Abstract
This research aimed to study the chemical composition of Aloysia citrodora methanolic extract and its biological activities as an antioxidant, and its antibacterial, antifungal and anti-inflammatory activities based on four bioclimatic collection stages. The contents of total phenols, total flavonoids and total tannins were determined. Nine phenolic compounds were identified by LC-DAD–ESI-MS/MS. The major compound was acteoside, a phenylpropanoid which represented about 80% of the methanolic fraction in the various regions. The antioxidant activities of different locations were measured by different analytical assays, such as DPPH, ABTS and iron reducing power. The results showed that phenolic compounds and antioxidant activities varied with climatic and environmental factors. Moreover, there was a significant dependency between regions and biological activities. The use of a principal component analysis showed that there was a close relationship among phenylpropanoids, phenolic compounds and the studied biological activities.
1. Introduction
Medicinal plants are an important source of secondary metabolites having therapeutic, cosmetic and food applications. Among the various plant protection products, phenolic compounds and essential oils generate a large group of secondary metabolites having a remarkable therapeutic effect and play the role of natural antioxidants and preservatives. In general, the effectiveness of the plant extracts depends on their yield, chemical composition and bioactivity. The latter could be generally affected by genetic and/or environmental factors [1]. Environmental factors, such as soil type, temperature, altitude and rainfall can affect the concentrations of phenolic compounds [2]. Therefore, phenolic compounds and other secondary metabolites represent a chemical interface between plants and the environment [3]. Variations in phenol quantity directly influence the quality of the plant for food products [4]. Food products rich in antioxidants are in demand because they act as scavengers of reactive oxygen species (ROS) [5].
Lemon verbena (Aloysia citrodora) belongs to the Verbenaceae family originating in South America and grows in North Africa, Southern Europe and various parts of Iran. Lemon verbena is one of the well-known aromatic species rich in phenylpropanoids and phenolic compounds [6] exerting the strongest antioxidant power [7]. Lemon verbena extract is extensively used in aromatherapy and perfumery applications. It is applied in traditional medicine for the cure of digestive problems, such as flatulence, indigestion and acidity [8]. The water infusion of verbena has been also ascribed for its antibacterial [9], antifungal [10], anti-inflammatory [11] and antioxidant [9] proprieties. Lemon verbena leaf extract is known for its high content of acteoside, named also verbascoside [12]. It has demonstrated potent biological effects, such as antioxidant, anxiolytic, neuroprotective, anticancer, anesthetic, antimicrobial and sedative properties [13]. Several studies reported that lemon verbena leaf extract contains a higher concentration of total phenols, tannins and flavonoids [14,15,16]. Wernert et al. [16] also described the presence of polyphenols (hydroxycinnamic acids and flavons) in lemon verbena leaf extract. On the other hand, various studies have been carried out on the antioxidant activity of the constituents of aqueous and alcoholic extract preparations of lemon verbena and antioxidant activity [17,18,19]. The aqueous and alcoholic extracts of the fresh aerial parts of Egyptian lemon verbena exhibited variable anti-inflammatory, antipyretic, analgesic and antioxidant properties [17]. However, there were few literature data concerning the effect of environmental conditions on the phenol composition and bioactivity of lemon verbena [20,21]. Rezig et al. [20] studied the variability of phenolics and the biological activities of Tunisian lemon verbena as affected by the geographic and climatic conditions of the collection areas. Chrysargyris et al. [21] investigated the fluctuation of polyphenols and antioxidant activities of Greece lemon verbena cultivated under two environmental conditions characterized by different altitudes.
In this way, this work was a comparative study of the variation of the chemical composition and antioxidant, antibacterial and antifungal potential among methanolic lemon verbena extracts extracted separately from four Tunisian climatic stages (Upper Arid, Middle Arid, Interior Sub-humid and Upper Semi-Arid). The detected variability was statistically interpreted and discussed according to environmental conditions of the studied sites underlining the environment’s effect on the diversify yield, chemical composition and biological activities of lemon verbena extract.
2. Materials and Methods
2.1. Plant Material
Lemon verbena aerial parts were randomly harvested in early to mid-October 2019 by hand from plants at the flowering stage cultivated in the Tunisian areas of Boussalem, Belli, Kairouan and Gabes (Table 1). The voucher specimen was deposited at the herbarium of our laboratory under the number “TC2011-2405”. The aerial parts of the lemon verbena were air-dried at room temperature for approximately seven days until reaching a constant mass of the plant material. Then, the lemon verbena aerial parts were ground and conserved at ~25 °C in darkness for further extractions.

Table 1.
Studied area data of Tunisian lemon verbena.
2.2. Methanolic Extract Preparation
The air-dried samples were finely ground (grain size 0.7 mm) with a blade mill (IKA-WERK Type: A: 10). Triplicate 1 g subsamples of crushed aerial parts were extracted by shaking with 10 mL of pure methanol for 30 min at room temperature (25 °C). The extract was then stored for 24 h at 4 °C, filtered through Whatman No. 4 filter paper, evaporated in a vacuum (Rotavapor® R-100, BUCHI, Flawil, Switzerland) to dryness and stored at 4 °C until analysis [22].
The extraction yield was 110 mg/g DW for Kairouan, 80 mg/g DW for Gabes, 60 mg/g DW for Boussalem and 180 mg/g DW for Belli. The extracts obtained will be used for the quantification of phenolic compounds and the evaluation of antioxidant activities.
2.3. HPLC-PDA-ESI-MS/MS Analysis
HPLC systems contained a photodiode array detector (PDA), a triple quadrupole mass spectrometer type Micromass Autospec Ultima Pt (Kelso, UK), and an ESI ion source working in negative mode was used for the identification of phenolic compounds. The latter was also done by a comparison of their retention time and fragmentation pattern with those of authentic standards and/or literature data [23,24].
2.4. Determination of Total Polyphenol, Flavonoid and Condensed Tannin Contents
The total polyphenol content was colorimetrically determined by using the Folin–Ciocalteu reagent [25,26]. The total polyphenol contents were expressed as mg of gallic acid equivalents per gram of dry weight (mg GAE/g DW).
The total flavonoid content was measured according to the method described by Kim et al. [27]. The absorbance of the mixture versus a prepared blank was read at 510 nm. These contents were expressed as mg of catechin equivalents per gram of dry weight (mg CE/g DW).
The total tannin content was measured using the modified vanillin assay described by Sun et al. [28]. The amount of total condensed tannins was expressed as mg (+)-catechin equivalent per gram of dry weight (mg CE/g DW).
Triplicates measurements were taken for total polyphenols, flavonoids and condensed tannins.
2.5. Antioxidant Activity
The electron donation ability of lemon verbena methanolic extract in relation to the growing region was determined using the DPPH assay according to Hatano et al. [29]. The antiradical activity was expressed as IC50 (μg/mL), the concentration required to cause 50% DPPH inhibition. BHT was used as the positive control.
The ABTS radical cation (ABTS+) was performed by the method described by Re et al. [30]. The ABTS scavenging capacity was expressed as IC50 (µg/mL), and BHT was used as the positive control.
The reducing power of methanolic lemon verbena extract was determined according to Amezouar et al. [31]. Ascorbic acid was used as the positive control. The results are expressed as IC50 values (µg/mL).
2.6. Antimicrobial Assay
Six bacterial strains: Staphylococcus aureus ATCC 6538, Listeria monocytogenes ATCC 19115, Enterococcus faecalis ATCC 29212, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 2134 and Salmonella arizonae DMB 560 and three yeast strains: Candida albicans ATCC 10231, Fusarium solani and Rhizoctonia solani were taken from the microorganism collection of our laboratory.
The disc diffusion method was used to determine the antibacterial activity by measuring the diameter of the growth inhibition zone (IZ) around the discs as described before by Rios and Recio [32]. The mycelial growth inhibitory effect was performed as described by Rguez et al. [33]. The minimum inhibitory concentration was determined by the microdilution method [34]. Gentamicin and nystatin were used as positive standards.
2.7. Anti-Inflammatory Activity
The cell line (RAW 264.7 murine macrophage), supplied from the American Type Culture Collection (ATCC, Manassas, VA, USA) was cultured and kept at 37 °C in a humidified environment of 5% CO2 [35].
To evaluate the in vitro cytotoxicity of the methanolic lemon verbena extract [36], RAW macrophage cells were seeded in 24-well plates at 5 × 104 cells/well and allowed to attach. Then, the cells were treated with increasing concentrations for 24 h. The proportionality of stained cells with viable cell numbers at the absorbance at 540 nm was determined. Cell viability in each well was calculated as a % of the control. The Resazurin reduction test was used to determine the extract cytotoxicity [37].
To determine the quantity of the nitrite production, RAW 264.7 cells were seeded in 24-well plates at a density of 2 × 105 for 24 h at 37 °C. Then, after 60 min, cells were treated with lemon verbena extracts, dissolved in the DMSO and stimulated with 100 mg/mL lipopolysaccharide (LPS). After 24 h, the quantity of nitrite accumulated in the culture supernatant was determined [38].
