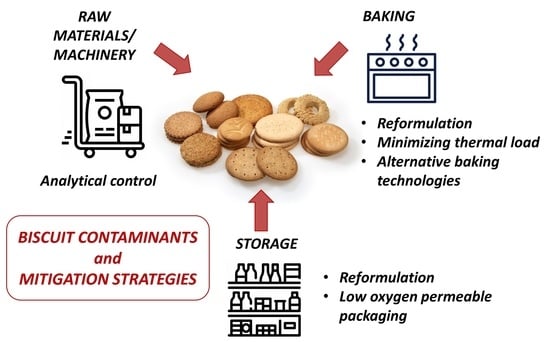
Biscuit Contaminants, Their Sources and Mitigation Strategies: A Review
Abstract
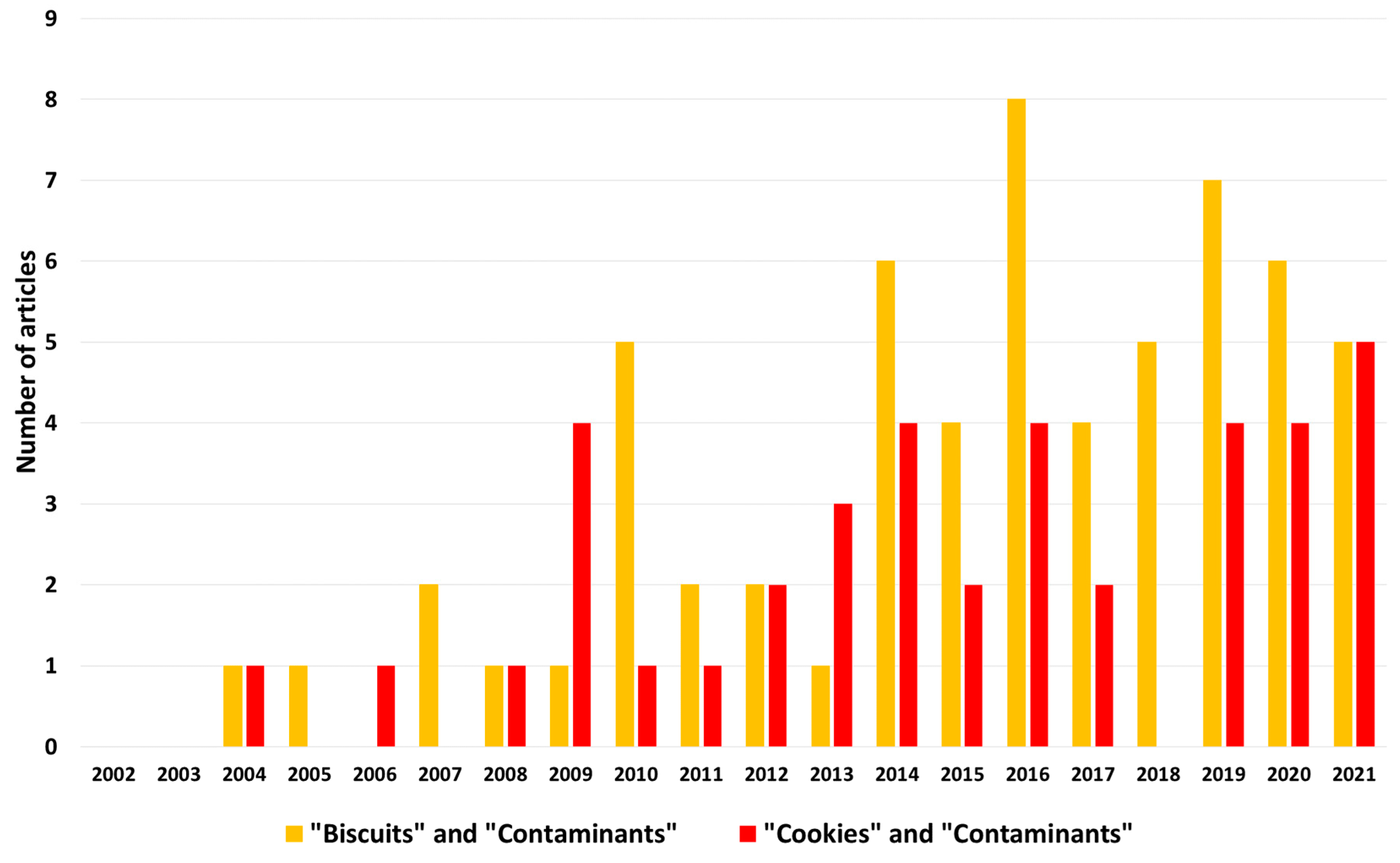
:1. Introduction
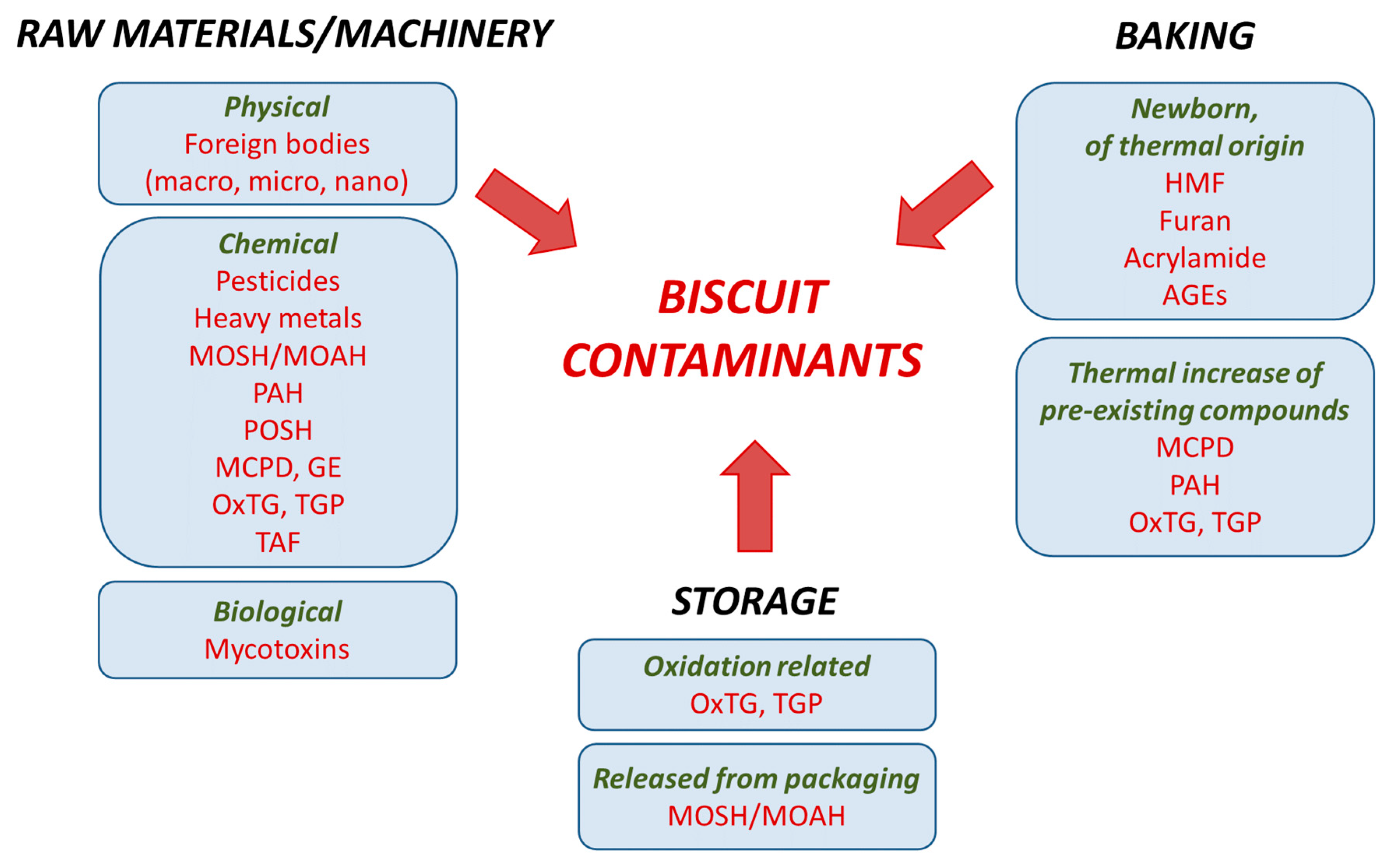
2. Contaminants Deriving from Raw Materials or Machinery
2.1. Physical Contaminants
2.2. Chemical Contaminants
2.3. Biological Contaminants
3. Contaminants Deriving from Baking
3.1. Neoformation of Contaminants of Thermal Origin
| Thermal Contaminant | Country | Year | Amount Detected | Reference |
|---|---|---|---|---|
| HMF | Slovakia | n.r. | 0.34–34.99 mg/kg | [110] |
| Spain | n.r. | 3.1–182.5 mg/kg | [111] | |
| Furan | Brazil | 2009–2011 | 38.1–105.3 μg/kg | [112] |
| UK | 2012 | 4–165 μg/kg | [113] | |
| UK | 2013 | 4–108 μg/kg | [113] | |
| Chile | n.r. | 91 μg/kg | [114] | |
| Acrylamide | Spain | 2007–2019 | 20–2144 μg/kg | [121] |
| Turkey | 2004–2006 | 78–486 μg/kg | [122] | |
| Slovenia | 2017–2018 | 20.5–3439 μg/kg | [123] | |
| Pakistan | n.r. | 52–507 μg/kg | [124] | |
| Syria | 2015 | 57–1433 μg/kg | [125] | |
| Colombia | n.r. | 35–753 μg/kg | [126] | |
| Saudi Arabia | 2015 | 90–182 μg/kg | [127] | |
| Czech Republic | n.r. | 571–787 μg/kg | [128] | |
| Belgium | 2002–2007 | 167 μg/kg (mean); 1514 μg/kg (maximum) | [129] | |
| Belgium | 2008–2013 | 142 μg/kg (mean); 1113 μg/kg (maximum) | [129] | |
| US | 2002–2006 | 119 μg/kg (mean) (n.d.–955 μg/kg) | [130] | |
| US | 2011–2015 | 181 μg/kg (mean) (5–1796 μg/kg) | [130] |
3.2. Thermal Increase of Pre-Existing Contaminants
3.3. Thermal Degradation of Pre-Existing Contaminants
4. Contaminants Deriving from Storage
4.1. Oxidation-Related Contaminants
4.2. Contaminants Released from Packaging
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Janssen, M.; Chang, B.P.; Hristov, H.; Pravst, I.; Profeta, A.; Millard, J. Changes in food consumption during the COVID-19 pandemic: Analysis of consumer survey data from the first lockdown period in Denmark, Germany, and Slovenia. Front. Nutr. 2021, 8, 635859. [Google Scholar] [CrossRef]
- Zwanka, R.J.; Buff, C. COVID-19 generation: A conceptual framework of the consumer behavioral shifts to be caused by the COVID-19 pandemic. J. Int. Consum. 2021, 33, 58–67. [Google Scholar] [CrossRef]
- Manley, D. Manley’s Technology of Biscuits, Crackers and Cookies; Woodhead Publishing: Sawston, UK, 2011; 632p. [Google Scholar]
