The Effects of Anthocyanin-Rich Bilberry Extract on Transintestinal Cholesterol Excretion
Abstract
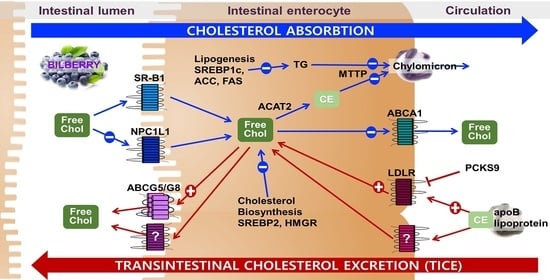
:1. Introduction
2. Materials and Methods
2.1. Total Anthocyanin, Phenolic, and Flavonoid Contents
2.2. Cell Culture and the Sample Treatment
2.3. Quantitative Real-Time PCR (qRT-PCR)
2.4. Western Blot Analysis
2.5. LDL Uptake and Immunochemistry of LDLR
2.6. Total Cholesterol Measurement
2.7. Statistical Analysis
3. Results
3.1. Contents of Polyphenols in BE
3.2. Cytotoxicity of BE in Caco-2 Cells
3.3. Effects of BE on the Genes for Cholesterol Absorption
3.4. Effects of BE on Cholesterol Biosynthesis and TICE
3.5. Effects of BE on Cellular LDL Uptake, LDLR Distribution, and Cholesterol Levels
3.6. Effects of BE on Fatty Acid Metabolism
3.7. Effects of BE on the Regulation of Sirtuins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Luo, J.; Yang, H.; Song, B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Afonso, M.S.; Machado, R.M.; Lavrador, M.S.; Quintao, E.C.R.; Moore, K.J.; Lottenberg, A.M. Molecular Pathways Underlying Cholesterol Homeostasis. Nutrients 2018, 10, 760. [Google Scholar] [CrossRef] [Green Version]
- Kapourchali, F.R.; Surendiran, G.; Goulet, A.; Moghadasian, M.H. The Role of Dietary Cholesterol in Lipoprotein Metabolism and Related Metabolic Abnormalities: A Mini-review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2408–2415. [Google Scholar] [CrossRef]
- Millar, J.S.; Cuchel, M. Cholesterol metabolism in humans: A review of methods and comparison of results. Curr. Opin. Lipidol. 2018, 29, 1–9. [Google Scholar] [CrossRef]
- Alphonse, P.A.S.; Jones, P.J.H. Revisiting Human Cholesterol Synthesis and Absorption: The Reciprocity Paradigm and its Key Regulators. Lipids 2016, 51, 519–536. [Google Scholar] [CrossRef] [PubMed]
- van der Velde, A.E.; Brufau, G.; Groen, A.K. Transintestinal cholesterol efflux. Curr. Opin. Lipidol. 2010, 21, 167–171. [Google Scholar] [CrossRef]
- Tietge, U.J.; Groen, A.K. Role the TICE?: Advancing the concept of transintestinal cholesterol excretion. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1452–1453. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.Q.; Portincasa, P.; Tso, P. Transintestinal cholesterol excretion: A secondary, nonbiliary pathway contributing to reverse cholesterol transport. Hepatology 2017, 66, 1337–1340. [Google Scholar] [CrossRef] [Green Version]
- Grefhorst, A.; Verkade, H.J.; Groen, A.K. The TICE Pathway: Mechanisms and Lipid-Lowering Therapies. Methodist Debakey Cardiovasc. J. 2019, 15, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Reeskamp, L.F.; Meessen, E.C.E.; Groen, A.K. Transintestinal cholesterol excretion in humans. Curr. Opin. Lipidol. 2018, 29, 10–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Velde, A.E.; Vrins, C.L.; van den Oever, K.; Seemann, I.; Oude Elferink, R.P.; van Eck, M.; Kuipers, F.; Groen, A.K. Regulation of direct transintestinal cholesterol excretion in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G203–G208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruit, J.K.; Plosch, T.; Havinga, R.; Boverhof, R.; Groot, P.H.; Groen, A.K.; Kuipers, F. Increased fecal neutral sterol loss upon liver X receptor activation is independent of biliary sterol secretion in mice. Gastroenterology 2005, 128, 147–156. [Google Scholar] [CrossRef]
