction in the industrially important red alga Gracilaria dura. FEMS Microbiol. Ecol. 2011, 76, 381–392. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Rajauria, G.; Abu-Ghannam, N. Study of the microbial diversity and antimicrobial properties of Irish edible brown seaweeds. Int. J. Food Sci. Technol. 2010, 45, 482–489. [Google Scholar] [CrossRef] [Green Version]
- Oh, D.H.; Ha, S.D.; Hong, C.H. Study on the Reduction of Foodborne Pathogenic Bacteria in Ready-to-Eat (RTE) Foods; Korea Food and Drug Administration: Seoul, Korea, 2004.
- Park, S.Y.; Choi, J.W.; Yeon, J.H.; Lee, M.J.; Oh, D.H.; Hong, C.H. Assessment of contamination level of foodborne pathogens isolated in Kimbab and its main ingredients in the process of preparation. Korean J. Food Sci. Technol. 2005, 37, 122–128. [Google Scholar]
- Bahk, G.J.; Todd, E.C.D.; Hong, C.H.; Oh, D.H.; Ha, S.D. Exposure assessment for Bacillus cereus in ready-to-eat Kimbab selling at stores. Food Control 2007, 18, 682–688. [Google Scholar] [CrossRef]
- Granum, P.E.; Braid-Parker, T.C. Bacillus species. In The Microbiological Safety and Quality of Food; Lund, B.M., Braid-Parker, T.C., Gould, G.W., Eds.; Aspen Publishers: Gaithersburg, MD, USA, 2000; Volume II, pp. 1029–1039. [Google Scholar]
- Granum, P.E. Bacillus cereus. In Food Microbiology. Fundamentals and Frontiers; Doyle, M.P., Beuchat, L.R., Eds.; ASM Press: Washington, DC, USA, 2007; pp. 445–455. [Google Scholar]
- Setlow, P. Spores of Bacillus subtilis: Their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 2006, 101, 514–525. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Bacteriological Analytical Manual; Association of Official Analytical Chemists: Washington DC, USA, 1998.
- Trunet, C.; Mtimet, N.; Mathot, A.G.; Postollec, F.; Leguerinel, I.; Sohier, D.; Couvert, O.; Carlin, F.; Coroller, L. Modeling the Recovery of Heat-Treated Bacillus licheniformis Ad978 and Bacillus weihenstephanensis KBAB4 Spores at Suboptimal Temperature and pH Using Growth Limits. Appl. Environ. Microbiol. 2015, 81, 562–568. [Google Scholar] [CrossRef] [Green Version]
- Samapundo, S.; Heyndrickx, M.; Xhaferi, R.; de Baenst, I.; Devlieghere, F. The combined effect of pasteurization intensity, water activity, pH and incubation temperature on the survival and outgrowth of spores of Bacillus cereus and Bacillus pumilus in artificial media and food products. Int. J. Food Microbiol. 2014, 181, 10–18. [Google Scholar] [CrossRef]
- Gauvry, E.; Mathot, A.-G.; Couvert, O.; Leguerinel, I.; Coroner, L. Effects of temperature, pH and water activity on the growth and the sporulation abilities of Bacillus subtilis BSB1. Int. J. Food Microbiol. 2021, 337, 108915. [Google Scholar] [CrossRef]
- International Organization of Standardization. Microbiology of Food and Animal Feeding Stuffs—Guidelines for the Estimation of Measurement Uncertainty for Quantitative Determinations; ISO/TS 19036; French Association for Normalization: La Plaine Saint-Denis, France, 2009. [Google Scholar]
- Glass, K.A.; Loeffelholz, J.M.; Ford, J.P.; Doyle, M.P. Fate of Escherichia coli O157/H7 as affected by pH or sodium chloride and in fermented dry sausage. Appl. Environ. Microbiol. 1992, 58, 2513–2516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clavero, M.R.S.; Beuchat, L.R. Survival of Escherichia coli O157:H7 in broth and processed salami as influenced by pH, water activity, and temperature and suitability of media for its recovery. Appl. Environ. Microbiol. 1996, 62, 2735–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, P.A.; Brayton, P.R.; Bryant, T.N.; Colwell, R.R. Numerical taxonomy of Vibrios isolated from aquatic environments. Int. J. Syst. Bacteriol. 1986, 36, 531–543. [Google Scholar] [CrossRef]
- Lunestad, B.T.; Rosnes, J.T.; Levsen, A. Tracing pathogens in fish production chains. In Tracing Patogens in the Food Chain; Brul, S., Fratamico, P., McMeekin, T.A., Eds.; Woodhead Publishing: Cambrigde, UK, 2011; pp. 433–464. [Google Scholar]
- Adams, M.R.; Moss, M.O.; McClure, P. Food Microbiology, 4th ed.; The Royal Society of Chemistry: Cambrigde, UK, 2016. [Google Scholar]
- Drake, S.L.; DePaola, A.; Jaykus, L.A. An overview of Vibrio vulnificus and Vibrio parahaemolyticus. Compr. Rev. Food Sci. Food Saf. 2007, 6, 120–144. [Google Scholar] [CrossRef]
