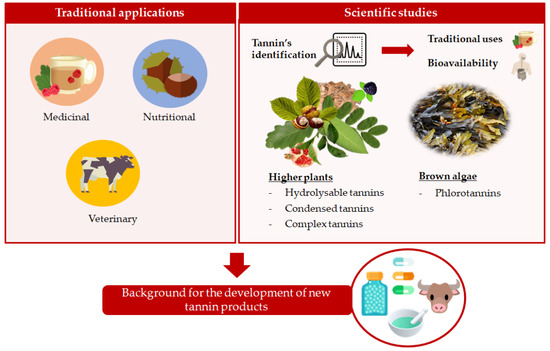
Traditional Applications of Tannin Rich Extracts Supported by Scientific Data: Chemical Composition, Bioavailability and Bioaccessibility
Abstract
:1. Introduction
2. Traditional Applications of Rich-Tannins Plants
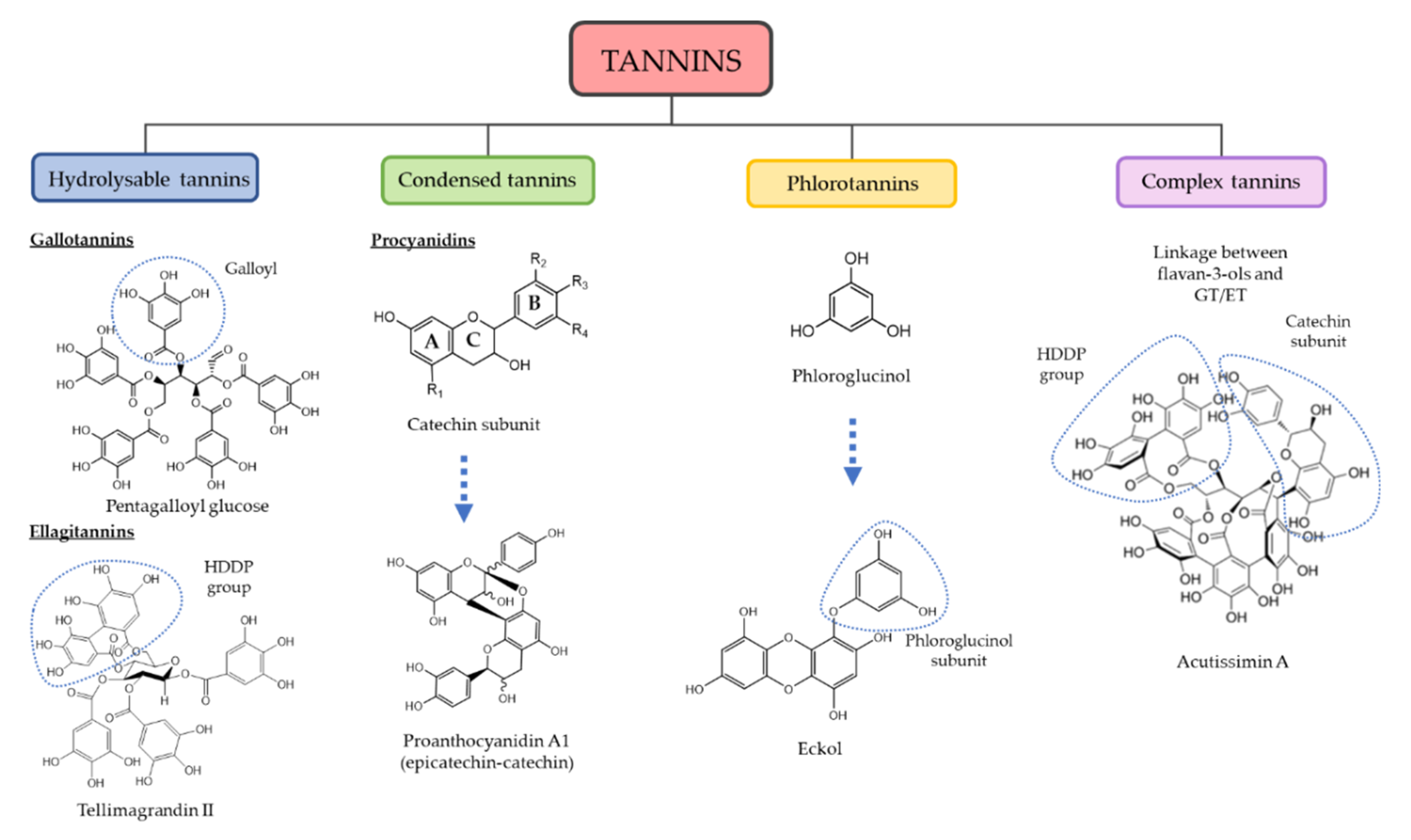
3. Chemical and Quantitative Composition of Rich Tannins Plants Traditionally Used
| Species | Tissue | Type | Method | Concentration (mg/g dw) | Ref. |
|---|---|---|---|---|---|
| Acacia sp. | Leaves | HT, CT | Folin–Ciocâlteu | 84–256 | [144] |
| Bark | CT | HPLC-UV-MS | 108 | [145] | |
| Betula sp. | Leaves | CT | Abs. 550 nm | 73–81 | [115] |
| Castanea sativa | Bark, heartwood, peel | HT | HPLC-DAD-MS | 47.5–167.3 (bark), 62.8 (heartwood), 4.9 (peel) | [22,146,147] |
| Ecklonia cava | Whole alga | PT | HPLC | 6.07 | [148] |
| Hedysarum sp. | Whole plant | CT | - | 68 | [149] |
| Juglans regia | Seeds | CT, ET | - | 35–87(CT), 36–59 (ET) | [5] |
| Lespedeza procumbens | Leaves | CT | Abs. 550 nm | 60–130 | [150] |
| Lotus sp. | Flowers, leaves, stems and roots | CT | Abs. 550 nm | 25–54 | [151] |
| Parietaria sp. | Whole plant | CT | Abs. 550 nm | 10 mg DE/g dw | [47] |
| Pistacia sp. | Leaves | CT | Folin–Ciocâlteu | 21.7–25.1 | [152] |
| Hulls | HT | HPLC-DAD-MS | 20.4–33.1 | [153] | |
| Prunus sp. | Fruits and leaves | CT | Abs. 550 nm | 2.2–37.6 (fruit), 74 (leaves) | [154,155] |
| Punica granatum | Whole fruit | HT | Abs. 550 nm | 62.71–139.63 mg TAE/g dw | [156] |
| Quercus sp. | Whole fruit | HT | Abs. 270–325 nm | 8.18–47.26 | [157,158] |
| Rhus sp. | Leaves | GT | Folin–Ciocâlteu | 13–550 mg GAE/g dw | [159] |
| Plant | HT | LC–MS/MS | 230.7 mg/kg | [160] | |
| Schinopsis sp. | Barks | CT | Folin–Ciocâlteu | 453 mg TAE/g dw | [161] |
| Heartwood | CT | HPLC | 164 | [145] | |
| Smilax sp. | Leaves | CT | Abs. 550 nm | 11.36 mg DE/g dw | [47] |
| Umbilicus sp. | Whole plant | CT | Abs. 550 nm | 5.45 mg DE/g dw | [47] |
| Urtica sp. | Whole plant | CT | Abs. 550 nm | 8 mg DE/g dw | [47] |
| Vitris sp. | Skins and seeds | CT | Abs. 500 nm | 6–165 mg CE/g dw (skins), 3–241 mg CE/g dw (seed) | [162] |
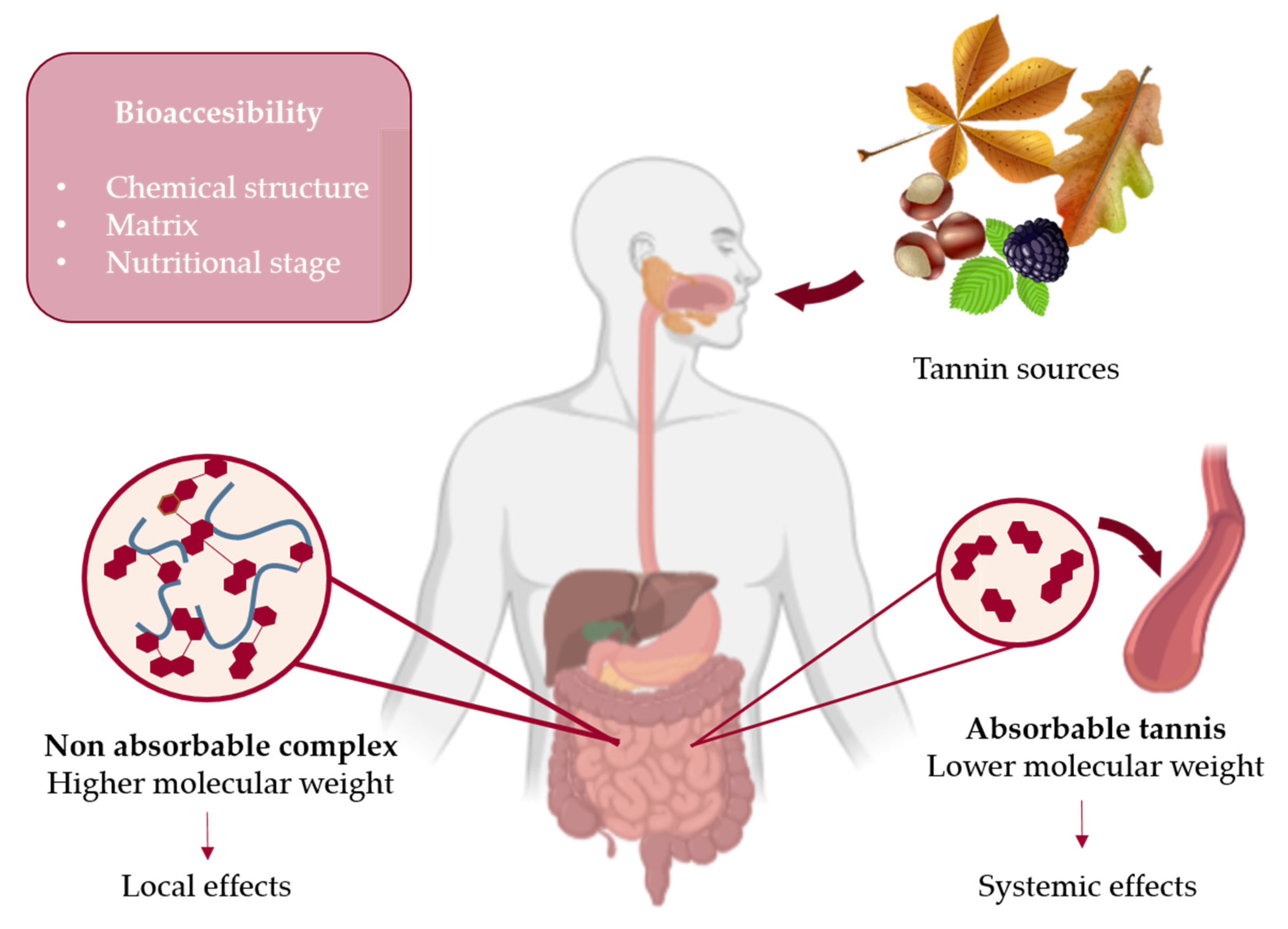
4. Bioavailability and Bioaccessibility of Tannins
5. Traditional and Scientific Knowledge: Building Bridges
6. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CoT | Complex tannin |
| CT | Condensed tannins |
| EA | Ellagic acid |
| ET | Ellagitannin |
| GA | Gallic acid |
| GT | Gallotannin |
| HHDP | Hexahydroxydiphenol |
| HT | Hydrolyzable tannins |
| NHTP | Nonahydroxytriphenoyl |
| PC | Procyanidin |
| PD | Prodelphinidin |
| PG | Phloroglucinol |
| TGG | Trigalloylglucose |
| PGG | Pentagalloylglucose |
| PT | Phlorotannins |
| GABA | Gamma-Aminobutyric acid |
| ICAM | Intercellular adhesion molecule |
| TNF-α | Tumor necrosis factor-α |
| VCAM | Vascular cell adhesion protein |
References
- Falcão, L.; Araújo, M.E.M. Vegetable tannins used in the manufacture of historic leathers. Molecules 2018, 23, 1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Phenolic Compounds: Structure, Classification, and Antioxidant Power; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128147757. [Google Scholar]
- Okuda, T.; Ito, H. Tannins of constant structure in medicinal and food plants-hydrolyzable tannins and polyphenols related to tannins. Molecules 2011, 16, 2191–2217. [Google Scholar] [CrossRef]
- Molino, S.; Casanova, N.A.; Rufián Henares, J.Á.; Fernandez Miyakawa, M.E. Natural Tannin Wood Extracts as a Potential Food Ingredient in the Food Industry. J. Agric. Food Chem. 2020, 68, 2836–2848. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salminen, J.P.; Karonen, M. Chemical ecology of tannins and other phenolics: We need a change in approach. Funct. Ecol. 2011, 25, 325–338. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological application of tannin-based extracts. Molecules 2020, 25, 614. [Google Scholar] [CrossRef] [Green Version]
- Hagerman, A.E. Hydrolyzable Tannin Structural Chemistry. Tann. Handb. 2010, 8, 1–8. [Google Scholar]
- Jiménez, N.; Esteban-Torres, M.; Mancheño, J.M.; De las Rivas, B.; Muñoza, R. Tannin degradation by a novel tannase enzyme present in some Lactobacillus plantarum strains. Appl. Environ. Microbiol. 2014, 80, 2991–2997. [Google Scholar] [CrossRef] [Green Version]
- Khanbabaee, K.; van Ree, T. Tannins: Classification and definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar] [CrossRef]
- Rousserie, P.; Rabot, A.; Geny-Denis, L. From Flavanols Biosynthesis to Wine Tannins: What Place for Grape Seeds? J. Agric. Food Chem. 2019, 67, 1325–1343. [Google Scholar] [CrossRef]
- Díaz, A.M.; Caldas, G.V.; Blair, M.W. Concentrations of condensed tannins and anthocyanins in common bean seed coats. Food Res. Int. 2010, 43, 595–601. [Google Scholar] [CrossRef]
- Sieniawska, E.; Baj, T. Tannins; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128020999. [Google Scholar]