- van der Veen, J.N.; van Dijk, T.H.; Vrins, C.L.; van Meer, H.; Havinga, R.; Bijsterveld, K.; Tietge, U.J.; Groen, A.K.; Kuipers, F. Activation of the liver X receptor stimulates trans-intestinal excretion of plasma cholesterol. J. Biol. Chem. 2009, 284, 19211–19219. [Google Scholar] [CrossRef] [Green Version]
- Vrins, C.L.; van der Velde, A.E.; van den Oever, K.; Levels, J.H.; Huet, S.; Oude Elferink, R.P.; Kuipers, F.; Groen, A.K. Peroxisome proliferator-activated receptor delta activation leads to increased transintestinal cholesterol efflux. J. Lipid Res. 2009, 50, 2046–2054. [Google Scholar] [CrossRef] [Green Version]
- de Boer, J.F.; Schonewille, M.; Boesjes, M.; Wolters, H.; Bloks, V.W.; Bos, T.; van Dijk, T.H.; Jurdzinski, A.; Boverhof, R.; Wolters, J.C.; et al. Intestinal Farnesoid X Receptor Controls Transintestinal Cholesterol Excretion in Mice. Gastroenterology 2017, 152, 1126–1138.e1126. [Google Scholar] [CrossRef] [Green Version]
- Jakulj, L.; van Dijk, T.H.; de Boer, J.F.; Kootte, R.S.; Schonewille, M.; Paalvast, Y.; Boer, T.; Bloks, V.W.; Boverhof, R.; Nieuwdorp, M.; et al. Transintestinal Cholesterol Transport Is Active in Mice and Humans and Controls Ezetimibe-Induced Fecal Neutral Sterol Excretion. Cell Metab. 2016, 24, 783–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, T.; Inoue, I.; Takenaka, Y.; Ono, H.; Katayama, S.; Awata, T.; Murakoshi, T. Ezetimibe Promotes Brush Border Membrane-to-Lumen Cholesterol Efflux in the Small Intestine. PLoS ONE 2016, 11, e0152207. [Google Scholar] [CrossRef]
- Lifsey, H.C.; Kaur, R.; Thompson, B.H.; Bennett, L.; Temel, R.E.; Graf, G.A. Stigmasterol stimulates transintestinal cholesterol excretion independent of liver X receptor activation in the small intestine. J. Nutr. Biochem. 2020, 76, 108263. [Google Scholar] [CrossRef]
- Xu, Z.; Xie, J.; Zhang, H.; Pang, J.; Li, Q.; Wang, X.; Xu, H.; Sun, X.; Zhao, H.; Yang, Y.; et al. Anthocyanin supplementation at different doses improves cholesterol efflux capacity in subjects with dyslipidemia—A randomized controlled trial. Eur. J. Clin. Nutr. 2021, 75, 345–354. [Google Scholar] [CrossRef]
- Zern, T.L.; Fernandez, M.L. Cardioprotective effects of dietary polyphenols. J. Nutr. 2005, 135, 2291–2294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, M.F.; Macdonald, R.; Lovegrove, J.A. Fruit polyphenols and CVD risk: A review of human intervention studies. Br. J. Nutr. 2010, 104 (Suppl. 3), S28–S39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, W.; Cheung, S.C.M.; Lau, R.A.W.; Benzie, I.F.F. Bilberry (Vaccinium myrtillus L.). In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Ulbricht, C.; Basch, E.; Basch, S.; Bent, S.; Boon, H.; Burke, D.; Costa, D.; Falkson, C.; Giese, N.; Goble, M.; et al. An evidence-based systematic review of bilberry (Vaccinium myrtillus) by the Natural Standard Research Collaboration. J. Diet. Suppl. 2009, 6, 162–200. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, A.; Paur, I.; Bohn, S.K.; Sakhi, A.K.; Borge, G.I.; Serafini, M.; Erlund, I.; Laake, P.; Tonstad, S.; Blomhoff, R. Bilberry juice modulates plasma concentration of NF-kappaB related inflammatory markers in subjects at increased risk of CVD. Eur. J. Nutr. 2010, 49, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Mauray, A.; Milenkovic, D.; Besson, C.; Caccia, N.; Morand, C.; Michel, F.; Mazur, A.; Scalbert, A.; Felgines, C. Atheroprotective effects of bilberry extracts in apo E-deficient mice. J. Agric. Food Chem. 2009, 57, 11106–11111. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, M.; Inoue, S.; Horio, F.; Tsuda, T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J. Nutr. 2010, 140, 527–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bornsek, S.M.; Ziberna, L.; Polak, T.; Vanzo, A.; Ulrih, N.P.; Abram, V.; Tramer, F.; Passamonti, S. Bilberry and blueberry anthocyanins act as powerful intracellular antioxidants in mammalian cells. Food Chem. 2012, 134, 1878–1884. [Google Scholar] [CrossRef]
- Mauray, A.; Felgines, C.; Morand, C.; Mazur, A.; Scalbert, A.; Milenkovic, D. Bilberry anthocyanin-rich extract alters expression of genes related to atherosclerosis development in aorta of apo E-deficient mice. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 72–80. [Google Scholar] [CrossRef]
- Kropat, C.; Mueller, D.; Boettler, U.; Zimmermann, K.; Heiss, E.H.; Dirsch, V.M.; Rogoll, D.; Melcher, R.; Richling, E.; Marko, D. Modulation of Nrf2-dependent gene transcription by bilberry anthocyanins in vivo. Mol. Nutr. Food Res. 2013, 57, 545–550. [Google Scholar] [CrossRef]
- Stefanut, M.N.; Cata, A.; Pop, R.; Tanasie, C.; Boc, D.; Ienascu, I.; Ordodi, V. Anti-hyperglycemic effect of bilberry, blackberry and mulberry ultrasonic extracts on diabetic rats. Plant Foods Hum. Nutr. 2013, 68, 378–384. [Google Scholar] [CrossRef]
- Roth, S.; Spalinger, M.R.; Muller, I.; Lang, S.; Rogler, G.; Scharl, M. Bilberry-derived anthocyanins prevent IFN-gamma-induced pro-inflammatory signalling and cytokine secretion in human THP-1 monocytic cells. Digestion 2014, 90, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Grohmann, T.; Litts, C.; Horgan, G.; Zhang, X.; Hoggard, N.; Russell, W.; de Roos, B. Efficacy of Bilberry and Grape Seed Extract Supplement Interventions to Improve Glucose and Cholesterol Metabolism and Blood Pressure in Different Populations—A Systematic Review of the Literature. Nutrients 2021, 13, 1692. [Google Scholar] [CrossRef]
- Heyman, L.; Axling, U.; Blanco, N.; Sterner, O.; Holm, C.; Berger, K. Evaluation of Beneficial Metabolic Effects of Berries in High-Fat Fed C57BL/6J Mice. J. Nutr. Metab. 2014, 2014, 403041. [Google Scholar] [CrossRef]
- Graf, D.; Seifert, S.; Jaudszus, A.; Bub, A.; Watzl, B. Anthocyanin-Rich Juice Lowers Serum Cholesterol, Leptin, and Resistin and Improves Plasma Fatty Acid Composition in Fischer Rats. PLoS ONE 2013, 8, e66690. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Li, J.; Hao, J.; Zhang, M.; Yin, J.; Geng, J.; Wu, T.; Lyv, X. Bilberry anthocyanin improves the serum cholesterol in aging perimenopausal rats via the estrogen receptor signaling pathway. Food Funct. 2019, 10, 3430–3438. [Google Scholar] [CrossRef]