- Forsythe, S.J. The Microbiology of Safe Food; John Wiley & Sons: Nottingham, UK, 2010; p. 496. [Google Scholar]
- Jorgensen, J.H.; Pfaller, M.A.; Carroll, K.C. Manual of Clinical Microbiology; ASM Press: Washington, DC, USA, 2015; p. 2892. [Google Scholar]
- Farmer, J.J. The family Vibrionaceae. In The Prokaryotes: Volume 6: Proteobacteria: Gamma Subclass; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Vezzulli, L.; Colwell, R.R.; Pruzzo, C. Ocean Warming and Spread of Pathogenic Vibrios in the Aquatic Environment. Microb. Ecol. 2013, 65, 817–825. [Google Scholar] [CrossRef]
- Egan, S.; Harder, T.; Burke, C.; Steinberg, P.; Kjelleberg, S.; Thomas, T. The seaweed holobiont: Understanding seaweed-bacteria interactions. FEMS Microbiol. Rev. 2013, 37, 462–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnin-Jusserand, M.; Copin, S.; Le Bris, C.; Brauge, T.; Gay, M.; Brisabois, A.; Grard, T.; Midelet-Bourdin, G. Vibrio species involved in seafood-borne outbreaks (Vibrio cholerae, V. parahaemolyticus and V. vulnificus): Review of microbiological versus recent molecular detection methods in seafood products. Crit. Rev. Food Sci. Nutr. 2019, 59, 597–610. [Google Scholar] [CrossRef]
- Kokashvili, T.; Whitehouse, C.A.; Tskhvediani, A.; Grim, C.J.; Elbakidze, T.; Mitaishvili, N.; Janelidze, N.; Jaiani, E.; Haley, B.J.; Lashkhi, N.; et al. Occurrence and diversity of clinically important Vibrio species in the aquatic environment of Georgia. Front. Public Health 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- West, P.A. The human pathogenic Vibrios—A public health update with environmental perspectives. Epidemiol. Infect. 1989, 103, 1–34. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. infections. Nat. Rev. Dis. Primers 2018, 4, 1–19. [Google Scholar] [CrossRef]
- Austin, B. Vibrios as causal agents of zoonoses. Vet. Microbiol. 2010, 140, 310–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CDC—Centers for Disease Control and Prevention. Cholera—Vibrio cholerae infection. Available online: https://www.cdc.gov/cholera/infection-sources.html (accessed on 28 May 2021).
- Vugia, D.J.; Shefer, A.M.; Douglas, J.; Greene, K.D.; Bryant, R.G.; Werner, S.B. Cholera from raw seaweed transported from the Philippines to California. J. Clin. Microbiol. 1997, 35, 284–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumner, J.; Ross, T. A semi-quantitative seafood safety risk assessment. Int. J. Food Microbiol. 2002, 77, 55–59. [Google Scholar] [CrossRef]
- Honda, T.; Iida, T. The pathogenicity of Vibrio parahaemolyticus and the role of the thermostable direct haemolysin and related haemolysins. Rev. Med Microbiol. 1993, 4, 106–113. [Google Scholar] [CrossRef]
- Mahmud, Z.H.; Neogi, S.B.; Kassu, A.; Wada, T.; Islam, M.S.; Nair, G.B.; Ota, F. Seaweeds as a reservoir for diverse Vibrio parahaemolyticus populations in Japan. Int. J. Food Microbiol. 2007, 118, 92–96. [Google Scholar] [CrossRef]
- Mahmud, Z.H.; Neogi, S.B.; Kassu, A.; Huong, B.T.M.; Jahid, I.K.; Islam, M.S.; Ota, F. Occurrence, seasonality and genetic diversity of Vibrio vulnificus in coastal seaweeds and water along the Kii Channel, Japan. FEMS Microbiol. Ecol. 2008, 64, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Barberi, O.N.; Byron, C.J.; Burkholder, K.M.; St Gelais, A.T.; Williams, A.K. Assessment of bacterial pathogens on edible macroalgae in coastal waters. J. Appl. Phycol. 2020, 32, 683–696. [Google Scholar] [CrossRef] [Green Version]
- Håkonsholm, F.; Lunestad, B.T.; Sanchez, J.R.A.; Martinez-Urtaza, J.; Marathe, N.P.; Svanevik, C.S. Vibrios from the Norwegian marine environment: Characterization of associated antibiotic resistance and virulence genes. MicrobiologyOpen 2020, 9. [Google Scholar] [CrossRef]
- Colwell, R.R.; Macdonell, M.T.; Deley, J. Proposal to recognize the family Aeromonadaceae fam. nov. Int. J. Syst. Bacteriol. 1986, 36, 473–477. [Google Scholar] [CrossRef] [Green Version]
- Martin-Carnahan, A.; Joseph, S.W. Aeromonadales ord. nov. In Bergey’s Manual of Systematic Bacteriology: Volume Two The Proteobacteria Part B The Gammaproteobacteria; Brenner, D.J., Krieg, N.R., Staley, J.T., Garrity, G.M., Boone, D.R., De Vos, P., Goodfellow, M., Rainey, F.A., Schleifer, K.-H., Eds.; Springer: Boston, MA, USA, 2005. [Google Scholar]
