Chemical Composition Analysis and Antioxidant Activity of Coffea robusta Monofloral Honeys from Vietnam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Collection of Coffee Honey Samples
2.3. Physicochemical Analysis
2.3.1. Total Sugar and Free Reducing Sugar Contents
- C: Total sugar concentration from the calibration curve (ppm);
- W: Weight of sample (g);
- V1: 1st titration volume (mL);
- V2: Second titration volume (mL);
- 106: Convert ppm concentration to g/mL;
- V: Analytical sample volume (mL).
- C: Concentration from the calibration curve (ppm);
- V1: Volumetric first time (mL);
- V2: Second titration volume (mL);
- 106: Convert concentration (ppm) to (g/mL);
- V: Analytical sample volume (mL);
- W: Weight of sample (g).
2.3.2. Hydroxymethylfurfural (HMF)
- D: dilution factor;
- W: sample weight (g).
2.3.3. Free Acid Contents
- VNaOH: volume of NaOH (ml);
- W: sample weight (g).
2.3.4. Mineral Content
2.4. Determination of Total Phenolic Content (TPC) and Flavonoid Content (TFC)
2.5. Antioxidant Activity
2.5.1. DPPH Free Radical Scavenging Assay
2.5.2. ABTS Free Radical Scavenging Assay
2.6. Phenolic Analysis
2.6.1. Extraction of Phenolic Compounds
2.6.2. Preparation of Phenolic Acid Standard Solutions
2.6.3. HPLC Analysis
2.7. Trigonelline and Caffein Analysis
2.7.1. Extraction of Alkaloid Compounds
2.7.2. HPLC analysis
2.8. Data Analysis
3. Results
3.1. Physicochemical Parameters
3.2. Total Phenolic Contents (TPC) and Total Flavonoid Contents (TFC)
3.3. Antioxidant Activity
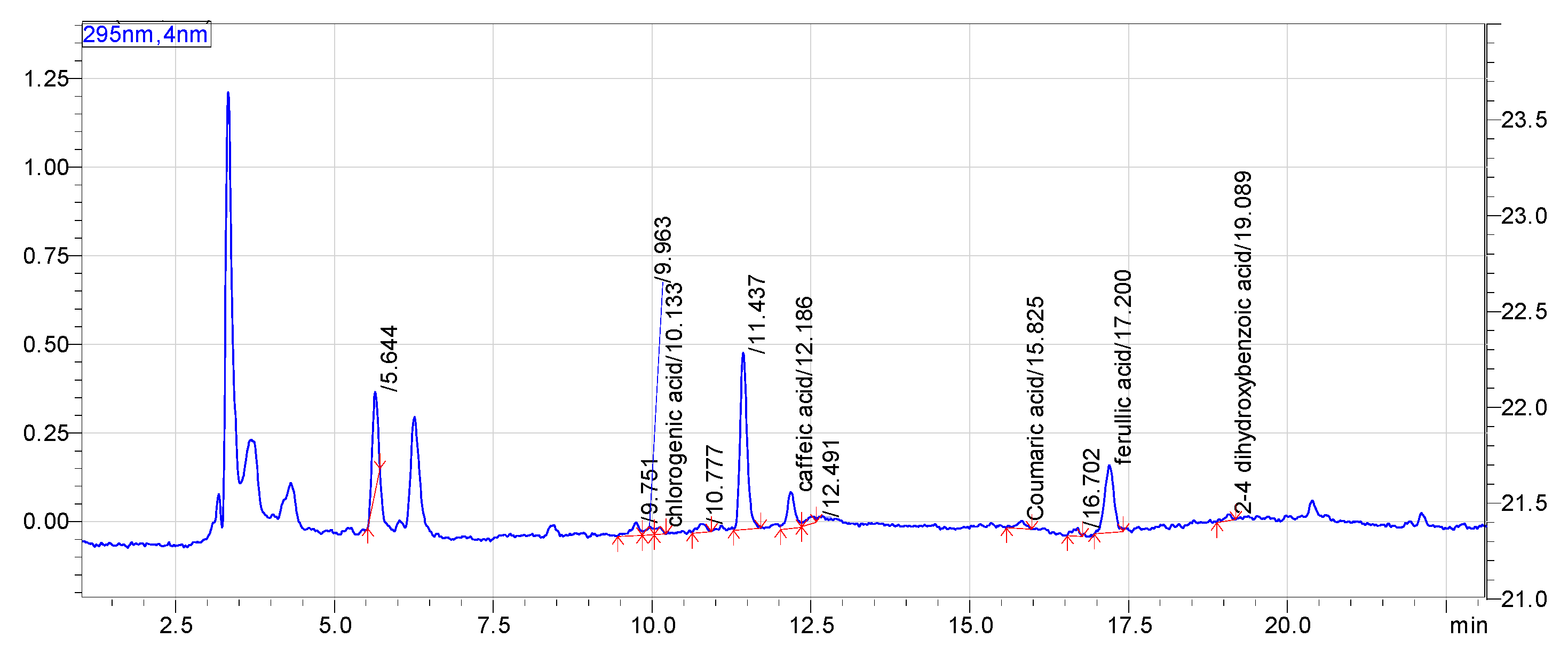
3.4. Phenolic Acids Analysis
3.5. Trigonelline and Caffeine Analysis
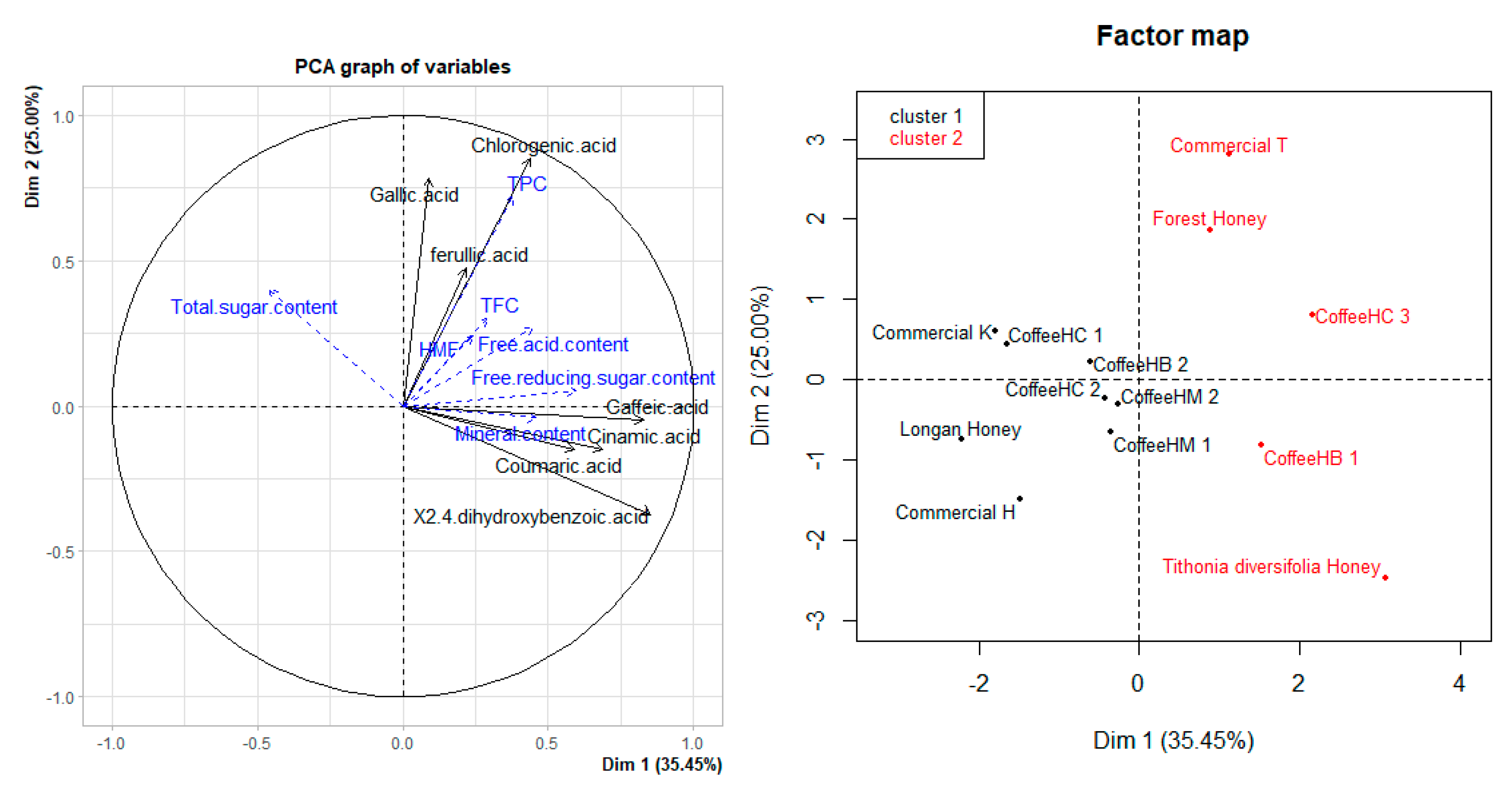
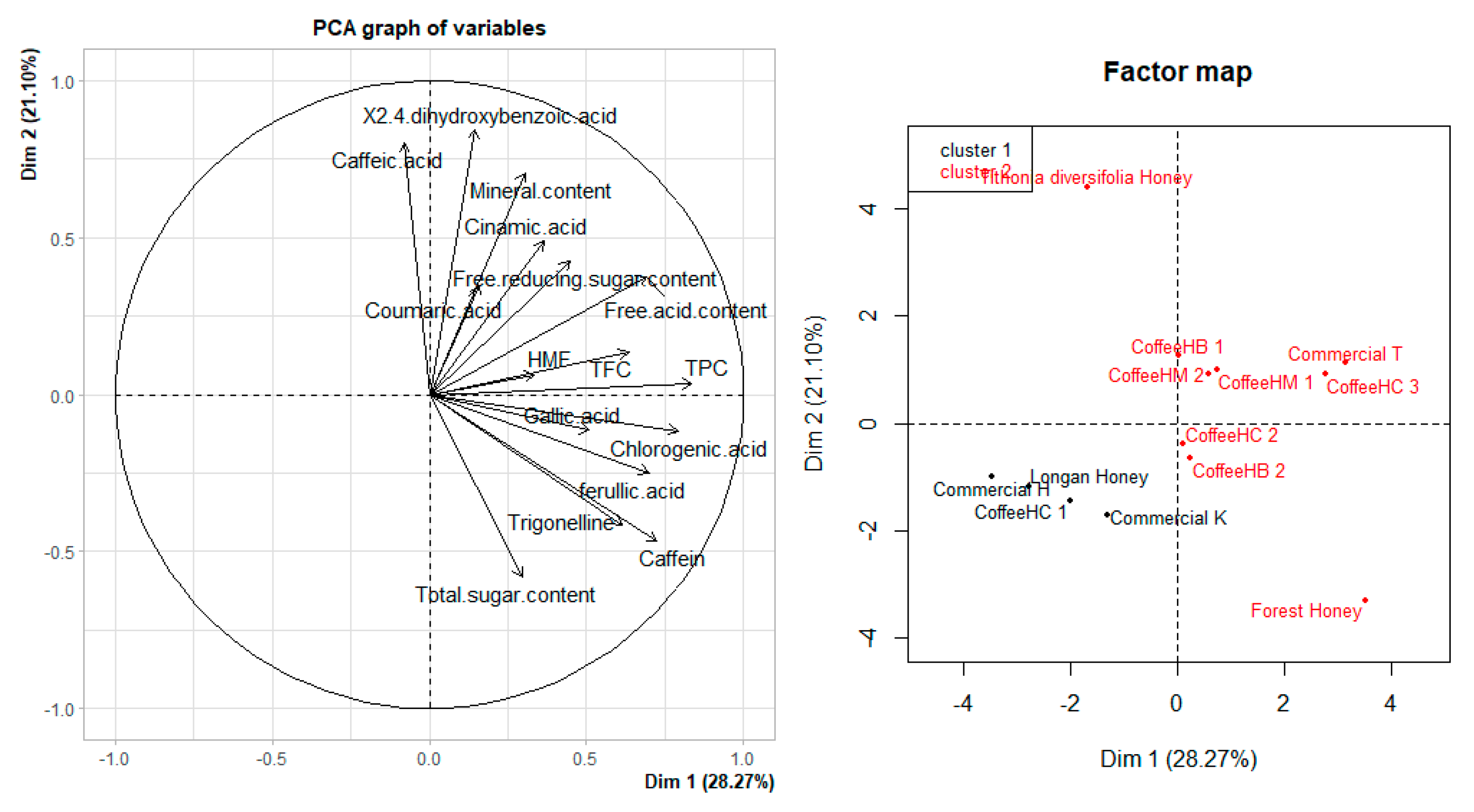
3.6. PCA Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Kliks, M.M.; Jun, S.; Li, Q.X. Residues of organochlorine pesticides in honeys from different geographic regions. Food Res. Int. 2010, 43, 2329–2334. [Google Scholar] [CrossRef]
- Omotayo, E.O.; Gurtu, S.; Sulaiman, S.A.; Wahab, M.S.A.; Sirajudeen, K.N.S.; Salleh, M.S.M. Hypoglycemic and antioxidant effects of honey supplementation in streptozotocin-induced diabetic rats. Int. J. Vitam. Nutr. Res. 2010, 80, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; Al-Asiri, S.A.; Mahesar, A.L.; Ansari, M.J. Role of honey in modern medicine. Saudi J. Biol. Sci. 2017, 24, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.R.; Abadin, Z.U.; Rauf, N. Honey: Nutritional and medicinal value. Int. J. Clin. Pract. 