- Macáková, K.; Kolečkář, V.; Cahlíková, L.; Chlebek, J.; Hošt’álková, A.; Kuča, K.; Jun, D.; Opletal, L.; Hoštálková, A.; Kuča, K.; et al. Tannins and their Influence on Health. In Recent Advances in Medicinal Chemistry; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1, pp. 159–208. ISBN 9780128039618. [Google Scholar]
- Cuong, D.X.; Hoan, N.X.; Dong, D.H.; Thuy, L.T.M.; Van Thanh, N.; Ha, H.T.; Tuyen, D.T.T.; Chinh, D.X.; Dang, X.C.; Hoan, N.X.; et al. Tannins: Extraction from Plants. In Tannins—Structural Properties, Biological Properties and Current Knowledge; IntechOpen: London, UK, 2019; pp. 1–20. [Google Scholar]
- Venkatesan, J.; Keekan, K.K.; Anil, S.; Bhatnagar, I.; Kim, S.-K. Phlorotannins. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 3, pp. 515–527. ISBN 9780081005965. [Google Scholar]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine Aroma Compounds in Grapes: A Critical Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, V.; Kaur, J.; Tanwar, B.; Goyal, A.; Sharma, R.; Gat, Y.; Kumar, A. Health effects, sources, utilization and safety of tannins: A critical review. Toxin Rev. 2019, 1–13. [Google Scholar] [CrossRef]
- EC. E.C. Commission Implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334. Off. J. Eur. Communities 2.10. 2012 L 2012, 267, 1–161. [Google Scholar]
- Sieniawska, E. Activities of tannins-From in vitro studies to clinical trials. Nat. Prod. Commun. 2015, 10, 1877–1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, J.; Paul, J. Consumer behavior and purchase intention for organic food: A review and research agenda. J. Retail. Consum. Serv. 2017, 38, 157–165. [Google Scholar] [CrossRef]
- Aires, A.; Carvalho, R.; Saavedra, M.J. Valorization of solid wastes from chestnut industry processing: Extraction and optimization of polyphenols, tannins and ellagitannins and its potential for adhesives, cosmetic and pharmaceutical industry. Waste Manag. 2016, 48, 457–464. [Google Scholar] [CrossRef]
- Grenda, K.; Arnold, J.; Hunkeler, D.; Gamelas, J.A.F.; Rasteiro, M.G. Tannin-based coagulants from laboratory to pilot plant scales for coloured wastewater treatment. BioResources 2018, 13, 2727–2747. [Google Scholar] [CrossRef] [Green Version]
- Laddha, A.P.; Kulkarni, Y.A. Tannins and vascular complications of Diabetes: An update. Phytomedicine 2019, 56, 229–245. [Google Scholar] [CrossRef]
- Redondo, L.M.; Chacana, P.A.; Dominguez, J.E.; Fernandez Miyakawa, M.E. Perspectives in the use of tannins as alternative to antimicrobial growth promoter factors in poultry. Front. Microbiol. 2014, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hassanat, F.; Benchaar, C. Assessment of the effect of condensed (acacia and quebracho) and hydrolysable (chestnut and valonea) tannins on rumen fermentation and methane production in vitro. J. Sci. Food Agric. 2013, 93, 332–339. [Google Scholar] [CrossRef]
- Cassani, L.; Gomez-Zavaglia, A.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Seaweed-based natural ingredients: Stability of phlorotannins during extraction, storage, passage through the gastrointestinal tract and potential incorporation into functional foods. Food Res. Int. 2020, 137, 109676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B.; Kitts, D.D. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Subhan, N.; Burrows, G.E.; Kerr, P.G.; Obied, H.K. Chapter 9—Phytochemistry, Ethnomedicine, and Pharmacology of Acacia. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 57, pp. 247–326. ISBN 1572-5995. [Google Scholar]
- Rather, L.J.; Shahid-ul-Islam; Mohammad, F. Acacia nilotica (L.): A review of its traditional uses, phytochemistry, and pharmacology. Sustain. Chem. Pharm. 2015, 2, 12–30. [Google Scholar] [CrossRef]
- Xiong, J.; Grace, M.H.; Esposito, D.; Wang, F.; Lila, M.A. Phytochemical Characterization and Anti-inflammatory Properties of Acacia mearnsii Leaves. Nat. Prod. Commun. 2016, 11, 1934578X1601100524. [Google Scholar] [CrossRef] [Green Version]
- Safari, V.Z.; Kamau, J.K.; Nthiga, P.M.; Ngugi, M.P.; Orinda, G.; Njagi, E.M. Antipyretic, antiinflammatory and antinociceptive activities of aqueous bark extract of Acacia nilotica (L.) Delile in albino mice. Pain Manag. Med 2016, 2, 2. [Google Scholar]
- Roqaiya, M.; Begum, W.; Jahufer, R. Acacia arabica (Babool)-A Review on Ethnobotanical and Unani Traditional Uses as well as Phytochemical and Pharmacological Properties. Int. J. Pharm. Phytopharm. Res. 2015, 4, 315–321. [Google Scholar]
- Jaouadi, W.; Mechergui, K.; Ammari, Y.; Hamrouni, L.; Hanana, M.; Khouja, M.L. Étude ethnobotanique et ethnopharmacologique d’Acacia tortilis (Forssk) Hayne subsp. raddiana (Savi) de la steppe arborée du Nord de l’Afrique. Phytothérapie 2016, 14, 285–292. [Google Scholar] [CrossRef]
