Nutritional and Volatile Characterisation of Milk Inoculated with Thermo-Tolerant Lactobacillus bulgaricus through Adaptive Laboratory Evolution
Abstract
:1. Introduction
2. Materials and Methods
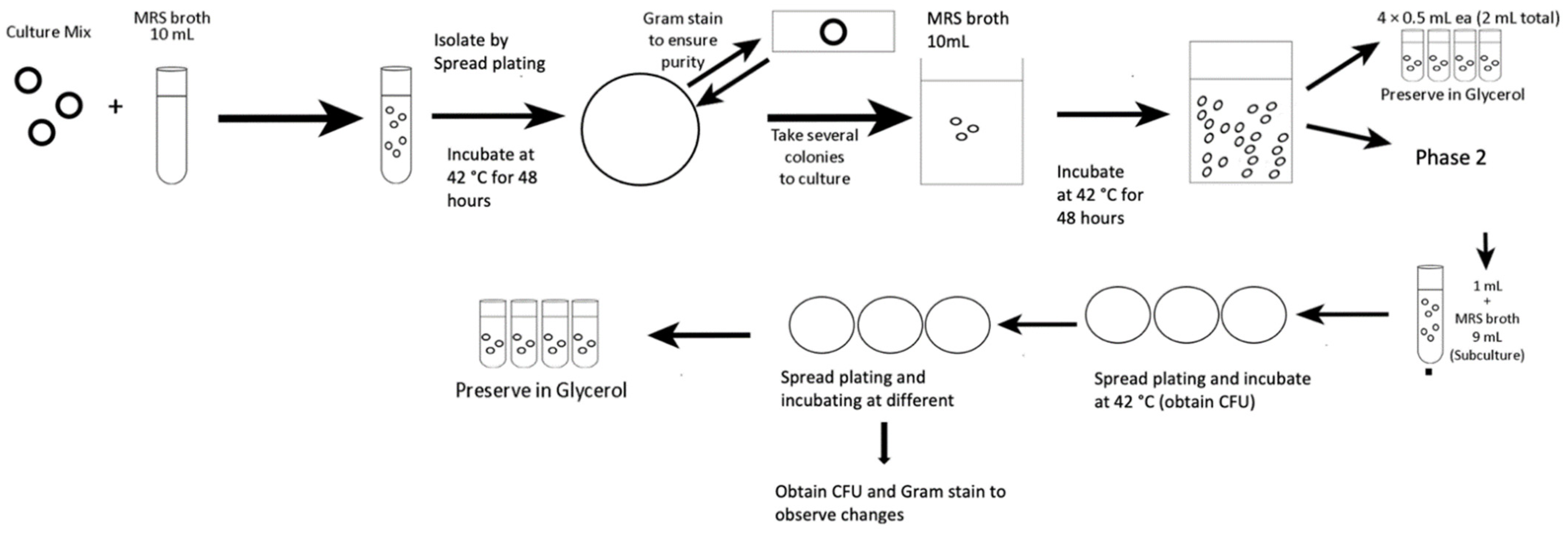
2.1. Isolation of L. bulgaricus
2.2. Application of Adaptive Laboratory Evolution to Generate Thermo-Tolerant Strain
Performance of Thermo-Tolerant L. bulgaricus
2.3. Milk Inoculation and Incubation with L. bulgaricus
2.4. Inoculated Milk Analyses
2.4.1. Determination of pH and Viscosity
2.4.2. Water Holding Capacity (WHC)
2.4.3. Determination of Free Amino Acids with Liquid Chromatography-Mass Spectrometry (LCMS)
2.4.4. Determination of Volatile Compounds with Solid Phase Microextraction—Gas Chromatography-Mass Spectrometry (SPME-GC-MS)
2.4.5. Determination of Odour Threshold and Relative Odour Activity Value (OAV)
2.5. Statistical Analysis
3. Result and Discussion
3.1. Generation of Thermo-Tolerant Strains of L. bulgaricus
3.2. Level of Fitness for Thermo-Tolerant L. bulgaricus and pH
3.3. Physicochemical Properties
3.3.1. Viscosity
3.3.2. WHC
3.3.3. Free Amino Acid Profile
3.3.4. Volatile Compounds
3.3.5. Odour Threshold and Relative OAV
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Puvanenthiran, A.; Stevovitch-Rykner, C.; McCann, T.H.; Day, L. Synergistic effect of milk solids and carrot cell wall particles on the rheology and texture of yoghurt gels. Food Res. Int. 2014, 62, 701–708. [Google Scholar] [CrossRef]
- Han, X.; Yang, Z.; Jing, X.; Yu, P.; Zhang, Y.; Yi, H.; Zhang, L. Improvement of the Texture of Yogurt by Use of Exopolysaccharide Producing Lactic Acid Bacteria. BioMed Res. Int. 2016, 2016, 7945675. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, D.; Waszkiewicz-Robak, B.; Kolanowski, W.; Swiderski, F. Relative importance of texture properties in the sensory quality and acceptance of natural yoghurts. Int. J. Dairy Technol. 2005, 58, 39–46. [Google Scholar] [CrossRef]
- Hekmat, S.; Reid, G. Sensory properties of probiotic yogurt is comparable to standard yogurt. Nutr. Res. 2006, 26, 163–166. [Google Scholar] [CrossRef]
- Ruiz-Avila, L.; Ming, D.; Margolskee, R.F. An In vitro assay useful to determine the potency of several bitter compounds. Chem. Senses 2000, 25, 361–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panesar, P.; Kennedy, J.F.; Gandhi, D.N.; Bunko, K. Bioutilisation of whey for lactic acid production. Food Chem. 2007, 105, 1–14. [Google Scholar] [CrossRef]
- Hatti-Kaul, R.; Chen, L.; Dishisha, T.; El Enshasy, H. Lactic acid bacteria: From starter cultures to producers of chemicals. FEMS Microbiol. Lett. 2018, 365, fny213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Zhao, S.; Hao, G.; Yu, H.; Tian, H.; Zhao, G. Role of lactic acid bacteria on the yogurt flavour: A review. Int. J. Food Prop. 2017, 20, S316–S330. [Google Scholar] [CrossRef] [Green Version]
- Guerra, E.J.; Estepa, R.G.; Rivas, I.R. Analysis of diacetyl in yogurt by two new spectrophotometric and fluorimetric methods. Food Chem. 1995, 53, 315–319. [Google Scholar]
- Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 2018, 4, 665–684. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chan, S.H.J.; Chen, J.; Solem, C.; Jensen, P.R. Systems Biology—A Guide for Understanding and Developing Improved Strains of Lactic Acid Bacteria. Front. Microbiol. 2019, 10, 876. [Google Scholar] [CrossRef] [PubMed]
