Chemical Characterization of Coffee Husks, a By-Product of Coffea arabica Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Flours Preparation
2.3. Extractable and Non-Extractable Phenolics
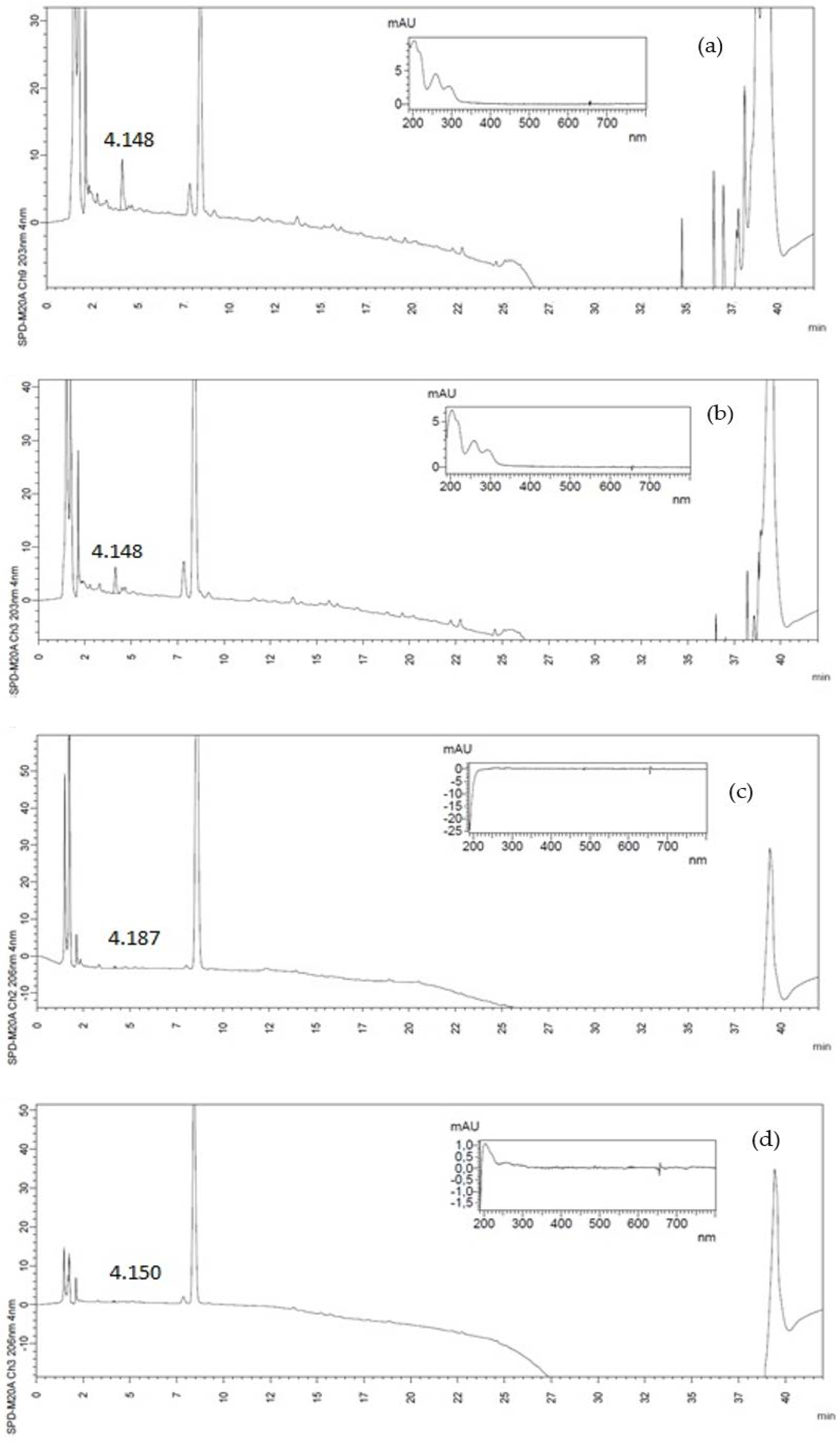
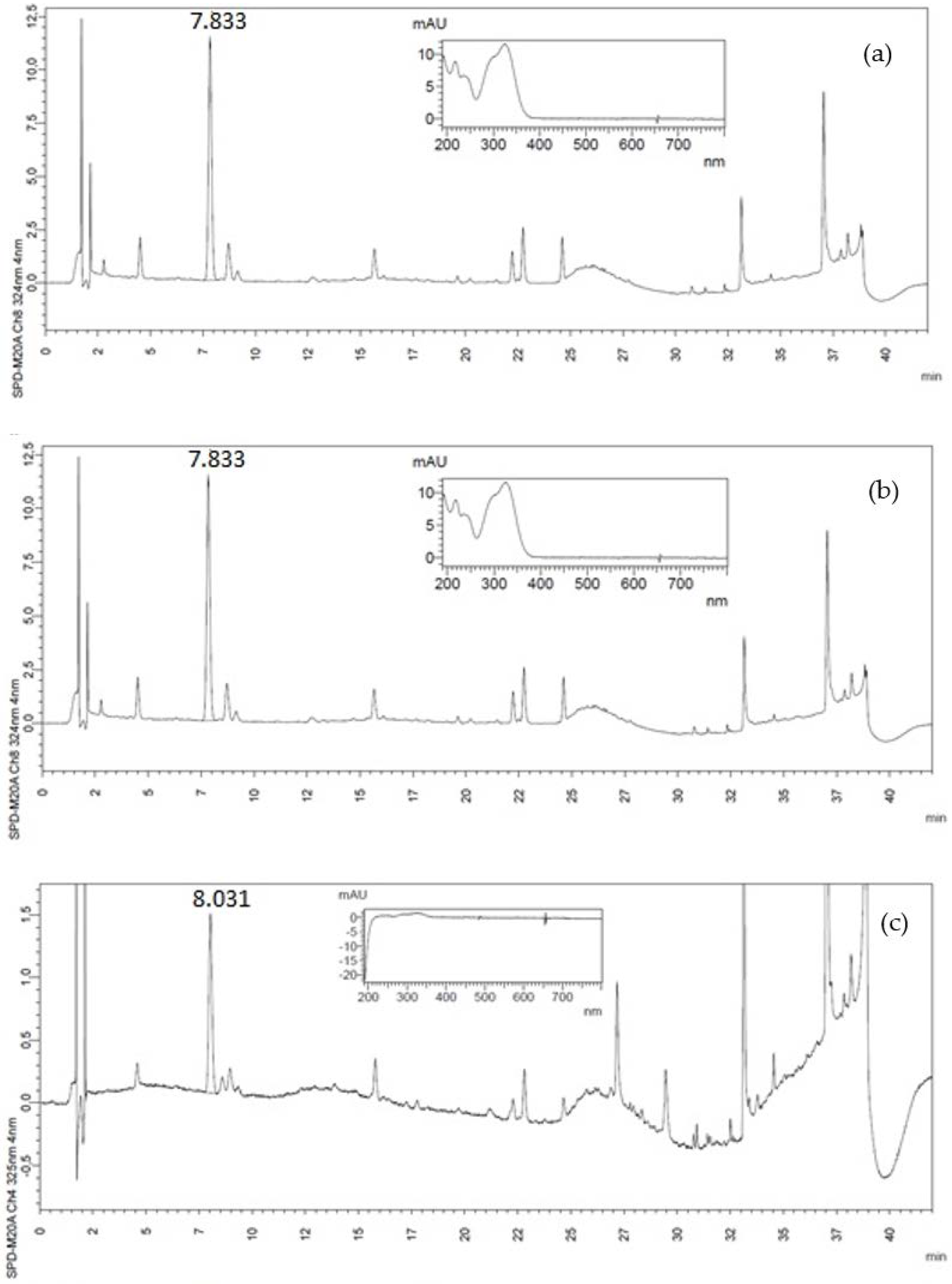
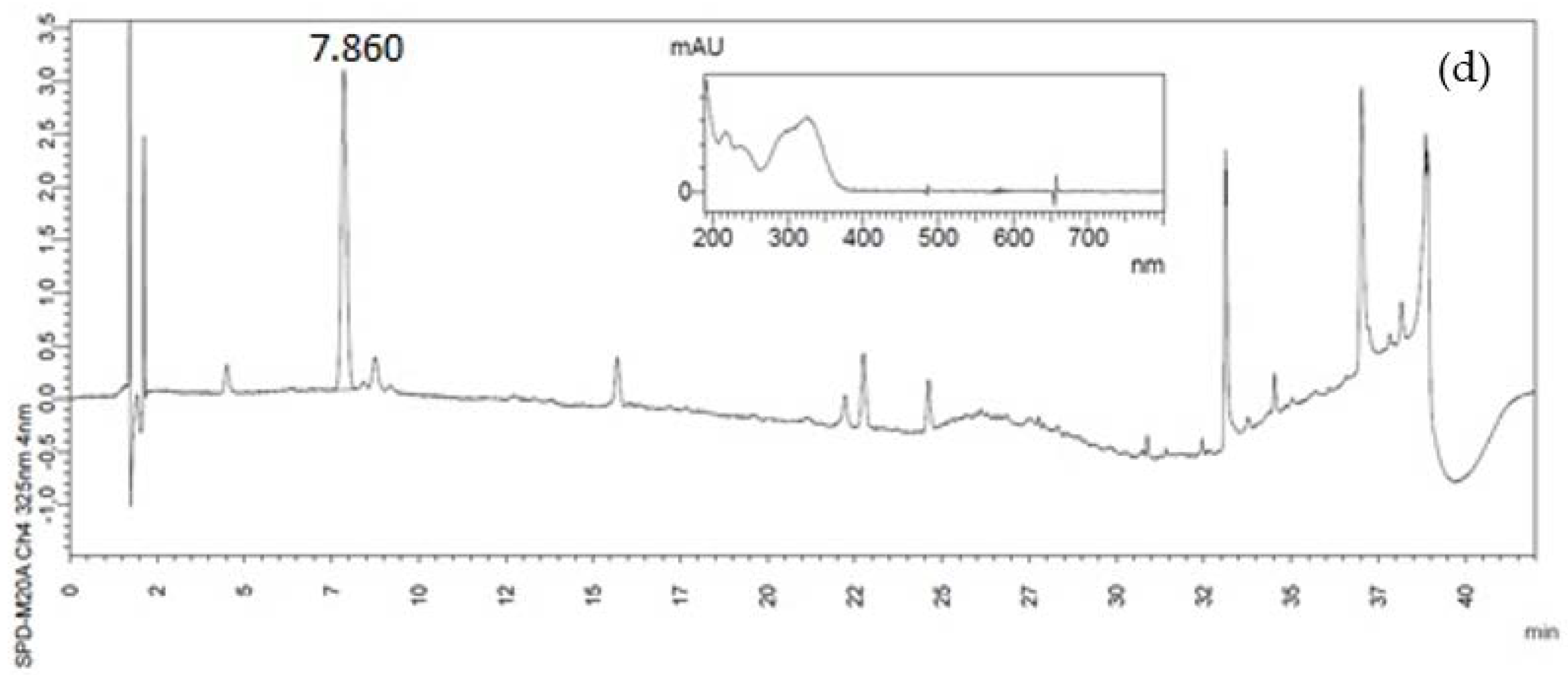
2.4. Identification and Quantification of Phenolic Compounds by High-Performance Liquid Chromatography (HPLC)
2.5. Quantification of Caffeine and Trigonelline
2.6. Monosacharides Profile
2.7. Chemical, Physical and Technological Properties
2.8. Statistical Analysis
3. Results
3.1. Phenolic Compounds
3.2. Trigonelline and Caffeine
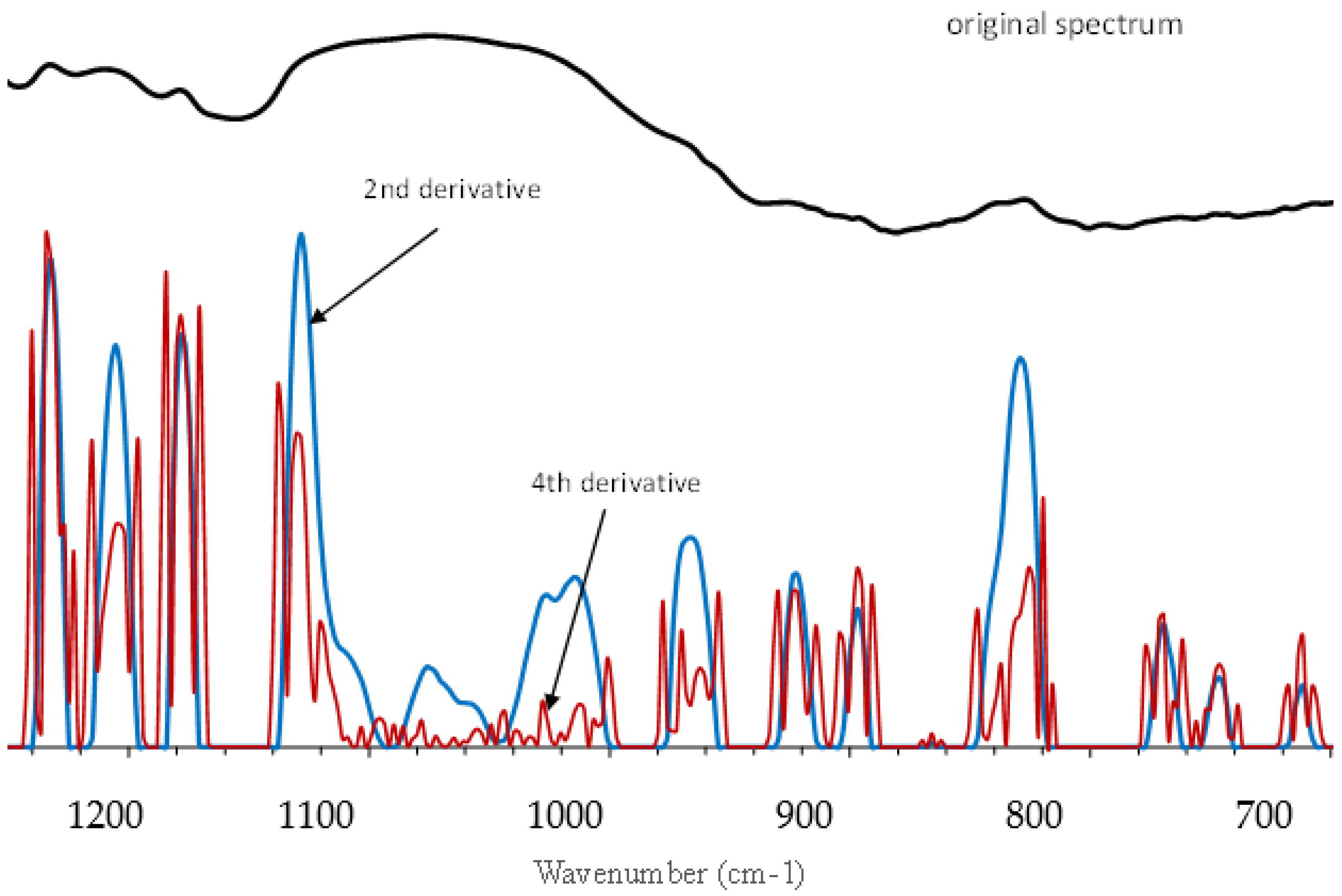
3.3. Characterization of Polysaccharides
3.4. Chemical, Physical, and Technological Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- ICO—International Coffee Organization. World Coffee Production. 2019. Available online: http://www.ico.org/pt/trade_statisticsp.asp (accessed on 1 December 2019).
- Franca, A.S.; Oliveira, L.S. Coffee processing solid wastes: Current uses and future perspectives. In Agricultural Wastes; Ashworth, G.S., Azevedo, P., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2009; pp. 155–189. [Google Scholar]
- Franca, A.S.; Oliveira, L.S. Coffee. In Integrated Processing Technologies for Food and Agricultural By-Products; Elsevier: London, UK, 2019; pp. 413–438. [Google Scholar] [CrossRef]
- Pleissner, D.; Neu, A.-K.; Mehlmann, K.; Schneider, R.; Quintero, G.I.P.; Venus, J. Fermentative lactic acid production from coffee pulp hydrolysate using Bacillus coagulans at laboratory and pilot scales. Bioresour. Technol. 2016, 218, 167–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, M.D.; Melo, M.M.; Coimbra, J.M.; dos Reis, K.C.; Schwan, R.; Silva, C.F. Solid coffee waste as alternative to produce carotenoids with antioxidant and antimicrobial activities. Waste Manag. 2018, 82, 93–99. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; García, N.A.; Fernandez-Gomez, B.; Guisantes-Batan, E.; Escobar, F.V.; Blanch, G.P.; Andres, M.I.S.; Sanchez-Fortun, S.; del Castillo, M.D. Validation of coffee by-products as novel food ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Parra-Campos, A.; Ordóñez-Santos, L.E. Natural pigment extraction optimization from coffee exocarp and its use as a natural dye in French meringue. Food Chem. 2019, 285, 59–66. [Google Scholar] [CrossRef]