- Rastogi, S.; Pandey, M.M.; Kumar Singh Rawat, A. Medicinal plants of the genus Betula--traditional uses and a phytochemical-pharmacological review. J. Ethnopharmacol. 2015, 159, 62–83. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Zheng, L.; Huang, X.; Wang, Y.; Chen, C.-H.; Cheng, Y.-Y.; Morris-Natschke, S.L.; Lee, K.-H. Novel Betulinic Acid–Nucleoside Hybrids with Potent Anti-HIV Activity. ACS Med. Chem. Lett. 2020. [Google Scholar] [CrossRef]
- Sargin, S.A.; Selvi, S.; López, V. Ethnomedicinal plants of Sarigöl district (Manisa), Turkey. J. Ethnopharmacol. 2015, 171, 64–84. [Google Scholar] [CrossRef] [PubMed]
- Quave, C.L.; Plano, L.R.W.; Pantuso, T.; Bennett, B.C. Effects of extracts from Italian medicinal plants on planktonic growth, biofilm formation and adherence of methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 2008, 118, 418–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regueiro, J.; Sánchez-González, C.; Vallverdú-Queralt, A.; Simal-Gándara, J.; Lamuela-Raventós, R.; Izquierdo-Pulido, M. Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap–Orbitrap mass spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Papoutsi, Z.; Kassi, E.; Chinou, I.; Halabalaki, M.; Skaltsounis, L.A.; Moutsatsou, P. Walnut extract (Juglans regia L.) and its component ellagic acid exhibit anti-inflammatory activity in human aorta endothelial cells and osteoblastic activity in the cell line KS483. Br. J. Nutr. 2008, 99, 715–722. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Qi, G.; Li, D.; Meng, H.; Zhu, Z.; Zhao, Y.; Qi, Y.; Zhang, X. Walnut (Juglans regia L.) Kernel Extracts Protect Against Isoproterenol-Induced Myocardial Infarction in Rats. Rejuvenation Res. 2018, 22, 306–312. [Google Scholar] [CrossRef]
- Guarrera, P.M.; Forti, G.; Marignoli, S. Ethnobotanical and ethnomedicinal uses of plants in the district of Acquapendente (Latium, Central Italy). J. Ethnopharmacol. 2005, 96, 429–444. [Google Scholar] [CrossRef]
- Raitanen, J.-E.; Järvenpää, E.; Korpinen, R.; Mäkinen, S.; Hellström, J.; Kilpeläinen, P.; Liimatainen, J.; Ora, A.; Tupasela, T.; Jyske, T. Tannins of Conifer Bark as Nordic Piquancy—Sustainable Preservative and Aroma? Molecules 2020, 25, 567. [Google Scholar] [CrossRef] [Green Version]
- Landau, S.; Azaizeh, H.; Muklada, H.; Glasser, T.; Ungar, E.D.; Baram, H.; Abbas, N.; Markovics, A. Anthelmintic activity of Pistacia lentiscus foliage in two Middle Eastern breeds of goats differing in their propensity to consume tannin-rich browse. Vet. Parasitol. 2010, 173, 280–286. [Google Scholar] [CrossRef]
- Markovics, A.; Cohen, I.; Muklada, H.; Glasser, T.A.; Dvash, L.; Ungar, E.D.; Azaizeh, H.; Landau, S.Y. Consumption of Pistacia lentiscus foliage alleviates coccidiosis in young goats. Vet. Parasitol. 2012, 186, 165–169. [Google Scholar] [CrossRef]
- Bullitta, S.; Piluzza, G.; Viegi, L. Plant resources used for traditional ethnoveterinary phytotherapy in Sardinia (Italy). Genet. Resour. Crop Evol. 2007, 54, 1447–1464. [Google Scholar] [CrossRef]
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Wu, L.-F.; Guo, H.-L.; Chen, W.-J.; Cui, Y.-P.; Qi, Q.; Li, S.; Liang, W.-Y.; Yang, G.-H.; Shao, Y.-Y.; et al. The Genus Phyllanthus: An Ethnopharmacological, Phytochemical, and Pharmacological Review. Evid.-Based Complement. Altern. Med. 2016, 2016, 7584952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.Y.S.; Khoo, W.K.S.; Adnan, M.A.; Mahalingam, T.P.; Fernandez, A.R.; Jeevaratnam, K. The pharmacological potential of Phyllanthus niruri. J. Pharm. Pharmacol. 2016, 68, 953–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muccilli, V.; Cardullo, N.; Spatafora, C.; Cunsolo, V.; Tringali, C. α-Glucosidase inhibition and antioxidant activity of an oenological commercial tannin. Extraction, fractionation and analysis by HPLC/ESI-MS/MS and 1H NMR. Food Chem. 2017, 215, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Horvathova, M.; Orszaghova, Z.; Laubertova, L.; Vavakova, M.; Sabaka, P.; Rohdewald, P.; Durackova, Z.; Muchova, J. Effect of the French oak wood extract robuvit on markers of oxidative stress and activity of antioxidant enzymes in healthy volunteers: A pilot study. Oxidative Med. Cell. Longev. 2014, 2014, 639868. [Google Scholar] [CrossRef] [Green Version]
- Natella, F.; Leoni, G.; Maldini, M.; Natarelli, L.; Comitato, R.; Schonlau, F.; Virgili, F.; Canali, R. Absorption, metabolism, and effects at transcriptome level of a standardized french oak wood extract, Robuvit, in healthy volunteers: Pilot study. J. Agric. Food Chem. 2014, 62, 443–453. [Google Scholar] [CrossRef]
- Isik, S.; Tayman, C.; Cakir, U.; Koyuncu, I.; Taskin Turkmenoglu, T.; Cakir, E. Sumac (Rhus coriaria) for the prevention and treatment of necrotizing enterocolitis. J. Food Biochem. 2019, 43, e13068. [Google Scholar] [CrossRef]
- Khalilpour, S.; Sangiovanni, E.; Piazza, S.; Fumagalli, M.; Beretta, G.; Dell’Agli, M. In vitro evidences of the traditional use of Rhus coriaria L. fruits against skin inflammatory conditions. J. Ethnopharmacol. 2019, 238, 111829. [Google Scholar] [CrossRef]