- Torino, M.I.; De Valdez, G.F.; Mozzi, F. Biopolymers from lactic acid bacteria. Novel applications in foods and beverages. Front. Microbiol. 2015, 6, 834. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, A.; Romano, G.H.; Groisman, B.; Yona, A.; Dekel, E.; Kupiec, M.; Dahan, O.; Pilpel, Y. Adaptive prediction of environmental changes by microorganisms. Nat. Cell Biol. 2009, 460, 220–224. [Google Scholar]
- Ibrahim, I.A.; Naufalin, R.; Dwiyanti, H. (Eds.) Effect of fermentation temperature and culture concentration on microbial and physicochemical properties of cow and goat milk yogurt. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019. [Google Scholar]
- GuhanNath, S.; Aaron, S.I.; Raj, A.A.; Ranganathan, T.V. Recent innovations in nanotechnology in food processing and its various applications—A review. Int. J. Pharm. Sci. Rev. Res. 2014, 29, 116–124. [Google Scholar]
- Dragosits, M.; Mattanovich, D. Adaptive laboratory evolution–principles and applications for biotechnology. Microb. Cell Factories 2013, 12, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandberg, T.E.; Salazar, M.J.; Weng, L.L.; Palsson, B.O.; Feist, A.M. The emergence of adaptive laboratory evolution as an efficient tool for biological discovery and industrial biotechnology. Metab. Eng. 2019, 56, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Herring, C.D.; Raghunathan, A.; Honisch, C.; Patel, T.; Applebee, M.K.; Joyce, A.R.; Albert, T.J.; Blattner, F.R.; Van den Boom, D.; Cantor, C.R.; et al. Comparative genome sequencing of Escherichia coli allows observation of bacterial evolution on a laboratory timescale. Nat. Genet. 2019, 38, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Ying, B.-W.; Matsumoto, Y.; Kitahara, K.; Suzuki, S.; Ono, N.; Furusawa, C.; Kishimoto, T.; Yomo, T. Bacterial transcriptome reorganization in thermal adaptive evolution. BMC Genom. 2015, 16, 802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, B.; Dahabieh, M.; Krogerus, K.; Jouhten, P.; Magalhães, F.; Pereira, R.; Siewers, V.; Vidgren, V. Adaptive Laboratory Evolution of Ale and Lager Yeasts for Improved Brewing Efficiency and Beer Quality. Annu. Rev. Food Sci. Technol. 2020, 11, 23–44. [Google Scholar] [CrossRef] [Green Version]
- Aguilera, J.; Andreu, P.; Randez-Gil, F.; Prieto, J.A. Adaptive evolution of baker’s yeast in a dough-like environment enhances freeze and salinity tolerance. Microb. Biotechnol. 2010, 3, 210–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, Y.W.; Bae, J.-H.; Kim, S.-A.; Han, N.S. Development of Freeze-Thaw Tolerant Lactobacillus rhamnosus GG by Adaptive Laboratory Evolution. Front. Microbiol. 2018, 9, 2781. [Google Scholar] [CrossRef]
- Pena-Miller, R.; Laehnemann, D.; Jansen, G.; Fuentes-Hernandez, A.; Rosenstiel, P.; Schulenburg, H.; Beardmore, R. When the Most Potent Combination of Antibiotics Selects for the Greatest Bacterial Load: The Smile-Frown Transition. PLoS Biol. 2013, 11, e1001540. [Google Scholar] [CrossRef] [Green Version]
- D-LACTIC ACID (D-LACTATE) (Rapid) and L-LACTIC ACID (L-LACTATE) ASSAY PROCEDURES. Available online: https://www.megazyme.com/documents/Assay_Protocol/K-DLATE_DATA.pdf (accessed on 26 October 2021).