- Tomás, J.M. The main Aeromonas pathogenic factors. ISRN Microbiol. 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, L.N.; Wang, L.X. The microbial safety of fish and fish products: Recent advances in understanding its significance, contamination sources, and control strategies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 738–786. [Google Scholar] [CrossRef]
- Di Pinto, A.; Terio, V.; Pinto, P.; Tantillo, G. Detection of potentially pathogenic Aeromonas isolates from ready-to-eat seafood products by PCR analysis. Int. J. Food Sci. Technol. 2012, 47, 269–273. [Google Scholar] [CrossRef]
- Hoel, S.; Vadstein, O.; Jakobsen, A.N. Species Distribution and Prevalence of Putative Virulence Factors in Mesophilic Aeromonas spp. Isolated from Fresh Retail Sushi. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Hoel, S.; Lunestad, B.T.; Lerfall, J.; Jakobsen, A.N. Aeromonas spp. isolated from ready-to-eat seafood on the Norwegian market: Prevalence, putative virulence factors and antimicrobial resistance. J. Appl. Microbiol. 2021, 130, 1380–1393. [Google Scholar] [CrossRef] [PubMed]
- Vairappan, C.S.; Suzuki, M. Dynamics of total surface bacteria and bacterial species counts during desiccation in the Malaysian sea lettuce, Ulva reticulata (Ulvales, Chlorophyta). Phycol. Res. 2000, 48, 55–61. [Google Scholar] [CrossRef]
- Liot, F.; Colin, A.; Mabeau, S. Microbiology and storage life of fresh edible seaweeds. J. Appl. Phycol. 1993, 5, 243–247. [Google Scholar] [CrossRef]
- Cho, K.M.; Kambiranda, D.M.; Kim, S.W.; Math, R.K.; Lim, W.J.; Hong, S.Y.; Yun, H.D. Simultaneous Detection of Food-borne Pathogenic Bacteria in Ready-to-eat Kimbab Using Multiplex PCR Method. Food Sci. Biotechnol. 2008, 17, 1240–1245. [Google Scholar]
- Rho, M.J.; Schaffner, D.W. Microbial risk assessment of staphylococcal food poisoning in Korean kimbab. Int. J. Food Microbiol. 2007, 116, 332–338. [Google Scholar] [CrossRef]
- Ahmed, F.E. Seafood Safety; Commitee on the Evaluation of the Safety of Fishery Products; National Academic Press: Washington, DC, USA, 1991. [Google Scholar]
- Gill, C.O.; Reichel, M.P. Growth of the cold-tolerant pathogens Yersinia enterocolitica, Aeromonas hydrophila and Listeria monocytogenes on high-pH beef packaged under vacuum or carbon dioxide. Food Microbiol. 1989, 6, 223–230. [Google Scholar] [CrossRef]
- Choi, E.S.; Kim, N.H.; Kim, H.W.; Kim, S.A.; Il Jo, J.I.; Kim, S.H.; Lee, S.H.; Ha, S.D.; Rhee, M.S. Microbiological Quality of Seasoned Roasted Layer and Potential Hazard Control in a Real Processing Line. J. Food Prot. 2014, 77, 2069–2075. [Google Scholar] [CrossRef]
- Stevant, P.; Olafsdottir, A.; Deleris, P.; Dumay, J.; Fleurence, J.; Ingadottir, B.; Jonsdottir, R.; Ragueneau, E.; Rebours, C.; Rustad, T. Semi-dry storage as a maturation process for improving the sensory characteristics of the edible red seaweed dulse (Palmaria palmata). Algal Research-Biomass Biofuels Bioprod. 2020, 51. [Google Scholar] [CrossRef]
- Hyun, J.-E.; Kim, J.-H.; Choi, Y.-S.; Kim, E.-M.; Kim, J.-C.; Lee, S.-Y. Evaluation of microbial quality of dried foods stored at different relative humidity and temperature, and effects of packaging methods. J. Food Saf. 2018, 38. [Google Scholar] [CrossRef]
- General Administration of Quality Supervision Inspection and Quarantine in China (AQSIQ). Hygienic Standard for Marine Algae and Algae Products. 2005. Available online: http://www.codeofchina.com/gb/medical/18524.html (accessed on 19 August 2021).
- General Administration of Quality Supervision Inspection and Quarantine in China (AQSIQ). Dried Laver. 2009. Available online: http://www.codeofchina.com/gb/food/57681.html (accessed on 19 August 2021).
- Bosch, A.; Gkogka, E.; Le Guyader, S.F.; Loisy-Hamon, F.; Lee, A.; van Lieshout, L.; Marthi, B.; Myrmel, M.; Sansom, A.; Schultz, A.C.; et al. Foodborne viruses: Detection, risk assessment, and control options in food processing. Int. J. Food Microbiol. 2018, 285, 110–128. [Google Scholar] [CrossRef]
- Rzezutka, A.; Cook, N. Survival of human enteric viruses in the environment and food. FEMS Microbiol. Rev. 2004, 28, 441–453. [Google Scholar] [CrossRef] [Green Version]
- CDC—Centers for Disease Control and Prevention. Norovirus Worldwide. Available online: https://www.cdc.gov/norovirus/trends-outbreaks/worldwide.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fnorovirus%2Fworldwide.html (accessed on 4 June 2021).