- Goubgou, M.; Songré-Ouattara, L.T.; Bationo, F.; Lingani-Sawadogo, H.; Traoré, Y.; Savadogo, A. Biscuits: A systematic review and meta-analysis of improving the nutritional quality and health benefits. Food Prod. Process. Nutr. 2021, 3, 1–18. [Google Scholar] [CrossRef]
- Bravo-Núñez, A.; Gómez, M. Enrichment of cakes and cookies with pulse flours. A review. Food Rev. Int. 2021, 1, 1–9. [Google Scholar] [CrossRef]
- Feyera, M. Review on some cereal and legume based composite biscuits. Int. J. Agric. Sci. Technol. 2020, 6, 101–109. [Google Scholar]
- Nogueira, A.D.C.; Steel, C.J. Protein enrichment of biscuits: A review. Food Rev. Int. 2018, 34, 796–809. [Google Scholar] [CrossRef]
- Pasqualone, A.; Makhlouf, F.Z.; Barkat, M.; Difonzo, G.; Summo, C.; Squeo, G.; Caponio, F. Effect of acorn flour on the physico-chemical and sensory properties of biscuits. Heliyon 2019, 5, e02242. [Google Scholar] [CrossRef] [Green Version]
- Salehi, F. Recent applications of powdered fruits and vegetables as novel ingredients in biscuits: A review. Nutrire 2020, 45, 1. [Google Scholar] [CrossRef]
- Pasqualone, A.; Bianco, A.M.; Paradiso, V.M. Production trials to improve the nutritional quality of biscuits and to enrich them with natural anthocyanins. CYTA J. Food 2013, 11, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Pasqualone, A.; Laddomada, B.; Boukid, F.; De Angelis, D.; Summo, C. Use of almond skins to improve nutritional and functional properties of biscuits: An example of upcycling. Foods 2020, 9, 1705. [Google Scholar] [CrossRef]
- Awobusuyi, T.D.; Pillay, K.; Siwela, M. Consumer acceptance of biscuits supplemented with a sorghum–insect meal. Nutrients 2020, 12, 895. [Google Scholar] [CrossRef] [Green Version]
- Forbes-Ewan, C.; Moon, T.; Stanley, R. Present and future of military food technology. J. Food Sci. Eng. 2016, 6, 308–315. [Google Scholar] [CrossRef] [Green Version]
- Roth, J.P. The Logistics of the Roman Army at War (264 B.C.–A.D. 235); Brill: Boston, MA, USA, 1999; pp. 1–422. [Google Scholar]
- Grant, T.T. No. xiii. Apparatus for making ship’s biscuits. Trans. Soc. Inst. Lond. Encourag. Arts Manuf. Commer. 1833–1834, 50, 97–106. Available online: https://www.jstor.org/stable/41327298 (accessed on 2 November 2021).