- Remeuf, F.; Mohammed, S.; Sodini, I.; Tissier, J. Preliminary observations on the effects of milk fortification and heating on microstructure and physical properties of stirred yogurt. Int. Dairy J. 2003, 13, 773–782. [Google Scholar] [CrossRef]
- Diep, T.T.; Pook, C.; Yoo, M.J.Y. Physicochemical properties and proximate composition of tamarillo (Solanum betaceum Cav.) fruits from New Zealand. J. Food Compos. Anal. 2020, 92, 103563. [Google Scholar] [CrossRef]
- Diep, T.T.; Yoo, M.J.Y.; Pook, C.; Sadooghy-Saraby, S.; Gite, A.; Rush, E. Volatile Components and Preliminary Antibacterial Activity of Tamarillo (Solanum betaceum Cav.). Foods 2021, 10, 2212. [Google Scholar] [CrossRef] [PubMed]
- Leffingwell & Associates. Odor & Flavor Detection Thresholds in Water (in Parts per Billion); Leffingwell & Associates: Canton, GA, USA, 1991. [Google Scholar]
- Cheng, H. Volatile Flavor Compounds in Yogurt: A Review. Crit. Rev. Food Sci. Nutr. 2010, 50, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Thierry, A.; Pogačić, T.; Weber, M.; Lortal, S. Production of Flavor Compounds by Lactic Acid Bacteria in Fermented Foods. In Biotechnology of Lactic Acid Bacteria; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 314–340. [Google Scholar]
- Grobben, G.J.; Sikkema, J.; Smith, M.R.; de Bont, J.A.M. Production of extracellular polysaccharides by Lactobacillus delbrueckii ssp. bulgaricus NCFB 2772 grown in a chemically defined medium. J. Appl. Bacteriol. 1995, 79, 103–107. [Google Scholar] [CrossRef]
- Chervaux, C.; Ehrlich, S.D.; Maguin, E. Physiological Study of Lactobacillus delbrueckii subsp. bulgaricus Strains in a Novel Chemically Defined Medium. Appl. Environ. Microbiol. 2000, 66, 5306–5311. [Google Scholar] [CrossRef] [Green Version]
- Costa, E.; Usall, J.; Teixidó, N.; Garcia, N.; Viñas, I. Effect of protective agents, rehydration media and initial cell concentration on viability of Pantoea agglomerans strain CPA-2 subjected to freeze-drying. J. Appl. Microbiol. 2000, 89, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Young, K.D. The Selective Value of Bacterial Shape. Microbiol. Mol. Biol. Rev. 2006, 70, 660–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindquist, S.; Craig, E.A. The heat-shock proteins. Annu. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Silva, J.; Ho, P.; Teixeira, P.; Malcata, F.X.; Gibbs, P. Effects of Various Sugars Added to Growth and Drying Media upon Thermotolerance and Survival throughout Storage of Freeze-Dried Lactobacillus delbrueckii ssp. bulgaricus. Biotechnol. Prog. 2008, 20, 248–254. [Google Scholar] [CrossRef] [PubMed]
- De Boer, P.; Crossley, R.; Rothfield, L. The essential bacterial cell-division protein FtsZ is a GTPase. Nature 1992, 359, 254–256. [Google Scholar] [CrossRef]
- Mercier, R.; Kawai, Y.; Errington, J. Wall proficient E. coli capable of sustained growth in the absence of the Z-ring division machine. Nat. Microbiol. 2016, 1, 16091. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; De Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress Physiology of Lactic Acid Bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837–890. [Google Scholar] [CrossRef] [Green Version]
