Disaccharide Type Affected Phenolic and Volatile Compounds of Citrus Fiber-Blackberry Cream Fillings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Blackberry Cream Fillings
2.3. Extraction of Phenols
2.4. Determination of the Total Phenolics
2.5. Determination of Total Proanthocyanidins
2.6. Determination of Antioxidant Activity
2.7. Color Measurement and Color Change
2.8. Volatile Compounds Analysis
2.9. Water Activity Measurement
2.10. Statistical Analysis
3. Results
3.1. Total Phenolic and Total Proanthocyanidin Content
3.2. Antioxidant Activity
3.3. Color Parameters
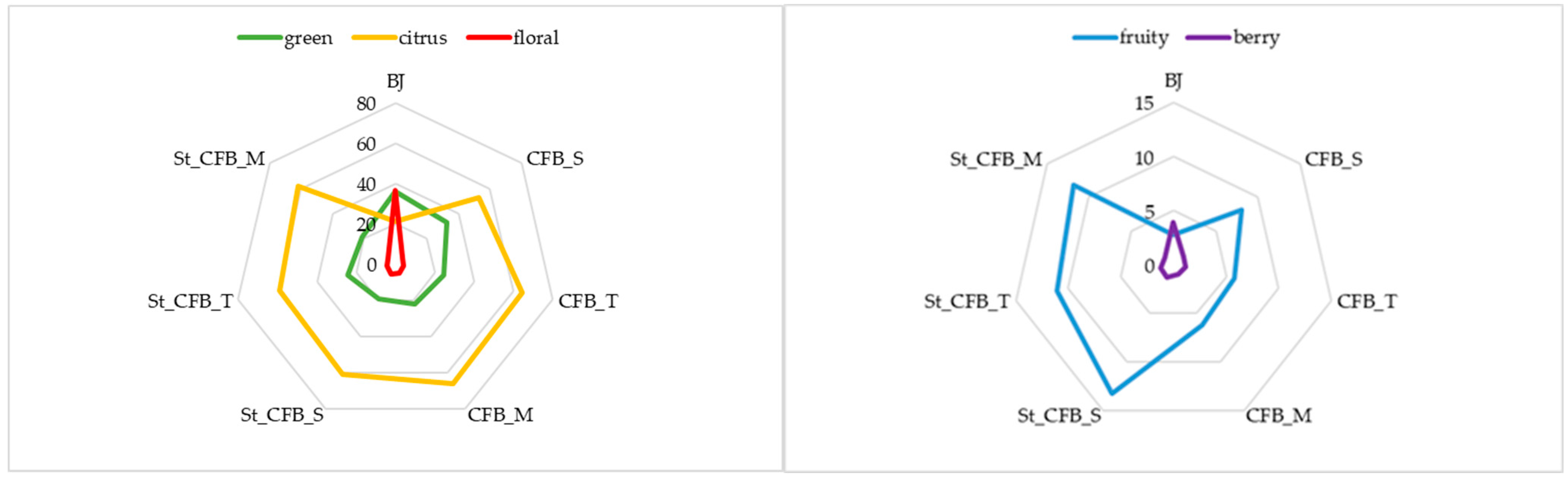
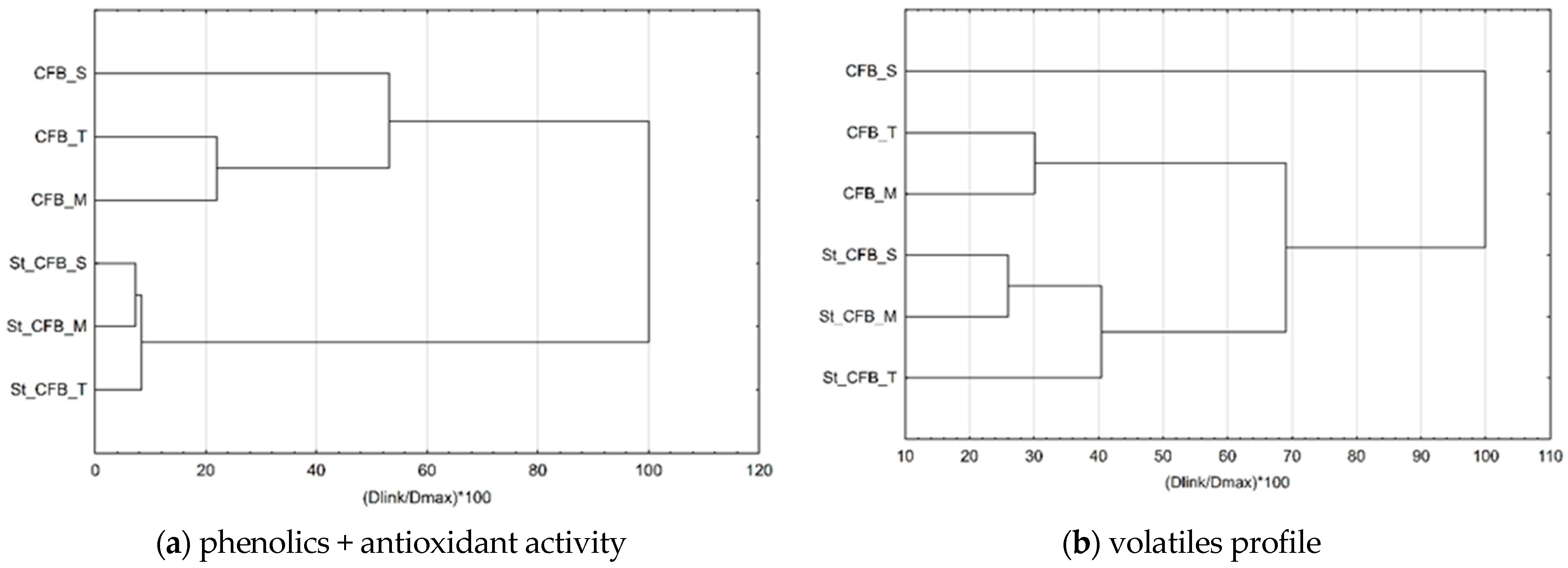
3.4. Volatile Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, D.; Martinez-Sanz, M.; Lopez-Sanchez, P.; Gilbert, E.P.; Gidley, M.J. Adsorption behavior of polyphenols on cellulose is affected by processing history. Food Hydrocoll. 2017, 63, 496–507. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, R.H., Jr.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef] [PubMed]
- Velderrain-Rodríguez, G.R.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Chen, C.Y.; Robles-Sánchez, M.; Astiazaran-García, H.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Phenolic compounds: Their journey after intake. Food Funct. 2014, 5, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.; Franceschi, P.; Feller, A.; Palmieri, L.; Wehrens, R.; Martens, S. A targeted metabolomics approach to understand differences in flavonoid biosynthesis in red and yellow raspberries. Plant Physiol. Biochem. 2013, 72, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Aghraz, A.; Albergamo, A.; Benameur, Q.; Salvo, A.; Larhsini, M.; Markouk, M.; Gervasi, T.; Cicero, N. Polyphenols contents, heavy metals analysis and in vitro antibacterial activity of extracts from Cladanthus arabicus and Bubonium imbricatum of Moroccan Origin. Nat. Prod. Res. 2020, 34, 63–70. [Google Scholar] [CrossRef]
- Capillo, G.; Savoca, S.; Costa, R.; Sanfilippo, M.; Rizzo, C.; Lo Giudice, A.; Albergamo, A.; Rando, R.; Bartolomeo, G.; Spano, N.; et al. New insights into the culture method and antibacterial potential of Gracilaria gracilis. Mar. Drugs 2018, 16, 492. [Google Scholar] [CrossRef]
- Lundberg, B.M. Using highly expanded citrus fiber to improve the quality and nutritional properties of foods. Cereal Foods World 2005, 50, 248–252. [Google Scholar]
- Lundberg, B.; Pan, X.; White, A.; Chau, H.; Hotchkiss, A. Rheology and composition of citrus fiber. J. Food Eng. 2014, 125, 97–104. [Google Scholar] [CrossRef]
- Jakobek, L.; Šeruga, M.; Šeruga, B.; Novak, I.; Medvidović-Kosanović, M. Phenolic compound composition and antioxidant activity of fruits of Rubus and Prunus species from Croatia. Int. J. Food Sci. Technol. 2009, 44, 860–868. [Google Scholar] [CrossRef]