- Sarrouh, B.; De Souza, R.O.A.; Florindo, R.H.D.S.; Lofrano, R.C.Z.; De Oliveira, A.M. Extraction and identification of biomolecules from lignin alkaline hydrolysate from coffee husk. Waste Biomass Valoriz. 2020, 12, 787–794. [Google Scholar] [CrossRef]
- Klingel, T.; Kremer, J.I.; Gottstein, V.; De Rezende, T.R.; Schwarz, S.; Lachenmeier, D.W. A review of coffee by-products including leaf, flower, cherry, husk, silver skin, and spent grounds as novel foods within the European Union. Foods 2020, 9, 665. [Google Scholar] [CrossRef]
- Gouvea, B.M.; Torres, C.; Franca, A.S.; Oliveira, L.S.; Oliveira, E.S. Feasibility of ethanol production from coffee husks. Biotechnol. Lett. 2009, 31, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Bekalo, S.A.; Reinhardt, H.-W. Fibers of coffee husk and hulls for the production of particleboard. Mater. Struct. 2010, 43, 1049–1060. [Google Scholar] [CrossRef]
- Cerda, A.; Gea, T.; García, M.D.C.V.; Sánchez, A. Towards a competitive solid state fermentation: Cellulases production from coffee husk by sequential batch operation and role of microbial diversity. Sci. Total Environ. 2017, 589, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, F.D.C.; Srinivas, K.; Helms, G.L.; Isern, N.G.; Cort, J.R.; Gonçalves, A.R.; Ahring, B.K. Characterization of coffee (Coffea arabica) husk lignin and degradation products obtained after oxygen and alkali addition. Bioresour. Technol. 2018, 257, 172–180. [Google Scholar] [CrossRef]
- Ramirez-Martinez, J.R. Phenolic compounds in coffee pulp: Quantitative determination by HPLC. J. Sci. Food Agric. 1988, 43, 135–144. [Google Scholar] [CrossRef]
- Clifford, M.; Ramirez-Martinez, J. Phenols and caffeine in wet-processed coffee beans and coffee pulp. Food Chem. 1991, 40, 35–42. [Google Scholar] [CrossRef]