2007, 61, 1705–1707. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.; Giampieri, F.; Battino, M. Honey as a Source of Dietary Antioxidants: Structures, Bioavailability and Evidence of Protective Effects against Human Chronic Diseases. Curr. Med. Chem. 2013, 20, 621–638. [Google Scholar] [CrossRef]
- Schramm, D.D.; Karim, M.; Schrader, H.R.; Holt, R.R.; Cardetti, M.; Keen, C.L. Honey with high levels of antioxidants can provide protection to healthy human subjects. J. Agric. Food Chem. 2003, 51, 1732–1735. [Google Scholar] [CrossRef]
- Rebane, R.; Herodes, K. Evaluation of the botanical origin of Estonian uni- and polyfloral honeys by amino acid content. J. Agric. Food Chem. 2008, 56, 10716–10720. [Google Scholar] [CrossRef]
- Kadri, S.M.; Zaluski, R.; Lima, G.P.P.; Mazzafera, P.; Orsi, R.d. Characterization of Coffea arabica monofloral honey from Espírito Santo, Brazil. Food Chem. 2016, 203, 252–257. [Google Scholar] [CrossRef]
- Jantakee, K.; Tragoolpua, Y. Activities of different types of thai honey on pathogenic bacteria causing skin diseases, tyrosinase enzyme and generating free radicals. Biol. Res. 2015, 48, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, S.S. Phenol-Sulfuric Acid Method for Total Carbohydrates. In Food Analysis Laboratory Manual; Springer: Boston, MA, USA, 2010; pp. 47–53. [Google Scholar] [CrossRef]
- de Almeida-Muradian, L.B.; Stramm, K.M.; Horita, A.; Barth, O.M.; de Freitas, A.d.; Estevinho, L.M. Comparative study of the physicochemical and palynological characteristics of honey from Melipona subnitida and Apis mellifera. Int. J. Food Sci. Technol. 2013, 48, 1698–1706. [Google Scholar] [CrossRef] [Green Version]
- Finola, M.S.; Lasagno, M.C.; Marioli, J.M. Microbiological and chemical characterization of honeys from central Argentina. Food Chem. 2007, 100, 1649–1653. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteau, V. Tyrosine and Tryptophane in Proteins. J. Biol. Chem. 1927, 73, 627–648. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Stanek, N.; Teper, D.; Kafarski, P.; Jasicka-Misiak, I. Authentication of phacelia honeys (Phacelia tanacetifolia) based on a combination of HPLC and HPTLC analyses as well as spectrophotometric measurements. LWT 2019, 107, 199–207. [Google Scholar] [CrossRef]