- Brader, L.; Overgaard, A.; Christensen, L.P.; Jeppesen, P.B.; Hermansen, K. Polyphenol-rich bilberry ameliorates total cholesterol and LDL-cholesterol when implemented in the diet of Zucker diabetic fatty rats. Rev. Diabet. Stud. 2013, 10, 270–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asgary, S.; RafieianKopaei, M.; Sahebkar, A.; Shamsi, F.; Goli-malekabadi, N. Anti-hyperglycemic and anti-hyperlipidemic effects of Vaccinium myrtillus fruit in experimentally induced diabetes (antidiabetic effect of Vaccinium myrtillus fruit). J. Sci. Food Agric. 2016, 96, 764–768. [Google Scholar] [CrossRef]
- Zhu, Y.; Xia, M.; Yang, Y.; Liu, F.; Li, Z.; Hao, Y.; Mi, M.; Jin, T.; Ling, W. Purified anthocyanin supplementation improves endothelial function via NO-cGMP activation in hypercholesterolemic individuals. Clin. Chem. 2011, 57, 1524–1533. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Huang, X.; Zhang, Y.; Wang, Y.; Liu, Y.; Sun, R.; Xia, M. Anthocyanin supplementation improves HDL-associated paraoxonase 1 activity and enhances cholesterol efflux capacity in subjects with hypercholesterolemia. J. Clin. Endocrinol. Metab. 2014, 99, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.W.; Tomlinson, B. Effects of Bilberry Supplementation on Metabolic and Cardiovascular Disease Risk. Molecules 2020, 25, 1653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.; Park, Y.; Wegner, C.J.; Bolling, B.W.; Lee, J. Polyphenol-rich black chokeberry (Aronia melanocarpa) extract regulates the expression of genes critical for intestinal cholesterol flux in Caco-2 cells. J. Nutr. Biochem. 2013, 24, 1564–1570. [Google Scholar] [CrossRef]
- Kim, B.; Bae, M.; Park, Y.K.; Ma, H.; Yuan, T.; Seeram, N.P.; Lee, J.Y. Blackcurrant anthocyanins stimulated cholesterol transport via post-transcriptional induction of LDL receptor in Caco-2 cells. Eur. J. Nutr. 2018, 57, 405–415. [Google Scholar] [CrossRef]
- Jeon, S.; Kim, M.; Kim, B. Polyphenol-Rich Black Elderberry Extract Stimulates Transintestinal Cholesterol Excretion. Appl. Sci. 2021, 11, 2790. [Google Scholar] [CrossRef]
- Pang, J.; Xu, H.; Wang, X.; Chen, X.; Li, Q.; Liu, Q.; You, Y.; Zhang, H.; Xu, Z.; Zhao, Y.; et al. Resveratrol enhances trans-intestinal cholesterol excretion through selective activation of intestinal liver X receptor alpha. Biochem. Pharmacol. 2021, 186, 114481. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Acylated anthocyanins from edible sources and their applications in food systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Jeon, S.; Lee, S.; Choi, Y.; Kim, B. The Effects of Polyphenol-Rich Black Elderberry on Oxidative Stress and Hepatic Cholesterol Metabolism. Appl. Sci. 2021, 11, 10018. [Google Scholar] [CrossRef]
- Hajar, R. Risk Factors for Coronary Artery Disease: Historical Perspectives. Heart Views 2017, 18, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Groen, A.K.; Bloks, V.W.; Verkade, H.; Kuipers, F. Cross-talk between liver and intestine in control of cholesterol and energy homeostasis. Mol. Asp. Med. 2014, 37, 77–88. [Google Scholar] [CrossRef]
- van der Velde, A.E. Reverse cholesterol transport: From classical view to new insights. World J. Gastroenterol. 2010, 16, 5908–5915. [Google Scholar] [CrossRef]