- Ramirez-Coronel, M.A.; Marnet, N.; Kolli, V.S.K.; Roussos, S.; Guyot, S.; Augur, C. Characterization and estimation of proanthocyanidins and other phenolics in coffee pulp (Coffea arabica) by thiolysis−high-performance liquid chromatography. J. Agric. Food Chem. 2004, 52, 1344–1349. [Google Scholar] [CrossRef] [PubMed]
- Prata, E.R.; Oliveira, L.S. Fresh coffee husks as potential sources of anthocyanins. LWT 2007, 40, 1555–1560. [Google Scholar] [CrossRef]
- Mullen, W.; Nemzer, B.; Stalmach, A.; Ali, S.; Combet, E. Polyphenolic and hydroxycinnamate contents of whole coffee fruits from China, India, and Mexico. J. Agric. Food Chem. 2013, 61, 5298–5309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodsamran, P.; Sothornvit, R. Extraction of phenolic compounds from lime peel waste using ultrasonic-assisted and microwave-assisted extractions. Food Biosci. 2019, 28, 66–73. [Google Scholar] [CrossRef]
- Arranz, S.; Saura-Calixto, F.; Shaha, S.; Kroon, P. High Contents of nonextractable polyphenols in fruits suggest that polyphenol contents of plant foods have been underestimated. J. Agric. Food Chem. 2009, 57, 7298–7303. [Google Scholar] [CrossRef]
- Zurita, J.; Díaz-Rubio, M.E.; Calixto, F.D.S. Improved procedure to determine non-extractable polymeric proanthocyanidins in plant foods. Int. J. Food Sci. Nutr. 2012, 63, 936–939. [Google Scholar] [CrossRef]
- Renard, C.M.; Watrelot, A.A.; Le Bourvellec, C. Interactions between polyphenols and polysaccharides: Mechanisms and consequences in food processing and digestion. Trends Food Sci. Technol. 2017, 60, 43–51. [Google Scholar] [CrossRef]
- Dutra, R.L.T.; Dantas, A.M.; Marques, D.D.A.; Batista, J.D.F.; Meireles, B.; Cordeiro, M.T.D.M.; Magnani, M.; Borges, G. Bioaccessibility and antioxidant activity of phenolic compounds in frozen pulps of Brazilian exotic fruits exposed to simulated gastrointestinal conditions. Food Res. Int. 2017, 100, 650–657. [Google Scholar] [CrossRef]
- Perrone, D.; Donangelo, C.M.; Farah, A. Fast simultaneous analysis of caffeine, trigonelline, nicotinic acid and sucrose in coffee by liquid chromatography-mass spectrometry. Food Chem. 2008, 110, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Leão, D.P.; Franca, A.S.; Oliveira, L.S.; Bastos, R.; Coimbra, M.A. Physicochemical characterization, antioxidant capacity, total phenolic and proanthocyanidin content of flours prepared from pequi (Caryocar brasilense Camb.) fruit by-products. Food Chem. 2017, 225, 146–153. [Google Scholar] [CrossRef]