- Kaume, L.; Howard, L.R.; Devareddy, L. The blackberry fruit: A review on its composition and chemistry, metabolism and bioavailability, and health benefits. J. Agric. Food Chem. 2012, 60, 5716–5727. [Google Scholar] [CrossRef] [PubMed]
- Kopjar, M.; Piližota, V. Blackberry Juice. In Handbook of Functional Beverages and Human Health; Shahidi, F., Alaalvar, C., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Oxfordshire, UK, 2016; pp. 135–145. [Google Scholar]
- Turemis, N.; Kafkas, E.; Kafkas, S.; Kurkcuoglu, M.; Baser, K.H.C. Determination of aroma compounds in blackberry by GC/MS analysis. Chem. Nat. Compd. 2003, 39, 174–176. [Google Scholar] [CrossRef]
- Georgilopoulos, D.N.; Gallois, A.N. Flavour compounds of a commercial concentrated blackberry juice. Food Chem. 1988, 28, 141–148. [Google Scholar] [CrossRef]
- Komes, D.; Lovrić, T.; Ganić, K.K.; Gracin, L. Study of trehalose addition on aroma retention in dehydrated strawberry puree. Food Technol. Biotechnol. 2003, 41, 111–119. [Google Scholar]
- Komes, D.; Lovrić, T.; Ganić, K.K.; Kljusurić, J.G.; Banović, M. Trehalose improves flavour retention in dehydrated apricot puree. Int. J. Food Sci. Technol. 2005, 40, 425–435. [Google Scholar] [CrossRef]
- Komes, D.; Lovrić, T.; Kovačević Ganić, K. Aroma of dehydrated pear products. LWT 2007, 40, 1578–1586. [Google Scholar] [CrossRef]
- Kopjar, M.; Hribar, J.; Simčič, M.; Zlatić, E.; Tomaž, P.; Piližota, V. Effect of trehalose addition on volatiles responsible for strawberry aroma. Nat. Prod. Commun. 2013, 8, 1767–1770. [Google Scholar] [CrossRef]
- Zlatić, E.; Pichler, A.; Lončarić, A.; Vidrih, R.; Požrl, T.; Hribar, J.; Piližota, V.; Kopjar, M. Volatile compounds of freeze-dried sour cherry puree affected by addition of sugars. Int. J. Food Prop. 2017, 20 (Suppl. 1), S449–S456. [Google Scholar]
- Galmarini, M.V.; van Baren, C.; Zamora, M.C.; Chirife, J.; Di Leo Lira, P.; Bandoni, A. Impact of trehalose, sucrose and/or maltodextrin addition on aroma retention in freeze dried strawberry puree. Int. J. Food Sci. Technol. 2011, 46, 1337–1345. [Google Scholar] [CrossRef]
- Kopjar, M.; Piližota, V.; Hribar, J.; Simčič, M.; Zlatič, E.; Nedić Tiban, N. Influence of trehalose addition and storage conditions on the quality of strawberry cream filling. J. Food Eng. 2008, 87, 341–350. [Google Scholar] [CrossRef]
- Zlatič, E.; Pichler, A.; Kopjar, M. Disaccharides: Influence on volatiles and phenolics of sour cherry juice. Molecules 2017, 22, 1939. [Google Scholar] [CrossRef] [PubMed]
- Betoret, E.; Calabuig-Jimenez, L.; Patrignani, F.; Lanciotti, R.; Dalla Rosa, M. Effect of high pressure processing and trehalose addition on functional properties of mandarin juice enriched with probiotic microorganisms. LWT 2016, 85, 418–422. [Google Scholar] [CrossRef]