- Saraiva, A.M.; Saraiva, C.L.; Cordeiro, R.P.; Soares, R.R.; Xavier, H.S.; Caetano, N. Atividade antimicrobiana e sinérgica das frações das folhas de Schinopsis brasiliensis Engl. frente a clones multirresistentes de Staphylococcus aureus. Rev. Bras. Plantas Med. 2013, 15, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Del Carrizo, E.V.; Palacio, M.O.; Roic, L.D. Uso medicinal de algunas especies nativas en Santiago del Estero (República Argentina). Dominguezia 2005, 21, 25–32. [Google Scholar]
- Barboza, G.E.; Cantero, J.J.; Núñez, C.; Ariza Espinar, L.; del Pacciaroni, A.V. Medicinal plants: A general review and a phytochemical and ethnopharmacological screening of the native Argentine Flora. Kurtiziana 2009, 34, 7–365. [Google Scholar]
- Venter, P.B.; Sisa, M.; Van Der Merwe, M.J.; Bonnet, S.L.; Van Der Westhuizen, J.H. Analysis of commercial proanthocyanidins. Part 1: The chemical composition of quebracho (Schinopsis lorentzii and Schinopsis balansae) heartwood extract. Phytochemistry 2012, 73, 95–105. [Google Scholar] [CrossRef]
- Cardullo, N.; Muccilli, V.; Cunsolo, V.; Tringali, C. Mass Spectrometry and 1H-NMR Study of Schinopsis lorentzii (Quebracho) Tannins as a Source of Hypoglycemic and Antioxidant Principles. Molecules 2020, 25, 3257. [Google Scholar] [CrossRef] [PubMed]
- Fruet, A.P.B.; Giotto, F.M.; Fonseca, M.A.; Nörnberg, J.L.; De Mello, A.S. Effects of the Incorporation of Tannin Extract from Quebracho Colorado Wood on Color Parameters, Lipid Oxidation, and Sensory Attributes of Beef Patties. Foods 2020, 9, 667. [Google Scholar] [CrossRef] [PubMed]
- Bonet, M.À.; Vallès, J. Ethnobotany of Montseny biosphere reserve (Catalonia, Iberian Peninsula): Plants used in veterinary medicine. J. Ethnopharmacol. 2007, 110, 130–147. [Google Scholar] [CrossRef] [PubMed]
- Bullitta, S.; Re, G.A.; Manunta, M.D.I.; Piluzza, G. Traditional knowledge about plant, animal, and mineral-based remedies to treat cattle, pigs, horses, and other domestic animals in the Mediterranean island of Sardinia. J. Ethnobiol. Ethnomed. 2018, 14, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhikari, B.M.; Bajracharya, A.; Shrestha, A.K. Comparison of nutritional properties of Stinging nettle (Urtica dioica) flour with wheat and barley flours. Food Sci. Nutr. 2015, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Dar, S.A.; Ganai, F.A.; Yousuf, A.R.; Balkhi, M.-H.; Bhat, T.M.; Sharma, P. Pharmacological and toxicological evaluation of Urtica dioica. Pharm. Biol. 2013, 51, 170–180. [Google Scholar] [CrossRef]
- Jan, K.N.; Zarafshan, K.; Singh, S. Stinging nettle (Urtica dioica L.): A reservoir of nutrition and bioactive components with great functional potential. J. Food Meas. Charact. 2017, 11, 423–433. [Google Scholar] [CrossRef]
- Guarrera, P.M. Traditional phytotherapy in Central Italy (Marche, Abruzzo, and Latium). Fitoterapia 2005, 76, 1–25. [Google Scholar] [CrossRef]
- Uncini Manganelli, R.E.; Camangi, F.; Tomei, P.E. Curing animals with plants: Traditional usage in Tuscany (Italy). J. Ethnopharmacol. 2001, 78, 171–191. [Google Scholar] [CrossRef]
- Viegi, L.; Pieroni, A.; Guarrera, P.M.; Vangelisti, R. A review of plants used in folk veterinary medicine in Italy as basis for a databank. J. Ethnopharmacol. 2003, 89, 221–244. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, C.; García-Villanova, B.; Guerra-Hernández, E.; Verardo, V. Grape seeds proanthocyanidins: An overview of in vivo bioactivity in animal models. Nutrients 2019, 11, 2435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Kumar, R.; Bhattacharyya, P.; Bishayee, A.; Pandey, A.K. Terminalia bellirica (Gaertn.) roxb. (Bahera) in health and disease: A systematic and comprehensive review. Phytomedicine 2020, 77, 153278. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Heinrich, M.; Myers, S.; Dworjanyn, S.A. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in Traditional Chinese Medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2012, 142, 591–619. [Google Scholar] [CrossRef]
- Casas, M.P.; Rodríguez-Hermida, V.; Pérez-Larrán, P.; Conde, E.; Liveri, M.T.; Ribeiro, D.; Fernandes, E.; Domínguez, H. In vitro bioactive properties of phlorotannins recovered from hydrothermal treatment of Sargassum muticum. Sep. Purif. Technol. 2016, 167, 117–126. [Google Scholar] [CrossRef]
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.; Gao, X. Extraction and Identification of Phlorotannins from the Brown Alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2018/460 of 20 March 2018 authorising the placing on the market of Ecklonia cava phlorotannins as a novel food under Regulation (EU) 2015/2283 of the European Parliament and of the Council and amending Commiss. Off. J. Eur. Union 2018, 2016, 48–119. [Google Scholar]
- Park, J.; Kim, J.H.; Kwon, J.M.; Kwon, H.; Jeong, H.J.; Kim, Y.M.; Kim, D.; Lee, W.S.; Ryu, Y.B. Dieckol, a SARS-CoV 3CLpro inhibitor, isolated from the edible brown algae Ecklonia cava. Bioorg. Med. Chem. 2013, 21, 3730–3737. [Google Scholar] [CrossRef]