- Takacs, C.N.; Poggio, S.; Charbon, G.; Pucheault, M.; Vollmer, W.; Jacobs-Wagner, C. MreB drives de novo rod morphogenesis in Caulobacter crescentus via remodeling of the cell wall. J. Bacteriol. 2010, 192, 1671–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, W.; Davit, Y.; Osborne, J.M.; Kim, W.; Foster, K.R.; Pitt-Francis, J.M. Cell morphology drives spatial patterning in microbial communities. Proc. Natl. Acad. Sci. USA 2017, 114, E280–E286. [Google Scholar] [CrossRef] [Green Version]
- Christensen, B.B.; Haagensen, J.A.J.; Heydorn, A.; Molin, S. Metabolic Commensalism and Competition in a Two-Species Microbial Consortium. Appl. Environ. Microbiol. 2002, 68, 2495–2502. [Google Scholar] [CrossRef] [Green Version]
- Hansen, S.K.; Rainey, P.; Haagensen, J.A.J.; Molin, S. Evolution of species interactions in a biofilm community. Nat. Cell Biol. 2007, 445, 533–536. [Google Scholar] [CrossRef]
- Slapkauskaite, J.; Sekmokiene, D.; Kabasinskiene, A.; Bartkiene, E.; Juodeikiene, G.; Sarkinas, A. Influence of lactic acid bacteria–fermented Helianthus tuberosus L. and Lupinus luteus on quality of milk products. CyTA—J. Food 2016, 14, 482–488. [Google Scholar]
- Gaspar, P.; Al-Bayati, F.A.Y.; Andrew, P.W.; Neves, A.R.; Yesilkaya, H. Lactate Dehydrogenase Is the Key Enzyme for Pneumococcal Pyruvate Metabolism and Pneumococcal Survival in Blood. Infect. Immun. 2014, 82, 5099–5109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Shanmugam, K.T.; Ingram, L.O. Functional Replacement of the Escherichia coli d-(−)-Lactate Dehydrogenase Gene (ldhA) with the l-(+)-Lactate Dehydrogenase Gene (ldhL) from Pediococcus acidilactici. Appl. Environ. Microbiol. 2003, 69, 2237–2244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucey, J.A. The relationship between rheological parameters and whey separation in milk gels. Food Hydrocoll. 2001, 15, 603–608. [Google Scholar] [CrossRef]
- Lobato-Calleros, C.; Ramírez-Santiago, C.; Vernon-Carter, E.; Alvarez-Ramirez, J. Impact of native and chemically modified starches addition as fat replacers in the viscoelasticity of reduced-fat stirred yogurt. J. Food Eng. 2014, 131, 110–115. [Google Scholar] [CrossRef]
- Hassan, A.; Frank, J.; Schmidt, K.; Shalabi, S. Textural Properties of Yogurt Made with Encapsulated Nonropy Lactic Cultures. J. Dairy Sci. 1996, 79, 2098–2103. [Google Scholar] [CrossRef]
- Butler, F.; McNulty, P. Time dependent rheological characterisation of buttermilk at 5 °C. J. Food Eng. 1995, 25, 569–580. [Google Scholar] [CrossRef]
- Saleh, A.; Mohamed, A.A.; Alamri, M.S.; Hussain, S.; Qasem, A.A.; Ibraheem, M.A. Effect of Different Starches on the Rheological, Sensory and Storage Attributes of Non-fat Set Yogurt. Foods 2020, 9, 61. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.-Y.; Wang, L.; McCarthy, K.L. Characterization of yogurts made with milk solids nonfat by rheological behavior and nuclear magnetic resonance spectroscopy. J. Food Drug Anal. 2016, 24, 804–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donkor, O.N.; Henriksson, A.; Vasiljevic, T.; Shah, N.P. Proteolytic activity of dairy lactic acid bacteria and probiotics as determinant of growth and in vitro angiotensin-converting enzyme inhibitory activity in fermented milk. Lait 2007, 87, 21–38. [Google Scholar] [CrossRef]