- Betoret, E.; Mannozzi, C.; Dellarosa, N.; Laghi, L.; Rocculi, P.; Dalla Rosa, M. Metabolomic studies after high pressure homogenization processed low pulp mandarin juice with trehalose addition. Functional and technological properties. J. Food Eng. 2017, 200, 22–28. [Google Scholar] [CrossRef]
- Kopjar, M.; Jakšić, K.; Piližota, V. Influence of sugars and chlorogenic acid addition on anthocyanin content, antioxidant activity and color of blackberry juice during storage. J. Food Process. Preserv. 2012, 36, 545–552. [Google Scholar] [CrossRef]
- Loncaric, A.; Dugalic, K.; Mihaljevic, I.; Jakobek, L.; Pilizota, V. Effects of sugar addition on total polyphenol content and antioxidant activity of frozen and freeze-dried apple puree. J. Agric. Food Chem. 2014, 62, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Lončarić, A.; Pichler, A.; Trtinjak, I.; Piližota, V.; Kopjar, M. Phenolics and antioxidant activity of freeze-dried sour cherry puree with addition of disaccharides. LWT 2016, 73, 391–396. [Google Scholar] [CrossRef]
- Pichler, A.; Pozderović, A.; Moslavac, T.; Popović, K. Influence of sugars, modified starches and hydrocolloids addition on colour and thermal properties of raspberry cream fillings. Pol. J. Food Nutr. Sci. 2017, 67, 49–58. [Google Scholar] [CrossRef][Green Version]
- Kopjar, M.; Pichler, A.; Turi, J.; Piližota, V. Influence of trehalose addition on antioxidant activity, colour and texture of orange jelly during storage. Int. J. Food Sci. Technol. 2016, 51, 2640–2646. [Google Scholar] [CrossRef]
- Neta, T.; Takada, K.; Hirasawa, M. Low-cariogenicity of trehalose as a substrate. J. Dent. 2000, 28, 571–576. [Google Scholar] [CrossRef]
- Van Can, J.G.P.; Van Loon, L.J.C.; Brouns, F.; Blaak, E.E. Reduced glycaemic and insulinaemic responses following trehalose and isomaltulose ingestion: Implications for postprandial substrate use in impaired glucose-tolerant subjects. Br. J. Nutr. 2012, 108, 1210–1217. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotonutric acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Prior, R.L.; Fan, E.; Ji, H.; Howell, A.; Nio, C.; Payne, M.J.; Reed, J. Multi-laboratory validation of a standard method for quantifying proanthocyanidins in cranberry powders. J. Sci. Food Agric. 2010, 90, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Apak, R.; Guculu, K.G.; Ozyurek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric iron reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–79. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Costa, R.; Salvo, A.; Rotondo, A.; Bartolomeo, G.; Pellizzeri, V.; Saija, E.; Arrigo, S.; Interdonato, M.; Trozzi, A.; Dugo, G. Combination of separation and spectroscopic analytical techniques: Application to compositional analysis of a minor citrus species. Nat. Prod. Res. 2018, 32, 2596–2602. [Google Scholar] [CrossRef]