- van der Velde, A.E. Reverse cholesterol transport revisited. World J. Gastroenterol. 2010, 16, 5907. [Google Scholar] [CrossRef] [PubMed]
- Temel, R.E.; Brown, J.M. A new model of reverse cholesterol transport: enTICEing strategies to stimulate intestinal cholesterol excretion. Trends Pharmacol. Sci. 2015, 36, 440–451. [Google Scholar] [CrossRef] [Green Version]
- Dietschy, J.M.; Siperstein, M.D. Cholesterol synthesis by the gastrointestinal tract: Localization and mechanisms of control. J. Clin. Investig. 1965, 44, 1311–1327. [Google Scholar] [CrossRef]
- Bandsma, R.H.; Stellaard, F.; Vonk, R.J.; Nagel, G.T.; Neese, R.A.; Hellerstein, M.K.; Kuipers, F. Contribution of newly synthesized cholesterol to rat plasma and bile determined by mass isotopomer distribution analysis: Bile-salt flux promotes secretion of newly synthesized cholesterol into bile. Biochem. J. 1998, 329 Pt 3, 699–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertsemlidis, D.; Kirchman, E.H.; Ahrens, E.H., Jr. Regulation of cholesterol metabolism in the dog. I. Effects of complete bile diversion and of cholesterol feeding on absorption, synthesis, accumulation, and excretion rates measured during life. J. Clin. Investig. 1973, 52, 2353–2367. [Google Scholar] [CrossRef] [Green Version]
- Pertsemlidis, D.; Kirchman, E.H.; Ahrens, E.H., Jr. Regulation of cholesterol metabolism in the dog. II. Effects of complete bile diversion and of cholesterol feeding on pool sizes of tissue cholesterol measured at autopsy. J. Clin. Investig. 1973, 52, 2368–2378. [Google Scholar] [CrossRef] [PubMed]
- Moreau, F.; Blanchard, C.; Perret, C.; Flet, L.; Douane, F.; Frampas, E.; Mirallie, E.; Croyal, M.; Aguesse, A.; Krempf, M.; et al. In vivo evidence for transintestinal cholesterol efflux in patients with complete common bile duct obstruction. J. Clin. Lipidol. 2019, 13, 213–217.e211. [Google Scholar] [CrossRef] [Green Version]
- Temel, R.E.; Brown, J.M. A new framework for reverse cholesterol transport: Non-biliary contributions to reverse cholesterol transport. World J. Gastroenterol. 2010, 16, 5946–5952. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Wang, D.Q.; Garruti, G.; Wang, H.H.; Grattagliano, I.; de Bari, O.; Portincasa, P. Therapeutic reflections in cholesterol homeostasis and gallstone disease: A review. Curr. Med. Chem. 2014, 21, 1435–1447. [Google Scholar] [CrossRef]
- Lee, S.; Youn, B. Hypolipidemic Roles of Casein-Derived Peptides by Regulation of Trans-Intestinal Cholesterol Excretion and Bile Acid Synthesis. Nutrients 2020, 12, 3058. [Google Scholar] [CrossRef]
- Mauray, A.; Felgines, C.; Morand, C.; Mazur, A.; Scalbert, A.; Milenkovic, D. Nutrigenomic analysis of the protective effects of bilberry anthocyanin-rich extract in apo E-deficient mice. Genes Nutr. 2010, 5, 343–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, D.Y.; Howles, P.N. Molecular mechanisms of cholesterol absorption and transport in the intestine. Semin. Cell Dev. Biol. 2005, 16, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Betters, J.L.; Yu, L. NPC1L1 and cholesterol transport. FEBS Lett. 2010, 584, 2740–2747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oram, J.F.; Vaughan, A.M. ATP-Binding cassette cholesterol transporters and cardiovascular disease. Circ. Res. 2006, 99, 1031–1043. [Google Scholar] [CrossRef]