- Senni, L.; Ricci, M.; Palazzi, A.; Burrascano, P.; Pennisi, P.; Ghirelli, F. On-line automatic detection of foreign bodies in biscuits by infrared thermography and image processing. J. Food Eng. 2014, 128, 146–156. [Google Scholar] [CrossRef]
- Gowen, A.A.; Tiwari, B.K.; Cullen, P.J.; McDonnell, K.; O’Donnell, C.P. Applications of thermal imaging in food quality and safety assessment. Trends Food Sci. Technol. 2010, 21, 190–200. [Google Scholar] [CrossRef]
- Amrani, M.A.; Alhomdi, M.; Ghaleb, A.M.; Al-Qubati, M.; Shameeri, M. Implementing an integrated maintenance management system for monitoring production lines: A case study for biscuit industry. J. Qual. Maint. Eng. 2020. [Google Scholar] [CrossRef]
- Gatti, A.M.; Tossini, D.; Gambarelli, A.; Montanari, S.; Capitani, F. Investigation of the presence of inorganic micro-and nanosized contaminants in bread and biscuits by environmental scanning electron microscopy. Crit. Rev. Food Sci. Nutr. 2008, 49, 275–282. [Google Scholar] [CrossRef]
- Ravikanth, L.; Singh, C.B.; Jayas, D.S.; White, N.D. Classification of contaminants from wheat using near-infrared hyperspectral imaging. Biosyst. Eng. 2015, 135, 73–86. [Google Scholar] [CrossRef]
- Thompson, L.A.; Darwish, W.S. Environmental chemical contaminants in food: Review of a global problem. J. Toxicol. 2019, 2019, 2345283. [Google Scholar] [CrossRef] [Green Version]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [Green Version]
- Walorczyk, S. Development of a multi-residue screening method for the determination of pesticides in cereals and dry animal feed using gas chromatography–triple quadrupole tandem mass spectrometry. J. Chromatogr. A 2007, 1165, 200–212. [Google Scholar] [CrossRef]
- He, Z.; Wang, L.; Peng, Y.; Luo, M.; Wang, W.; Liu, X. Multiresidue analysis of over 200 pesticides in cereals using a QuEChERS and gas chromatography–tandem mass spectrometry-based method. Food Chem. 2015, 169, 372–380. [Google Scholar] [CrossRef]
- Mastovska, K.; Dorweiler, K.J.; Lehotay, S.J.; Wegscheid, J.S.; Szpylka, K.A. Pesticide multiresidue analysis in cereal grains using modified QuEChERS method combined with automated direct sample introduction GC-TOFMS and UPLC-MS/MS techniques. J. Agric. Food Chem. 2010, 58, 5959–5972. [Google Scholar] [CrossRef]
- European Parliament and Council. Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. Off. J. Eur. Union 2005, L70, 1–16. [Google Scholar]
- European Food Safety Authority (EFSA); Carrasco Cabrera, L.; Medina Pastor, P. The 2019 European Union report on pesticide residues in food. EFSA J. 2021, 19, e06491. [Google Scholar]
- Łozowicka, B.; Miciński, J.; Zwierzchowski, G.; Kowalski, I.M.; Szarek, J. Monitoring study of pesticide residues in cereals and foodstuff from Poland. Pol. J. Environ. Stud. 2012, 21, 1703–1712. [Google Scholar]
- Kovač, M.; Bulaić, M.; Jakovljević, J.; Nevistić, A.; Rot, T.; Kovač, T.; Šarkanj, B. Mycotoxins, pesticide residues, and heavy metals analysis of Croatian cereals. Microorganisms 2021, 9, 216. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Neal, A.P.; Guilarte, T.R. Mechanisms of heavy metal neurotoxicity: Lead and manganese. J. Drug Metab. Toxicol. 2012, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Dada, E.O.; Ojo, O.N.; Njoku, K.L.; Akinola, M.O. Assessing the levels of Pb, Cd, Zn and Cu in biscuits and home-made snacks obtained from vendors in two tertiary institutions in Lagos, Nigeria. J. Appl. Sci. Environ. Manag. 2017, 21, 521–524. [Google Scholar] [CrossRef] [Green Version]
- Iwegbue, C.M. Metal contents in some brands of biscuits consumed in southern Nigeria. Am. J. Food Technol. 2012, 7, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Oyekunle, J.A.O.; Durodola, S.S.; Adekunle, A.S.; Afolabi, F.P.; Ore, O.T.; Lawal, M.O.; Ojo, O.S. Potentially toxic metals and polycyclic aromatic hydrocarbons composition of some popular biscuits in Nigeria. Chem. Afr. 2012, 4, 399–410. [Google Scholar] [CrossRef]
- Gopalani, M.; Shahare, M.; Ramteke, D.S.; Wate, S.R. Heavy metal content of potato chips and biscuits from Nagpur city, India. Bull. Environ. Contam. Toxicol. 2007, 97, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.K.; Radwan, M.A. Heavy metals (Cd, Pb) and trace elements (Cu, Zn) contents in some foodstuffs from the Egyptian market. Emirate J. Food Agric. 2005, 17, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Karavoltsos, S.; Sakellari, A.; Dimopoulos, M.; Dasenakis, M.; Scoullos, M. Cadmium content in foodstuffs from the Greek market. Food Addit. Contam. 2002, 19, 954–962. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- World Health Organization (WHO); Food and Agriculture Organization (FAO). General Standard for Contaminants and Toxins in Food and Feed. Codex Stan 193–1995. 2019, pp. 1–66. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B193-1995%252FCXS_193e.pdf (accessed on 2 November 2021).
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Nielsen, E. Update of the risk assessment of nickel in food and drinking water. EFSA J. 2020, 18, e06268. [Google Scholar] [CrossRef]
- Dahiya, S.; Karpe, R.; Hegde, A.G.; Sharma, R.M. Lead, cadmium and nickel in chocolates and candies from suburban areas of Mumbai, India. J. Food Compost. Anal. 2005, 18, 517–522. [Google Scholar] [CrossRef]
- Santos, A.P.; Dos Santos, M.J.; das Graças Andrade Korn, M.; Lemos, V.A. Determination of cadmium in bread and biscuit samples using ultrasound-assisted temperature-controlled ionic liquid microextraction. J. Sci. Food Agric. 2019, 99, 4609–4614. [Google Scholar] [CrossRef]
- EFSA. Scientific opinion on mineral oil hydrocarbons in food. EFSA J. 2012, 10, 2704–2889. [Google Scholar] [CrossRef]
- Bevan, R.; Harrison, P.T.C.; Jeffery, B.; Mitchell, D. Evaluating the risk to humans from mineral oils in foods: Current state of the evidence. Food Chem. Toxicol. 2020, 136, 110966. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, B.; Yang, D.; Ouyang, J.; Sui, H.; Wu, Y. Survey of mineral oil hydrocarbons in Chinese commercial complementary foods for infants and young children. Food Addit. Contam. Part A 2021, 38, 1441–1455. [Google Scholar] [CrossRef] [PubMed]
- Neukom, H.P.; Grob, K.; Biedermann, M.; Noti, A. Food contamination by C20–C50 mineral paraffins from the atmosphere. Atmos. Environ. 2002, 36, 4839–4847. [Google Scholar] [CrossRef]
- Grob, K.; Artho, A.; Biedermann, M.; Mikle, H. Contamination of hazel nuts and chocolate by mineral oil from jute and sisal bags. Z. Lebensm. Unters. Forsch. 1993, 197, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Biedermann-Brem, S.; Kasprick, N.; Simat, T.; Grob, K. Migration of polyolefin oligomeric saturated hydrocarbons (POSH) into food. Food Addit. Contam. Part A 2012, 29, 449–460. [Google Scholar] [CrossRef]
- EFSA. Scientific opinion of the panel on contaminants in the food chain on polycyclic aromatic hydrocarbons in food. EFSA J. 2008, 724, 1–114. [Google Scholar] [CrossRef]