- Salvo, A.; Costa, R.; Albergamo, A.; Arrigo, S.; Rotondo, A.; La Torre, G.L.; Mangano, V.; Dugo, G. An in-depth study of the volatile variability of chinotto (Citrus myrtifolia Raf.) induced by the extraction procedure. Eur. Food Res. Technol. 2019, 245, 873–883. [Google Scholar] [CrossRef]
- Syamaladevi, R.M.; Sablani, S.S.; Tang, J.; Powers, J.; Swanson, B.G. Stability of anthocyanins in frozen and freeze-dried raspberries during long-term storage: In relation to glass transition. J. Food Sci. 2011, 76, 414–421. [Google Scholar] [CrossRef]
- Da Porto, C.; Calligaris, S.; Celotti, E.; Nicoli, M.C. Antiradical properties of commercial cognacs assessed by the DPPH test. J. Agric. Food Chem. 2000, 48, 4241–4245. [Google Scholar] [CrossRef]
- Manzocco, L.; Calligaris, S.; Mastrocola, D.; Nicoli, M.C.; Lerici, C.R. Review of nonenzymatic browning and antioxidant capacity in processed foods. Trends Food Sci. Technol. 2001, 11, 340–346. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Calligaris, S.; Manzocco, L. Effect of enzymatic and chemical oxidation on the antioxidant capacity of catechin model systems and apple derivatives. J. Agric. Food Chem. 2000, 48, 4576–4580. [Google Scholar] [CrossRef] [PubMed]
- Vukoja, J.; Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Formulation and Stability of Cellulose-Based Delivery Systems of Raspberry Phenolics. Processes 2021, 9, 90. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R. Antioxidant activity of water-soluble Maillard reaction products. Food Chem. 2005, 93, 273–278. [Google Scholar] [CrossRef]
- Howard, L.R.; Castrodale, C.; Brownmiller, C.; Mauromoustakos, A. Jam Processing and Storage Effects on Blueberry Polyphenolics and Antioxidant Capacity†. J. Agric. Food Chem. 2010, 58, 4022–4029. [Google Scholar] [CrossRef]
- Schebor, C.; Burin, L.; del Pilar Bueras, M.; Chirife, J. Stability to hydrolysis and browning of trehalose, sucrose and raffinose in low-moisture systems in relation to their use as protectants of dry biomaterials. LWT-Food Sci. Technol. 1999, 32, 481–485. [Google Scholar] [CrossRef]
- O’Brien, J. Stability of trehalose, sucrose and glucose to nonenzymatic browning in model systems. J. Food Sci. 1996, 61, 679–682. [Google Scholar] [CrossRef]
- Licciardello, F.; Muratore, G. Effect of temperature and some added compounds on the stability of blood orange marmalade. J. Food Sci. 2011, 76, C1094–C1100. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A. Stability of phenolic compounds, antioxidant activity and colour through natural sweeteners addition during storage of sour cherry puree. Food Chem. 2016, 196, 925–934. [Google Scholar] [CrossRef]