2.8. Statistical Analysis
All results of these assays were repeated three-fold and expressed as mean values and standard deviation. A one-way and multivariate analysis of variance (ANOVA) followed by Duncan’s multiple range test and Tukey’s test were performed by SPSS 15 (SPSS Inc. Chicago, IL, USA), Pearson and PCA tests were performed by XLSTAT (XLSTAT Version 2014.5.03 Copyright Addinsoft, Paris, France).
3. Results and Discussion
3.1. Methanolic Extract Composition
3.1.1. Identification of Phenolic Compounds
The fingerprinting of methanolic lemon verbena extract led to the detection of nine phenolic compounds (Table 2).

Table 2.
Retention time (Rt), spectral data and tentative identification of methanolic lemon verbena extract.
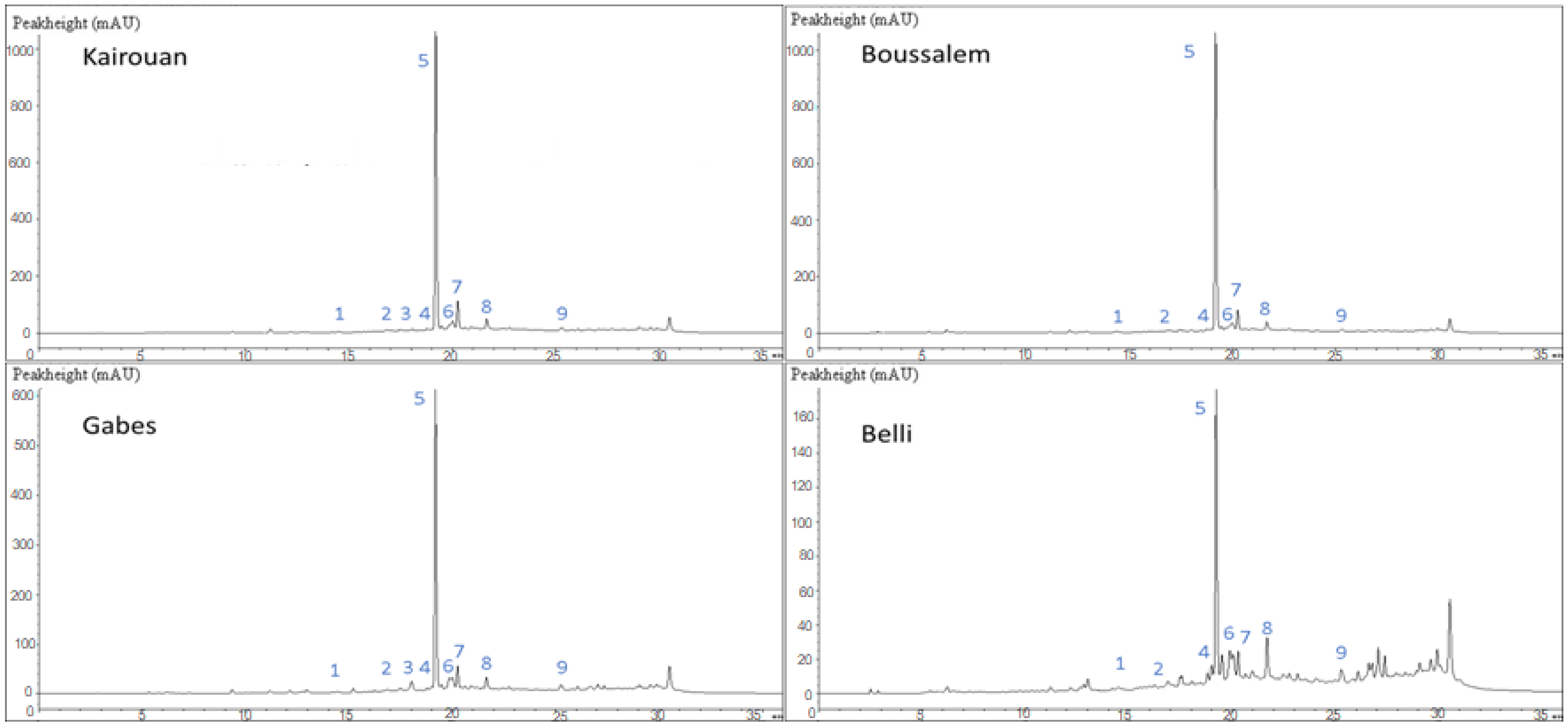
Peak 1 (Rt 11.17 min; λmax, 326 nm) exhibited a pseudomolecular ion [M-H]− at m/z 335, yielding an intense fragment ion at m/z 179 (caffeoyl residue) through the loss of shikimic moiety detected as caffeoylshikimic acid [24].
Peak 2, eluted at 14.08 min, presented a pseudomolecular ion [M-H]− at m/z 305 and a fragment ion at m/z 289 indicative of the catechin or (epi)-catechin aglycone. This compound was tentatively identified as a catechin- or an (epi)-catechin gallate [39].
Peaks 3 and 4 (λmax, 326 nm), eluted at tR, 16.02 and 16.7 min, respectively displayed a pseudomolecular ion [M-H]− at m/z 515 with an intense MS2 fragment ion at m/z 353 (caffeoylquinate residues). This fragmentation pattern was consistent with Clifford et al. [23] for 3,4-di-caffeoylquinic and 3,5-di-caffeoylquinic acids.
Peaks 5, 6 and 7 showed a characteristic UV spectrum of phenylethanoid glycosides with absorption peaks at 249, 289 and 331 nm. Peak 5 (Rt 24.26 min) exhibited a pseudomolecular ion [M-H]− at m/z 623, and its fragmentation gave an intense fragment ion at m/z 461 [M-H-162]− (loss of caffeoyl moiety). This component was the most abundant one, and it was identified as acteoside, also known as verbascoside [40]. Peak 6 showed similar UV (λmax, 249, 289 and 328 nm) and mass spectral ([M-H]− at m/z 623; MS2 at m/z 461) characteristics, with peak 5 suggesting that both compounds (peaks 5 and 6) were isomers. Consequently, compound 6 was tentatively identified as an isoacteoside (isoverbascoside). Peak 7 (Rt 27.83 min; λmax, 281, 325 nm) presented a pseudomolecular ion [M-H]− at m/z 651, and an intense fragment at m/z 475 was owed to the removal of feruloyl moiety (176 amu) and identified as martynoside [41].
Peak 8 (Rt 39.44 min) showed a characteristic UV spectrum of flavone (λmax, 337 nm), exhibited a deprotonated molecular ion [M-H]− at m/z 299 and was tentatively identified as diosmetin.
Peak 9 (Rt 40.72 min; λmax, 339 nm) showed similar mass spectral data ([M-H]− at m/z 299) to that observed for the commercial standard apigenin.
3.1.2. Phenolic Composition of Methanolic Lemon Verbena Extracts
According to Table 3, the phenolic composition of the methanolic lemon verbena extracts was influenced by the bioclimatic stage effect. These extracts were characterized by a high acteoside content. The methanolic extract from Boussalem region was the richest in acteoside with a rate of 83.54%, followed by that extracted from the Kairouan region with a percentage of 81.94%, the other two regions were marked by 76.07% for Belli and 76.15% for Gabes. This variation was statistically significant with a p-value equal to 0.000.

Table 3.
Variation in the chemical composition of methanolic lemon verbena extracts depending on the collection area.
Acteoside (also known as verbascoside) is a phenylpropanoid widely distributed in the plant kingdom and especially in the Aloysia species [12,41]. The second major compounds were 3,4-Di-caffeoylquinic acid, isoacteoside, martynoside and diosmetin with significantly varied percentages. The Belli region was marked by the highest levels of 3,4-Di-caffeoylquinic acid (4.81%) and diosmetin (4.58%). 3,4-Dicaffeoylquinic acid (3,4-Di-O-caféoylquinic acid), naturally isolated from Laggera alata, has antioxidant, DNA protective, neuroprotective and hepatoprotective properties. 3,4-Dicaffeoylquinic acid exerts apoptosis-induced cytotoxicity and glucosidase inhibitory effects [42].
Diosmetin is a bioflavonoid abundant in citrus fruits, legume leaves and spermine. It exerts antioxidant, anti-inflammatory, antiapoptotic, antimutagenic and antibacterial effects. Likewise, this flavone promotes strong cellular antioxidant activity in human monocytes by preventing the generation of intracellular ROS and the formation of malondialdehyde and by increasing the effects of the intracellular antioxidant enzymes superoxide dismutase, catalase and glutathione peroxidase. [43]. Isoacteoside is a phenylethanoide glycoside isomer of acteoside, its percentage was 4.19% for Gabes and 4.70% for Boussalem having the same biological activities (Figure 1).