- Sawada, K.; Koyano, H.; Yamamoto, N.; Yamada, T. The relationships between microbiota and the amino acids and organic acids in commercial vegetable pickle fermented in rice-bran beds. Sci. Rep. 2021, 11, 1791. [Google Scholar] [CrossRef]
- Asano, M.; Nakamura, N.; Kawai, M.; Miwa, T.; Nio, N. Purification and Characterization of an N-Terminal Acidic Amino Acid-Specific Aminopeptidase from Soybean Cotyledons (Glycine max). Biosci. Biotechnol. Biochem. 2010, 74, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Li, X.; Chen, H.; Guan, K.; Qi, X.; Yang, L.; Ma, Y. Evaluation of FAAs and FFAs in yogurts fermented with different starter cultures during storage. J. Food Compos. Anal. 2021, 96, 103666. [Google Scholar] [CrossRef]
- Mann, B.; Athira, S.; Sharma, R.; Bajaj, R. Chapter 24-Bioactive Peptides in Yogurt. In Yogurt in Health and Disease Prevention; Shah, N.P., Ed.; Academic Press: London, UK, 2017; pp. 411–426. [Google Scholar]
- Utagawa, T. Production of Arginine by Fermentation. J. Nutr. 2004, 134, 2854S–2857S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, H.; Lee, J.-H. Characterization of Arginine Catabolism by Lactic Acid Bacteria Isolated from Kimchi. Molecules 2018, 23, 3049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, F.L.; Kim, W.K. Secondary Functions of Arginine and Sulfur Amino Acids in Poultry Health: Review. Animals 2020, 10, 2106. [Google Scholar] [CrossRef]
- Ardö, Y. Flavour formation by amino acid catabolism. Biotechnol. Adv. 2006, 24, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Smit, G.; Smit, B.A.; Engels, W.J. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol. Rev. 2005, 29, 591–610. [Google Scholar] [CrossRef] [PubMed]
- Simova, E.; Simov, Z.; Beshkova, D.; Frengova, G.; Dimitrov, Z.; Spasov, Z. Amino acid profiles of lactic acid bacteria, isolated from kefir grains and kefir starter made from them. Int. J. Food Microbiol. 2006, 107, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mi, S.; Liu, R.B.; Sang, Y.X.; Wang, X.H. Evaluation of Volatile Compounds during the Fermentation Process of Yogurts by Streptococcus thermophilus Based on Odor Activity Value and Heat Map Analysis. Int. J. Anal. Chem. 2020, 2020, 3242854. [Google Scholar] [CrossRef] [PubMed]
- Erkaya, T.; Şengül, M. Comparison of volatile compounds in yoghurts made from cows’, buffaloes’, ewes’ and goats’ milks. Int. J. Dairy Technol. 2011, 64, 240–246. [Google Scholar] [CrossRef]
- Tamime, A.Y.; Robinson, R.K. Nutritional value of yoghurt. In Tamime and Robinson’s Yoghurt, 3rd ed.; Tamime, A.Y., Robinson, R.K., Eds.; Woodhead Publishing: Cambridge, UK, 2007; pp. 646–684. [Google Scholar]
- Gyosheva, B. Regression analysis applied to evaluation of aroma and flavour of Bulgarian sour milk. Food/Nahrung 1985, 29, 185–190. [Google Scholar] [CrossRef]
- Raya, R.R.; de Nadra, M.C.M.; de Ruiz Holgado, A.P.; Oliver, G. Acetaldehyde metabolism in lactic acid bacteria. In Milchwissenschaft; AVA: Kempten, Germany, 1986; Volume 41, pp. 397–399. [Google Scholar]
- Curioni, P.M.G.; Bosset, J.O. Key odorants in various cheese types as determined by gas chromatography-olfactometry. Int. Dairy J. 2002, 12, 959–984. [Google Scholar] [CrossRef]