- Singh, K.N.; Lal, B. Notes on traditional uses of khair (Acacia catechu Willd.) by inhabitants of shivalik range in Western Himalaya. Ethnobot. Leafl. 2006, 2006, 12. [Google Scholar]
- Ghalandari, S.; Kariman, N.; Sheikhan, Z.; Mojab, F.; Mirzaei, M.; Shahrahmani, H. Effect of Hydroalcoholic Extract of Capsella bursa pastoris on Early Postpartum Hemorrhage: A Clinical Trial Study. J. Altern. Complement. Med. 2017, 23, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Conedera, M.; Krebs, P.; Tinner, W.; Pradella, M.; Torriani, D. The cultivation of Castanea sativa (Mill.) in Europe, from its origin to its diffusion on a continental scale. Veg. Hist. Archaeobot. 2004, 13, 161–179. [Google Scholar] [CrossRef] [Green Version]
- Aguerre, M.J.; Duval, B.; Powell, J.M.; Vadas, P.A.; Wattiaux, M.A. Effects of feeding a quebracho–chestnut tannin extract on lactating cow performance and nitrogen utilization efficiency. J. Dairy Sci. 2020, 103, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Meagher, L.P.; Lane, G.; Sivakumaran, S.; Tavendale, M.H.; Fraser, K. Characterization of condensed tannins from Lotus species by thiolytic degradation and electrospray mass spectrometry. Anim. Feed Sci. Technol. 2004, 117, 151–163. [Google Scholar] [CrossRef]
- Christensen, R.G.; Eun, J.S.; Yang, S.Y.; Min, B.R.; MacAdam, J.W. In vitro effects of birdsfoot trefoil (Lotus corniculatus L.) pasture on ruminal fermentation, microbial population, and methane production. Prof. Anim. Sci. 2017, 33, 451–460. [Google Scholar] [CrossRef]
- Lange, K.C.; Olcott, D.D.; Miller, J.E.; Mosjidis, J.A.; Terrill, T.H.; Burke, J.M.; Kearney, M.T. Effect of sericea lespedeza (Lespedeza cuneata) fed as hay, on natural and experimental Haemonchus contortus infections in lambs. Vet. Parasitol. 2006, 141, 273–278. [Google Scholar] [CrossRef]
- Katiki, L.M.; Ferreira, J.F.S.; Gonzalez, J.M.; Zajac, A.M.; Lindsay, D.S.; Chagas, A.C.S.; Amarante, A.F.T. Anthelmintic effect of plant extracts containing condensed and hydrolyzable tannins on Caenorhabditis elegans, and their antioxidant capacity. Vet. Parasitol. 2013, 192, 218–227. [Google Scholar] [CrossRef]
- Jyske, T.; Kuroda, K.; Keriö, S.; Pranovich, A.; Linnakoski, R.; Hayashi, N.; Aoki, D.; Fukushima, K. Localization of (+)-Catechin in Picea abies Phloem: Responses to Wounding and Fungal Inoculation. Molecules 2020, 25, 2952. [Google Scholar] [CrossRef]
- Disler, M.; Ivemeyer, S.; Hamburger, M.; Vogl, C.R.; Tesic, A.; Klarer, F.; Meier, B.; Walkenhorst, M. Ethnoveterinary herbal remedies used by farmers in four north-eastern Swiss cantons (St. Gallen, Thurgau, Appenzell Innerrhoden and Appenzell Ausserrhoden). J. Ethnobiol. Ethnomed. 2014, 10, 32. [Google Scholar] [CrossRef] [Green Version]
- Agency, E.M.; Medicines, V. EMA 2004 Committee for Medicinal Products for Veterinary Use; EMA: Amsterdam, The Netherlands, 2005; pp. 1–3. [Google Scholar]
- Landau, S.Y.; Muklada, H.; Abu-Rabia, A.; Kaadan, S.; Azaizeh, H. Traditional Arab ethno-veterinary practices in small ruminant breeding in Israel. Small Rumin. Res. 2014, 119, 161–171. [Google Scholar] [CrossRef]
- Piluzza, G.; Virdis, S.; Serralutzu, F.; Bullitta, S. Uses of plants, animal and mineral substances in Mediterranean ethno-veterinary practices for the care of small ruminants. J. Ethnopharmacol. 2015, 168, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Lansky, E.P.; Newman, R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007, 109, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Lansky, E.; Shubert, S.; Neeman, I. Pharmacological and therapeutic properties of pomegranate. In Production, Processing and Marketing of Pomegranate in the Mediterranean Region: Advances in Research and Technology; Options Méditerranéennes: Série A. Séminaires Méditerranéens; CIHEAM: Zaragoza, Spain, 2000; pp. 231–235. [Google Scholar]
- Ballabh, B.; Chaurasia, O.P. Traditional medicinal plants of cold desert Ladakh—Used in treatment of cold, cough and fever. J. Ethnopharmacol. 2007, 112, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Al Muqarrabun, L.M.R.; Ahmat, N.; Aris, S.R.S. A review of the medicinal uses, phytochemistry and pharmacology of the genus Sapium. J. Ethnopharmacol. 2014, 155, 9–20. [Google Scholar] [CrossRef]
- Zhang, X.-R.; Kaunda, J.S.; Zhu, H.-T.; Wang, D.; Yang, C.-R.; Zhang, Y.-J. The Genus Terminalia (Combretaceae): An Ethnopharmacological, Phytochemical and Pharmacological Review. Nat. Prod. Bioprospect. 2019, 9, 357–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahmy, N.M.; Al-Sayed, E.; Singab, A.N. Genus Terminalia: A phytochemical and biological review. Med. Aromat Plants 2015, 4, 1–22. [Google Scholar]
- Bag, A.; Bhattacharyya, S.K.; Chattopadhyay, R.R. The development of Terminalia chebula Retz. (Combretaceae) in clinical research. Asian Pac. J. Trop. Biomed. 2013, 3, 244–252. [Google Scholar] [CrossRef] [Green Version]