- AOAC—Association of Official Analytical Chemists. Official Methods of Analysis of the Association of Analytical Chemists, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Resende, L.M.; Franca, A.S.; Oliveira, L.S. Buriti (Mauritia flexuosa L. f.) fruit by-products flours: Evaluation as source of dietary fibers and natural antioxidants. Food Chem. 2019, 270, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Vasco, C.; Ruales, J.; Kamal-Eldin, A. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008, 111, 816–823. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Saura-Calixto, F. Fruit peels as sources of non-extractable polyphenols or macromolecular antioxidants: Analysis and nutritional implications. Food Res. Int. 2018, 111, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Guo, J.; Zhang, Y.; Shi, S. efficient determination of protocatechuic acid in fruit juices by selective and rapid magnetic molecular imprinted solid phase extraction coupled with HPLC. J. Agric. Food Chem. 2014, 62, 8221–8228. [Google Scholar] [CrossRef]
- Yun, N.; Kang, J.; Lee, S. Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: Molecular evidence of its antioxidant and anti-inflammatory properties. J. Nutr. Biochem. 2012, 23, 1249–1255. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; De Mejia, E.G. Relationship of the phytochemicals from coffee and cocoa by-products with their potential to modulate biomarkers of metabolic syndrome in vitro. Antioxidants 2019, 8, 279. [Google Scholar] [CrossRef] [Green Version]
- Phan, A.; Netzel, G.; Wang, D.; Flanagan, B.M.; D’Arcy, B.R.; Gidley, M.J. Binding of dietary polyphenols to cellulose: Structural and nutritional aspects. Food Chem. 2016, 171, 388–396. [Google Scholar] [CrossRef]
- Liu, X.; Le Bourvellec, C.; Renard, C.M.G.C. Interactions between cell wall polysaccharides and polyphenols: Effect of molecular internal structure. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3574–3617. [Google Scholar] [CrossRef]