- Smit, B.A.; Engels, W.J.; Smit, G. Branched chain aldehydes: Production and breakdown pathways and relevance for flavour in foods. Appl. Microbiol. Biotechnol. 2009, 81, 987–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.I.; Yoon, K.-H.; Kalichamy, S.S.; Yoon, S.-S.; Lee, J.I. A natural odor attraction between lactic acid bacteria and the nematode Caenorhabditis elegans. ISME J. 2015, 10, 558–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleerebezemab, M.; Hols, P.; Hugenholtz, J. Lactic acid bacteria as a cell factory: Rerouting of carbon metabolism in Lactococcus lactis by metabolic engineering. Enzym. Microb. Technol. 2000, 26, 840–848. [Google Scholar] [CrossRef]
- Bassit, N.; Boquien, C.-Y.; Picque, D.; Corrieu, G. Effect of Initial Oxygen Concentration on Diacetyl and Acetoin Production by Lactococcus lactis subsp. lactis biovar diacetylactis. Appl. Environ. Microbiol. 1993, 59, 1893–1897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristiani, G.; Monnet, V. Food micro-organisms and aromatic ester synthesis. Sci. Aliments 2001, 21, 211–230. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Holland, R.; Crow, V.L. Esters and their biosynthesis in fermented dairy products: A review. Int. Dairy J. 2004, 14, 923–945. [Google Scholar] [CrossRef]


| Control (μg/100 g) | Thermo-Tolerant (μg/100 g) | |
|---|---|---|
| Essential amino acid | ||
| Lysine | 22.73 ± 3.22 a | 174.79 ± 1.90 b |
| Phenylalanine | 6.09 ± 1.05 a | 137.45 ± 2.25 b |
| Threonine | 4.80 ± 0.78 a | 49.49 ± 1.17 b |
| Histidine | 4.63 ± 0.23 a | 92.36 ± 2.4 b |
| Valine | 4.62 ± 0.87 a | 89.02 ± 2.05 b |
| Leucine | 4.59 ± 0.77 a | 218.79 ± 4.36 b |
| Isoleucine | 2.78 ± 0.46 a | 41.66 ± 1.12 b |
| Tryptophan | 1.20 ± 0.20 a | 23.55 ± 0.31 b |
| Methionine | 0.36 ± 0.07 a | 98.83 ± 1.78 b |
| Non-essential amino acid | ||
| Glutamic acid | 55.27 ± 9.67 a | 265.88 ± 5.08 b |
| Proline | 40.76 ± 7.19 a | 17.52 ± 0.42 a |
| Ethanolamine | 22.72 ± 3.93 a | 4.07 ± 0.14 a |
| Alanine | 22.29 ± 4.02 a | 79.98 ± 2.63 b |
| Glycine | 9.57 ± 1.58 a | 14.31 ± 0.34 a |
| Taurine | 8.34 ± 1.44 a | 6.52 ± 0.26 a |
| Serine | 2.65 ± 0.32 a | 0.28 ± 0.04 b |
| Aspartic acid | 2.59 ± 0.47 a | 1.11 ± 0.05 a |
| Tyrosine | 2.35 ± 0.35 a | 87.59 ± 2.89 b |
| Arginine | 2.05 ± 0.35 a | 237.24 ± 5.94 b |
| Ornithine | 1.86 ± 0.16 a | 103.49 ± 2.09 b |
| Citrulline | 1.03 ± 0.10 a | 2.30 ± 0.04 b |
| Alanine | 0.59 ± 0.09 a | 0.19 ± 2.63 a |
| L_α_Amino-n-butyric acid | 0.57 ± 0.11 a | 0.26 ± 0.02 a |
| δ_Hydroxylysine | 0.31 ± 0.08 a | 0 a |
| Hydroxy-L-proline | 0.19 ± 0.03 a | 0.18 ± 0.01 a |
| Γ-amino-n-butyric acid | 0.19 ± 0.03 a | 0.42 ± 0.02 a |
| Sarcosine | 0.07 ± 0.01 a | 0.04 ± 0.01 a |
| DL-β-aminoisobutryic acid | 0.03 ± 0.01 a | 0.11 ± 0.01 b |
| Total free amino acids | 225.25 ± 37.45 | 1747.42 ± 34.69 |
| Compound | m/z | RT (min) | RI | Concentration (µg/L) | |
|---|---|---|---|---|---|
| Control | Thermo-Tolerant | ||||
| Carboxylic acid | |||||
| Oxalic acid | 89.90 | 3.17 | 929.00 | 158.34 ± 0.19 | 7973.73 ± 3.45 |
| Acetic acid | 60.02 | 11.75 | 1443.27 | 1619.33 ± 1.12 | 1198.05 ± 2.13 |
| Propanoic acid, 2-methyl- | 88.05 | 13.66 | 1563.69 | 14.06 ± 0.33 | 130.81 ± 0.34 |
| Butanoic acid | 88.05 | 14.52 | 1620.85 | 4675.15 ± 3.76 | 3523.13 ± 1.21 |
| Pentanoic acid | 102.07 | 16.18 | 1735.18 | 30.38 ± 0.61 | 18.97 ± 0.31 |
| Hexanoic acid | 116.08 | 17.60 | 1838.63 | 9195.56 ± 4.32 | 5026.78 ± 0.73 |