- CDC—Centers for Disease Control and Prevention. Viral Hepatitis. Available online: https://www.cdc.gov/hepatitis/hav/index.htm (accessed on 4 June 2021).
- Tian, P.; Engelbrektson, A.L.; Jiang, X.; Zhong, W.M.; Mandrelli, R.E. Norovirus recognizes histo-blood group antigens on gastrointestinal cells of clams, mussels, and oysters: A possible mechanism of bioaccumulation. J. Food Prot. 2007, 70, 2140–2147. [Google Scholar] [CrossRef] [Green Version]
- Esseili, M.A.; Gao, X.; Boley, P.; Hou, Y.X.; Saif, L.J.; Brewer-Jensen, P.; Lindesmith, L.C.; Baric, R.S.; Atmar, R.L.; Wang, Q.H. Human Norovirus Histo-Blood Group Antigen (HBGA) Binding Sites Mediate the Virus Specific Interactions with Lettuce Carbohydrates. Viruses-Basel 2019, 11, 833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusumi, E.; Tanimoto, T.; Hosoda, K.; Tsubokura, M.; Hamaki, T.; Takahashi, K.; Kami, M. Multiple Norovirus Outbreaks Due to Shredded, Dried, Laver Seaweed in Japan. Infect. Control Hosp. Epidemiol. 2017, 38, 885–886. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Jeong, H.S.; Lee, J.S.; Lee, S.W.; Choi, Y.H.; Choi, S.J.; Joo, I.S.; Kim, Y.R.; Park, Y.K.; Youn, S.K. First norovirus outbreaks associated with consumption of green seaweed (Enteromorpha spp.) in South Korea. Epidemiol. Infect. 2015, 143, 515–521. [Google Scholar] [CrossRef]
- Donaldson, E.F.; Lindesmith, L.C.; Lobue, A.D.; Baric, R.S. Norovirus pathogenesis: Mechanisms of persistence and immune evasion in human populations. Immunol. Rev. 2008, 225, 190–211. [Google Scholar] [CrossRef]
- Lopman, B.; Gastanaduy, P.; Park, G.W.; Hall, A.J.; Parashar, U.D.; Vinje, J. Environmental transmission of norovirus gastroenteritis. Curr. Opin. Virol. 2012, 2, 96–102. [Google Scholar] [CrossRef]
- Whitworth, J. Norway Norovirus Outbreaks Linked to Seaweed Salad from China. Food Safety News. 2019. Available online: https://www.foodsafetynews.com/2019/09/norway-norovirus-outbreaks-linked-to-seaweed-salad-from-china/ (accessed on 10 July 2021).
- Martinez, J.L. Antibiotics and antibiotic resistance genes in natural environments. Science 2008, 321, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Burgmann, H.; Sorum, H.; Norstrom, M.; Pons, M.N.; et al. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef]
- Amarasiri, M.; Sano, D.; Suzuki, S. Understanding human health risks caused by antibiotic resistant bacteria (ARB) and antibiotic resistance genes (ARG) in water environments: Current knowledge and questions to be answered. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2016–2059. [Google Scholar] [CrossRef]
- Hatosy, S.M.; Martiny, A.C. The Ocean as a Global Reservoir of Antibiotic Resistance Genes. Appl. Environ. Microbiol. 2015, 81, 7593–7599. [Google Scholar] [CrossRef] [Green Version]
- Håkonsholm, F.; Hetland, M.A.K.; Svanevik, C.S.; Sundsfjord, A.; Lunestad, B.T.; Marathe, N.P. Antibiotic Sensitivity Screening of Klebsiella spp. and Raoultella spp. Isolated from Marine Bivalve Molluscs Reveal Presence of CTX-M-Producing K. pneumoniae. Microorganisms 2020, 8, 1909. [Google Scholar] [CrossRef]
- Grevskott, D.H.; Svanevik, C.S.; Sunde, M.; Wester, A.L.; Lunestad, B.T. Marine Bivalve Mollusks As Possible Indicators of Multidrug-Resistant Escherichia coli and Other Species of the Enterobacteriaceae Family. Front. Microbiol. 2017, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Canica, M.; Manageiro, V.; Abriouel, H.; Moran-Gilad, J.; Franz, C.M.A.P. Antibiotic resistance in foodborne bacteria. Trends Food Sci. Technol. 2019, 84, 41–44. [Google Scholar] [CrossRef]
- Bergspica, I.; Kaprou, G.; Alexa, E.A.; Prieto-Maradona, M.; Alvarez-Ordonez, A. Identification of risk factors and hotspots of antibiotic resistance along the food chain using next-generation sequencing. EFSA J. 2020, 18. [Google Scholar] [CrossRef]
- Morcom, T. The Role of Seaweed Antimicrobials in Selection for Antibiotic Resistance. University of Exeter, UK, Exeter Medical School. 2018. Available online: https://ore.exeter.ac.uk/repository/handle/10871/34642 (accessed on 17 August 2021).