- Oroian, M.; Ropciuc, S. Honey authentication based on physicochemical parameters and phenolic compounds. Comput. Electron. Agric. 2017, 138, 148–156. [Google Scholar] [CrossRef]
- Melendez-Martinez, A.J.; Stinco, C.M.; Liu, C.; Wang, X.-D. Fast HPLC-DAD quantification of nine polyphenols in honey by using second-order calibration method based on trilinear decomposition algorithm. Food Chem. 2013, 138, 62–69. [Google Scholar] [CrossRef]
- Doukani, K.; Gacem, N.; Benlarbi, H. Physicochemical and Phytochemical Characterization of Some Algerian Honeys Types. Int. J. Appl. Phys. Bio Chem. Res. (IJAPBCR) 2014, 4, 1–16. Available online: http://www.tjprc.org/view-archives.php?year=2014_48_2&id=46&jtype=2&page=2 (accessed on 1 December 2014).
- Fahim, H.; Dasti, J.I.; Ali, I.; Ahmed, S.; Nadeem, M. Physico-chemical analysis and antimicrobial potential of Apis dorsata, Apis mellifera and Ziziphus jujube honey samples from Pakistan. Asian Pac. J. Trop. Biomed. 2014, 4, 633–641. [Google Scholar] [CrossRef] [Green Version]
- Azonwade, F.E.; Paraiso, A.; Agbangnan Dossa, C.P.; Dougnon, V.T.; N’tcha, C.; Mousse, W.; Baba-Moussa, L. Physicochemical Characteristics and Microbiological Quality of Honey Produced in Benin. J. Food Qual. 2018, 2018, 1896057. [Google Scholar] [CrossRef] [Green Version]
- Bahrami, M.; Ataie-Jafari, A.; Hosseini, S.; Foruzanfar, M.H.; Rahmani, M.; Pajouhi, M. Effects of natural honey consumption in diabetic patients: An 8-week randomized clinical trial. Int. J. Food Sci. Nutr. 2009, 60, 618–626. [Google Scholar] [CrossRef]
- Ramli, N.Z.; Chin, K.Y.; Zarkasi, K.A.; Ahmad, F. A review on the protective effects of honey against metabolic syndrome. Nutrients 2018, 10, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moniruzzaman, M.; Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. Physicochemical and antioxidant properties of Malaysian honeys produced by Apis cerana, Apis dorsata and Apis mellifera. BMC Complementary Altern. 2013, 13, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.; Kim, Y.J.; Kim, M.K. Effect of fructose or sucrose feeding with different levels on oral glucose tolerance test in normal and type 2 diabetic rats. Nutr. Res. Pract. 2008, 2, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.; Sirajudeen, K.N.S.; Salleh, M.S.M.D.; Gurtu, S. Action protectrice anti-oxydante du miel malésien Tualang sur le pancréas de rats normaux ou diabétiques induits par la streptozotocine. Ann. Endocrinol. 