- Burris, T.P.; Eacho, P.I.; Cao, G. Genetic disorders associated with ATP binding cassette cholesterol transporters. Mol. Genet. Metab. 2002, 77, 13–20. [Google Scholar] [CrossRef]
- Jia, L.; Betters, J.L.; Yu, L. Niemann-pick C1-like 1 (NPC1L1) protein in intestinal and hepatic cholesterol transport. Annu. Rev. Physiol. 2011, 73, 239–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Calvo, M.; Lisnock, J.M.; Bull, H.G.; Hawes, B.E.; Burnett, D.A.; Braun, M.P.; Crona, J.H.; Davis, H.R.; Dean, D.C.; Detmers, P.A.; et al. The target of ezetimibe is Niemann-Pick Cl-Like 1 (NPC1L1). Proc. Natl. Acad. Sci. USA 2005, 102, 8132–8137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, S. The Effect of Polyphenols on Hypercholesterolemia through Inhibiting the Transport and Expression of Niemann-Pick C1-Like 1. Int. J. Mol. Sci. 2019, 20, 4939. [Google Scholar] [CrossRef] [Green Version]
- Field, F.J.; Watt, K.; Mathur, S.N. Origins of intestinal ABCA1-mediated HDL-cholesterol. J. Lipid Res. 2008, 49, 2605–2619. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.M. Intestinal lipid absorption and lipoprotein formation. Curr. Opin. Lipidol. 2014, 25, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.M.; Sawyer, J.K.; Kelley, K.L.; Davis, M.A.; Rudel, L.L. Cholesterol esterification by ACAT2 is essential for efficient intestinal cholesterol absorption: Evidence from thoracic lymph duct cannulation. J. Lipid Res. 2012, 53, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, J.; Boutjdir, M.; Rudel, L.L.; Hussain, M.M. Intestine-specific MTP and global ACAT2 deficiency lowers acute cholesterol absorption with chylomicrons and HDLs. J. Lipid Res. 2014, 55, 2261–2275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, R. Sterol metabolism and SREBP activation. Arch. Biochem. Biophys. 2010, 501, 177–181. [Google Scholar] [CrossRef]
- Sakai, J. Cholesterol feedback: The SREBP pathway. Nihon Rinsho. Jpn. J. Clin. Med. 2011, 69 (Suppl. S1), 241–258. [Google Scholar]
- DeBose-Boyd, R.A. Feedback regulation of cholesterol synthesis: Sterol-accelerated ubiquitination and degradation of HMG CoA reductase. Cell Res. 2008, 18, 609–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, J.L.; Brown, M.S. Regulation of the mevalonate pathway. Nature 1990, 343, 425–430. [Google Scholar] [CrossRef]
- Yu, D.; Liao, J.K. Emerging views of statin pleiotropy and cholesterol lowering. Cardiovasc. Res. 2021. [Google Scholar] [CrossRef]
- Ojha, S.; Islam, B.; Charu, C.; Adem, A.; Aburawi, E. Insight into the mechanism of polyphenols on the activity of HMGR by molecular docking. Drug Des. Dev. Ther. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bura, K.S.; Lord, C.; Marshall, S.; McDaniel, A.; Thomas, G.; Warrier, M.; Zhang, J.; Davis, M.A.; Sawyer, J.K.; Shah, R.; et al. Intestinal SR-BI does not impact cholesterol absorption or transintestinal cholesterol efflux in mice. J. Lipid Res. 2013, 54, 1567–1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le May, C.; Berger, J.M.; Lespine, A.; Pillot, B.; Prieur, X.; Letessier, E.; Hussain, M.M.; Collet, X.; Cariou, B.; Costet, P. Transintestinal cholesterol excretion is an active metabolic process modulated by PCSK9 and statin involving ABCB1. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1484–1493. [Google Scholar] [CrossRef] [Green Version]