- Mathlouthi, M. Water content, water activity, water structure and the stability of foodstuffs. Food Control 2001, 12, 409–417. [Google Scholar] [CrossRef]
- Da Silva, V.M.; Silva, L.A.; de Andrade, J.B.; da Cunha Veloso, M.C.; Santos, G.V. Determination of moisture content and water activity in algae and fish by thermoanalytical techniques. Quim. Nova 2008, 31, 901–905. [Google Scholar] [CrossRef] [Green Version]
- Ho, K.K.H.Y.; Redan, B.W. Impact of thermal processing on the nutrients, phytochemicals, and metal contaminants in edible algae. Crit. Rev. Food Sci. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.S. A Study on the Consumption Pattern of Laver. Korean J. Food Mark. Econ. 2010, 27, 1–23. [Google Scholar]
- Kim, N.H.; Yun, A.R.; Rhee, M.S. Prevalence and classification of toxigenic Staphylococcus aureus isolated from refrigerated ready-to-eat foods (sushi, kimbab and California rolls) in Korea. J. Appl. Microbiol. 2011, 111, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.; Ocio, M.J.; Fernandez, P.S.; Rodrigo, M.; Martinez, A. Application of nonlinear regression analysis to the estimation of kinetic parameters for two enterotoxigenic strains of Bacillus cereus spores. Food Microbiol. 1999, 16, 607–613. [Google Scholar] [CrossRef]
- Akomea-Frempong, S.; Skonberg, D.I.; Camire, M.E.; Perry, J.J. Impact of blanching, freezing, and fermentation on physicochemical, microbial, and sensory quality of sugar kelp (Saccharina latissima). Foods 2021, 10, 2258. [Google Scholar] [CrossRef]
- Skipnes, D.; Øines, S.; Rosnes, J.T.; Skåra, T. Heat transfer in vacuum packed mussels (Mytilus edulis) during thermal processing. J. Aquat. Food Prod. Technol. 2002, 11, 5–19. [Google Scholar] [CrossRef]
- Uchida, M.; Murata, M.; Ishikawa, F. Lactic acid bacteria effective for regulating the growth of contaminant bacteria during the fermentation of Undaria pinnatifida (Phaeophyta). Fish. Sci. 2007, 73, 694–704. [Google Scholar] [CrossRef]
- Skonberg, D.I.; Fader, S.; Perkins, L.B.; Perry, J.J. Lactic acid fermentation in the development of a seaweed sauerkraut-style product: Microbiological, physicochemical, and sensory evaluation. J. Food Sci. 2021, 86, 334–342. [Google Scholar] [CrossRef]
- Bruhn, A.; Brynning, G.; Johansen, A.; Lindegaard, M.S.; Sveigaard, H.H.; Aarup, B.; Fonager, L.; Andersen, L.L.; Rasmussen, M.B.; Larsen, M.M.; et al. Fermentation of sugar kelp (Saccharina latissima)-effects on sensory properties, and content of minerals and metals. J. Appl. Phycol. 2019, 31, 3175–3187. [Google Scholar] [CrossRef] [Green Version]
- Tolstorebrov, I.; Eikevik, T.M.; Saether, M. Influence of thermal properties of brown seaweeds (Saccharina latissima) on atmospheric freeze-drying process in fluidized bed. In Proceedings of the 25th International Congress of Refrigeration, Montreal, Canada, 24–30 August 2019. [Google Scholar]
- Del Olmo, A.; Picon, A.; Nunez, M. High pressure processing for the extension of Laminaria ochroleuca (kombu) shelf-life: A comparative study with seaweed salting and freezing. Innov. Food Sci. Emerg. Technol. 2019, 52, 420–428. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, X.; Hou, Z.; Hu, X.; Wang, Y.; Wang, C.; Yang, S.; Cui, H.; Zhu, L. Microbial Regulation of Deterioration and Preservation of Salted Kelp under Different Temperature and Salinity Conditions. Foods 2021, 10, 1723. [Google Scholar] [CrossRef]