2010, 71, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Bobiş, O.; Dezmirean, D.S.; Moise, A.R. Honey and Diabetes: The Importance of Natural Simple Sugars in Diet for Preventing and Treating Different Type of Diabetes. Oxidative Med. Cell. Longev. 2018, 4757893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 1–18. [Google Scholar] [CrossRef]
- Gomes, S.; Dias, L.G.; Moreira, L.L.; Rodrigues, P.; Estevinho, L. Physicochemical, microbiological and antimicrobial properties of commercial honeys from Portugal. Food Chem. Toxicol. 2010, 48, 544–548. [Google Scholar] [CrossRef] [Green Version]
- Fallico, B.; Arena, E.; Verzera, A.; Zappalà, M. The European Food Legislation and its impact on honey sector. Accredit. Qual. Assur. 2006, 11, 49–54. [Google Scholar] [CrossRef]
- Bhattarai, S.; Subedi, U.; Bhattarai, U.K.; Karki, R.; Ojha, P. Study on Chemical and Bioactive Components of Different Floral Sources’ Honey in Nepal. J. Food Sci. Technol. Nepal 2019, 11, 51–59. [Google Scholar] [CrossRef]
- Živkov Baloš, M.; Popov, N.; Vidakovic, S.; Ljubojevic-Pelic, D.; Pelic, M.; Mihaljev, Z.; Kaksic, S. Electrical conductivity and acidity of honey. Arch. Vet. Med. 2018, 11, 91–101. [Google Scholar] [CrossRef]
- Benaziza-Bouchema, D.; Schweitzer, P. Caractérisation des principaux miels des régions du Nord de l’Algérie. Cah. Agric. 2010, 19, 432–438. [Google Scholar] [CrossRef]
- Ajlouni, S.; Sujirapinyokul, P. Hydroxymethylfurfuraldehyde and amylase contents in Australian honey. Food Chem. 2010, 119, 1000–1005. [Google Scholar] [CrossRef]
- Belay, A.; Solomon, W.K.; Bultossa, G.; Adgaba, N.; Melaku, S. Physicochemical properties of the Harenna forest honey, Bale, Ethiopia. Food Chem. 2013, 141, 3386–3392. [Google Scholar] [CrossRef] [PubMed]
- Vanhanen, L.P.; Emmertz, A.; Savage, G.P. Mineral analysis of mono-floral New Zealand honey. Food Chem. 2011, 128, 236–240. [Google Scholar] [CrossRef]
- Schievano, E.; Finotello, C.; Mammi, S.; Belci, A.I.; Colomban, S.; Navarini, L. Preliminary Characterization of Monofloral Coffea spp. Honey: Correlation between Potential Biomarkers and Pollen Content. J. Agric. Food Chem. 2015, 63, 5858–5863. [Google Scholar] [CrossRef]
- Khalil, M.I.; Alam, N.; Moniruzzaman, M.; Sulaiman, S.A.; Gan, S.H. Phenolic Acid Composition and Antioxidant Properties of Malaysian Honeys. J. Food Sci. 2011, 76, 921–928. [Google Scholar] [CrossRef]