- Lerbret, A.; Bordat, P.; Affouard, F.; Descamps, M.; Migliardo, F. How homogeneous are the trehalose, maltose, and sucrose water solutions? An insight from molecular dynamics simulations. J. Phys. Chem. B 2005, 109, 11046–11057. [Google Scholar] [CrossRef] [PubMed]
- Bordat, P.; Lerbret, A.; Demaret, J.-P.; Affouard, F.; Descamps, M. Comparative study of trehalose, sucrose and maltose in water solutions by molecular modelling. Europhys. Lett. 2004, 65, 41–47. [Google Scholar] [CrossRef]
- Heid, E.; Honegger, P.; Braun, D.; Szabadi, A.; Stankovic, T.; Steinhauser, O.; Schröder, C. Computational spectroscopy of trehalose, sucrose, maltose, and glucose: A comprehensive study of TDSS, NQR, NOE, and DRS. J. Chem. Phys. 2019, 150, 175102. [Google Scholar] [CrossRef] [PubMed]
- Olsson, C.; Swenson, J. Structural comparison between sucrose and trehalose in aqueous solution. J. Phys. Chem. B 2020, 124, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Oku, K.; Watanabe, H.; Kubota, M.; Fukuda, S.; Kurimoto, M.; Tujisaka, Y.; Komori, M.; Inoue, Y.; Sakurai, M. NMR and quantum chemical study on the OH...pi and CH...O interactions between trehalose and unsaturated fatty acids: Implication for the mechanism of antioxidant function of trehalose. J. Am. Chem. Soc. 2003, 125, 12739–12748. [Google Scholar] [CrossRef]
- Oku, K.; Kurose, M.; Kubota, M.; Fukuda, S.; Kurimoto, M.; Tujisaka, Y.; Okabe, A.; Sakurai, M. Combined NMR and Quantum Chemical Studies on the Interaction between Trehalose and Dienes Relevant to the Antioxidant Function of Trehalose. J. Phys. Chem. B 2005, 109, 3032–3040. [Google Scholar] [CrossRef]
- Sakakura, K.; Okabe, A.; Oku, K.; Sakurai, M. Experimental and theoretical study on the intermolecular complex formation between trehalose and benzene compounds in aqueous solution. J. Phys. Chem. B 2011, 115, 9823–9830. [Google Scholar] [CrossRef]
- Engelsena, S.B.; Monteiro, C.; de Penhoat, C.H.; Pérez, S. The diluted aqueous solvation of carbohydrates as inferred from molecular dynamics simulations and NMR spectroscopy. Biophys. Chem. 2001, 93, 103–127. [Google Scholar] [CrossRef]
| Samples | TPC (g/100 g) | PAC (mg/100 g) |
|---|---|---|
| After preparation | ||
| CFB_sucrose | 4.249 ± 0.015 c | 299.93 ± 8.72 c |
| CFB_maltose | 4.495 ± 0.029 b | 473.05 ± 8.72 a |
| CFB_trehalose | 4.977 ± 0.006 a | 422.40 ± 6.54 b |
| After storage | ||
| CFB_sucrose | 3.715 ± 0.018 e | 69.511 ± 0.584 e |
| CFB_maltose | 4.152 ± 0.011 d | 70.164 ± 0.693 e |
| CFB_trehalose | 4.758 ± 0.019 d | 80.221 ± 0.507 d |
| Samples | DPPH (nmoL TE/100 g) | ABTS (µmoL TE/100 g) | FRAP (nmoL TE/100 g) | CUPRAC (nmoL TE/100 g) |
|---|---|---|---|---|
| After preparation | ||||
| CFB_sucrose | 164.30 ± 0.37 c | 2.786 ± 0.029 a | 224.49 ± 0.68 a | 181.34 ± 1.88 a |