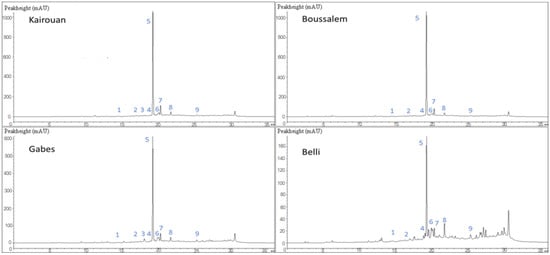
Figure 1.
Chemical composition chromatographic profile of methanolic lemon verbena extracts depending on the location of collection by LC-DAD. The affiliation of the numbered peaks is reported in Table 3.
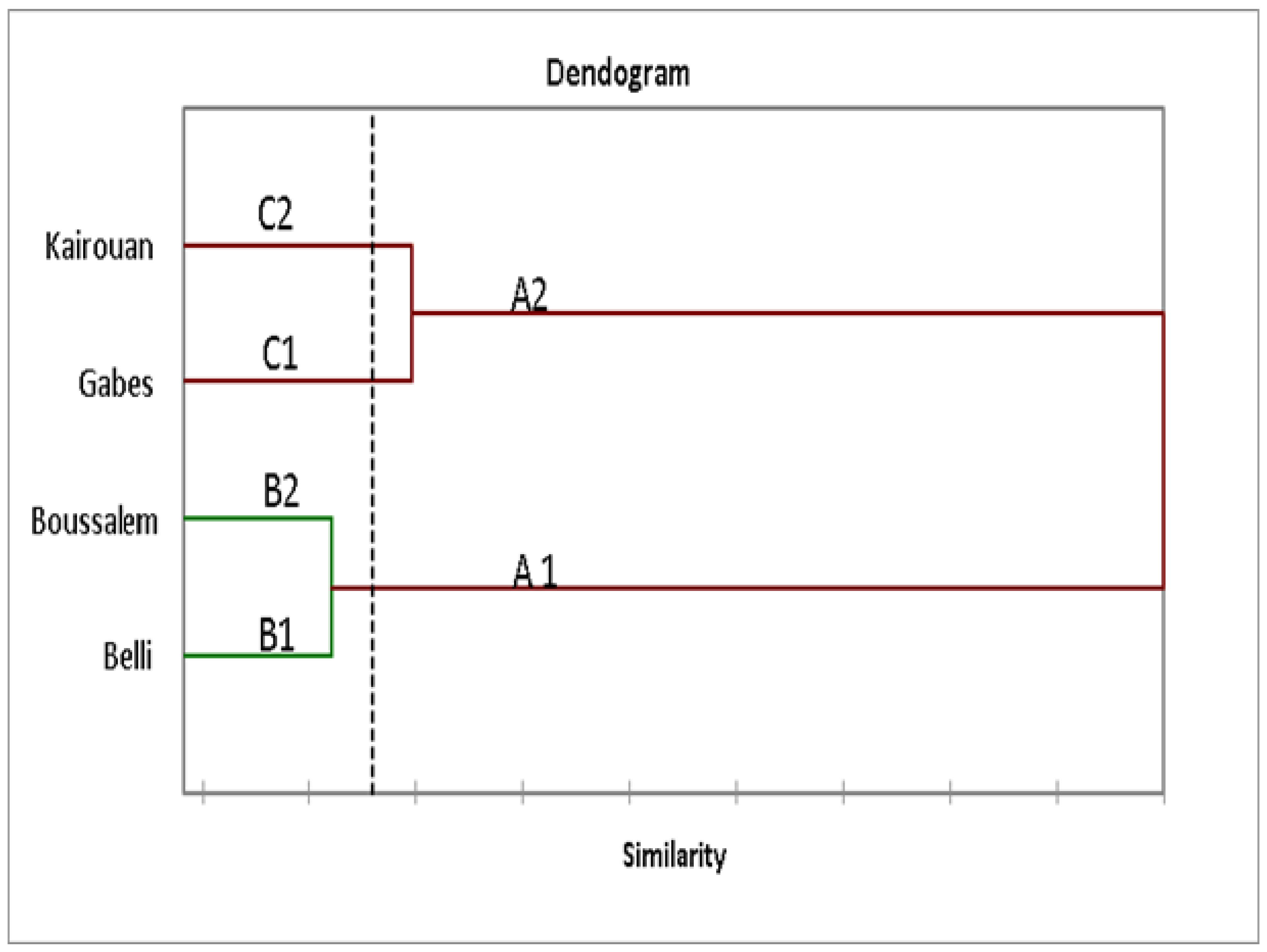
The phenolic compounds analyzed by LC-DAD were subjected to a hierarchical ascending classification (HAC) of the lemon verbena populations according to the composition of phenolic compounds and climatic conditions which grouped regions according to their similar composition. As shown in Figure 2, the dendrogram sorted the regions into two main groups, namely A1 and A2. The first group A1 consisted of two lemon verbena populations located in the Upper Semi-Arid and Interior Sub-Humid regions. The second (A2) grouped together four populations located in the Upper Arid and Semi-Arid regions. This distribution was visibly influenced by the rainfall and soil (Table 1) which could have caused oxidative stress in plant species by producing antioxidants. Phenolic compounds are among the main substances responsible for antioxidant activities.
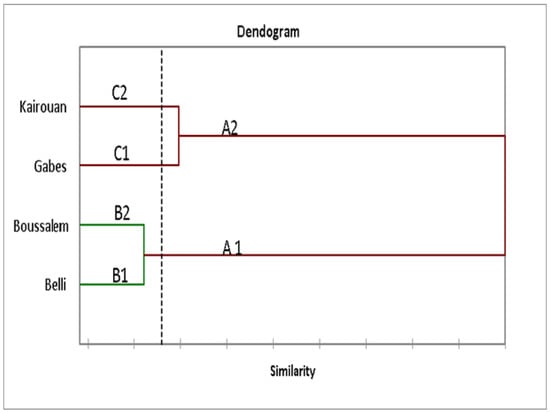
Figure 2.
Relative positions of lemon verbena collection regions according to their phenolic composition using a hierarchical ascending classification (HAC).
3.2. Phenolic Contents of Methanolic Lemon Verbena Extracts
From Table 3, lemon verbena had an appreciable content of total phenols ranging from 29.16 mg GAE/g for Boussalem to 11.66 mg GAE/g for Gabes. Similar results were obtained by Rezig et al. [20] who reported that the content of the total polyphenols of Tunisian lemon verbena varied from 14.25 to 26.58 mg GAE/g. Lemon verbena extracted with a phosphate buffer had a lower total phenol content of 1.55 mg GAE/g [44]. Likewise, Cheurfa and Allem [45] and Choupani et al. [46] determined the effect of the extraction solvent on the content of the total polyphenols of lemon verbena extracts. The highest total phenol content was observed in the methanolic extract having 25.94 mg GAE/g [46].
In descending order, the total flavonoid content was 39.86 mg CE/g in Boussalem, 38.86 mg CE/g in Kairouan, 37, 20 mg CE/g in Gabes and 27.53 mg CE/g in Belli. Lower results were detected by Rezig et al. [20] who reported that the content of total flavonoid of Tunisian lemon verbena varied from 10.59 to 19.26 mg CE/g using methanol as a solvent. Additionally, the aqueous extract of Aloysia triphylla had a weak content of total flavonoids (0.43 mg CE/g) as reported by Vinha et al. [47].
The lemon verbena of Kairouan and Boussalem showed the highest condensed total tannins (0.04 mg EC/g DM), followed by that of Belli (0.03 mg EC/g) and Gabes (0.02 mg EC/g). A higher content of total condensed tannins was observed by Rezig et al. [20] in the case of Tunisian lemon verbena which varied from 0.89 to 1.10 mg CE/g.
3.3. Antioxidant Activity
The results of the antioxidant capacities of the four collection regions are presented in Table 4. The methanolic extract of the Boussalem sample showed the highest DPPH activity (IC50 = 12.71 µg/mL) and the lowest in the Gabes sample (14.90 µg/mL). BHT had an IC50 = 17.00 µg/mL. Similar results were obtained by Rezig et al. [20] in the case of Tunisian lemon verbena. The aqueous extract of Algerian lemon verbena leaves had a lower antiradical activity (IC50 = 27.4 mg/mL) than that of the hydroalcoholic extract (IC50 = 23.52 mg/mL) [44]. A decoction of verbena (Verbena officinalis L.) had an IC50 = 15.76 mg/mL [48]. Likewise, the lemon verbena of the Boussalem region was characterized by a notable activity for ABTS (IC50 = 4.54 µg/mL) and a reducing power (IC50 = 10.37 µg/mL).

Table 4.
Antioxidant properties against DPPH and ABTS radicals and the reducing power (RP) of methanolic lemon verbena extract.