- Perna, A.; Intaglietta, I.; Simonetti, A.; Gambacorta, E. A comparative study on phenolic profile, vitamin C content and antioxidant activity of Italian honeys of different botanical origin. Int. J. Food Sci. Technol. 2013, 48, 1899–1908. [Google Scholar] [CrossRef]
- Bertoncelj, J.; Golob, T.; Kropf, U.; Korošec, M. Characterisation of Slovenian honeys on the basis of sensory and physicochemical analysis with a chemometric approach. Int. J. Food Sci. Technol. 2011, 46, 1661–1671. [Google Scholar] [CrossRef]
- Attanzio, A.; Tesoriere, L.; Allegra, M.; Livrea, M.A. Monofloral honeys by Sicilian black honeybee (Apis mellifera ssp. sicula) have high reducing power and antioxidant capacity. Heliyon 2016, 2, e00193. [Google Scholar] [CrossRef] [Green Version]
- Kishore, G.; Ranjan, S.; Pandey, A.; Gupta, S. Influence of altitudinal variation on the antioxidant potential of tartar buckwheat of Western Himalaya. Food Sci. Biotechnol. 2010, 19, 1355–1363. [Google Scholar] [CrossRef]
- Kishore, R.K.; Halim, A.S.; Syazana, M.S.N.; Sirajudeen, K.N.S. Tualang honey has higher phenolic content and greater radical scavenging activity compared with other honey sources. Nutr. Res. 2011, 31, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a Potential Natural Antioxidant Medicine: An Insight into Its Molecular Mechanisms of Action. Oxidative Med. Cell. Longev. 2018, 2018, 8367846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.A. Honey: A novel antioxidant. Molecules 2012, 17, 4400–4423. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Tulipani, S.; Díaz, D.; Estevez, Y.; Romandini, S.; Giampieri, F.; Battino, M. Antioxidant and antimicrobial capacity of several monofloral Cuban honeys and their correlation with color, polyphenol content and other chemical compounds. Food Chem. Toxicol. 2010, 48, 2490–2499. [Google Scholar] [CrossRef]
- Bertoncelj, J.; Dobersek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Silici, S.; Sagdic, O.; Ekici, L. Total phenolic content, antiradical, antioxidant and antimicrobial activities of Rhododendron honeys. Food Chem. 2010, 121, 238–243. [Google Scholar] [CrossRef]
| Name Sample | Physicochemical Parameters | ||||
|---|---|---|---|---|---|
| Free Acid Content (mg/kg) | Mineral Content (%) | HMF (mg/kg) | Total Sugar Content (mg/kg) | Free Reducing Sugar Content (mg/kg) | |
| CoffeeHC 1 | 20.326 ± 0.105 b | 0.051 ± 0.013 ac | 0.048 ± 0.016 a | 811.660 ± 2.746 bc | 669.964 ± 1.335 bc |