| Octanoic acid | 144.12 | 20.35 | 2053.31 | 4251.74 ± 1.11 | 2209.72 ± 2.13 |
| Nonanoic acid | 158.13 | 21.68 | 2164.51 | 0.76 ± 0.00 | 9.71 ± 0.20 |
| Benzoic acid | 122.04 | 24.62 | 2430.28 | 2304.57 ± 0.52 | 822.18 ± 0.78 |
| Dodecanoic acid | 200.18 | 25.14 | 2479.94 | 118.84 ± 0.94 | 177.05 ± 0.64 |
| Aldehyde | |||||
| Pentanal | 86.07 | 3.68 | 969.00 | 249.09 ± 0.83 | 69.44 ± 0.63 |
| Hexanal | 100.09 | 5.30 | 1073.77 | 107.97 ± 0.74 | ND |
| Heptanal | 114.10 | 7.13 | 1177.34 | 152.60 ± 0.37 | 26.64 ± 0.32 |
| 4-Heptenal, (Z)- | 112.09 | 8.17 | 1235.22 | 12.37 ± 0.27 | 154.09 ± 0.62 |
| Nonanal | 142.14 | 10.87 | 1390.06 | 57.98 ± 0.08 | 33.29 ± 0.34 |
| Methional | 104.03 | 11.82 | 1447.77 | 12.02 ± 0,61 | 10.79 ± 0.12 |
| Benzaldehyde | 106.04 | 12.92 | 1515.97 | 602.83 ± 0.26 | 195.73 ± 0.12 |
| 2-Nonenal, (E)- | 104.12 | 13.19 | 1533.35 | 50.58 ± 0.27 | 12.50 ± 0.77 |
| Ketone | |||||
| Butanone | 72.06 | 2.73 | 891.00 | 170.66 ± 0.51 | 459.19 ± 0.40 |
| 2,3-Butanedione | 86.04 | 3.65 | 967.00 | 313.62 ± 0.20 | 844.79 ± 0.13 |
| 2-Pentanone | 86.07 | 3.66 | 968.16 | 313.62 ± 0.63 | 845.02 ± 0.13 |
| 2,3-Pentanedione | 100.05 | 4.92 | 1051.51 | 75.49 ± 0.12 | 8.27 ± 0.32 |
| 3-Heptanone | 114.10 | 6.55 | 1145.18 | 28.69 ± 0.01 | 9.11 ± 0.01 |
| 2-Heptanone | 114.10 | 7.10 | 1175.84 | 126.81 ± 0.04 | 2045.73 ± 0.35 |
| 2-Nonanone | 142.14 | 10.80 | 1385.67 | 98.23 ± 0.51 | 1881.56 ± 0.87 |
| 3-Octen-2-one | 126.10 | 11.11 | 1404.45 | 6.09 ± 0.12 | ND |
| 1-Propanone, 1-(2-furanyl)- | 124.05 | 13.71 | 1567.32 | 59.12 ± 0.68 | 18.65 ± 0.76 |
| 2-Undecanone | 107.17 | 14.17 | 1597.26 | 54.96 ± 0.32 | 222.63 ± 0.49 |
| 2-Tridecanone | 198.20 | 17.20 | 1809.13 | 12.67 ± 0.12 | 49.41 ± 0.77 |
| Alcohol | |||||
| 2-Butanol | 74.07 | 4.48 | 1024.77 | 2.34 ± 0.15 | 16.94 ± 0.64 |
| 1-Propanol | 60.06 | 4.69 | 1037.41 | 2.48 ± 0.01 | 61.96 ± 0.70 |
| 1-Butanol | 74.07 | 6.69 | 1153.23 | 7.27 ± 0.34 | 33.59 ± 0.41 |
| 1-Pentanol | 88.09 | 8.45 | 1250.99 | 380.14 ± 0.91 | 127.93 ± 0.18 |
| 2-Heptanol | 116.12 | 9.69 | 1320.80 | ND | 419.95 ± 0.02 |
| 1-Hexanol | 102.10 | 10.24 | 1353.39 | 159.70 ± 0.73 | 181.99 ± 0.91 |
| 1-Octen-3-ol | 128.12 | 11.84 | 1448.93 | 45.45 ± 0.82 | 19.08 ± 0. 19 |
| 1-Heptanol | 116.12 | 11.94 | 1455.01 | 117.50 ± 0.38 | 58.30 ± 0.23 |
| 1-Hexanol, 2-ethyl- | 130.14 | 12.49 | 1488.77 | 754.34 ± 0.94 | 1945.54 ± 0.76 |
| 2-Nonanol | 144.15 | 12.97 | 1519.23 | ND | 189.36 ± 0.83 |
| 2,3-Butanediol | 90.07 | 13.25 | 1537.43 | 24.49 ± 0.42 | 6641.79 ± 0.18 |
| Linalool | 154.14 | 13.37 | 1545.42 | 46.18 ± 0.85 | 10.41 ± 0.62 |
| 1-Octanol | 130.14 | 13.56 | 1557.23 | 31.98 ± 0.76 | 158.46 ± 0.10 |
| 2-Undecanol | 172.18 | 15.95 | 1718.99 | 21.66 ± 0.37 | 8.74 ± 0.99 |
| 1-Decanol | 158.17 | 16.54 | 1761.46 | 6.05 ± 0.17 | 109.51 ± 0.57 |
| Benzyl alcohol | 108.06 | 18.01 | 1869.40 | 28.03 ± 0.82 | 38.95 ± 0.35 |
| 1-Dodecanol | 186.20 | 19.26 | 1966.08 | 9.24 ± 0.74 | 9.10 ± 0.68 |
| Ester | |||||
| Methyl butyrate | 102.07 | 3.79 | 978.00 | 37.91 ± 0.27 | 8.19 ± 0.97 |
| Ethyl butyrate | 116.08 | 4.60 | 1031.81 | 51.60 ± 0.89 | 607.84 ± 0.47 |
| Butanoic acid, 2-methylpropyl ester | 144.12 | 6.64 | 1150.09 | ND | 26.88 ± 0.30 |
| Ethyl hexanoate | 144.12 | 8.03 | 1227.60 | 4.25 ± 0.51 | 168.82 ± 0.63 |