3.4. Antibacterial Activity
As can be seen in Table 5, the zone of inhibition diameter (IZ) was strongly influenced by the area factor for S. aureus, L. monocytogenes, P. aeuroginosa and S. arizonae (p < 0.001). In contrast, the methanolic lemon verbena extracts did not show a significant difference in antibacterial activity against E. coli. The highest antibacterial activity was observed against L. monocytogenes showing an inhibition zone equal to 32.5 mm in methanolic extracts of lemon verbena from Kairouan, followed by Gabes (IZ = 17.33 mm) against S. arizonae, Kairouan (IZ = 16.33 mm) against E. faecalis and Boussalem (IZ = 14 mm) against P. aeuroginosa. The lower antibacterial activity of methanolic lemon verbena extracts was measured against E coli for the Belli region.

Table 5.
In vitro antimicrobial activities of methanolic lemon verbena extracts collected in four localities.
Similar results were found by Kumar et al. [49] who studied the antibacterial activity of aqueous and organic extracts of lemon verbena aerial parts against Gram-positive (B. subtilis, S. aureus) and Gram-negative (E. coli, K. pneumoniae and P. vulgaris) bacteria. They found that all the bacterial strains were resistant to the aqueous extract, while the methanol and ethanol extracts showed moderate activities (IZ = 10.0–32 mm). A slight inhibition of growth was observed with extracts of chloroform, diethyl ether and chloroform-methanol (3:1) against all these studied strains (IZ = 6.2–8.4 mm). The phenolic compounds and phenylpropanoids were responsible for the antibacterial effect by the inactivation of microbial adhesins. The transport proteins and cell envelope may be related to the mechanism of inhibition of hydrolytic enzymes, such as proteases, carbohydrases and others interactions [41]. Indeed, the major compound of this extract was acteoside (verbascoside), this compound had significant antibacterial activity. Bazzaz et al. [50] reported that verbascoside and caffeine were able to decrease the MIC of gentamicin against standard resistant strains and two clinical isolates, Staphylococcus aureus and Escherichia coli. Among these associations, the co-administration of verbascoside and gentamicin was more effective, and synergistic activities (FICI < 1) against clinical isolates were observed.
3.5. Antifungal Activity
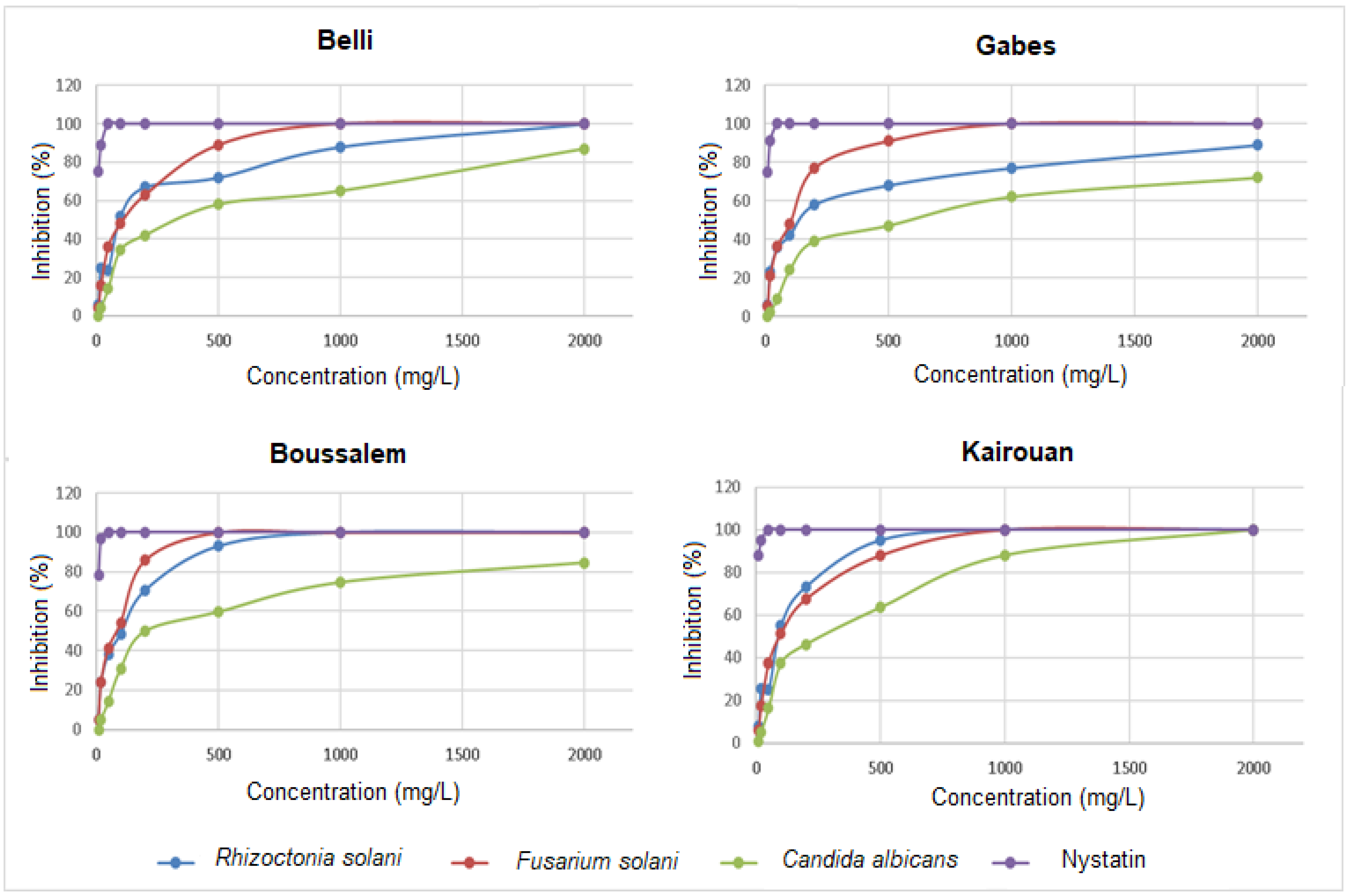
The antifungal activity evaluation consisted in measuring the percentage of the mycelial growth inhibition of C. albicans, F. solani and R. solani in the presence of methanolic lemon verbena extracts (Figure 3).
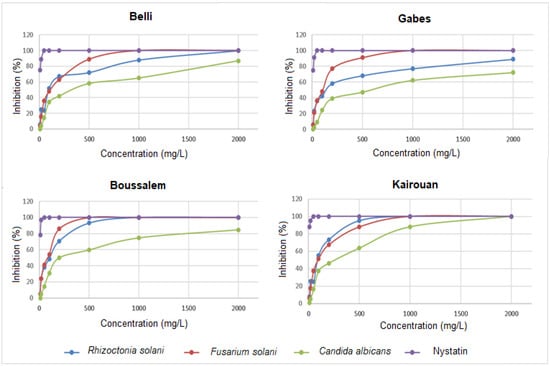
Figure 3.
Variation in the percentages of inhibition of Rhizoctonia solani, Fusarium solani, Candida albicans and Nystatin depending on the location of collection. The values are the average of three replicates.
The methanolic lemon verbena extracts had a significant variation in antifungal activity. The results revealed that F. solani and R. solani were the strains most affected by the methanolic fractions extracted from the regions of Boussalem and Kairouan, where the percentages of inhibition reached 100% at the dose of 500 mg/L. C. albicans appeared less sensitive than the two previous strains, the extract from Kairouan was the most active on this strain with a dose of 2000 mg/L. The methanolic lemon verbena extract was characterized by a high content of phenylpropanoid glycoside, acteoside (verbascoside) and its isomers isoacteoside and martynoside (about 90%). These molecules are known for their important biological activities including antibacterial and antifungal potential. In fact, Ali et al. [51] demonstrated that acteoside significantly reduced the inhibitory concentration of amphotericin B against clinically important fungal pathogens (the Cryptococcus neoformans, Candida species and Aspergillus species). Nikonorova et al. [52] proved that verbascoside inhibited the in vitro growth of F. culmorum, B. sorokiniana and B. cinerea but not of R. solani and A. solani. At low concentrations, verbascoside influenced the morphological development of pathogenic fungi.