| CoffeeHC 2 | 23.902 ± 0.055 ef | 0.089 ± 0.044 bce | 0.245 ± 0.003 b | 884.718 ± 1.762 e | 712.129 ± 6.301 d |
| CoffeeHC 3 | 29.485 ± 0.259 h | 0.131 ± 0.032 e | 2.933 ± 0.022 h | 804.128 ± 9.591 ab | 722.881 ± 5.844 de |
| CoffeeHB 1 | 25.692 ± 0.122 g | 0.064 ± 0.003 acd | 0.108 ± 0.056 a | 814.759 ± 3.719 bc | 741.375 ± 17.496 ef |
| CoffeeHB 2 | 24.327 ± 0.031 f | 0.097 ± 0.026 ce | 2.722 ± 0.017 fg | 848.308 ± 1.093 be | 655.251 ± 4.348 b |
| CoffeeHM 1 | 31.163 ± 0.779 i | 0.095 ± 0.003 bce | 2.113 ± 0.047 d | 879.702 ± 7.681 de | 757.769 ± 1.339 f |
| CoffeeHM 2 | 29.455 ± 0.564 h | 0.101 ± 0.003 ce | 2.037 ± 0.017 d | 818.946 ± 15.456 bcd | 685.185 ± 2.500 c |
| Commercial H | 16.142 ± 0.296 a | 0.041 ± 0.011 ab | 2.607 ± 0.036 f | 795.115 ± 0.994 ab | 625.62 ± 15.746 a |
| Commercial K | 23.185 ± 0.254 de | 0.054 ± 0.005 acd | 2.712 ± 0.022 fg | 828.385 ± 56.895 be | 666.972 ± 5.335 bc |
| Commercial T | 26.140 ± 0.179 g | 0.107 ± 0.003 de | 2.781 ± 0.037 g | 831.394 ± 18.746 be | 741.878 ± 5.685 ef |
| Forest Honey | 22.318 ± 0.172 cd | 0.026 ± 0.007 a | 2.624 ± 0.008 f | 870.097 ± 12.347 ce | 745.118 ± 2.3 ef |
| Tithonia diversifolia Honey | 21.647 ± 0.526 c | 0.106 ± 0.013 de | 2.449 ± 0.038 e | 742.860 ± 14.554 a | 760.964 ± 1.375 f |
| Longan Honey | 15.220 ± 0.146 a | 0.079 ± 0.005 ace | 1.123 ± 0.091 c | 868.913 ± 37.668 ce | 741.414 ± 2.295 ef |
| Name Sample | Total Polyphenol (mg GAE/g) | Total Flavonoid (mg QE/g) | IC50 of DPPH Inhibition (mg/mL) | IC50 of ABTS Inhibition (mg/mL) |
|---|---|---|---|---|
| CoffeeHC 1 | 0.519 ± 0.0083 a | 0.032 ± 0.0005 d | 9.769 ± 0.83 f | 175.519 ± 1.440 i |
| CoffeeHC 2 | 0.596 ± 0.0048 b | 0.037 ± 0.0021 f | 10.235 ± 0.86 | 175.776 ± 1.665 i |
| CoffeeHC 3 | 0.704 ± 0.016 d | 0.037 ± 0.001 f | 4.753 ± 0.60 d | 142.955 ± 2.835 f |
| CoffeeHB 1 | 0.730 ± 0.025 de | 0.030 ± 0.0005 c | 3.587 ± 0.89 cd | 130.424 ± 1.570 e |
| CoffeeHB 2 | 0.657 ± 0.014 c | 0.033 ± 0.0007 d | 9.669 ± 0.73 f | 167.471 ± 2.001 h |
| CoffeeHM 1 | 0.749 ± 0.003 e | 0.036 ± 0.0003 ef | 2.831 ± 0.44 bcd | 128.616 ± 0.668 e |
| CoffeeHM 2 | 0.863 ± 0.012 f | 0.034 ± 0.0003 de | 1.589 ± 0.38 abc | 123.013 ± 0.721 d |
| Commercial H | 0.508 ± 0.010 a | 0.028 ± 0.0003 ac | 17.031 ± 0.27 g | 213.769 ± 1.614 k |
| Commercial K | 0.699 ± 0.008 cd | 0.026 ± 0.0005 a | 10.135 ± 0.516 f | 158.65 ± 0.286 g |