| Butanoic acid, 3-methylbutyl ester | 158.13 | 8.62 | 1260.50 | 3.29 ± 0.58 | 50.87 ± 0.07 |
| Ethyl octanoate | 172.15 | 11.64 | 1436.68 | 55.99 ± 0.21 | 182.17 ± 0.16 |
| Ethyl decanoate | 200.18 | 14.78 | 1638.23 | 23.60 ± 0.74 | 313.61 ± 0.11 |
| Ethyl dodecanoate | 228.21 | 17.67 | 1844.22 | 112.40 ± 0.14 | 107.41 ± 0.28 |
| Methyl hexadecanoate | 270.26 | 22.29 | 2217.59 | 39.96 ± 0.47 | 30.74 ± 0.20 |
| Ethyl hexadecanoate | 284.27 | 22.72 | 2255.91 | 42.95 ± 0.71 | 40.25 ± 0.79 |
| Compound | Odour Description | Threshold in Water (ppb) * | OAVs | |
|---|---|---|---|---|
| Control | Thermo-Tolerant | |||
| Carboxylic acid | ||||
| Acetic acid | Vinegar, pungent acidic | 800 | 2.02 | 1.49 |
| Propanoic acid | Vinegar, pungent, sour milk | 20,000 | >1 | >1 |
| Butanoic acid | Fruity, dairy, cheesy | 240 | 19.47 | 14.69 |
| Hexanoic acid | Fatty, cheesy | 3000 | 3.07 | 1.68 |
| Octanoic acid | Fatty, cheesy | 3000 | 1.41 | >1 |
| Nonanoic acid | Fatty, green | 3000 | >1 | >1 |
| Dodecanoic acid | Fatty, coconut, bay oil | 10,000 | >1 | >1 |
| Aldehyde | ||||
| Pentanal | Berry, nutty | 1500 | >1 | >1 |
| Heptanal | Green, sweet | 3 | 50.87 | 8.88 |
| 4-Heptenal, (Z)- | Cream and butter | 0.8–10 | 15.46 | 2.48 |
| Nonanal | Fatty, citrus, green | 1 | 57.98 | 33.29 |
| 2-Nonenal, (E)- | Fatty, green, mushroom | 0.08–0.1 | 632.25 | 156.25 |
| Ketone | ||||
| 2-Butanone | Varnish-like, sweet, fruity | 50,000 | >1 | >1 |
| 2-Pentanone | Fruity, acetone | 70,000 | >1 | >1 |
| 2-Heptanone | Banana-like, fruity | 140–3000 | >1 | 14.61 |
| 2-Nonanone | Fruity, cheesy, buttery | 5–200 | 19.65 | 376.31 |
| 3-Octen-2-one | Mushroom, fruity | 28 | >1 | ND |
| 2-Undecanone | Floral, rose-like, herbaceous | 14.17 | 3.87 | 15.71 |
| Alcohol | ||||
| 2-Butanol | wine | 500 | >1 | >1 |
| 1-Propanol | Alcoholic, pungent | 9000 | >1 | >1 |
| 1-Butanol | Fruity, alcoholic | 500 | >1 | >1 |
| 1-Pentanol | Alcoholic, iodoform-like | 4000 | >1 | >1 |
| 2-Heptanol | Earthy oily | 3 | ND | 139.98 |
| 1-Octen-3-ol | Mushroom-like | 1 | 45.45 | 19.08 |
| 1-Heptanol | Earthy, oily | 3 | 39.17 | 19.43 |
| 1-Octanol | pungent | 110–130 | >1 | 1.44 |
| Ester | ||||
| Methyl butyrate | Fruity, apple, pineapple | 60–76 | >1 | >1 |
| Ethyl butyrate | Pineapple-like | 1 | 51.60 | 607.84 |
| Ethyl hexanoate | Fruity, apple, banana | 1 | 4.25 | 168.82 |
| Ethyl hexadecanoate | Fruity, creamy, waxy | >2000 | >1 | >1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Yoo, M.J.Y.; Seale, B.; Grazioli, G. Nutritional and Volatile Characterisation of Milk Inoculated with Thermo-Tolerant Lactobacillus bulgaricus through Adaptive Laboratory Evolution. Foods 2021, 10, 2944. https://doi.org/10.3390/foods10122944
Liang J, Yoo MJY, Seale B, Grazioli G. Nutritional and Volatile Characterisation of Milk Inoculated with Thermo-Tolerant Lactobacillus bulgaricus through Adaptive Laboratory Evolution. Foods. 2021; 10(12):2944. https://doi.org/10.3390/foods10122944
Chicago/Turabian StyleLiang, Jiahui, Michelle Ji Yeon Yoo, Brent Seale, and Gianpaolo Grazioli. 2021. "Nutritional and Volatile Characterisation of Milk Inoculated with Thermo-Tolerant Lactobacillus bulgaricus through Adaptive Laboratory Evolution" Foods 10, no. 12: 2944. https://doi.org/10.3390/foods10122944