| CFB_maltose | 170.95 ± 0.21 a | 2.564 ± 0.018 b | 223.94 ± 0.78 a | 169.58 ± 1.42 c |
| CFB_trehalose | 168.22 ± 0.13 b | 2.597 ± 0.054 b | 219.69 ± 0.50 b | 173.01 ± 1.24 b |
| After storage | ||||
| CFB_sucrose | 139.32 ± 0.61 e | 1.114 ± 0.037 c | 194.19 ± 0.86 c | 110.43 ± 0.91 f |
| CFB_maltose | 138.83 ± 0.54 e | 1.118 ± 0.043 c | 180.48 ± 0.49 d | 120.11 ± 0.83 e |
| CFB_trehalose | 153.30 ± 0.47 d | 0.828 ± 0.027 d | 178.07 ± 0.25 e | 127.52 ± 0.77 c |
| Samples | L* | a* | b* | ΔE | ΔEs | °h | C* |
|---|---|---|---|---|---|---|---|
| After preparation | |||||||
| CFB_sucrose | 28.64 ± 0.02 a | 9.47 ± 0.05 a | 2.35 ± 0.05 a | 13.95 ± 0.21 d | 9.76 ± 0.06 c | ||
| CFB_maltose | 28.36 ± 0.03 b | 9.94 ± 0.02 b | 2.59 ± 0.02 b | 0.60 | 14.60 ± 0.11 c | 10.27 ± 0.02 b | |
| CFB_trehalose | 28.67 ± 0.01 a | 10.39 ± 0.01 c | 2.72 ± 0.02 c | 0.99 | 14.68 ± 0.11 c | 10.74 ± 0.01 a | |
| After storage | |||||||
| CFB_sucrose | 32.10 ± 0.08 c | 4.60 ± 0.07 d | 3.97 ± 0.06 d | 6.19 | 40.76 ± 0.29 b | 6.07 ± 0.02 e | |
| CFB_maltose | 32.87 ± 0.02 d | 4.17 ± 0.04 e | 4.66 ± 0.06 e | 7.61 | 1.13 | 48.18 ± 0.15 a | 6.25 ± 0.07 d |
| CFB_trehalose | 32.96 ± 0.08 d | 4.46 ± 0.05 d | 3.88 ± 0.06 d | 7.41 | 0.88 | 40.97 ± 0.13 b | 5.91 ± 0.07 f |
| Samples | After Preparation | After Storage |
|---|---|---|
| CFB_sucrose | 0.94 | 0.95 |
| CFB_maltose | 0.95 | 0.96 |
| CFB_trehalose | 0.96 | 0.97 |
| Compounds | BJ * | RT 1 | RI 2 | MW 3 | log P 4 | Vp 5 | OD 6 |
|---|---|---|---|---|---|---|---|
| 2-ethylhexanol | + | 19.956 | 1030 | 139 | 3.10 | 0.207 | citrus |
| Benzyl alcohol | − | 20.111 | 1037 | 192.3 | 3.2 | 0.008 | fruity |
| 1-octanol | + | 22.48 | 1071 | 130.2 | 3.00 | 0.079 | green |
| Phenethyl alcohol | + | 24.6 | 1103 | 122.2 | 1.40 | 0.087 | floral |
| Perillyl alcohol | + | 33.93 | 1290 | 152.2 | 2.10 | 0.006 | woody |
| Hexanoic acid | − | 18.535 | 1005 | 116.1 | 1.90 | 0.158 | fatty |
| Nonanoic acid | + | 33.490 | 1281 | 158.2 | 3.50 | 0.009 | fatty |
| Hexanal | − | 5.132 | 800 | 100.2 | 1.80 | 10.888 | green |
| Heptanal | + | 10.761 | 897 | 114.2 | 2.30 | 3.854 | green |
| 2-heptenal | − | 14.953 | 956 | 112.1 | 2.10 | 1.823 | green |
| 1-octen-3-one | − | 16.464 | 982 | 126.2 | 2.40 | 1.063 | fruity |
| 6-methyl-5-hepten-2-one | − | 17.106 | 987 | 126.2 | 1.90 | 1.277 | citrus |
| Octanal | − | 18.080 | 998 | 128.2 | 2.70 | 2.068 | green |
| 2-octenal | + | 21.492 | 1054 | 126.2 | 2.60 | 0.552 | fatty |
| 2-nonenal | − | 27.113 | 1155 | 140.2 | 3.10 | 0.256 | green |
| Decanal | − | 29.502 | 1200 | 156.3 | 3.80 | 0.207 | floral |
| 2,4-nonadienal | + | 29.859 | 1205 | 138.2 | 2.70 | 0.102 | fatty |
| 2-decenal | + | 32.231 | 1255 | 154.3 | 3.70 | 0.067 | fatty |
| Geranylacetone | + | 39.598 | 1448 | 194.3 | 3.834 | 0.016 | floral |