| Commercial T | 1.090 ± 0.034 h | 0.033 ± 0.0005 d | 1.134 ± 0.34 ab | 115.381 ± 1.665 c |
| Forest Honey | 0.913 ± 0.0053 g | 0.032 ± 0.0003 d | 1.255 ± 0.236 ab | 90.0196 ± 1.188 b |
| Tithonia diversifolia Honey | 0.570 ± 0.0025 b | 0.029 ± 0.0003 bc | 11.855 ± 0.88 f | 159.11 ± 1.363 g |
| Longan Honey | 0.512 ± 0.0026 a | 0.027 ± 0.0003 ab | 15.645 ± 0.572 g | 158.535 ± 0.881 g |
| Ascorbic acid | - | - | 0.0000234667 ± 0.00005 a | 0.0000164333 ± 0.0000083 a |
| Peak Number | Phenolic Acid | Linear Range (mg/L) | R2 | LOD (mg/L) | LOQ (mg/L) |
|---|---|---|---|---|---|
| 1 | gallic acid | 0.1–5 | 0.9996 | 0.0063 | 0.0191 |
| 2 | chlorogenic acid | 0.1–5 | 0.9989 | 0.0066 | 0.020 |
| 3 | caffeic acid | 0.1–5 | 0.9997 | 0.0048 | 0.0146 |
| 4 | coumaric acid | 0.1–5 | 0.9998 | 0.0040 | 0.0123 |
| 5 | ferulic acid | 0.1–5 | 0.9990 | 0.0049 | 0.0149 |
| 6 | 2,4-dihydroxy-benzoic acid | 0.1–5 | 0.9992 | 0.0322 | 0.0977 |
| 7 | cinnamic acid | 0.1–5 | 0.9994 | 0.0021 | 0.0066 |
| Name Sample | Phenolic Acids Concentration (mg/kg) | ||||||
|---|---|---|---|---|---|---|---|
| Gallic Acid | Cinnamic Acid | Chlorogenic Acid | Caffeic Acid | Coumaric Acid | Ferulic Acid | 2,4 Dihydroxy-benzoic Acid | |
| CoffeeHC 1 | 1.067 ± 0.005 ac | 0.074 ± 0.001 ab | 0.794 ± 0.009 b | 0.521 ± 0.007 ab | 0.223 ± 0.006 ab | 1.927 ± 0.015 c | 0.406 ± 0.010 a |
| CoffeeHC 2 | 1.213 ± 0.008 ac | 0.089 ± 0.002 ac | 0.44 ± 0.007 ab | 1.082 ± 009 ce | 0.278 ± 0.011 ab | 2.701 ± 0.013 de | 0.68 ± 0.003 a |
| CoffeeHC 3 | 1.857 ± 0.02 cd | 0.134 ± 0.002 bc | 1.285 ± 0.02 c | 0.220 ± 0.025 f | 0.561 ± 0.005 b | 2.766 ± 0.013 e | 0.858 ± 0.026 ab |
| CoffeeHB 1 | 1.935 ± 0.046 cd | 0.158 ± 0.003 bc | 0.501 ± 0.019 ab | 0.996 ± 0.01 cd | 1.11 ± 0.005 c | 2.141 ± 0.016 cd | 0.79 ± 0.036 ab |
| CoffeeHB 2 | 1.645 ± 0.027 bc | 0.092 ± 0.004 ac | 0.79 ± 0.020 b | 0.965 ± 0.02 cd | 0.279 ± 0.007 ab | 1.866 ± 0.013 c | 0.59 ± 0.009 a |
| CoffeeHM 1 | 1.333 ± 0.034 ac | 0.118 ± 0.001 ac | 0.344 ± 0.005 ab | 1.436 ± 0.005 e | 0.149 ± 0.004 a | 2.036 ± 0.011 c | 0.638 ± 0.010 a |
| CoffeeHM 2 | 1.087 ± 0.021 ac | 0.133 ± 0.003 bc | 0.525 ± 0.024 ab | 1.178 ± 0.009 de | 0.142 ± 0.0008 a | 2.147 ± 0.016 cd | 0.687 ± 0.025 a |
| Commercial H | 0.560 ± 0.012 ab | 0.147 ± 0.002 bc | 0.329 ± 0.002 ab | 0.258 ± 0.001 a | 0.144 ± 0.009 a | NA | 0.578 ± 0.012 a |