- Ćurko, N.; Tomašević, M.; Bubalo, M.C.; Gracin, L.; Redovniković, I.R.; Ganić, K.K. Extraction of proanthocyanidins and anthocyanins from grape skin by using ionic liquids. Food Technol. Biotechnol. 2017, 55, 429–437. [Google Scholar] [CrossRef]
- Caimari, A.; del Bas, J.M.; Crescenti, A.; Arola, L. Low doses of grape seed procyanidins reduce adiposity and improve the plasma lipid profile in hamsters. Int. J. Obes. 2013, 37, 576–583. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Yin, M.; Liu, Y.; Han, X.; Guo, J.; Ren, C.; Wang, W.; Huang, W.; Zhan, J.; You, Y. Grape seed flour intake decreases adiposity gain in high-fat-diet induced obese mice by activating thermogenesis. J. Funct. Foods 2019, 62, 103509. [Google Scholar] [CrossRef]
- Griffin, L.E.; Witrick, K.A.; Klotz, C.; Dorenkott, M.R.; Goodrich, K.M.; Fundaro, G.; McMillan, R.P.; Hulver, M.W.; Ponder, M.A.; Neilson, A.P. Alterations to metabolically active bacteria in the mucosa of the small intestine predict anti-obesity and anti-diabetic activities of grape seed extract in mice. Food Funct. 2017, 8, 3510–3522. [Google Scholar] [CrossRef] [PubMed]
- Cardullo, N.; Muccilli, V.; Saletti, R.; Giovando, S.; Tringali, C. A mass spectrometry and 1H NMR study of hypoglycemic and antioxidant principles from a Castanea sativa tannin employed in oenology. Food Chem. 2018, 268, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, G.P.; Subramanian, S.P. Biochemical studies on the effect of Terminalia chebula on the levels of glycoproteins in streptozotocin-induced experimental diabetes in rats. J. Appl. Biomed. 2008, 6, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Turck, D.; Bresson, J.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Ecklonia cava phlorotannins as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Z.J.; Ryu, B.M.; Lee, S.H.; Kim, M.M.; Kim, S.K. Chemical components and its antioxidant properties in vitro: An edible marine brown alga, Ecklonia cava. Bioorg. Med. Chem. 2009, 17, 1963–1973. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, D.Y.; Jung, W.K.; Kim, J.H.; Choi, I.; Park, S.G.; Seo, S.K.; Lee, S.W.; Lee, C.M.; Yea, S.S.; et al. Effects of Ecklonia cava ethanolic extracts on airway hyperresponsiveness and inflammation in a murine asthma model: Role of suppressor of cytokine signaling. Biomed. Pharmacother. 2008, 62, 289–296. [Google Scholar] [CrossRef]
- Kang, M.C.; Ahn, G.; Yang, X.; Kim, K.N.; Kang, S.M.; Lee, S.H.; Ko, S.C.; Ko, J.Y.; Kim, D.; Kim, Y.T.; et al. Hepatoprotective effects of dieckol-rich phlorotannins from Ecklonia cava, a brown seaweed, against ethanol induced liver damage in BALB/c mice. Food Chem. Toxicol. 2012, 50, 1986–1991. [Google Scholar] [CrossRef]
- Roy, M.C.; Anguenot, R.; Fillion, C.; Beaulieu, M.; Bérubé, J.; Richard, D. Effect of a commercially-available algal phlorotannins extract on digestive enzymes and carbohydrate absorption in vivo. Food Res. Int. 2011, 44, 3026–3029. [Google Scholar] [CrossRef]
- Wang, T.; Li, Q.; Bi, K. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Hooper, B.; Frazier, R. Polyphenols in the diet: Friend or foe? Nutr. Bull. 2012, 37, 297–308. [Google Scholar] [CrossRef]
- Duval, A.; Avérous, L. Characterization and Physicochemical Properties of Condensed Tannins from Acacia catechu. J. Agric. Food Chem. 2016, 64, 1751–1760. [Google Scholar] [CrossRef]
- Ogawa, S.; Yazaki, Y. Tannins from Acacia mearnsii De Wild. Bark: Tannin determination and biological activities. Molecules 2018, 23, 837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crestini, C.; Lange, H.; Bianchetti, G. Detailed Chemical Composition of Condensed Tannins via Quantitative 31P NMR and HSQC Analyses: Acacia catechu, Schinopsis balansae, and Acacia mearnsii. J. Nat. Prod. 2016, 79, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Elgailani, I.E.H.; Ishak, C.Y. Determination of Tannins of Three Common Acacia Species of Sudan. Adv. Chem. 2014, 2014, 192708. [Google Scholar] [CrossRef] [Green Version]
- Julkunen-Tiitto, R.; Sorsa, S. Testing the effects of drying methods on willow flavonoids, tannins, and salicylates. J. Chem. Ecol. 2001, 27, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, K.; Julkunen-Tiitto, R.; Rousi, M.; Freiwald, V.; Oksanen, E. Ozone exposure over two growing seasons alters root-to-shoot ratio and chemical composition of birch (Betula pendula Roth). Glob. Chang. Biol. 2003, 9, 1363–1377. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Bioactive Compounds of Chestnut (Castanea sativa Mill.) BT. In Bioactive Compounds in Underutilized Fruits and Nuts; Murthy, H.N., Bapat, V.A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 303–313. ISBN 978-3-030-30182-8. [Google Scholar]