- Bansal, V.; Kumar, P.; Kwon, E.E.; Kim, K.H. Review of the quantification techniques for polycyclic aromatic hydrocarbons (PAHs) in food products. Crit. Rev. Food Sci. Nutr. 2017, 57, 3297–3312. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 835/2011 of 19 August 2011 amending Regulation (EC) No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in foodstuffs. Off. J. Eur. Union 2011, L215, 4–8. [Google Scholar]
- Kang, B.; Lee, B.M.; Shin, H.S. Determination of polycyclic aromatic hydrocarbon (PAH) content and risk assessment from edible oils in Korea. J. Toxicol. Environ. Health 2014, 77, 1359–1371. [Google Scholar] [CrossRef]
- Gharbi, I.; Moret, S.; Chaari, O.; Issaoui, M.; Conte, L.S.; Lucci, P.; Hammami, M. Evaluation of hydrocarbon contaminants in olives and virgin olive oils from Tunisia. Food Control 2017, 75, 160–166. [Google Scholar] [CrossRef]
- Pandey, M.K.; Mishra, K.K.; Khanna, S.K.; Das, M. Detection of polycyclic aromatic hydrocarbons in commonly consumed edible oils and their likely intake in the Indian population. J. Am. Oil Chem. Soc. 2004, 81, 1131–1136. [Google Scholar] [CrossRef]
- Bansal, V.; Kim, K. Review of PAH contamination in food products and their health hazards. Environ. Int. 2015, 84, 26–38. [Google Scholar] [CrossRef]
- Singh, L.; Varshney, J.G.; Agarwal, T. Polycyclic aromatic hydrocarbons’ formation and occurrence in processed food. Food Chem. 2016, 199, 768–781. [Google Scholar] [CrossRef]
- Dennis, M.J.; Massey, R.C.; Cripps, G.; Venn, I.; Howarth, N.; Lee, G. Factors affecting the polycyclic aromatic hydrocarbon content of cereals, fats and other food products. Food Addit. Contam. 1991, 8, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Iwegbue, C.M.; Onyonyewoma, U.A.; Bassey, F.I.; Nwajei, G.E.; Martincigh, B.S. Concentrations and health risk of polycyclic aromatic hydrocarbons in some brands of biscuits in the Nigerian market. Hum. Ecol. Risk Assess. 2015, 21, 338–357. [Google Scholar] [CrossRef]
- Arisseto, A.P.; Silva, W.C.; Tivanello, R.G.; Sampaio, K.A.; Vicente, E. Recent advances in toxicity and analytical methods of monochloropropanediols and glycidyl fatty acid esters in foods. Curr. Opin. Food Sci. 2018, 24, 36–42. [Google Scholar] [CrossRef]
- Yan, J.; Oey, S.B.; van Leeuwen, S.P.; Van Ruth, S.M. Discrimination of processing grades of olive oil and other vegetable oils by monochloropropanediol esters and glycidyl esters. Food Chem. 2018, 248, 93–100. [Google Scholar] [CrossRef]
- Dingel, A.; Matissek, R. No Endogenous formation of MCPD fatty acid esters and glycidyl fatty acid esters during the baking process of fine bakery wares. Dtsch. Lebensm. 2017, 113, 511–515. [Google Scholar]
- Mogol, B.A.; Pye, C.; Anderson, W.; Crews, C.; Gökmen, V. Formation of monochloropropane-1, 2-diol and its esters in biscuits during baking. J. Agric. Food Chem. 2014, 62, 7297–7301. [Google Scholar] [CrossRef]
- Redeuil, K.; Theurillat, X.; Nicolas, M.; Nagy, K. Recommendations for oil extraction and refining process to prevent the formation of monochloropropane-diol esters in sunflower oil. J. Agric. Food Chem. 2021, 69, 6043–6053. [Google Scholar] [CrossRef]
- Nguyen, K.H.; Fromberg, A. Monochloropropanediol and glycidyl esters in infant formula and baby food products on the Danish market: Occurrence and preliminary risk assessment. Food Control 2020, 110, 106980. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, L.; Zhou, P.; Liu, Z.; Fan, S.; Yang, D.; Li, J.; Liu, Q. Dietary exposure of general Chinese population to fatty acid esters of 3-monochloropropane-1, 2-diol (3-MCPD) from edible oils and oil-containing foods. Food Addit. Contam. Part A 2021, 38, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Di Campi, E.; Di Pasquale, M.; Coni, E. Contamination of some foodstuffs marketed in Italy by fatty acid esters of monochloropropanediols and glycidol. Food Addit. Contam. Part A 2020, 37, 753–762. [Google Scholar] [CrossRef]
- Sadowska-Rociek, A.; Surma, M.; Cieslik, E. Analysis of acrylamide, 3-monochloropropane-1, 2-diol, its esters and glycidyl esters in carbohydrate-rich products available on the Polish market. Rocz. Panstw. Zakl. Hig. 2018, 69, 127–136. [Google Scholar]
- Gomes, T.; Caponio, F.; Delcuratolo, D. Fate of oxidized triglycerides during refining of seed oils. J. Agric. Food Chem. 2003, 51, 4647–4651. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Li, Y.; Zhang, N.; Gao, Y.; Yu, X. The formation, determination and health implications of polar compounds in edible oils: Current status, challenges and perspectives. Food Chem. 2021, 364, 130451. [Google Scholar] [CrossRef] [PubMed]
- Billek, G. Health aspects of thermoxidized oils and fats. Eur. J. Lipid Sci. Technol. 2020, 102, 587–593. [Google Scholar] [CrossRef]
- Caponio, F.; Summo, C.; Delcuratolo, D.; Pasqualone, A. Quality of the lipid fraction of Italian biscuits. J. Sci. Food Agric. 2006, 86, 356–361. [Google Scholar] [CrossRef]
- Caponio, F.; Gomes, T.; Pasqualone, A.; Summo, C. Use of the high performance size exclusion chromatography analysis for the measurement of the degree of hydrolytic and oxidative degradation of the lipid fraction of biscuits. Food Chem. 2007, 102, 232–236. [Google Scholar] [CrossRef]
- Caponio, F.; Summo, C.; Paradiso, V.M.; Pasqualone, A.; Gomes, T. Evolution of the oxidative and hydrolytic degradation of biscuits’ fatty fraction during storage. J. Sci. Food Agric. 2009, 89, 1392–1396. [Google Scholar] [CrossRef]
- Teegala, S.M.; Willett, W.C.; Mozaffarian, D. Consumption and health effects of trans fatty acids: A review. J. AOAC Int. 2009, 92, 1250–1257. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Commission Regulation (EU) 2019/649 of 24 April 2019 amending Annex III to Regulation (EC) No 1925/2006 of the European Parliament and of the Council as regards trans fat, other than trans fat naturally occurring in fat of animal origin. Off. J. Eur. Union 2019, L110, 17–20. [Google Scholar]
- Costa, N.; Cruz, R.; Graça, P.; Breda, J.; Casal, S. Trans fatty acids in the Portuguese food market. Food Control 2016, 64, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Mencin, M.; Abramovič, H.; Zlatić, E.; Demšar, L.; Piskernik, S.; Schreiner, M.; Žmitekc, K.; Kušar, A.; Pravst, I.; Vidrih, R. Content of trans-fatty acid isomers in bakery products on the Slovenian market. LWT-Food Sci. Technol. 2021, 143, 111095. [Google Scholar] [CrossRef]
- Aued-Pimentel, S.; Kus-Yamashita, M.M. Analysis of the fat profile of industrialized food in Brazil with emphasis on trans-fatty acids. J. Food Comp. Anal. 2021, 97, 103799. [Google Scholar] [CrossRef]
- Egmond, H.P.; Schothorst, R.C.; Jonker, M.A. Regulations relating to mycotoxins in food. Anal. Bioanal. Chem. 2007, 1, 147–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Commission. Commission Recommendation (2013/165/EU) of 27 March 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products. Off. J. Eur. Union 2013, L91, 12–15. [Google Scholar]