- Pinarli, B.; Karliga, E.S.; Ozkan, G.; Capanoglu, E. Interaction of phenolics with food matrix: In vitro and in vivo approaches. Mediterr. J. Nutr. Metab. 2020, 13, 63–74. [Google Scholar] [CrossRef]
- Benitez, V.; Rebollo-Hernanz, M.; Hernanz, S.; Chantres, S.; Aguilera, Y.; Martin-Cabrejas, M.A. Coffee parchment as a new dietary fiber ingredient: Functional and physiological characterization. Food Res. Int. 2019, 122, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Reichembach, L.H.; Petkowicz, C.L.D.O. Extraction and characterization of a pectin from coffee (Coffea arabica L.) pulp with gelling properties. Carbohydr. Polym. 2020, 245, 116473. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi, N.; Sharififar, F.; Pournamdari, M.; Ansari, M. A review on biosynthesis, analytical techniques, and pharmacological activities of trigonelline as a plant alkaloid. J. Diet. Suppl. 2017, 15, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Simões, J.; Maricato, E.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Thermal stability of spent coffee ground polysaccharides: Galactomannans and arabinogalactans. Carbohydr. Polym. 2014, 101, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, L.F.; Cerqueira, M.; Teixeira, J.; Mussatto, S.I. Characterization of polysaccharides extracted from spent coffee grounds by alkali pretreatment. Carbohydr. Polym. 2015, 127, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Masuda, T.; Kawano, A.; Kitahara, K.-I.; Nagashima, K.; Aikawa, Y.; Arai, S. Quantitative determination of sugars and myo-inositol in citrus fruits grown in Japan using high-performance anion-exchange chromatography. J. Nutr. Sci. Vitaminol. 2003, 49, 64–68. [Google Scholar] [CrossRef]
- Ruiz-Aceituno, L.; Rodríguez-Sánchez, S.; Sanz, J.; Sanz, M.; Ramos, L. Optimization of pressurized liquid extraction of inositols from pine nuts (Pinus pinea L.). Food Chem. 2014, 153, 450–456. [Google Scholar] [CrossRef] [Green Version]
- Harvey, B.H.; Brink, C.B.; Seedat, S.; Stein, D. Defining the neuromolecular action of myo-inositol application to obsessive-compulsive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2002, 26, 21–32. [Google Scholar] [CrossRef]