- Park, S.Y.; Kang, S.; Ha, S.-D. Inactivation of murine norovirus-1 in the edible seaweeds Capsosiphon fulvescens and Hizikia fusiforme using gamma radiation. Food Microbiol. 2016, 56, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.; Lee, N.Y.; Kang, H.J.; Hong, S.P.; Kim, Y.H.; Kim, J.K.; Byun, M.W. Inactivation of pathogens inoculated into prepared seafood products for manufacturing kimbab, steamed rice rolled in dried seaweed, by gamma irradiation. J. Food Prot. 2005, 68, 396–402. [Google Scholar] [CrossRef]
- Chawla, S.P.; Kim, D.H.; Jo, C.; Lee, J.W.; Song, H.P.; Byun, M.W. Effect of gamma irradiation on the survival of pathogens in kwamegi, a traditional Korean semidried seafood. J. Food Prot. 2003, 66, 2093–2096. [Google Scholar] [CrossRef] [PubMed]
- Sommers, C.H.; Rajkowski, K.T. Radiation Inactivation of Foodborne Pathogens on Frozen Seafood Products. J. Food Prot. 2011, 74, 641–644. [Google Scholar] [CrossRef]
- Song, H.P.; Kim, B.; Jung, S.; Choe, J.H.; Yun, H.J.; Kim, Y.J.; Jo, C. Effect of gamma and electron beam irradiation on the survival of pathogens inoculated into salted, seasoned, and fermented oyster. Lwt-Food Sci. Technol. 2009, 42, 1320–1324. [Google Scholar] [CrossRef]
- Bai, Y.; Muhammad, A.I.; Hu, Y.; Koseki, S.; Liao, X.; Chen, S.; Ye, X.; Liu, D.; Ding, T. Inactivation kinetics of Bacillus cereus spores by Plasma activated water (PAW). Food Res. Int. 2020, 131. [Google Scholar] [CrossRef]
- Serment-Moreno, V.; Barbosa-Canovas, G.; Torres, J.A.; Welti-Chanes, J. High-pressure Processing: Kinetic Models for Microbial and Enzyme Inactivation. Food Eng. Rev. 2014, 6, 56–88. [Google Scholar] [CrossRef]
- Picon, A.; del Olmo, A.; Nunez, M. Bacterial diversity in six species of fresh edible seaweeds submitted to high pressure processing and long-term refrigerated storage. Food Microbiol. 2021, 94. [Google Scholar] [CrossRef]
- Pal, M. Pulsed electric field processing: An emerging technology for food preservation. J. Exp. Food Process. 2017, 3, 1000126. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Song, H.-H.; Ha, S.-D. Synergistic Effects of NaOCl and Ultrasound Combination on the Reduction of Escherichia coli and Bacillus cereus in Raw Laver. Foodborne Pathog. Dis. 2014, 11, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrasonics Sonochemistry 2021, 70. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.V.M. Use of power ultrasound to enhance the thermal inactivation of Clostridium perfringens spores in beef slurry. Int. J. Food Microbiol. 2015, 206, 17–23. [Google Scholar] [CrossRef]
- Noriega-Fernandez, E.; Sone, I.; Astrain-Redin, L.; Prabhu, L.; Sivertsvik, M.; Alvarez, I.; Cebrian, G. Innovative Ultrasound-Assisted Approaches towards Reduction of Heavy Metals and Iodine in Macroalgal Biomass. Foods 2021, 10, 649. [Google Scholar] [CrossRef] [PubMed]
- CEVA. Règlementation Algues Alimentaries Synthèse CEVA au 10/02/2014; Centre D’etude et de Valorisation des Algues: Pleubian, France, 2014. [Google Scholar]
- Korean Food and Drug Administration (KFDA). Food Code; Korean Food and Drug Administration (KFDA): Seoul, Korea, 2008.
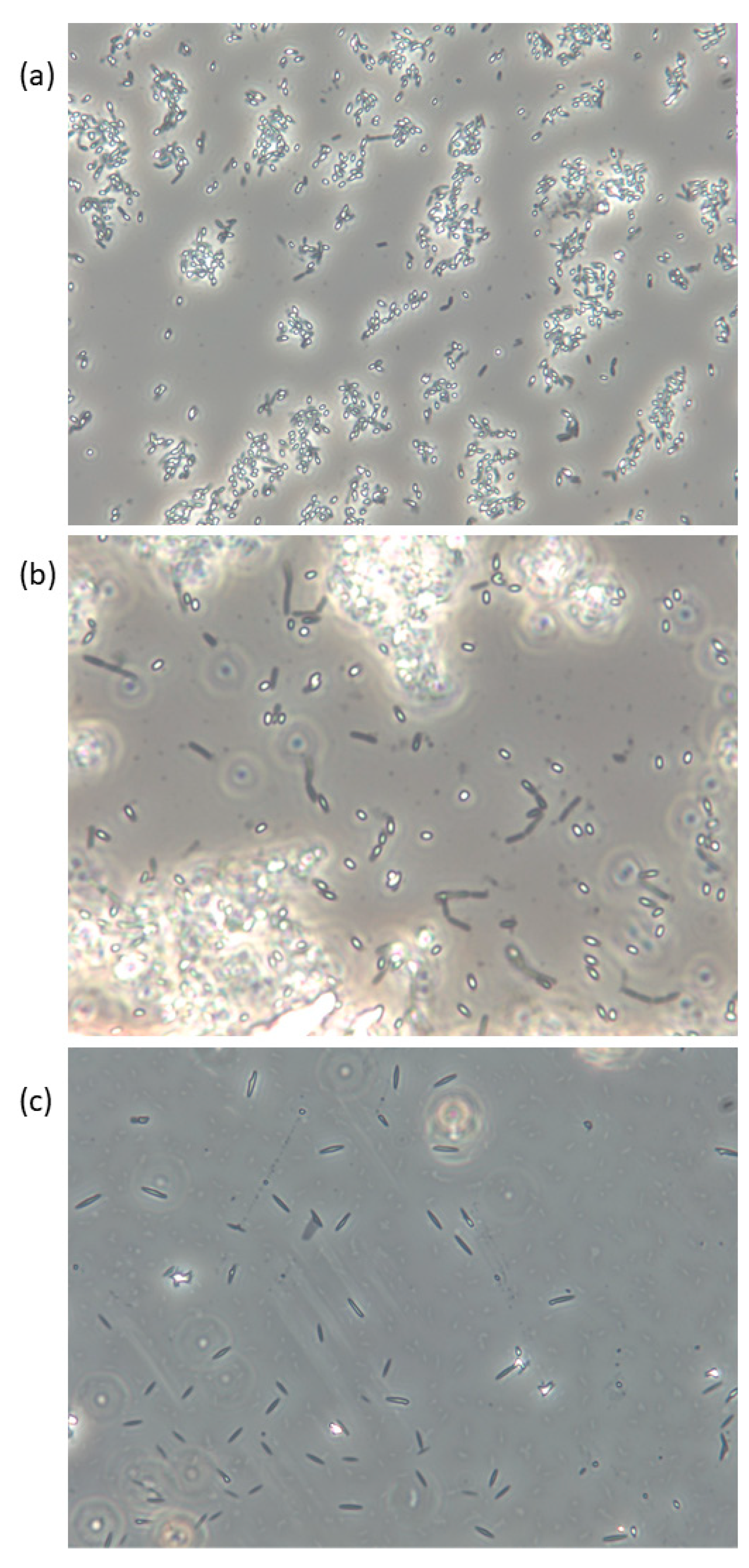