3.6. Anti-Inflammatory Activity
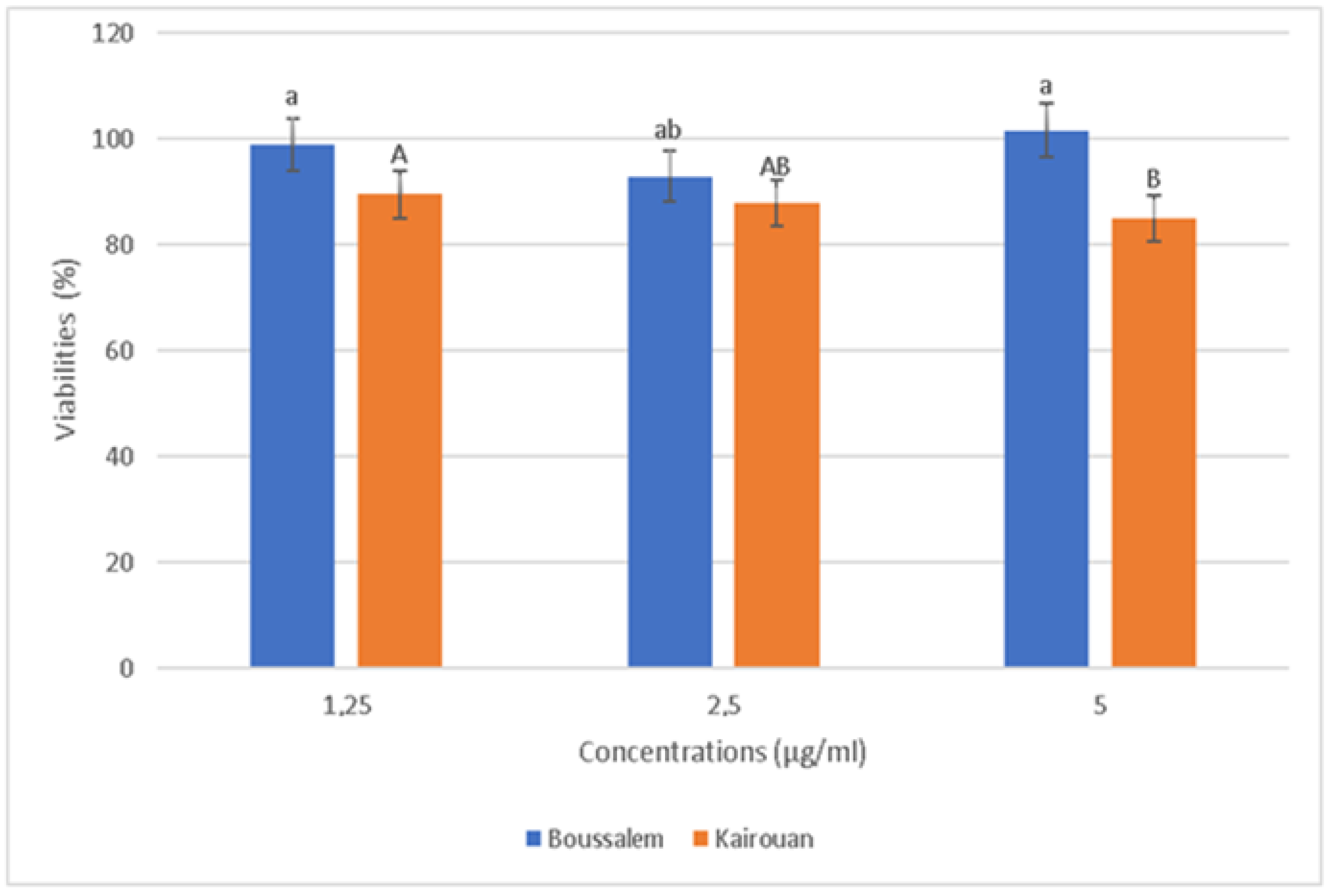
The relative positions of the lemon verbena collection regions according to their phenolic composition using a hierarchical ascending classification showed that the methanolic lemon verbena extracts were grouped into two clusters (A1: Belli/Boussalem and A2: Gabes/Kairouan) according to their similarities in composition in phenolic compounds (Figure 1). In fact, a region of each cluster, namely Kairouan and Boussalem, was selected to determine the anti-inflammatory activity of the methanolic extracts. In addition, according to the results of the activities and assays carried out previously, these two regions are characterized by an important antioxidant potential. The cytotoxicity effect of methanolic lemon verbena extracts was evaluated (Figure 4).
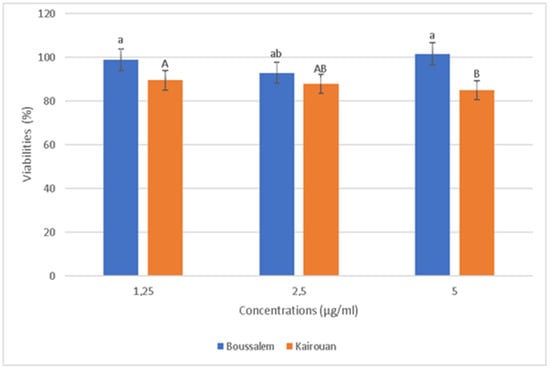
Figure 4.
Evolution of cell viability as a function of the concentrations of lemon verbena extracts in different collection regions. Percentages with different letters (a,A,b,B) were significantly different at p < 0.05.
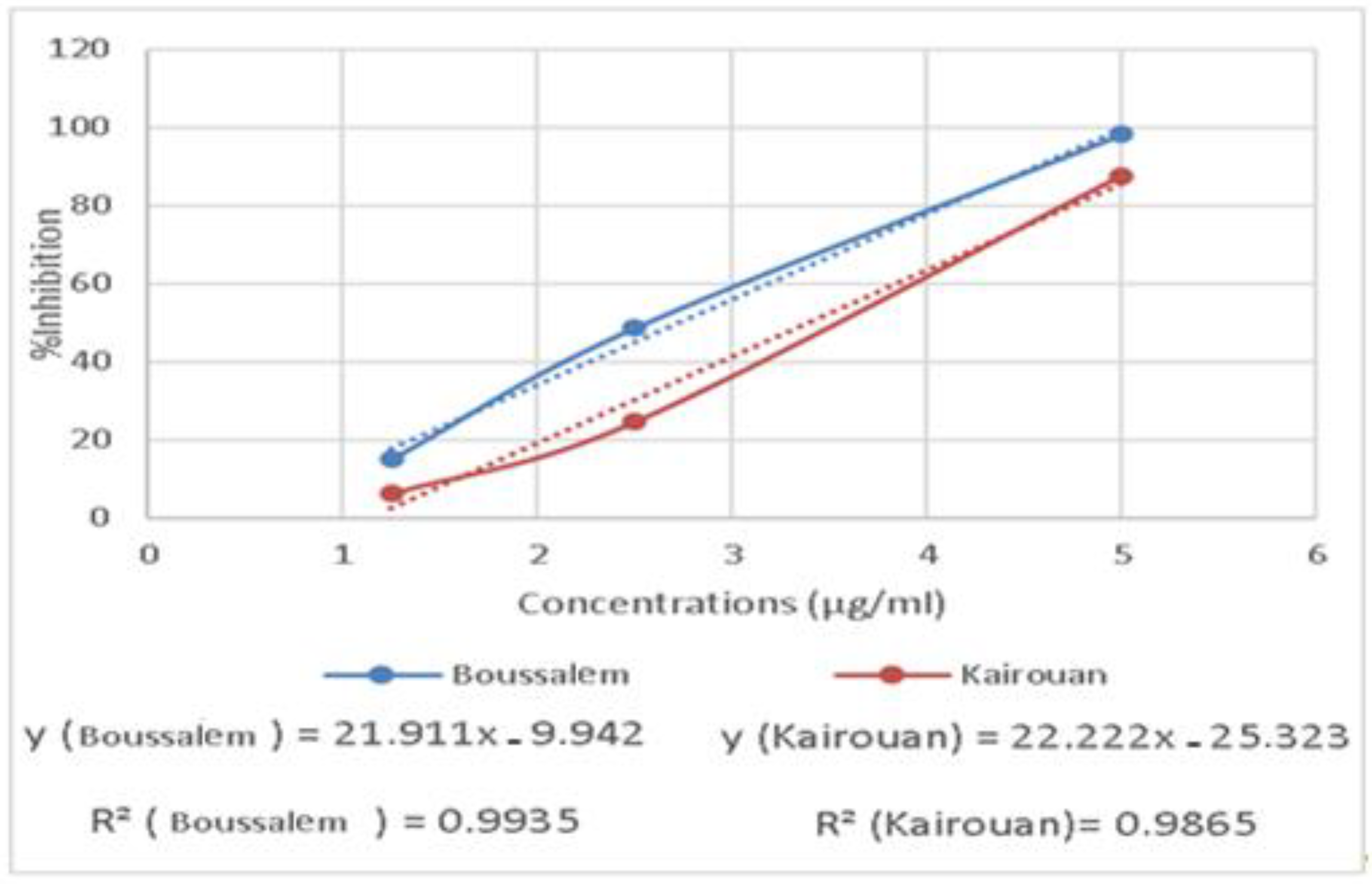
The RAW 267.4 cells were treated with increased extract concentrations (1.25 to 5 µg/mL). The extracts showed no significant cytotoxicity against the RAW 267.4 macrophage cells up to 5 µg/mL. Indeed, our experiments were carried out at nontoxic concentrations of 1.25, 2.5 and 5 µg/mL. In general, inflammation is a natural response that initiates the destruction of pathogens, tissue repair processes and restoration of homeostasis to infected or damaged sites [53]. The effect of the methanolic extracts from Kairouan and Boussalem on the production of NO • is illustrated in Figure 5.