- Farahnak, Z.; Chapados, N.; Lavoie, J.M. Exercise training increased gene expression of LDL-R and PCSK9 in intestine: Link to transintestinal cholesterol excretion. Gen. Physiol. Biophys. 2018, 37, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Silbernagel, G.; Steiner, L.K.; Hollstein, T.; Fauler, G.; Scharnagl, H.; Stojakovic, T.; Schumann, F.; Bolukbasi, B.; Marz, W.; Steinhagen-Thiessen, E.; et al. The interrelations between PCSK9 metabolism and cholesterol synthesis and absorption. J. Lipid Res. 2019, 60, 161–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Cao, X.; Yin, M.; Wang, J. Soluble dietary fiber from Qing Ke (highland barley) brewers spent grain could alter the intestinal cholesterol efflux in Caco-2 cells. J. Funct. Foods 2018, 47, 100–106. [Google Scholar] [CrossRef]
- Brasanac-Vukanovic, S.; Mutic, J.; Stankovic, D.M.; Arsic, I.; Blagojevic, N.; Vukasinovic-Pesic, V.; Tadic, V.M. Wild Bilberry (Vaccinium myrtillus L., Ericaceae) from Montenegro as a Source of Antioxidants for Use in the Production of Nutraceuticals. Molecules 2018, 23, 1864. [Google Scholar] [CrossRef] [Green Version]
- Ferre, P.; Foufelle, F. SREBP-1c transcription factor and lipid homeostasis: Clinical perspective. Horm. Res. 2007, 68, 72–82. [Google Scholar] [CrossRef]
- Pal, S.; Ho, S.S.; Takechi, R. Red Wine Polyphenolics Suppress the Secretion of ApoB48 from Human Intestinal CaCo-2 Cells. J. Agric. Food Chem. 2005, 53, 2767–2772. [Google Scholar] [CrossRef]
- Takechi, R.; Hiramatsu, N.; Mamo, J.C.L.; Pal, S. Red wine polyphenolics suppress the secretion and the synthesis of Apo B48 from human intestinal Caco-2 cells. BioFactors 2004, 22, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Sygitowicz, G.; Sitkiewicz, D. Sirtuins and their role as physiological modulators of metabolism. Postep. Hig. Med. Dosw. 2020, 74, 489–496. [Google Scholar] [CrossRef]
- Ye, X.; Li, M.T.; Hou, T.Y.; Gao, T.; Zhu, W.G.; Yang, Y. Sirtuins in glucose and lipid metabolism. Oncotarget 2017, 8, 1845–1859. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhang, S.; Blander, G.; Tse, J.G.; Krieger, M.; Guarente, L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol. Cell 2007, 28, 91–106. [Google Scholar] [CrossRef]
- Deng, Z.; Li, Y.; Liu, H.; Xiao, S.; Li, L.; Tian, J.; Cheng, C.; Zhang, G.; Zhang, F. The role of sirtuin 1 and its activator, resveratrol in osteoarthritis. Biosci. Rep. 2019, 39, BSR20190189. [Google Scholar] [CrossRef] [Green Version]






| Genes | GenBank No. | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|---|
| SR-B1 | NM_005505.5 | AGAATAAGCCCATGACCCTGAA | CGCCGAGGGTGGTGAA |
| NPC1L1 | NM_013389.3 | CACTGGATCACTCGAGGTGTTG | CCAGTCCCACGCTGATGTG |
| ABCA1 | NM_005502 | TTTCTCAGACAACACTTGACCAAGTA | GGTTTTTGTGTAATGAGAGGTCTTTTAA |
| MTTP | NM_000253.4 | TCCCCGTTCGGCATCTAC | CTTAGAATGCCAGAACCCGAGTA |
| ACAT2 | NM_005891 | TGGGCCACCCTCTTGGA | CCAGTGTGTGTAACAGGGTCACA |
| SREBP2 | BC056158.1 | TCCGCCTGTTCCGATGTAC | TGCACATTCAGCCAGGTTCA |