| Seaweed Species [Wild (w), Cultivated (c), or Unknown (u)] | Sampling Location/Region | Month and Year of Sampling | Bacterial Density (Log cfu/g) * | Method [PC; Platecounting, M; Microscopy] | Reference |
|---|---|---|---|---|---|
| Polysiphonia [Vertebrata] lanosa (Sea Truffle) (w) | Point Lepreau, Bay of Fundy, New Brunswick, Canada | May–September, 1964 | ~6–7 | PC: Seawater Tryptone Soy Agar, Aerobic, 10 °C | [12] |
| Ascophyllum nodosum (w) | ~4–7 | ||||
| Ascophyllum nodosum (w) | Nahant, Massachusetts, USA | February, 1976 | ~8 † | M: Scanning Electron Microscopy | [14] |
| Palmaria palmata (c) | Bristol, ME, USA | February (year not given) | ~3 | PC: Petrifilm Aerobic Count Plate, 37 °C | [15] |
| Gracilaria tikvahiae (c) | September (year not given) | ~4 | |||
| Gracillaria spp. (u) | Mactan Island, Cebu, Philippines | March, 1996 | ~8–9 | M: DAPI-staining | [16] |
| Kappaphycus alvarezii (c) | Uranouchi Inlet, Tosa Bay, Southern Japan | Not given | ~5 | ||
| Ulva lactuca (c) | Izmir, Turkey | March and April, 2005 | 4.94 | PC: Plate Count Agar, aerobic, 37 °C | [17] |
| Palmaria palmata (w) | Cushendall, County Antrim, Northern Ireland (55 °N) | Not given | 5.11 | PC: Plate Count Agar, aerobic, 30 °C | [18] |
| Caulerpa lentillifera (Sea grape) (c) | Okinawa, Japan | August–September, 2006 | ~7 | PC: Marine Agar, aerobic, 25 °C | [19] |
| Gracilaria changii (c) | Mengabang Telipot, Malaysia | December, 2007 | 8.46 | PC: Tryptic soy agar with 2% NaCl, room temperature | [20] |
| Alaria esculenta (c) | West Norway (61° N) | March and April, 2016 | 2.01 | PC: Marine Agar, aerobic, 25 °C | [21] |
| Saccharina latissima (c) | 1.10 | ||||
| Alaria esculenta (c) | Port-a-Bhuiltin seaweed farm, Scotland | 2019 | 5.2 | PC: Marine Agar, aerobic, 30 °C | [8] |
| 2020 | 3.2 | ||||
| Saccharina latissima (c) | 2019 | 3.7 | |||
| 2020 | <2 | ||||
| Laminaria hyperborea (w) | Outside Bergen, West Norway (60° N) | March, 2007 | ~4 † | M: DAPI-staining | [10] |
| May, 2007 | ~6 † | ||||
| July–February, 2007 | ~7 † | ||||
| Fucus serratus (w) | Millport, Scotland | July and August, 1990 | 7.7 † | M: Scanning Electron Microscopy | [22] |
| Porphyra umbilicalis (w) | 7.2 † | ||||
| Ulva lactuca (w) | 7.6 † | ||||
| Chaetomorpha sp. (w) | Vellar Estuary, Parangipettai, India | September, 1978 | 6.54 | PC: Estuarine Peptone Yeast Extract Agar | [11] |
| October, 1978 | 6.96 | ||||
| March, 1979 | 6.58 | ||||
| April, 1979 | 6.24 | ||||
| May, 1979 | 6.06 | ||||
| Enteromorpha sp. (w) | September, 1978 | 6.50 | |||
| October, 1978 | 7.27 | ||||
| March, 1979 | 6.47 | ||||
| April, 1979 | 6.13 | ||||
| May, 1979 | 6.05 | ||||
| Hypnea sp. (w) | September, 1978 | 6.63 | |||
| October, 1978 | 7.14 | ||||
| March, 1979 | 6.61 | ||||
| April, 1979 | 6.17 | ||||
| May, 1979 | 6.06 | ||||
| Chondrus crispus (u) | A Coruna Province, North-western Spain | Not given | 4.21 | PC: Marine Agar, aerobic, 25 °C | [23] |
| Himanthalia elongata (u) | 1.67 | ||||
| Laminaria ocroleuca (u) | 4.36 | ||||
| Palmaria palmata (u) | 3.71 | ||||
| Porphyra umbillicalis (u) | 3.56 | ||||
| Saccharina latissima (u) | 3.09 | ||||
| Ulva lactuca (u) | 3.15 | ||||
| Undaria pinnatifida (u) | 4.99 | ||||
| Undaria pinnatifida (u) | Commercial products purchased in Italy | Not given | 3.39–5.49 | PC: Marine Agar, aerobic, 30 °C | [24] |