- Coimbra, M.A.; Barros, A.; Rutledge, D.N.; Delgadillo, I. FTIR spectroscopy as a tool for the analysis of olive pulp cell-wall polysaccharide extracts. Carbohydr. Res. 1999, 317, 145–154. [Google Scholar] [CrossRef]
- Kacuráková, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerova, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Szymanska-Chargot, M.; Chylinska, M.; Kruk, B.; Zdunek, A. Combining FT-IR spectroscopy and multivariate analysis for qualitative and quantitative analysis of the cell wall composition changes during apples development. Carbohydr. Polym. 2015, 115, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Robert, P.; Marquis, M.; Barron, C.; Guillon, F.; Saulnier, L. FT-IR investigation of cell wall polysaccharides from cereal grains. Arabinoxylan infrared assignment. J. Agric. Food Chem. 2005, 53, 7014–7018. [Google Scholar] [CrossRef]
- Fernando, S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.-S.; Kim, E.-A.; Gunasekara, U.K.D.S.S.; Abeytunga, D.T.U.; Nanayakkara, C.; De Silva, E.D.; et al. FTIR characterization and antioxidant activity of water soluble crude polysaccharides of Sri Lankan marine algae. ALGAE 2017, 32, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Estevez, J.M.; Fernández, P.V.; Kasulin, L.; Dupree, P.; Ciancia, M. Chemical and in situ characterization of macromolecular components of the cell walls from the green seaweed Codium fragile. Glycobiology 2009, 19, 212–228. [Google Scholar] [CrossRef] [Green Version]
- Pawar, H.A.; Lalitha, K. Isolation, purification and characterization of galactomannans as an excipient from Senna tora seeds. Int. J. Biol. Macromol. 2014, 65, 167–175. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Recovery of phenolic antioxidants and functional compounds from coffee industry by-products. Food Bioprocess. Technol. 2010, 5, 897–903. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Páscoa, R.; Magalhaes, L.; Lopes, J. FT-NIR spectroscopy as a tool for valorization of spent coffee grounds: Application to assessment of antioxidant properties. Food Res. Int. 2013, 51, 579–586. [Google Scholar] [CrossRef]