| Commercial K | 3.846 ± 0.029 e | NA | 0.63 ± 0.018 ab | 0.448 ± 0.0063 ab | 0.316 ± 0.01 ab | 1.599 ± 0.011 bc | 0.58 ± 0.006 a |
| Commercial T | 10.124 ± 0.145 f | 0.214 ± 0.006 c | 1.584 ± 0.02 c | 0.695 ± 0.014 bc | 0.221 ± 0.0006 ab | 1.133 ± 0.02 b | 0.761 ± 0.007 ab |
| Forest Honey | 3.796 ± 0.019 e | 0.096 ± 0.008 ac | 1.294 ± 0.022 c | 0.204 ± 0.036 f | 0.297 ± 0.001 ab | 2.977 ± 0.014 e | 0.615 ± 0.009 a |
| Tithonia diversifolia Honey | 0.367 ± 0.014 a | 0.119 ± 0.009 bc | 0.498 ± 0.002 ab | 2.381 ± 0.17 f | 0.477 ± 0.05 ab | 0.377 ± 0.030 a | 1.349 ± 0.005 b |
| Longan Honey | 2.981 ± 0.009 de | 0.089 ± 0.002 ac | 0.23 ± 0.009 a | 0.202 ± 0.004 a | 0.13 ± 0.004 a | 0.236 ± 0.005 a | 0.497 ± 0.014 a |
| Name Sample | Trigonelline | Caffein |
|---|---|---|
| CoffeeHC 1 | 0.314 ± 0.003 a | 8.946 ± 0.227 d |
| CoffeeHC 2 | 1.244 ± 0.012 f | 25.736 ± 0.008 j |
| CoffeeHC 3 | 2.327 ± 0.007 g | 37.977 ± 0.003 l |
| CoffeeHB 1 | 0.579 ± 0.013 b | 29.954 ± 0.103 k |
| CoffeeHB 2 | 2.399 ± 0.007 h | 22.319 ± 0.025 i |
| CoffeeHM 1 | 0.353 ± 0.015 a | 16.597 ± 0.026 e |
| CoffeeHM 2 | 0.678 ± 0.020 c | 19.163 ± 0.002 g |
| Commercial H | 0.627 ± 0.017 bc | 4.556 ± 0.003 c |
| Commercial K | 0.803 ± 0.008 d | 21.94 ± 0.010 h |
| Commercial T | 0.928 ± 0.005 e | 17.412 ± 0.028 f |
| Forest Honey | 4.541 ± 0.032 i | 90.258 ± 0.07 m |
| Tithonia diversifolia Honey | 0.957 ± 0.007 e | 0.591 ± 0.004 a |
| Longan Honey | 0.763 ± 0.049 d | 0.915 ± 0.015 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinh, N.T.N.; Tuan, N.N.; Thang, T.D.; Kuo, P.-C.; Thanh, N.B.; Tam, L.N.; Tuoi, L.H.; Nguyen, T.H.D.; Vu, D.C.; Ho, T.L.; et al. Chemical Composition Analysis and Antioxidant Activity of Coffea robusta Monofloral Honeys from Vietnam. Foods 2022, 11, 388. https://doi.org/10.3390/foods11030388
Trinh NTN, Tuan NN, Thang TD, Kuo P-C, Thanh NB, Tam LN, Tuoi LH, Nguyen THD, Vu DC, Ho TL, et al. Chemical Composition Analysis and Antioxidant Activity of Coffea robusta Monofloral Honeys from Vietnam. Foods. 2022; 11(3):388. https://doi.org/10.3390/foods11030388
Chicago/Turabian StyleTrinh, Nguyen Thi Nu, Nguyen Ngoc Tuan, Tran Dinh Thang, Ping-Chung Kuo, Nguyen Ba Thanh, Le Nhat Tam, Le Hong Tuoi, Trang H. D. Nguyen, Danh C. Vu, Thi L. Ho, and et al. 2022. "Chemical Composition Analysis and Antioxidant Activity of Coffea robusta Monofloral Honeys from Vietnam" Foods 11, no. 3: 388. https://doi.org/10.3390/foods11030388