| Lily aldehyde | + | 41.150 | 1519 | 204.3 | 4.216 | 0.005 | floral |
| D-limonene | + | 19.413 | 1018 | 136.2 | 4.57 | 0.198 | citrus |
| Guaiacol | + | 23.166 | 1080 | 124.1 | 1.32 | 0.179 | green |
| Linalool | + | 23.962 | 1096 | 154.3 | 2.970 | 0.016 | citrus |
| Trans-verbenol | + | 25.7163 | 1129 | 152.2 | 1.60 | 0.033 | herbal |
| Nerol | + | 30.631 | 1222 | 154.3 | 3.47 | 0.013 | citrus |
| Phellandral | − | 32.613 | 1264 | 152.2 | 2.70 | 0.098 | floral |
| α-ionone | + | 38.949 | 1420 | 192.3 | 3.995 | 0.014 | berry |
| γ-ionone | + | 40.183 | 1473 | 192.3 | 3.2 | 0.008 | berry |
| β-ionone | + | 40.346 | 1480 | 192.3 | 3.995 | 0.017 | berry |
| Compounds | After Preparation | After Storage | ||||
|---|---|---|---|---|---|---|
| CFB_S | CFB_M | CFB_T | CFB_S | CFB_M | CFB_T | |
| Alcohols | 57.25 ± 0.66 | 44.41 ± 0.69 | 45.30 ± 0.67 | 53.51 ± 0.41 | 49.88 ± 0.39 | 49.31 ± 0.37 |
| 2-ethylhexanol | 14.36 ± 0.20 d | 9.65 ± 0.31 e | 10.45 ± 0.22 e | 19.38 ± 0.15 a | 17.36 ± 0.02 b | 16.83 ± 0.19 c |
| Benzyl alcohol | 17.72 ± 0.14 a | 11.76 ± 0.04 c | 13.47 ± 0.20 b | 7.95 ± 0.06 d | 6.54 ± 0.08 e | 8.16 ± 0.06 d |
| 1-octanol | 15.64 ± 0.03 c | 12.58 ± 0.21 d | 12.21 ± 0.08 d | 17.98 ± 0.06 b | 18.25 ± 0.02 a | 17.59 ± 0.05 b |
| Phenethyl alcohol | 5.16 ± 0.14 b | 6.42 ± 0.07 a | 4.95 ± 0.08 b | 4.88 ± 0.13 b | 4.98 ± 0.20 b | 4.10 ± 0.01 c |
| Perillyl alcohol | 4.38 ± 0.15 c | 4.00 ± 0.08 b | 4.23 ± 0.09 a, b | 3.32 ± 0.01 c | 2.76 ± 0.07 d | 2.64 ± 0.05 d |
| Acids | 23.99 ± 0.24 | 21.65± 0.08 | 24.10± 0.14 | 20.88± 0.05 | 22.22± 0.27 | 25.57± 0.29 |
| Hexanoi c acid | 21.01 ± 0.23 c | 18.06 ± 0.05 e | 21.48 ± 0.10 c | 18.49 ± 0.02 d | 22.22 ± 0.27 b | 23.18 ± 0.27 a |
| Nonanoic acid | 2.98 ± 0.02 b | 3.59 ± 0.03 a | 2.62 ± 0.05 c | 2.39 ± 0.03 d | - | 2.39 ± 0.02 d |
| Aldehydes and ketones | 250.87 ± 3.14 | 156.98± 2.17 | 203.62± 3.74 | 214.38± 5.16 | 171.69± 2.54 | 196.67± 4.19 |
| Hexanal | 74.05 ± 0.13 a | 46.23 ± 0.32 d | 54.81 ± 0.75 b | 50.04 ± 1.08 c | 34.41 ± 0.15 e | 34.48 ± 1.61 e |
| Heptanal | 2.75 ± 0.10 b | 2.65 ± 0.01 c | 3.25 ± 0.11 a | - | 1.63 ± 0.01 d | - |
| 2-heptenal | 48.00 ± 0.56 a | 16.68 ± 0.03 d | 32.79 ± 0.97 c | 40.61 ± 1.28 b | 14.07 ± 0.35 e | 33.79 ± 0.36 c |
| 1-octen-3-one | 25.70 ± 0.67 c | 19.13 ± 0.14 d | 19.00 ± 0.31 d | 43.53 ± 1.13 b | 48.77 ± 1.04 a | 45.86 ± 1.24 a,b |
| 6-methyl-5-hepten-2-one | 10.73 ± 0.05 c | 9.52 ± 0.11 d | 9.51 ± 0.07 d | 11.38 ± 0.46 b | 11.30 ± 0.13 b | 15.57 ± 0.21 a |
| Octanal | 24.09 ± 0.11 a | 20.78 ± 0.17 d | 21.97 ± 0.08 c | 23.77 ± 0.95 a,b | 19.80 ± 0.21 e | 22.86 ± 0.15 b |
| 2-octenal | 34.94 ± 1.14 a | 16.44 ± 0.01 d | 35.33 ± 0.78 a | 20.86 ± 0.01 b | 18.28 ± 0.18 c | 18.71 ± 0.19 c |
| 2-nonenal | 5.57 ± 0.05 a | 4.90 ± 0.04 b | 5.08 ± 0.12 b | 4.34 ± 0.05 d | 4.89 ± 0.03 b | 4.55 ± 0.06 c |