| Palmaria palmata (u) | 3.09–5.31 | ||||
| Laminaria spp. (u) | 2.23–4.54 | ||||
| Ulva spp. (u) | 2.88–5.58 | ||||
| Hizikia fusiformis (u) | 2.23–4.35 | ||||
| Alaria esculenta (c) | West Norway (61° N) | March, 2015 | 3.59 | PC: Marine Agar, aerobic, 25 °C | Unpublished results, the authors |
| Laminaria digitata (c) | 2.79 | ||||
| Saccharina latissima (c) | 2.75 | ||||
| Saccharina latissima (w) | West Norway | February, 2020 | 3.63 | PC: Plate Count Agar, aerobic, 30 °C |
| Pathogen | Temperature | pH | aw (min) | Max. Water Phase NaCl (%) | Reference | |||
|---|---|---|---|---|---|---|---|---|
| Min. | Max. | Min. | Max. | |||||
| B. cereus | 4 | 55 | 4.3 | 9.3 | 0.92 | 10 | [47] | |
| B. licheniformis | 11–15 | 50–55 | 4.6 | 9.8 | 0.91 | 7 | [32,48] | |
| B. pumilus | >5–15 | 40–50 | ≤6 (Some strains grow at 4.5) | ≥9.5 | <0.96 | >10 | [35,49] | |
| B. subtilis | 5.5 | 55.7 | 4.8 | 9.2 | 0.93 | >5–10 | [32,50] | |
| C. botulinum (growth only, proteolytic) | 10 | 48 | 4.6 | 9 | 0.93 | 10 | [47] | |
| C. botulinum (growth only, non-proteolytic) | 3.3 | 45 | 5.0 | 9 | 0.97 | 5 | ||
| C. perfringens | 10 | 52 | 5 | 9 | 0.93 | 7 | ||
| Pathogenic E. coli | 6.5 | 49.4 | 4 | 9 | 0.95 | 6.5 | [47,51,52,53] | |
| L. monocytogenes | −0.4 | 45 | 4.4 | 9.4 | 0.92 | 10 | [47] | |
| S. aureus | aerobe | 7 | 50 | 4 | 10 | 0.83 | 20 | |
| anaerobe | 5.0 | 0.90 | [51] | |||||
| Salmonella | 5.2 | 42.6 | 3.7 | 9.5 | 0.94 | 8 | [47] | |
| V. cholerae | 10 | ~44 | 5.0 | ~10 | 0.97 | 3 | [47,54,55,56] | |
| V. parahaemolyticus | 5 | ~44 | 4.8 | ~11 | 0.94 | 8 | [47,54,55,56,57] | |
| V. vulnificus | 10 | ~44 | 4.4 | ~9 | 0.96 | 6 | [47,57,58] | |
| Aeromonas hydrophila | 0 | 42 | 6 | 7.2 (optimum) | 0.97 | 5 | [47,59] | |
| Pathogen | Limit (cfu per g) | Comment | Reference |
|---|---|---|---|
| Aerobe mesophiles | ≤105 | French guidelines that apply to dry seaweed products | [141] |
| Coliforms (fecal) | ≤10 | ||
| Anaerobe sulfite reducers | ≤102 | ||
| S. aureus | ≤102 | ||
| C. perfringens | ≤1 | ||
| Salmonella | Not present per 25 g | ||
| S. aureus | <102 | Korean legislation that applies to RTE foods, including RTE seaweed | [85,142] |
| B. cereus | <103 | ||
| Salmonella spp. | 0 | ||
| Shigella spp. | 0 | ||
| L. monocytogenes | 0 | ||
| Aerobic plate counts | <3 × 104 cfu/g | Chinese hygienic standard for marine algae and algae products. Applies also to dried laver | [89,92,93] |
| Coliforms | <30 MPN/100 g | ||
| Mold | <300 cfu/g | ||
| Salmonella spp. | 0 | ||
| Shigella spp. | 0 | ||
| V. parahemolyticus | 0 | ||
| S. aureus | 0 | ||
| E. coli | <100 cfu/100 g | Guidelines for seaweed collected in Danish waters | [26] |
| Salmonella | Not present per 25 g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Løvdal, T.; Lunestad, B.T.; Myrmel, M.; Rosnes, J.T.; Skipnes, D. Microbiological Food Safety of Seaweeds. Foods 2021, 10, 2719. https://doi.org/10.3390/foods10112719
Løvdal T, Lunestad BT, Myrmel M, Rosnes JT, Skipnes D. Microbiological Food Safety of Seaweeds. Foods. 2021; 10(11):2719. https://doi.org/10.3390/foods10112719
Chicago/Turabian StyleLøvdal, Trond, Bjørn Tore Lunestad, Mette Myrmel, Jan Thomas Rosnes, and Dagbjørn Skipnes. 2021. "Microbiological Food Safety of Seaweeds" Foods 10, no. 11: 2719. https://doi.org/10.3390/foods10112719