| HMGR | NM_000859.3 | CCCAGTTGTGCGTCTTCCA | TTCGAGCCAGGCTTTCACTT |
| LDLR | AY114155.1 | ACTGGGTTGACTCCAAACTTCAC | GGTTGCCCCCGTTGACA |
| PCSK9 | NM_174936.3 | TTCCTGGTGAAGATGAGT | TTCCTGGTGAAGATGAGT |
| ABCG5 | NM_022436.3 | GCGTAGGTCTCCTTTACCAGTTTG | GGAAACAGATTCACAGCGTTCA |
| ABCG8 | NM_022437.3 | GCCGCCCTCTTGTTCATG | TAACATTTGGAGATGACATCCAGAA |
| SREBP1c | NM-001005291 | TCAGCGAGGCGGCTTTGGAGCAG | CATGTCTTCGATGTCGGTCAG |
| ACC | BC137287.1 | GGATCCGGCGCCTTACTT | CTCCGATCCACCTCATAGTTGAC |
| FAS | AY451392.1 | CGCTCGGCATGGCTATCT | CTCGTTGAAGAACGCATCCA |
| SCD-1 | NM_005063 | CCGACGTGGCTTTTTCTTCT | TGGGTGTTTGCGCACAAG |
| CPT1 | NM_001876.4 | TTATCGCCAAGGATGGCTCTA | CCACACCATCACCCCAAGA |
| ACOX | BC008767.2 | CTTGCTTCACCAGGCAACTG | TTCCAGGCGGGCATGA |
| SIRT1 | NM_012238.4 | TAGTTCTTGTGGCAGTAA | CATCAGGCTCATCTTCTA |
| SIRT2 | NM_012237.3 | AACCATCTGTCACTACTT | TATCTATGTTCTGCGTGTA |
| SIRT3 | NM_012239.5 | GCTCCCAGTTTCTTCTTT | CCACTTCCAACAACACTT |
| SIRT4 | NM_012240.2 | CTTCATCACCCTTTCCAA | ACCTGTAGTCTGGTATCC |
| SIRT5 | NM_012241.3 | AAGCACATAGTCATCATCT | TTCTCCAATAACCTCCAG |
| SIRT6 | NM_016539.2 | AGGGACAAACTGGCAGAG | TGTGTCTCGGACGTACTG |
| SIRT7 | NM_016538.2 | AATACTTGGTCGTCTACAC | TGTCCACACTCCATTAGG |
| GAPDH | NM_002046.7 | GGTGGTCTCCTCTGACTTCAACA | GTTGCTGTAGCCAAATTCGTTGT |
| Antibodies | Company | Catalog No. |
|---|---|---|
| Rabbit polyclonal Anti-Scavenging Receptor SR-BI antibody | Abcam | ab106572 |
| Rabbit monoclonal Anti-Niemann Pick C1-Like 1 antibody | Abcam | ab124801 |
| Mouse monoclonal Anti-ABCA1 antibody | Abcam | ab18180 |
| Rabbit polyclonal Anti-SREBP2 antibody | Abcam | ab30682 |
| Rabbit monoclonal Anti-HMGCR antibody | Abcam | ab174830 |
| Rabbit monoclonal Anti-LDL Receptor antibody | Abcam | ab52818 |
| Goat polyclonal Anti-PCSK9 antibody | Abcam | ab28770 |
| Rabbit polyclonal Anti-ABCG8 antibody | Abcam | ab126493 |
| Monoclonal Anti-β-Actin antibody | Sigma | A5441 |
| Goat anti-Mouse IgG (H + L) secondary antibody, HRP | Invitrogen | 31430 |
| Goat anti-Rabbit IgG (H + L) secondary antibody, HRP | Invitrogen | 31460 |
| Rabbit anti-Goat IgG (H + L) secondary antibody, HRP | Invitrogen | 31402 |
| Total Anthocyanin (mg CGE/g) | Total Phenolics (mg GAE/g) | Total Flavonoid (mg QE/g) | |
|---|---|---|---|
| Anthocyanin-rich bilberry extract (BE) | 237.9 ± 17.1 | 338.5 ± 28.0 | 735.4 ± 18.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.; Kim, M.; Kim, B. The Effects of Anthocyanin-Rich Bilberry Extract on Transintestinal Cholesterol Excretion. Foods 2021, 10, 2852. https://doi.org/10.3390/foods10112852
Hong J, Kim M, Kim B. The Effects of Anthocyanin-Rich Bilberry Extract on Transintestinal Cholesterol Excretion. Foods. 2021; 10(11):2852. https://doi.org/10.3390/foods10112852
Chicago/Turabian StyleHong, Jimin, Minji Kim, and Bohkyung Kim. 2021. "The Effects of Anthocyanin-Rich Bilberry Extract on Transintestinal Cholesterol Excretion" Foods 10, no. 11: 2852. https://doi.org/10.3390/foods10112852
APA StyleHong, J., Kim, M., & Kim, B. (2021). The Effects of Anthocyanin-Rich Bilberry Extract on Transintestinal Cholesterol Excretion. Foods, 10(11), 2852. https://doi.org/10.3390/foods10112852