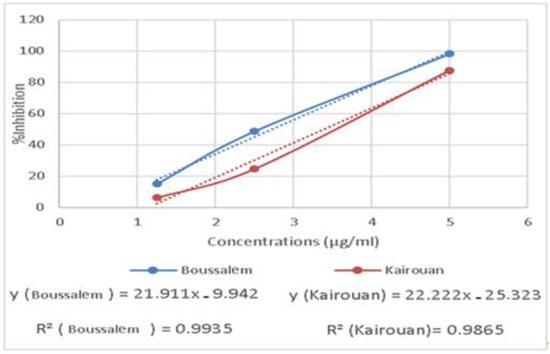
Figure 5.
Variation of anti-inflammatory activity of the methanolic lemon verbena extracts depending on the location of collection.
The results show that the various lemon verbena extracts exhibited a significant anti-inflammatory activity. The extracts from the Boussalem region showed the best activity inhibiting the production of NO • at an IC50 = 2.74 µg/mL, the lemon verbena collected in Kairouan gave an activity of an IC50 = 3.39 µg/mL. In line with our results, Lenoir et al. [54] showed that the preventative consumption of lemon verbena infusion could help manage oxidative stress by stimulating antioxidant enzymes, such as SOD, and reducing lipid peroxidation in a colonic inflammation model in rats.
3.7. Principle Component Analysis
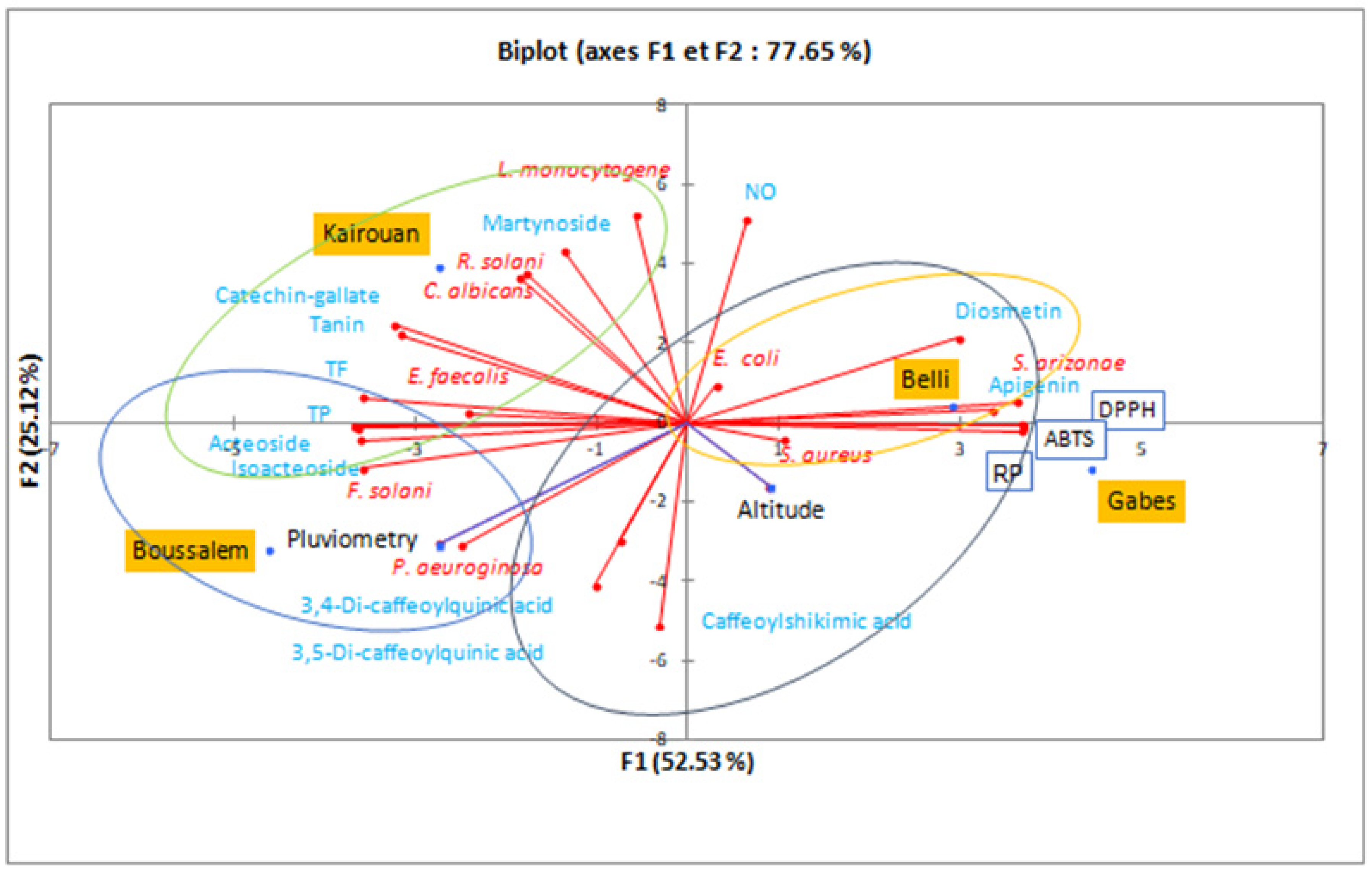
To better understand the climatic effects on the composition and the biological activities of the methanolic lemon verbena extracts, a principal component analysis allowed the analysis of the interactions between phenolic compounds and the results of biological activities (Figure 6).
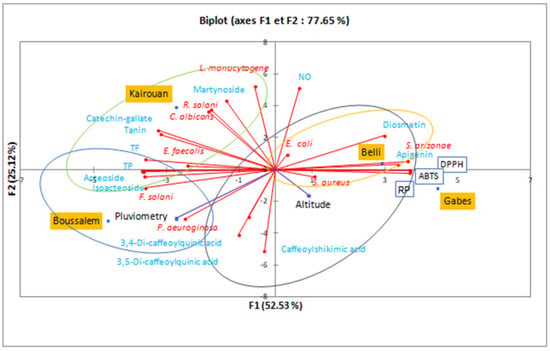
Figure 6.
Principal component analysis in a biplot of the lemon verbena populations according to the composition of methanolic extracts, their activities and climatic conditions. TP: total polyphenols, TF: total flavonoids, NO: anti-inflammatory, DPPH, ABTS: antifree radical activity, RP: reducing power.
The variability percentages associated with the axes of the representation space were presented by F1 (52.53%) and F2 (25.12%) with a sum of 77.65%, which shows that the representation is reliable. The region distribution showed that Gabes and Belli were statistically very close, with the highest IC50 of antioxidant activities (DPPH, ABTS and reducing power). However, they were characterized by bactericidal activity against S. aureus, E. coli and S. arizonae. These interpretations were approved by a similarity in phenolic composition for diosmetin and apigenin (4.58% for Belli and 2.35% for Gabes). Similar results were reported by Nayaka et al. [55], Morimoto et al. [56] and Cheng et al. [57] which proved the antibacterial potential of these flavones. The Upper Arid stage represented by Kairouan was marked by martynoside (8.18%) and catechin-galtate (1.01%). The lemon verbena extract from this region was characterized by antibacterial activity against L. monocytogen and E. faecalis, as well as antifungal activity against R. solani and C. albicans. According to Pendota et al. [58], martynoside is a phenylpropanoid glycoside isolated from the butanol fraction of Boscia albitrunca, it was the most active with the lowest MIC values of 7.81 and 31.2 µg/mL against B. subtilis and K. pneumoniae, respectively. However, it showed moderate activity against C. albicans with MIC values ranging from 62.5 to 250.0 µg/mL. The Lower Sub-Humid stage, represented by the Boussalem region, was associated with the highest mean rainfall factor. A chromatographic analysis of the methanolic extract from Boussalem showed that it was the richest in phenylpropanoid glycoside. These results were consolidated by Mechri et al. [59] and Mechri et al. [60]; Verbascoside and oleuropein were potential indicators of drought resistance in olive trees (Olea europaea L.). Although Boussalem and Kairouan have different bioclimatic stages, they are located at a close altitude (143 and 122 m, respectively). It is possible that this factor explained their similarity in phenylpropanoid glycoside contents and consequently a similarity in antioxidant activity. Chrysargyris et al. [20] found that the altitude factor affected the antioxidant capacity of lemon verbena, as plain plants presented higher flavonoids and DPPH than mountainous grown plants. This observation was consolidated by the correlation matrix (Pearson (n)) which studied the statistical interaction between composition and biological activities. This study clearly showed that acteoside had a significant correlation with the IC50 of antifree radical activities DPPH and ABTS and the iron reducing power with very remarkable coefficients: −0.988, −0.980 and −0.992. The antioxidant potential of acteoside and isoacteoside has been shown by several previous studies [61,62]. Likewise, Vertuani et al. [63] showed that the addition of verbacoside increases the oxidative stability of cosmetic formulas.