| Decanal | 7.24 ± 0.14 b | 7.29 ± 1.13 a,b | 6.44 ± 0.08 c | 7.68 ± 0.05 a | 7.89 ± 0.17 a | 7.69 ± 0.06 a |
| 2,4-nonadienal | 5.17 ± 0.10 a | 3.62 ± 0.10 b | 3.25 ± 0.09 c | 2.76 ± 0.08 d | 2.45 ± 0.04 e | 3.10 ± 0.07c |
| 2-decenal | 4.77 ± 0.03 a | 3.69 ± 0.07 c | 4.71 ± 0.06 a | 4.00 ± 0.05 b | 3.39 ± 0.16 c | 3.50 ± 0.14 c |
| Geranyl acetone | 3.97 ± 0.03 a | 3.82 ± 0.05 b | 3.94 ± 0.16 a | 3.12 ± 0.00 d | 3.62 ± 0.06 b | 3.29 ± 0.03 c |
| Lily aldehyde | 3.88 ± 0.02 a | 2.22 ± 0.00 c | 3.53 ± 0.16 b | 2.29 ± 0.01 c | 1.19 ± 0.00 d | 3.28 ± 0.07 b |
| Terpenes | 281.91 ± 1.53 | 328.39± 1.93 | 358.05± 8.01 | 223.44± 6.67 | 273.50± 4.67 | 271.42± 3.01 |
| D-limonene | 240.29 ± 0.89 c | 293.81 ± 1.47 b | 320.03 ± 7.46 a | 189.56 ± 5.68 d | 241.51 ± 3.95 c | 237.80 ± 2.60 c |
| Guaiacol | 6.97 ± 0.06 a | 5.30 ± 0.10 d | 6.09 ± 0.10 b | 5.22 ± 0.21 c, d | 5.21 ± 0.37 c, d | 5.72 ± 0.19 c |
| Linalool | 16.26 ± 0.07 a | 14.78 ± 0.03 c | 15.63 ± 0.02 b | 13.83 ± 0.24 d | 13.73 ± 0.16 d | 13.47 ± 0.04 e |
| Trans-verbenol | 3.41 ± 0.12 a | 2.58 ± 0.04 c | 2.68 ± 0.03 c | 3.12 ± 0.03 b | 2.69 ± 0.02 c | 2.35 ± 0.01 d |
| Nerol | 3.42 ± 0.18 a | 2.60 ± 0.16 c | 2.89 ± 0.20 b, c | 3.00 ± 0.10 b | 2.30 ± 0.08 d | 2.79 ± 0.01 c |
| Phellandral | 4.99 ± 0.04 a | 4.14 ± 0.03 b | 4.39 ± 0.18 b | 3.48 ± 0.19 c | 3.08 ± 0.03 d | 3.35 ± 0.01 c |
| α-ionone | 1.62 ± 0.03 a | 0.83 ± 0.01 c | 1.68 ± 0.00 a | 1.37 ± 0.02 b | 1.33 ± 0.02 b | 1.38 ± 0.00 b |
| γ-ionone | 2.80 ± 0.13 a | 2.52 ± 0.05 b | 2.56 ± 0.01 b | 2.42 ± 0.19 b,c | 2.41 ± 0.03 c | 2.91 ± 0.14 a |
| β-ionone | 2.15 ± 0.03 a | 1.83 ± 0.02 b | 2.10 ± 0.01 a | 1.43 ± 0.02 d | 1.25 ± 0.00 e | 1.65 ± 0.01 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vukoja, J.; Buljeta, I.; Ivić, I.; Šimunović, J.; Pichler, A.; Kopjar, M. Disaccharide Type Affected Phenolic and Volatile Compounds of Citrus Fiber-Blackberry Cream Fillings. Foods 2021, 10, 243. https://doi.org/10.3390/foods10020243
Vukoja J, Buljeta I, Ivić I, Šimunović J, Pichler A, Kopjar M. Disaccharide Type Affected Phenolic and Volatile Compounds of Citrus Fiber-Blackberry Cream Fillings. Foods. 2021; 10(2):243. https://doi.org/10.3390/foods10020243
Chicago/Turabian StyleVukoja, Josipa, Ivana Buljeta, Ivana Ivić, Josip Šimunović, Anita Pichler, and Mirela Kopjar. 2021. "Disaccharide Type Affected Phenolic and Volatile Compounds of Citrus Fiber-Blackberry Cream Fillings" Foods 10, no. 2: 243. https://doi.org/10.3390/foods10020243
APA StyleVukoja, J., Buljeta, I., Ivić, I., Šimunović, J., Pichler, A., & Kopjar, M. (2021). Disaccharide Type Affected Phenolic and Volatile Compounds of Citrus Fiber-Blackberry Cream Fillings. Foods, 10(2), 243. https://doi.org/10.3390/foods10020243