- Chiarini, A.; Micucci, M.; Malaguti, M.; Budriesi, R.; Ioan, P.; Lenzi, M.; Fimognari, C.; Gallina Toschi, T.; Comandini, P.; Hrelia, S. Sweet Chestnut (Castanea sativa Mill.) Bark Extract: Cardiovascular Activity and Myocyte Protection against Oxidative Damage. Oxidative Med. Cell. Longev. 2013, 2013, 471790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, T.; Celano, R.; Pane, C.; Piccinelli, A.L.; Sansone, F.; Picerno, P.; Zaccardelli, M.; Aquino, R.P.; Mencherini, T. Chestnut (Castanea sativa Miller.) Burs Extracts and Functional Compounds: UHPLC-UV-HRMS Profiling, Antioxidant Activity, and Inhibitory Effects on Phytopathogenic Fungi. Molecules 2019, 24, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Ko, J.Y.; Oh, J.Y.; Kim, C.Y.; Lee, H.J.; Kim, J.; Jeon, Y.J. Preparative isolation and purification of phlorotannins from Ecklonia cava using centrifugal partition chromatography by one-step. Food Chem. 2014, 158, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Hedqvist, H.; Mueller-Harvey, I.; Reed, J.D.; Krueger, C.G.; Murphy, M. Characterisation of tannins and in vitro protein digestibility of several Lotus corniculatus varieties. Anim. Feed Sci. Technol. 2000, 87, 41–56. [Google Scholar] [CrossRef]
- Behrens, A.; Maie, N.; Knicker, H.; Kögel-Knabner, I. MALDI-TOF mass spectrometry and PSD fragmentation as means for the analysis of condensed tannins in plant leaves and needles. Phytochemistry 2003, 62, 1159–1170. [Google Scholar] [CrossRef]
- Bianchi, S.; Gloess, A.N.; Kroslakova, I.; Mayer, I.; Pichelin, F. Analysis of the structure of condensed tannins in water extracts from bark tissues of Norway spruce (Picea abies [Karst.]) and Silver fir (Abies alba [Mill.]) using MALDI-TOF mass spectrometry. Ind. Crops Prod. 2014, 61, 430–437. [Google Scholar] [CrossRef]
- Sulaiman, T.; Kakakhan, H.; Sijam, K. Antifungal effects of Rhus coriaria L. fruit extracts against tomato anthracnose caused by Colletotrichum acutatum. Ind. Crops Prod. 2018, 113, 391–397. [Google Scholar] [CrossRef]
- Monforte, M.T.; Smeriglio, A.; Germanò, M.P.; Pergolizzi, S.; Circosta, C.; Galati, E.M. Evaluation of antioxidant, antiinflammatory, and gastroprotective properties of Rubus fruticosus L. fruit juice. Phyther. Res. 2018, 32, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.T.; Kim, H.; Tran, V.K.; Vu, H.D.; Hoang, T.X.; Han, J.W.; Choi, Y.H.; Jang, K.S.; Choi, G.J.; Kim, J. Antibacterial activity of tannins isolated from Sapium baccatum extract and use for control of tomato bacterial wilt. PLoS ONE 2017, 12, e0181499. [Google Scholar] [CrossRef]
- Isaza Martínez, J.H.; Torres Castañeda, H.G. Preparation and chromatographic analysis of phlorotannins. J. Chromatogr. Sci. 2013, 51, 825–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivas, N.; Nonier, M.F.; De Gaulejac, N.V.; Absalon, C.; Bertrand, A.; Mirabel, M. Differentiation of proanthocyanidin tannins from seeds, skins and stems of grapes (Vitis vinifera) and heartwood of Quebracho (Schinopsis balansae) by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and thioacidolysis/liquid c. Anal. Chim. Acta 2004, 513, 247–256. [Google Scholar] [CrossRef]
- Pfundstein, B.; El Desouky, S.K.; Hull, W.E.; Haubner, R.; Erben, G.; Owen, R.W. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry 2010, 71, 1132–1148. [Google Scholar] [CrossRef]
- Kyraleou, M.; Kallithraka, S.; Theodorou, N.; Teissedre, P.-L.; Kotseridis, Y.; Koundouras, S. Changes in Tannin Composition of Syrah Grape Skins and Seeds during Fruit Ripening under Contrasting Water Conditions. Molecules 2017, 22, 1453. [Google Scholar] [CrossRef] [Green Version]
- Ricci, A.; Parpinello, G.P.; Palma, A.S.; Teslić, N.; Brilli, C.; Pizzi, A.; Versari, A. Analytical profiling of food-grade extracts from grape (Vitis vinifera sp.) seeds and skins, green tea (Camellia sinensis) leaves and Limousin oak (Quercus robur) heartwood using MALDI-TOF-MS, ICP-MS and spectrophotometric methods. J. Food Compos. Anal. 2017, 59, 95–104. [Google Scholar] [CrossRef]
- Stark, S.; Julkunen-Tiitto, R.; Holappa, E.; Mikkola, K.; Nikula, A. Concentrations of foliar quercetin in natural populations of white birch (Betula pubescens) increase with latitude. J. Chem. Ecol. 2008, 34, 1382–1391. [Google Scholar] [CrossRef]
- Malayaman, V.; Sisubalan, N.; Senthilkumar, R.P.; Sheik Mohamed, S.; Ranjithkumar, R.; Ghouse Basha, M. Chitosan mediated enhancement of hydrolysable tannin in Phyllanthus debilis Klein ex Willd via plant cell suspension culture. Int. J. Biol. Macromol. 2017, 104, 1656–1663. [Google Scholar] [CrossRef]
- Sprenger, F.; Cass, Q.B. Characterization of four Phyllanthus species using liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2013, 1291, 97–103. [Google Scholar] [CrossRef]
- Cechinel, V.; Moreira, J.; Klein-ju, L.C. Anti-hyperalgesic activity of corilagin, a tannin isolated from Phyllanthus niruri L. (Euphorbiaceae). J. Ethnopharmacol. 2013, 146, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Ram, J.; Tripathi, P.; Sharma, V.; Singh, N.; Kumar, V. Phyllanthus amarus: Ethnomedicinal uses, phytochemistry and pharmacology: A review. J. Ethnopharmacol. 2011, 138, 286–313. [Google Scholar] [CrossRef]