- Richard, J.L. Some major mycotoxins and their mycotoxicoses—An overview. Int. J. Food Microbiol. 2007, 119, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Suman, M. Last decade studies on mycotoxins’ fate during food processing: An overview. Curr. Opin. Food Sci. 2021, 41, 70–80. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Cavaliere, C.; Foglia, P.; Samperi, R.; Stampachiacchiere, S.; Ventura, S.; Laganà, A. Multiclass analysis of mycotoxins in biscuits by high performance liquid chromatography–tandem mass spectrometry. Comparison of different extraction procedures. J. Chromatogr. A 2014, 1343, 69–78. [Google Scholar] [CrossRef]
- Tanaka, H.; Sugita-Konishi, Y.; Takino, M.; Tanaka, T.; Toriba, A.; Hayakawa, K. A survey of the occurrence of Fusarium mycotoxins in biscuits in Japan by using LC/MS. J. Health Sci. 2010, 56, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Škrbić, B.; Antić, I.; Cvejanov, J. Determination of mycotoxins in biscuits, dried fruits and fruit jams: An assessment of human exposure. Food Addit. Contam. 2017, 34, 1012–1025. [Google Scholar] [CrossRef] [PubMed]
- Oueslati, S.; Berrada, H.; Juan-García, A.; Mañes, J.; Juan, C. Multiple mycotoxin determination on Tunisian cereals-based food and evaluation of the population exposure. Food Anal. Methods 2020, 13, 1271–1281. [Google Scholar] [CrossRef]
- Dänicke, S.; Kersten, S.; Valenta, H.; Breves, G. Inactivation of deoxynivalenol-contaminated cereal grains with sodium metabisulfite: A review of procedures and toxicological aspects. Mycotoxin Res. 2012, 28, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hassan, Y.I.; Watts, C.; Zhou, T. Innovative technologies for the mitigation of mycotoxins in animal feed and ingredients—A review of recent patents. Anim. Feed Sci. Technol. 2016, 216, 19–29. [Google Scholar] [CrossRef]
- Zhu, F. Effect of ozone treatment on the quality of grain products. Food Chem. 2018, 264, 358–366. [Google Scholar] [CrossRef]
- Li, M.M.; Guan, E.Q.; Bian, K. Effect of ozone treatment on deoxynivalenol and quality evaluation of ozonised wheat. Food Addit. Contam. Part A 2015, 32, 544–553. [Google Scholar] [CrossRef]
- Wang, B.; Mahoney, N.E.; Pan, Z.; Khir, R.; Wu, B.; Ma, H. Effectiveness of pulsed light treatment for degradation and detoxification of aflatoxin B1 and B2 in rough rice and rice bran. Food Control 2016, 59, 461–467. [Google Scholar] [CrossRef]
- Ten Bosch, L.; Pfohl, K.; Avramidis, G.; Wieneke, S.; Viol, W.; Karlovsky, P. Plasma-based degradation of mycotoxins produced by Fusarium, Aspergillus and alternaria species. Toxins 2018, 9, 97. [Google Scholar] [CrossRef] [Green Version]
- Hojnik, N.; Cvelbar, U.; Tavčar-Kalcher, G.; Walsh, J.L.; Križaj, I. Mycotoxin decontamination of food: Cold atmospheric pressure plasma versus “classic” decontamination. Toxins 2017, 9, 151. [Google Scholar] [CrossRef]
- Červenka, L.; Brožková, I.; Vytřasová, J. Effects of the principal ingredients of biscuits upon water activity. J. Food Nutr. Res. 2006, 45, 39–43. [Google Scholar]
- European Commission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, L338, 1–26. [Google Scholar]
- World Food Program (WFP). Technical Specifications of High Energy Biscuits (HEB); WFP: Rome, Italy, 2016; pp. 1–7. Available online: https://documents.wfp.org/stellent/groups/public/documents/manual_guide_proced/wfp291333.pdf (accessed on 2 November 2021).
- Shuvo, S.D.; Zahid, M.A. Comparative study on nutritional and microbiological quality analysis of supplied fortified high energy biscuit for school feeding in poverty prone areas in Bangladesh with World Food Programme nutritional requirements. Sci. Technol. 2016, 2, 451–458. [Google Scholar]
- Aly, A.A. Chemical, Rheological, Sensorial and Microbial Evaluation of Supplemented Wheat Flour Biscuit with Guava Seeds Powder. J. Food Dairy Sci. 2019, 10, 147–152. [Google Scholar] [CrossRef]
- Saranraj, P.; Geetha, M. Microbial spoilage of bakery products and its control by preservatives. Int. J. Pharm. Biol. Sci. Arc. 2012, 3, 38–48. [Google Scholar]
- Sangeetha, A.V.; Mahadevamma, S.; Begum, K.; Sudha, M.L. Influence of processed sugarcane bagasse on the microbial, nutritional, rheological and quality characteristics of biscuits. Int. J. Food Sci. Nutr. 2011, 62, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Neil, K.P.; Biggerstaff, G.; MacDonald, J.K.; Trees, E.; Medus, C.; Musser, K.A.; Stroika, S.G.; Zink, D.; Sotir, M.J. A novel vehicle for transmission of Escherichia coli O157: H7 to humans: Multistate outbreak of E. coli O157: H7 infections associated with consumption of ready-to-bake commercial prepackaged cookie dough—United States, 2009. Clin. Infect. Dis. 2012, 54, 511–518. [Google Scholar] [CrossRef]
- Sabillón, L.; Stratton, J.; Rose, D.; Eskridge, K.; Bianchini, A. Effect of high-pressure processing on the microbial load and functionality of sugar-cookie dough. Cereal Chem. 2021, 98, 70–80. [Google Scholar] [CrossRef]
- Arepally, D.; Reddy, R.S.; Goswami, T.K.; Datta, A.K. Biscuit baking: A review. LWT-Food Sci. Technol. 2020, 131, 109726. [Google Scholar] [CrossRef]
- Erbersdobler, H.F.; Somoza, V. Forty years of furosine—Forty years of using Maillard reaction products as indicators of the nutritional quality of foods. Mol. Nutr. Food Res. 2007, 51, 423–430. [Google Scholar] [CrossRef]
- ALjahdali, N.; Carbonero, F. Impact of Maillard reaction products on nutrition and health: Current knowledge and need to understand their fate in the human digestive system. Crit. Rev. Food Sci. Nutr. 2019, 59, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Mesías, M.; Olombrada, E.; González-Mulero, L.; Morales, F.J.; Delgado-Andrade, C. Investigation on heat-induced chemical indexes in traditional and reformulated biscuits. J. Food Compost. Anal. 2021, 101, 103963. [Google Scholar] [CrossRef]
- Capuano, E.; Fogliano, V. Acrylamide and 5 hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT-Food Sci. Technol. 2011, 44, 793–810. [Google Scholar] [CrossRef]
- Kowalski, S.; Lukasiewicz, M.; Duda-Chodak, A.; Zięć, G. 5-Hydroxymethyl-2-furfural (HMF)-heat-induced formation, occurrence in food and biotransformation—A Review. Polish J. Food Nutr. Sci. 2013, 63, 207–225. [Google Scholar] [CrossRef] [Green Version]
- Janzowski, C.; Glaab, V.; Samimi, E.; Schlatter, J.; Eisenbrand, G. 5-Hydroxymethylfurfural: Assessment of mutagenicity, DNA-damaging potential and reactivity towards cellular glutathione. Food Chem. Toxicol. 2000, 38, 801–809. [Google Scholar] [CrossRef]
- Švecová, B.; Mojmír, M.A.C.H. Content of 5-hydroxymethyl-2-furfural in biscuits for kids. Interdiscip. Toxicol. 2017, 10, 66–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado-Andrade, C.; Rufian-Henares, J.A.; Morales, F.J. Hydroxymethylfurfural in commercial biscuits marketed in Spain. J. Food Nutr. Res. 2009, 48, 14–19. [Google Scholar]