4. Conclusions
In conclusion, the analysis of the biochemical variability of Tunisian verbena species showed that the chemical composition of methanolic extracts is similar to variations in the amounts of each compound identified. This trend was also recorded for all biological activities; the best data are associated with the region of Boussalem, this region is located in a lower Sub-Humid stage with an important rainfall and an average altitude. Antioxidant activity is correlated with the amount of phenylpropanoid including acteoside and isoacteoside. The other activities have no significant correlation with climatic factors. Regardless, these extracts are endowed with a remarkable antibacterial, antifungal and anti-inflammatory power, which shows that this extract can play the role of an important cosmetic additive to increase shelf life dates.
Author Contributions
Conceptualization, S.T.; writing, S.T., W.A.W., N.F., S.B. and S.D.; methodology, S.T., S.K. and M.H.; investigation, S.T., N.S. and H.L., formal analysis, S.T., W.A.W. and K.M.; data curation, W.A.W.; resources, S.T.; supervision, K.M. and G.D.R.; writing—review editing, K.M.; funding acquisition and final revision, K.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Tunisian Ministry of Higher Education and Scientific Research (LR15CBBC06). This research was also funded by Taif University Researchers Supporting Project number (TURSP-2020/94), Taif University, Taif, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
All authors declare no conflict of interest.
References
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Monteiro, J.M.; Albuquerque, U.P.; Lins Neto, E.M.; Araújo, E.L.; Albuquerque, M.M.; Amorim, E.L. The effects of seasonal climate changes in the Caatinga on tannin levels in Myracrodruon urundeuva (Engl.) Fr. All. and Anadenanthera colubrina (Vell.) brenan. Rev. Bras. Farmacogn. 2006, 16, 338–344. [Google Scholar] [CrossRef]
- Gobbo-Neto, L.; Lopes, N.P. Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Quim. Nova 2007, 30, 374–381. [Google Scholar] [CrossRef]
- Santos, S.C.; Costa, W.F.; Batista, F.; Santos, L.R.; Ferri, P.H.; Ferreira, H.D.; Seraphin, J.C. Seasonal variation tannins in barks of barbatimao. Rev. Bras. Farmacogn. 2006, 16, 552–556. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Bono, A. Phytochemical antioxidants for health and medicine—A move towards nature. Biotechnol. Mol. Biol. Rev. 2007, 1, 97–104. [Google Scholar]
- Leyva-Jiménez, F.J.; Lozano-Sánchez, J.; Fernández-Ochoa, Á.; de la Luz Cádiz-Gurrea, M.; Arráez-Román, D.; Segura-Carretero, A. Optimized extraction of phenylpropanoids and flavonoids from lemon verbena leaves by supercritical fluid system using response surface methodology. Foods 2020, 9, 931. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Marzo, N.; Lozano-Sánchez, J.; Cádiz-Gurrea, M.L.; Herranz-López, M.; Micol, V.; Segura-Carretero, A. Relationships between chemical structure and antioxidant activity of isolated phytocompounds from lemon verbena. Antioxidants 2019, 8, 324. [Google Scholar] [CrossRef]
- Guimarães, R.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Infusions and decoctions of mixed herbs used in folk medicine: Synergism in antioxidant potential. Phytother. Res. 2011, 25, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, M.; Serravalle, L.; Nadra, M.C.M.; Saad, A.D. Antioxidant capacity and antibacterial activity of phenolic compounds from argentinean herbs infusions. Food Control. 2010, 21, 779–785. [Google Scholar] [CrossRef]
- Maliki, I.; Almehdi, A.M.; El Moussaoui, A.; Abdel-Rahman, I.; Ouahbi, A. Phytochemical screening and the antioxidant, antibacterial and antifungal activities of aqueous extracts from the leaves of Lippia triphylla planted in Morocco. Moroc. J. Chem. 2020, 8, 2943–2956. [Google Scholar]
- Amin, B.; Noorani, R.; Razavi, B.M.; Hosseinzadeh, H. The effect of ethanolic extract of Lippia citrodora on rats with chronic constriction injury of neuropathic pain. Cell J. 2018, 19, 528–536. [Google Scholar] [PubMed]
- Etemad, L.; Zafari, R.; Seyed, A.M.; Vahdati-Mashhadian, N.; Skouei Shirvan, Z.; Hosseinzadeh, H. Teratogenic effect of verbascoside, main constituent of Lippia citrodora leaves, in mice. Iran J. Pharm. Res. 2016, 15, 521–525. [Google Scholar] [PubMed]
- Bahramsoltani, R.; Rostamiasrabadi, P.; Shahpiri, Z.; Marques, A.M.; Rahimi, R.; Farzaei, M.H. Aloysia citrodora Paláu (Lemon verbena): A review of phytochemistry and pharmacology. J. Ethnopharmacol. 2018, 222, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Carnat, A.; Carnat, A.P.; Fraisse, D. The aromatic and polyphenolic composition of lemon verbena tea. Fitoterapia 1999, 70, 44–49. [Google Scholar] [CrossRef]
- Sartoratto, A.; Machado, A.L.M.; Delamerlina, C. Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Braz. J. Microbiol. 2004, 35, 275–280. [Google Scholar] [CrossRef]
- Wernert, M.F.; Wagner, M.L.; Gurni, A.A. Estudio de polifenoles de infusiones y cocimientos de hojas de “Cedrón” (Aloysia citrodora Palau) y “Poleo” (Lippia turbinata Griseb.)–Verbenaceae. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas 2009, 84, 308–311. [Google Scholar]
- El-Hawary, S.S.; Yousif, M.F.; Abdel Motaal, A.A.; Abd-El Hamid, L.M. Bioactivities, phenolic compounds and in-vitro propagation of Lippia citriodora Kunth cultivated in Egypt. Bull. Fac. Pharm. Cairo Univ. 2012, 50, 1–6. [Google Scholar] [CrossRef][Green Version]
- Bilia, A.R.; Giomi, M.; Innocent, M.; Gallori, S.; Vincier, F.F. HPLC-DAD- ESI-MS analysis of the constituents of aqueous preparations of verbena and Lemon verbena and evaluation of the antioxidant activity. J. Pharm. Biomed. Anal. 2008, 46, 463–470. [Google Scholar] [CrossRef]
- Yoo, K.M.; Lee, C.H.; Lee, H.; Moon, B.; Lee, C.Y. Relative antioxidant and cytoprotective activities of common herbs. Food Chem. 2008, 106, 929–936. [Google Scholar] [CrossRef]
- Rezig, L.; Saada, M.; Trabelsi, N.; Tammar, S.; Limam, H.; Bettaieb Rebey, I.; Smaoui, A.; Sghaier, G.; Del Re, G.; Ksouri, R.; et al. Chemical composition, antioxidant and antimicrobial activities of Aloysia triphylla L. essential oils and methanolic extract. Ital. J. Food Sci. 2019, 31, 556–572. [Google Scholar]
- Chrysargyris, A.; Mikallou, M.; Petropoulos, S.; Tzortzakis, N. Profiling of essential oils components and polyphenols for their antioxidant activity of medicinal and aromatic plants grown in different environmental conditions. Agronomy 2020, 10, 727. [Google Scholar] [CrossRef]
- Mau, J.L.; Chao, G.R.; Wu, K.T. Antioxidant properties of methanolic extracts from several ear mushrooms. J. Agric. Food Chem. 2001, 49, 5461–5467. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the six Bioactivities, phenolic compounds and in-vitro propagation of Lippia citriodora Kunth cultivated in Egypt isomers of dicaffeoylquinic acid by LC-MS n. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Nebehaj, E.; Albert, L. Antioxidant properties and detailed polyphenol profiling of European hornbeam (Carpinus betulus L.) leaves by multiple antioxidant capacity assays and high-performance liquid chromatography/multistage electrospray mass spectrometry. Ind. Crop. Prod. 2016, 87, 340–349. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Wang, W.; Salvaterra, P.M. Functional analysis and tissue-specific expression of Drosophila Na+, K+-ATPase subunits. J. Neurochem. 1998, 71, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Hatano, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two new flavonoids and other constituents in licorice root: Their relative astringency and radical scavenging effects. Chem. Pharm. Bull. 1988, 36, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Amezouar, F.; Hsaine, M.; Bourhim, N.; Fougrach, H. Évaluation des activités antioxydante et anti-inflammatoire de Erica arborea L. du Maroc. J. Pathol. Biol. 2013, 61, 254–258. [Google Scholar] [CrossRef]