- Rufino, M.D.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid. Med. Cell. Longev. 2016, 2016, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Martínez, R.; Torres, P.; Meneses, M.; Figueroa, J.G.; Pérez-Alvarez, J.A.; Viuda-Martos, M. Chemical, technological and in vitro antioxidant properties of mango, guava, pineapple and passion fruit dietary fibre concentrate. Food Chem. 2012, 135, 1520–1526. [Google Scholar] [CrossRef] [PubMed]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Martin-Sánchez, A.; Sánchez-Zapata, E.; Fernández-López, J.; Sendra, E.; Sayas-Barberá, E.; Navarro, C.; Pérez-Álvarez, J. Chemical, physico-chemical and functional properties of pomegranate (Punica granatum L.) bagasses powder co-product. J. Food Eng. 2012, 110, 220–224. [Google Scholar] [CrossRef]
- Kaczmarczyk, M.M.; Miller, M.; Freund, G.G. The health benefits of dietary fiber: Beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism 2012, 61, 1058–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishna, K.A.; Vishalakshi, B. Gellan gum-based novel composite hydrogel: Evaluation as adsorbent for cationic dyes. J. Appl. Polym. Sci. 2017, 134, 45527. [Google Scholar] [CrossRef]
- Song, X.; Zhu, W.; Pei, Y.; Ai, Z.; Chen, J. Effects of wheat bran with different colors on the qualities of dry noodles. J. Cereal Sci. 2013, 58, 400–407. [Google Scholar] [CrossRef]

| Sample | TEP (mg GAE/100 g) | NEP (mg/100 g) | CGA (mg/100 g) | PCA (mg/100 g) | Bioaccessibility TEP % | Caffeine (mg/100 g) | Trigonelline (mg/100 g) |
|---|---|---|---|---|---|---|---|
| CH1 | 983.05 ± 32.68 a | 2025.85 ± 55.68 c | 121.55 ± 0.18 b | 28.24 ± 0.18 a | 70.76 | 618.10 ± 6.66 b | 542.80 ± 6.54 a |
| CH1b | 904.74 ± 9.47 b | 2703.33 ± 10.73 a | 174.27 ± 0.26 a | 14.42 ± 0.01 b | 68.64 | 522.09 ± 0.62 c | 285.58 ± 4.83 b |
| CH2 | 274.77 ± 6.35 c | 1837.77 ± 23.73 d | 17.19 ± 0.13 d | - | 65.74 | 696.22 ± 4.86 a | 246.21 ± 2.56 c |
| CH2b | 196.31 ± 12.54 d | 2387.34 ± 32.88 b | 43.09 ± 0.16 c | - | 64.10 | 418.13 ± 0.65 d | 120.16 ± 1.20 d |
| Monosaccharides | CH1 (% mol) | CH1b (% mol) | CH2 (% mol) | CH2b (% mol) |
|---|---|---|---|---|