- Arisseto, A.P.; Vicente, E.; Furlani, R.P.Z.; Ueno, M.S.; Pereira, A.L.D.; Toledo, M.C.F. Occurrence of furan in commercial processed foods in Brazil. Food Addit. Contam. Part A 2012, 29, 1832–1839. [Google Scholar] [CrossRef]
- Hamlet, C.G.; Asuncion, L.; Liang, L. Survey of Acrylamide and Furan in UK Retail Products: Summary Report for Samples Purchased Between November 2011 and December 2013; Premier Analytical Services: High Wycombe, UK, 2014; pp. 1–74. [Google Scholar]
- Mariotti, M.S.; Toledo, C.; Hevia, K.; Gomez, J.P.; Fromberg, A.; Granby, K.; Rosowski, J.; Castillo, O.; Pedreschi, F. Are Chileans exposed to dietary furan? Food Addit. Contam. Part A 2013, 30, 1715–1721. [Google Scholar] [CrossRef] [Green Version]
- Mogol, B.A.; Gökmen, V. Thermal process contaminants: Acrylamide, chloropropanols and furan. Curr. Opin. Food Sci. 2016, 7, 86–92. [Google Scholar] [CrossRef]
- Gökmen, V. A perspective on the evaluation of safety risks in thermal processing of foods with an example for acrylamide formation in biscuits. Qual. Assur. Saf. Crop. 2014, 6, 319–325. [Google Scholar] [CrossRef]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef]
- Sarion, C.; Codină, G.G.; Dabija, A. Acrylamide in bakery products: A review on health risks, legal regulations and strategies to reduce its formation. Int. J. Environ. Res. 2021, 18, 4332. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Agency). Scientific opinion on acrylamide in food. EFSA J. 2015, 13, 4014. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Commission Regulation 2017/2158 of 20 November 2017 establishing mitigation measures and benchmark levels for the reduction of the presence of acrylamide in food. Off. J. Eur. Union 2017, L304, 24–44. [Google Scholar]
- Mesías, M.; Morales, F.J.; Delgado-Andrade, C. Acrylamide in biscuits commercialized in Spain: A view of the Spanish market from 2007 to 2019. Food Funct. 2019, 10, 6624–6632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ölmez, H.; Tuncay, F.; Özcan, N.; Demirel, S. A survey of acrylamide levels in foods from the Turkish market. J. Food Compost. Anal. 2008, 21, 564–568. [Google Scholar] [CrossRef]
- Mencin, M.; Abramovič, H.; Vidrih, R.; Schreiner, M. Acrylamide levels in food products on the Slovenian market. Food Control 2020, 114, 107267. [Google Scholar] [CrossRef]
- Razia, S.; Bertrand, M.; Klaus, V.; Meinolf, G.L. Investigation of acrylamide levels in branded biscuits, cakes and potato chips commonly consumed in Pakistan. Int. Food Res. J. 2016, 23, 2187–2192. [Google Scholar]
- Alyousef, H.A.; Wang, H.; Al-Hajj, N.Q.M.; Koko, M.Y. Determination of acrylamide levels in selected commercial and traditional foods in Syria. Trop. J. Pharm. Res. 2016, 15, 1275–1281. [Google Scholar] [CrossRef]
- Cortés, W.R.B.; Mejía, S.M.V.; Mahecha, H.S. Consumption study and margin of exposure of acrylamide in food consumed by the Bogotá population in Colombia. J. Food Compost. Anal. 2021, 100, 103934. [Google Scholar] [CrossRef]
- El Tawila, M.M.; Al-Ansari, A.M.; Alrasheedi, A.A.; Neamatallah, A.A. Dietary exposure to acrylamide from cafeteria foods in Jeddah schools and associated risk assessment. J. Sci. Food Agric. 2017, 97, 4494–4500. [Google Scholar] [CrossRef] [PubMed]
- Bušová, M.; Bencko, V.; Kromerová, K.; Nadjo, I.; Babjaková, J. Occurrence of acrylamide in selected food products. Cent. Eur. J. Public Health 2020, 28, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Claeys, W.; De Meulenaer, B.; Huyghebaert, A.; Scippo, M.L.; Hoet, P.; Matthys, C. Reassessment of the acrylamide risk: Belgium as a case-study. Food Control 2016, 59, 628–635. [Google Scholar] [CrossRef]
- Abt, E.; Robin, L.P.; McGrath, S.; Srinivasan, J.; DiNovi, M.; Adachi, Y.; Chirtel, S. Acrylamide levels and dietary exposure from foods in the United States, an update based on 2011-2015 data. Food Addit. Contam. Part A 2019, 36, 1475–1490. [Google Scholar] [CrossRef]
- Žilić, S.; Aktağ, I.G.; Dodig, D.; Filipović, M.; Gökmen, V. Acrylamide formation in biscuits made of different wholegrain flours depending on their free asparagine content and baking conditions. Food Res. Int. 2020, 132, 109109. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Van Boekel, M.A.J.S. Acrylamide and 5-hydroxymethylfurfural formation during biscuit baking. Part II: Effect of the ratio of reducing sugars and asparagine. Food Chem. 2017, 230, 14–23. [Google Scholar] [CrossRef]
- Ohm, J.B.; Simsek, S.; Mergoum, M. Variation of protein MWD parameters and their associations with free asparagine concentration and quality characteristics in hard red spring wheat. J. Cereal Sci. 2018, 79, 154–159. [Google Scholar] [CrossRef]
- Raffan, S.; Halford, N.G. Acrylamide in food: Progress in and prospects for genetic and agronomic solutions. Ann. Appl. Biol. 2019, 175, 259–281. [Google Scholar] [CrossRef] [Green Version]
- Anese, M.; Quarta, B.; Peloux, L.; Calligaris, S. Effect of formulation on the capacity of l-asparaginase to minimize acrylamide formation in short dough biscuits. Food Res. Int. 2011, 44, 2837–2842. [Google Scholar] [CrossRef]
- Vass, M.; Amrein, T.M.; Schönbächler, B.; Esher, F.; Amadòm, R. Ways to reduce the acrylamide formation in Cracker Products. Czech J. Food Sci. 2004, 22, 19–21. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Fu, J.; Fan, Q. Comparison of different rice flour-and wheat flour-based butter cookies for acrylamide formation. J. Cereal Sci. 2020, 95, 103086. [Google Scholar] [CrossRef]
- Shewry, P.R.; Zhao, F.J.; Gowa, G.B.; Hawkins, N.D.; Ward, J.L.; Beale, M.H.; Halford, N.G.; Parry, M.A.; Abécassis, J. Sulphur nutrition differentially affects the distribution of asparagine in wheat grain. J. Cereal Sci. 2009, 50, 407–409. [Google Scholar] [CrossRef]
- Konings, E.J.; Ashby, P.; Hamlet, C.G.; Thompson, G.A. Acrylamide in cereal and cereal products: A review on progress in level reduction. Food Addit. Contam. 2007, 24, 47–59. [Google Scholar] [CrossRef]
- Miskiewicz, K.; Nebesny, E.; Rosicka-Kaczmarek, J.; Zyzelewicz, D.; Budryn, G. The effects of baking conditions on acrylamide content in shortcrust cookies with added freeze-dried aqueous rosemary extract. J. Food Sci. Technol. 2018, 55, 4184–4196. [Google Scholar] [CrossRef] [Green Version]
- Açar, Ö.Ç.; Pollio, M.; Di Monaco, R.; Fogliano, V.; Gökmen, V. Effect of calcium on acrylamide level and sensory properties of cookies. Food Bioprocess Technol. 2012, 5, 519–526. [Google Scholar] [CrossRef] [Green Version]