- Rios, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Rguez, S.; Ben Slimene, I.; Abid, G.; Hammemi, M.; Kefi, A.; Elkahoui, S.; Ksouri, R.; Hamrouni Sellami, I.; Djebali, N. Tetraclinis articulata essential oil reduces Botrytis cinerea infections on tomato. Sci. Hortic. 2020, 266, 109291. [Google Scholar] [CrossRef]
- Fehlberg, L.C.C.; Gianinni Nicoletti, A.; Ramos, A.C.; Rodrigues-Costa, F.; Pereira de Matos, A.; Girardello, R.; Andrade Marques, E.; Gales, A.C. In vitro susceptibility of Burkholderia cepacia complex isolates: Comparison of disk diffusion, Etest®, agar dilution, and broth microdilution methods. Diagn. Microbiol. Infect. Dis. 2016, 86, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Schröter, D.; Neugart, S.; Schreiner, M.; Grune, T.; Rohn, S.; Ott, C. Amaranth’s 2-caffeoylisocitric acid—An anti-inflammatory caffeic acid derivative that impairs NF-κB signaling in LPS-challenged RAW 264.7 macrophages. Nutrients 2019, 11, 571. [Google Scholar] [CrossRef]
- Borra, R.C.; Lotufo, M.A.; Gagioti, S.M.; de Mesquita Barros, F.; Andrade, P.M. A simple method to measure cell viability in proliferation and cytotoxicity assays. Braz. Oral Res. 2009, 23, 255–262. [Google Scholar] [CrossRef] [PubMed]
- O’brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Griess, P. Bemerkungen zu der Abhandlung der HH. Weselsky und Benedikt, Ueber einige Azoverbindungen. Ber. Dtsch. Chem. Ges. 1879, 12, 426–428. [Google Scholar] [CrossRef]
- Kelebek, H. LC-DAD–ESI-MS/MS characterization of phenolic constituents in Turkish black tea: Effect of infusion time and temperature. Food Chem. 2016, 204, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Li, X.N.; Sun, J.; Shi, H.; Yu, L.L.; Ridge, C.D.; Mazzola, E.P.; Okunji, C.; Iwu, M.M.; Michel, T.K.; Chen, P. Profiling hydroxycinnamic acid glycosides, iridoid glycosides, and phenylethanoid glycosides in baobab fruit pulp (Adansonia digitata). Food Res. Int. 2017, 99, 755–761. [Google Scholar] [CrossRef]
- Funes, L.; Laporta, O.; Cerdán-Calero, M.; Micol, V. Effects of verbascoside, a phenylpropanoid glycoside from lemon verbena, on phospholipid model membranes. Chem. Phys. Lipids 2010, 163, 190–199. [Google Scholar] [CrossRef]
- Liu, X.; Huang, K.; Ziran, N.; Dan, M.; Bo, Z. Protective effect of isochlorogenic acid B on liver fibrosis in non-alcoholic steatohepatitis of mice. Basic Clin. Pharmacol. Toxicol. 2019, 124, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gong, X.B.; Huang, L.G.; Wang, Z.X.; Wan, R.Z.; Zhang, P.; Zhang, Q.Y.; Chen, Z.; Zhang, B.S. Diosmetin exerts anti-oxidative, anti-inflammatory and anti-apoptotic effects to protect against endotoxin-induced acute hepatic failure in mice. Oncotarget 2017, 8, 30723. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Cheurfa, M.; Allem, R. Evaluation of antioxidant activity of different extracts of Aloysia triphylla leaves (L’Herit.) from Algeria in vitro. Phytotherapy 2016, 14, 181–187. [Google Scholar] [CrossRef]
- Choupani, M.; Delouee, S.A.; Alami, M. Antioxidant properties of various solvent extracts of lemon verbena (Lippia Citrodora) leaves. Int. J. Adv. Biol. Biomed. Res. 2014, 2, 494–500. [Google Scholar]
- Vinha, A.F.; Soares, M.O.; Castro, A.; Santos, A.; Oliveira Maria, P.P.B.; Machado, M. Phytochemical characterization and radical scavenging activity of aqueous extracts of medicinal plants from Portugal. Eur. J. Med. Plants 2012, 2, 335–347. [Google Scholar] [CrossRef]
- Babili, F.E.; Babili, M.E.L.; Souchard, J.P.; Chatelain, C. Culinary decoctions: Spectrophotometric determination of various polyphenols coupled with their antioxidant activities. Pharm. Crop. 2013, 4, 15–20. [Google Scholar] [CrossRef]
- Kumar, N.K.; Kumar, K.S.; Raman, B.V.; Reddy, I.B.; Ramarao, M.; Rajagopal, S.V. Antibacterial activity of Lippia citrodora a folklore plant. J. Pure Appl. Microbiol. 2008, 2, 249–252. [Google Scholar]
- Bazzaz, B.S.F.; Khameneh, B.; Ostad, M.R.Z.; Hosseinzadeh, H. In vitro evaluation of antibacterial activity of verbascoside, lemon verbena extract and caffeine in combination with gentamicin against drug-resistant Staphylococcus aureus and Escherichia coli clinical isolates. Avicenna J. Phytomed. 2018, 8, 246. [Google Scholar]
- Ali, I.; Sharma, P.; Suri, K.A.; Satti, N.K.; Dutt, P.; Afrin, F.; Khan, I.A. In vitro antifungal activities of amphotericin B in combination with acteoside, a phenylethanoid glycoside from Colebrookea oppositifolia. J. Med. Microbiol. 2011, 60, 1326–1336. [Google Scholar] [CrossRef] [PubMed]
- Nikonorova, A.; Egorov, C.A.; Galikina, T.G.; Grishin, E.V.; Babakov, A.V. Antifungal activity of phenolic glicozide verbascoside from Plantago major seeds. Mikol. Fitopatol. 2009, 43, 52–57. [Google Scholar]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Lenoir, L.; Rossary, A.; Joubert-Zakeyh, J.; Vergnaud-Gauduchon, J.; Farges, M.C.; Fraisse, D.; Texier, O.; Lamaison, J.L.; Vasson, M.P.; Felgines, C. Lemon verbena infusion consumption attenuates oxidative stress in dextran sulfate sodium-induced colitis in the rat. Digest. Dis. Sci. 2011, 56, 3534–3545. [Google Scholar] [CrossRef] [PubMed]
- Nayaka, H.B.; Londonkar Ramesh, L.; Umesh Madire, K.; Tukappa, A. Antibacterial attributes of apigenin, isolated from Portulaca oleracea L. Int. J. Bacteriol. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Baba, T.; Sasaki, T.; Hiramatsu, K. Apigenin as an anti-quinolone-resistance antibiotic. Int. J. Antimicrob. Agents 2015, 46, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Zhang, L.; Zhang, H.; Li, X.; Wang, Y.; Xia, F.; Wang, B.; Cai, R.; Guo, Z.; Zhang, Y.; et al. An ointment consisting of the phage lysin LysGH15 and apigenin for decolonization of methicillin-resistant Staphylococcus aureus from skin wounds. Viruses 2018, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Pendota, S.; Aderogba, M.A.; Van Staden, J. In vitro antimicrobial activity of extracts and an isolated compound from Boscia albitrunca leaves. S. Afr. J. Bot. 2015, 96, 91–93. [Google Scholar] [CrossRef]
- Mechri, B.; Tekaya, M.; Attia, F.; Hammami, M.; Chehab, H. Drought stress improved the capacity of Rhizophagus irregularis for inducing the accumulation of oleuropein and mannitol in olive (Olea europaea) roots. Plant Physiol. Biochem. 2020, 156, 178–191. [Google Scholar] [CrossRef]
- Mechri, B.; Tekaya, M.; Hammami, M.; Chehab, H. Root verbascoside and oleuropein are potential indicators of drought resistance in olive trees (Olea europaea L.). Plant Physiol. Biochem. 2019, 141, 407–414. [Google Scholar] [CrossRef]
- Funes, L.; Fernández-Arroyo, S.; Laporta, O.; Pons, A.; Roche, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Micol, V. Correlation between plasma antioxidant capacity and verbascoside levels in rats after oral administration of lemon verbena extract. Food Chem. 2009, 117, 589–598. [Google Scholar] [CrossRef]
- Gonçalves, S.; Grevenstuk, T.; Martins, N.; Romano, A. Antioxidant activity and verbascoside content in extracts from two uninvestigated endemic Plantago spp. Ind. Crop. Prod. 2015, 65, 198–202. [Google Scholar] [CrossRef]
- Vertuani, S.; Beghelli, E.; Scalambra, E.; Malisardi, G.; Copetti, S.; Dal Toso, R.; Baldisserotto, A.; Manfredini, S. Activity and stability studies of verbascoside, a novel antioxidant, in dermo-cosmetic and pharmaceutical topical formulations. Molecules 2011, 16, 7068–7080. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).