| Rhamnose | 3.04 ± 0.60 a | 2.98 ± 0.52 a | 2.66 ± 0.38 a | 2.46 ± 0.58 a |
| Arabinose | 25.24 ± 1.37 a | 25.17 ± 1.26 a | 17.91 ± 1.77 b | 16.14 ± 1.77 b |
| Xylose | 32.92 ± 1.46 b | 23.69 ± 1.17 c | 55.53 ± 1.89 a | 53.66 ± 1.87 a |
| Mannose | 9.67 ± 0.90 a | 10.00 ± 1.10 a | 5.33 ± 0.96 b | 4.47 ± 0.86 b |
| Galactose | 11.91 ± 2.40 ab | 13.69 ± 1.78 a | 9.59 ± 2.32 b | 10.52 ± 1.39 b |
| Glucose | 15.84 ± 1.32 b | 22.92 ± 1.28 a | 8.68 ± 0.92 d | 12.74 ± 1.23 c |
| Myo-inositol | 1.38 ± 0.12 a | 1.54 ± 0.17 a | 0.30 ± 0.04 b | 0 |
| Centensimal Composition | ||||||||
|---|---|---|---|---|---|---|---|---|
| Moisture (g.100 g−1) | Protein (g.100 g−1) | Fat (g.100 g−1) | Ash (g.100 g−1) | TDF (g.100 g−1) | IDF (g.100 g−1) | SDF (g.100 g−1) | Pectin Content (g.100 g−1) | Pectin in SDF (%) |
| 5.50 ± 0.05 | 10.03 ± 0.01 | 5.01 ± 0.12 | 5.20 ± 0.08 | 65.83 ± 1.68 | 58.69 ± 0.45 | 7.97 ± 0.36 | 6.58 ± 0.01 | 82.91 ± 2.44 |
| Technological Properties | Antioxidant Capacity | ||||||
|---|---|---|---|---|---|---|---|
| Luminosity (L) | Hue Angle (h) | Color Saturation (c) | OHC (g oil. g1) | SWC (mL.g−1) | WHC (g water.g−1) | ABTS (µmol Trolox.g−1) | FRAP (µmol Fe2SO4. g−1) |
| 44.93 ± 0.18 | 67.28 ± 0.09 | 25.79 ± 0.10 | 5.21 ± 0.15 | 8.75 ± 0.35 | 4.08 ± 0.05 | 755.9 ± 47.97 | 175.78 ± 0.49 |
| (pH 1.5) 6.87 ± 0.03 a | (pH 1.5) 4.12 ± 0.02 a | ||||||
| (pH 7.0) 9.02 ± 0.37 b | (pH 7.0) 4.16 ± 0.01 b | ||||||
| (pH 8.5) 10.60 ± 0.52 c | (pH 8.5) 4.19 ± 0.04 b | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cangussu, L.B.; Melo, J.C.; Franca, A.S.; Oliveira, L.S. Chemical Characterization of Coffee Husks, a By-Product of Coffea arabica Production. Foods 2021, 10, 3125. https://doi.org/10.3390/foods10123125
Cangussu LB, Melo JC, Franca AS, Oliveira LS. Chemical Characterization of Coffee Husks, a By-Product of Coffea arabica Production. Foods. 2021; 10(12):3125. https://doi.org/10.3390/foods10123125
Chicago/Turabian StyleCangussu, Lais B., Jean Carlos Melo, Adriana S. Franca, and Leandro S. Oliveira. 2021. "Chemical Characterization of Coffee Husks, a By-Product of Coffea arabica Production" Foods 10, no. 12: 3125. https://doi.org/10.3390/foods10123125