- Kukurová, K.; Ciesarová, Z.; Mogol, B.A.; Açar, Ö.Ç.; Gökmen, V. Raising agents strongly influence acrylamide and HMF formation in cookies and conditions for asparaginase activity in dough. Eur. Food Res. Technol. 2013, 237, 1–8. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Peters, R.J.; Van Boekel, M.A. Acrylamide and 5-hydroxymethylfurfural formation during baking of biscuits: Part I: Effects of sugar type. Food Chem. 2016, 192, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Van Der Fels-Klerx, H.J.; Capuano, E.; Nguyen, H.T.; Mogol, B.A.; Kocadağlı, T.; Taş, N.G.; Hamzalıoğlu, A.; Van Boekel, M.A.J.S.; Gökmen, V.U.R.A.L. Acrylamide and 5-hydroxymethylfurfural formation during baking of biscuits: NaCl and temperature–time profile effects and kinetics. Food Res. Int. 2014, 57, 210–217. [Google Scholar] [CrossRef]
- Zou, Y.; Huang, C.; Pei, K.; Cai, Y.; Zhang, G.; Hu, C.; Ou, S. Cysteine alone or in combination with glycine simultaneously reduced the contents of acrylamide and hydroxymethylfurfural. LWT-Food Sci. Technol. 2015, 63, 275–280. [Google Scholar] [CrossRef]
- Suman, M.; Generotti, S.; Cirlini, M.; Dall’Asta, C. Acrylamide reduction strategy in combination with deoxynivalenol mitigation in industrial biscuits production. Toxins 2019, 11, 499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogol, B.A.; Gökmen, V. Mitigation of acrylamide and hydroxymethylfurfural in biscuits using a combined partial conventional baking and vacuum post-baking process: Preliminary study at the lab scale. Innov. Food Sci. Emerg. Technol. 2014, 26, 265–270. [Google Scholar] [CrossRef]
- Koray Palazoğlu, T.; Coşkun, Y.; Kocadağlı, T.; Gökmen, V. Effect of radio frequency postdrying of partially baked cookies on acrylamide content, texture, and colour of the final product. J. Food Sci. 2012, 77, E113–E117. [Google Scholar] [CrossRef] [PubMed]
- Michalak, J.; Czarnowska-Kujawska, M.; Klepacka, J.; Gujska, E. Effect of microwave heating on the acrylamide formation in foods. Molecules 2020, 25, 4140. [Google Scholar] [CrossRef] [PubMed]
- Gülcan, Ü.; Uslu, C.C.; Mutlu, C.; Arslan-Tontul, S.; Erba, S.M. Impact of inert and inhibitor baking atmosphere on HMF and acrylamide formation in bread. Food Chem. 2020, 332, 127434. [Google Scholar] [CrossRef]
- Goldberg, T.; Cai, W.; Peppa, M.; Dardaine, V.; Baliga, B.S.; Uribarri, J.; Vlassara, H. Advanced glycoxidation end products in commonly consumed foods. J. Am. Diet. Assoc. 2004, 104, 1287–1291. [Google Scholar] [CrossRef]
- Cordova, R.; Knaze, V.; Viallon, V.; Rust, P.; Schalkwijk, C.G.; Weiderpass, E.; Wagner, K.-H.; Mayen-Chacon, A.-L.; Aglago, E.K.; Dahm, C.C.; et al. Dietary intake of advanced glycation end products (AGEs) and changes in body weight in European adults. Eur. J. Nutr. 2020, 59, 2893–2904. [Google Scholar] [CrossRef]
- Van Nguyen, C. Toxicity of the AGEs generated from the Maillard reaction: On the relationship of food-AGEs and biological-AGEs. Mol. Nut. Food Res. 2006, 50, 1140–1149. [Google Scholar] [CrossRef]
- Scheijen, J.L.; Clevers, E.; Engelen, L.; Dagnelie, P.C.; Brouns, F.; Stehouwer, C.D.; Schalkwijk, C.G. Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database. Food Chem. 2016, 190, 1145–1150. [Google Scholar] [CrossRef]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation end products in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef]
- Delgado-Andrade, C. Maillard reaction products: Some considerations on their health effects. Clin. Chem. Lab. Med. 2014, 52, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Mesías, M.; Navarro, M.; Gökmen, V.; Morales, F.J. Antiglycative effect of fruit and vegetable seed extracts: Inhibition of AGE formation and carbonyl-trapping abilities. J. Sci. Food Agric. 2013, 93, 2037–2044. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Ma, J.; Chen, F.; Wang, M. Naturally occurring inhibitors against the formation of advanced glycation end-products. Food Funct. 2011, 2, 289–301. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Yang, L.; Peng, X.; Zheng, J.; Ou, S. Effect of maize bran feruloylated oligosaccharides on the formation of endogenous contaminants and the appearance and textural properties of biscuits. Food Chem. 2018, 245, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Caponio, F.; Summo, C.; Pasqualone, A.; Bilancia, M.T. Effect of kneading and baking on the degradation of the lipid fraction of biscuits. J. Cereal Sci. 2008, 48, 407–412. [Google Scholar] [CrossRef]
- Hakme, E.; Herrmann, S.S.; Poulsen, M.E. Processing factors of pesticide residues in biscuits and their relation to the physicochemical properties of pesticides. Food Addit. Contam. Part A 2020, 37, 1695–1706. [Google Scholar] [CrossRef] [PubMed]
- Khaneghah, A.M.; Fakhri, Y.; Sant’Ana, A.S. Impact of unit operations during processing of cereal-based products on the levels of deoxynivalenol, total aflatoxin, ochratoxin A, and zearalenone: A systematic review and meta-analysis. Food Chem. 2018, 268, 611–624. [Google Scholar] [CrossRef]
- Stadler, D.; Lambertini, F.; Woelflingseder, L.; Schwartz-Zimmermann, H.; Marko, D.; Suman, M.; Berthiller, F.; Krska, R. The influence of processing parameters on the mitigation of deoxynivalenol during industrial baking. Toxins 2019, 11, 317. [Google Scholar] [CrossRef] [Green Version]
- Stadler, D.; Lambertini, F.; Bueschl, C.; Wiesenberger, G.; Hametner, C.; Schwartz-Zimmermann, H.; Hellinger, R.; Sulyok, M.; Lemmens, M.; Schuhmacher, R.; et al. Untargeted LC–MS based 13C labelling provides a full mass balance of deoxynivalenol and its degradation products formed during baking of crackers, biscuits and bread. Food Chem. 2019, 279, 303–311. [Google Scholar] [CrossRef]
- Vidal, A.; Sanchis, V.; Ramos, A.J.; Marìn, S. Stability of DON and DON3G during baking as affected by the presence of food additives. Food Addit. Contam. Part A 2018, 35, 529–537. [Google Scholar] [CrossRef] [Green Version]
- Kuchenbuch, H.S.; Becker, S.; Schulz, M.; Cramer, B.; Humpf, H.U. Thermal stability of T-2 and HT-2 toxins during biscuit-and crunchy muesli-making and roasting. Food Addit. Contam. Part A 2018, 35, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
- Hozová, B.; Buchtová, V.; Dodok, L.; Zemanovič, J. Microbiological, nutritional and sensory aspects of stored amaranth biscuits and amaranth crackers. Food/Nahrung 1997, 41, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Koczoń, P.; Lipińska, E.; Czerniawska-Piątkowska, E.; Mikuła, M.; Bartyzel, B.J. The change of fatty acids composition of Polish biscuits during storage. Food Chem. 2016, 202, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Galić, K.; Ćurić, D.; Gabrić, D. Shelf life of packaged bakery goods—A review. Crit. Rev. Food Sci. Nutr. 2009, 49, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Piergiovanni, L.; Limbo, S. The protective effect of film metallization against oxidative deterioration and discoloration of sensitive foods. Packag. Technol. Sci. 2004, 17, 155–164. [Google Scholar] [CrossRef]
- Balestra, F.; Verardo, V.; Tappi, S.; Caboni, M.F.; Dalla Rosa, M.; Romani, S. Chemical and physical changes during storage of differently packed biscuits formulated with sunflower oil. J. Food Sci. Tech. 2019, 56, 4714–4721. [Google Scholar] [CrossRef] [PubMed]
- Mesías, M.; Holgado, F.; Márquez-Ruiz, G.; Morales, F.J. Effect of sodium replacement in cookies on the formation of process contaminants and lipid oxidation. LWT-Food Sci. Technol. 2015, 62, 633–639. [Google Scholar] [CrossRef] [Green Version]
- Reddy, V.; Urooj, A.; Kumar, A. Evaluation of antioxidant activity of some plants extracts and their application in biscuits. Food Chem. 2005, 90, 317–321. [Google Scholar] [CrossRef] [Green Version]
- Ismail, T.; Akhtar, S.; Riaz, M.; Ismail, A. Effect of pomegranate peel supplementation on nutritional, organoleptic and stability properties of cookies. Int. J. Food Sci. Nutr. 2014, 65, 661–666. [Google Scholar] [CrossRef]
- Ajila, C.M.; Leelavathi, K.U.J.S.; Rao, U.P. Improvement of dietary fiber content and antioxidant properties in soft dough biscuits with the incorporation of mango peel powder. J. Cereal Sci. 2008, 48, 319–326. [Google Scholar] [CrossRef]
- Ajila, C.M.; Naidu, K.A.; Bhat, S.G.; Rao, U.P. Bioactive compounds and antioxidant potential of mango peel extract. Food Chem. 2007, 105, 982–988. [Google Scholar] [CrossRef]
- Caleja, C.; Barros, L.; Antonio, A.L.; Oliveira, M.B.P.; Ferreira, I.C. A comparative study between natural and synthetic antioxidants: Evaluation of their performance after incorporation into biscuits. Food Chem. 2017, 216, 342–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mildner-Szkudlarz, S.; Zawirska-Wojtasiak, R.; Obuchowski, W.; Gośliński, M. Evaluation of antioxidant activity of green tea extract and its effect on the biscuits lipid fraction oxidative stability. J. Food Sci. 2009, 74, S362–S370. [Google Scholar] [CrossRef]
- Difonzo, G.; Pasqualone, A.; Silletti, R.; Cosmai, L.; Summo, C.; Paradiso, V.M.; Caponio, F. Use of olive leaf extract to reduce lipid oxidation of baked snacks. Food Res. Int. 2018, 108, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Difonzo, G.; Squeo, G.; Pasqualone, A.; Summo, C.; Paradiso, V.M.; Caponio, F. The challenge of exploiting polyphenols from olive leaves: Addition to foods to improve their shelf-life and nutritional value. J. Sci. Food Agric. 2021, 101, 3099–3116. [Google Scholar] [CrossRef]
- Vollmer, A.; Biedermann, M.; Grundböck, F.; Ingenhoff, J.E.; Biedermann-Brem, S.; Altkofer, W.; Grob, K. Migration of mineral oil from printed paperboard into dry foods: Survey of the German market. Eur. Food Res. Technol. 2011, 232, 175–182. [Google Scholar] [CrossRef]
- Baele, M.; Vermeulen, A.; Claes, M.; Ragaert, P.; De Meulenaer, B. Migration of surrogate contaminants from paperboard to foods: Effect of food and surrogate properties. Food Addit. Contam. Part A 2020, 37, 2165–2183. [Google Scholar] [CrossRef]
- Biedermann, M.; Schum, R.; Grob, K. Activated carbon added to recycled paperboard to prevent migration into food: Approach for determining efficacy, and first results. Food Addit. Contam. Part A 2018, 35, 1832–1844. [Google Scholar] [CrossRef]
- Buist, H.; van Harmelen, T.; van den Berg, C.; Leeman, W.; Meima, M.; Krul, L. Evaluation of measures to mitigate mineral oil migration from recycled paper in food packaging. Packag. Technol. Sci. 2020, 33, 531–546. [Google Scholar] [CrossRef]
- Gowen, A.A.; O’Donnell, C.P.; Cullen, P.J.; Downey, G.; Frias, J.M. Hyperspectral imaging–an emerging process analytical tool for food quality and safety control. Trends Food Sci. Technol. 2007, 18, 590–598. [Google Scholar] [CrossRef]
- Nazari, M.; Rubio-Martinez, M.; Babarao, R.; Younis, A.A.; Collins, S.F.; Hill, M.R.; Duke, M.C. Aqueous contaminant detection via UiO-66 thin film optical fiber sensor platform with fast Fourier transform based spectrum analysis. J. Phys. D Appl. Phys. 2017, 51, 025601. [Google Scholar] [CrossRef]

| Element | Country | Amount Detected (mg/kg) | Reference |
|---|---|---|---|
| Lead | Nigeria | 0.10–0.16 1 | [32] |
| Nigeria | 2.8 | [33,34] | |
| India | 0.13 | [35] | |
| Egypt | 0.12 | [36] | |
| Cadmium | Nigeria | 0.02–0.03 2 | [32] |
| Nigeria | 0.35 | [33,34] | |
| India | n.d. | [35] | |
| Greece | 0.01 | [36] | |
| Egypt | 0.01–0.12 | [37] |
| Mycotoxin | Food Product | Maximum Level (μg/kg) |
|---|---|---|
| B1 Aflatoxin | All cereals and all processed cereal products, with the exemption of maize | 2.0 |
| Maize | 5.0 | |
| Processed cereal-based foods for infants and young children | 0.10 | |
| Sum of B1, B2, G1 and G2 Aflatoxins | All cereals and all processed cereal products, with the exemption of maize | 4.0 |
| Maize | 10.0 | |
| Ochratoxin A | Unprocessed cereals | 5.0 |
| All processed cereal products | 3.0 | |
| Processed cereal-based foods for infants and young children | 0.50 | |
| Deoxynivalenol | Unprocessed cereals other than durum wheat, oats, and maize | 1250 |
| Unprocessed durum wheat and oats | 1750 | |
| Unprocessed maize | 1750 | |
| Cereal flour | 750 | |
| Bread, pastries, biscuits, cereal snacks, and breakfast cereals | 500 | |
| Processed cereal-based foods for infants and young children | 200 | |
| Zearalenone | Unprocessed cereals other than maize | 100 |
| Unprocessed maize | 200 | |
| Cereal flour | 75 | |
| Bread, pastries, biscuits, cereal snacks, and breakfast cereals | 50 | |
| Processed cereal-based foods for infants and young children | 20 | |
| Sum of T-2 and HT-2 | Unprocessed barley and maize | 200 |
| Unprocessed wheat and rye | 100 | |
| Bread, pastries, biscuits, cereal snacks and breakfast cereals, pasta | 25 | |
| Cereal-based foods for infants and young children | 15 |
| Microorganism | Maximum Level |
|---|---|
| Aerobic mesophilic bacteria | 104 cfu/g |
| Coliforms | 10 cfu/g |
| Escherichia coli | 0 cfu/10 g |
| Salmonella | 0 cfu/25 g |
| Staphylococcus aureus | 10 cfu/g |
| Bacillus cereus | 10 cfu/g |
| Yeasts and molds | 102 cfu/g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasqualone, A.; Haider, N.N.; Summo, C.; Coldea, T.E.; George, S.S.; Altemimi, A.B. Biscuit Contaminants, Their Sources and Mitigation Strategies: A Review. Foods 2021, 10, 2751. https://doi.org/10.3390/foods10112751
Pasqualone A, Haider NN, Summo C, Coldea TE, George SS, Altemimi AB. Biscuit Contaminants, Their Sources and Mitigation Strategies: A Review. Foods. 2021; 10(11):2751. https://doi.org/10.3390/foods10112751
Chicago/Turabian StylePasqualone, Antonella, Noor N. Haider, Carmine Summo, Teodora Emilia Coldea, Saher S. George, and Ammar B. Altemimi. 2021. "Biscuit Contaminants, Their Sources and Mitigation Strategies: A Review" Foods 10, no. 11: 2751. https://doi.org/10.3390/foods10112751
APA StylePasqualone, A., Haider, N. N., Summo, C., Coldea, T. E., George, S. S., & Altemimi, A. B. (2021). Biscuit Contaminants, Their Sources and Mitigation Strategies: A Review. Foods, 10(11), 2751. https://doi.org/10.3390/foods10112751