- Fawzi, M.; Yerlikaya, S.; Llorent-martínez, E.J.; Uğurlu, A. Pharmacological and polyphenolic profiles of Phyllanthus phillyreifolius var. commersonii Müll. Arg: An unexplored endemic species from Mauritius. Food Res. Int. 2019, 115, 425–438. [Google Scholar] [CrossRef]
- Rodríguez-pérez, C.; Quirantes-piné, R.; Amessis-ouchemoukh, N.; Madani, K. Journal of Pharmaceutical and Biomedical Analysis A metabolite-profiling approach allows the identification of new compounds from Pistacia lentiscus leaves. J. Pharm. Biomed. Anal. 2013, 77, 167–174. [Google Scholar] [CrossRef]
- Marchelak, A.; Owczarek, A.; Matczak, M.; Pawlak, A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. Bioactivity Potential of Prunus spinosa L. Flower Extracts: Phytochemical Profiling, Cellular Safety, Pro-inflammatory Enzymes Inhibition and Protective Effects Against Oxidative Stress In Vitro. Front. Pharmacol. 2017, 8, 680. [Google Scholar] [CrossRef] [Green Version]
- König, M.; Scholz, E.; Hartmann, R.; Lehmann, W.; Rimpler, H. Ellagitannins and complex tannins from Quercus petraea bark. J. Nat. Prod. 1994, 57, 1411–1415. [Google Scholar] [CrossRef]
- Park, M.; Cho, H.; Jung, H.; Lee, H.; Hwang, K.T. Antioxidant and anti-inflammatory activities of tannin fraction of the extract from black raspberry seeds compared to grape seeds. J. Food Biochem. 2014, 38, 259–270. [Google Scholar] [CrossRef]
- Kowalska, K.; Olejnik, A.; Zielińska-Wasielica, J.; Olkowicz, M. Raspberry (Rubus idaeus L.) fruit extract decreases oxidation markers, improves lipid metabolism and reduces adipose tissue inflammation in hypertrophied 3T3-L1 adipocytes. J. Funct. Foods 2019, 62, 103568. [Google Scholar] [CrossRef]
- Elhouchet, Z.B.; Autour, M.S.; Iyamoto, T.M.; Ubois, M.L.A. Steroidal Saponins from the Roots of Smilax aspera subsp. mauritanica. Chem. Pharm. Bull. 2008, 56, 1324–1327. [Google Scholar] [CrossRef] [Green Version]
- Harumi, J.; Fernandes, Â.; Calhelha, R.C.; José, M.; Dias, F.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Nutritional composition and bioactivity of Umbilicus rupestris (Salisb.) Dandy: An underexploited edible wild plant. Food Chem. 2019, 295, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Rubanza, C.D.K.; Shem, M.N.; Otsyina, R.; Bakengesa, S.S.; Ichinohe, T.; Fujihara, T. Polyphenolics and tannins effect on in vitro digestibility of selected Acacia species leaves. Anim. Feed Sci. Technol. 2005, 119, 129–142. [Google Scholar] [CrossRef]
- Kardel, M.; Taube, F.; Schulz, H.; Schütze, W.; Gierus, M. Different approaches to evaluate tannin content and structure of selected plant extracts—Review and new aspects. J. Appl. Bot. Food Qual. 2013, 86, 154–166. [Google Scholar]
- Comandini, P.; Lerma-García, M.J.; Simó-Alfonso, E.F.; Toschi, T.G. Tannin analysis of chestnut bark samples (Castanea sativa Mill.) by HPLC-DAD–MS. Food Chem. 2014, 157, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Cadahía, E.; Esteruelas, E.; Muñoz, Á.M.; De Simón, B.F.; Hernández, T.; Estrella, I. Phenolic compounds in chestnut (Castanea sativa Mill.) heartwood. Effect of toasting at cooperage. J. Agric. Food Chem. 2010, 58, 9631–9640. [Google Scholar] [CrossRef]
- Chowdhury, M.T.H.; Bangoura, I.; Kang, J.Y.; Park, N.G.; Ahn, D.H.; Hong, Y.K. Distribution of phlorotannins in the brown alga Ecklonia cava and comparison of pretreatments for extraction. Fish. Aquat. Sci. 2011, 14, 198–204. [Google Scholar] [CrossRef] [Green Version]
- Waghorn, G.C.; Tavendale, M.H.; Woodfield, D.R. Methanogenesis from forages fed to sheep. Proc. N. Z. Grassl. Assoc. 2002, 167–171. [Google Scholar] [CrossRef]
- Pawelek, D.L.; Muir, J.P.; Lambert, B.D.; Wittie, R.D. In sacco rumen disappearance of condensed tannins, fiber, and nitrogen from herbaceous native Texas legumes in goats. Anim. Feed Sci. Technol. 2008, 142, 1–16. [Google Scholar] [CrossRef]
- Häring, D.A.; Suter, D.; Amrhein, N.; Lüscher, A. Biomass allocation is an important determinant of the tannin concentration in growing plants. Ann. Bot. 2007, 99, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Decandia, M.; Sitzia, M.; Cabiddu, A.; Kababya, D.; Molle, G. The use of polyethylene glycol to reduce the anti-nutritional effects of tannins in goats fed woody species. Small Rumin. Res. 2000, 38, 157–164. [Google Scholar] [CrossRef]
- Erşan, S.; Güçlü Üstündağ, Ö.; Carle, R.; Schweiggert, R.M. Subcritical water extraction of phenolic and antioxidant constituents from pistachio (Pistacia vera L.) hulls. Food Chem. 2018, 253, 46–54. [Google Scholar] [CrossRef]
- Song, W.; Qin, S.-T.; Fang, F.-X.; Gao, Z.-J.; Liang, D.-D.; Liu, L.-L.; Tian, H.-T.; Yang, H.-B. Isolation and purification of condensed tannin from the leaves and branches of Prunus cerasifera and its structure and bioactivities. Appl. Biochem. Biotechnol. 2018, 185, 464–475. [Google Scholar] [CrossRef]
- Cao, J.; Jiang, Q.; Lin, J.; Li, X.; Sun, C.; Chen, K. Physicochemical characterisation of four cherry species (Prunus spp.) grown in China. Food Chem. 2015, 173, 855–863. [Google Scholar] [CrossRef]
- Elfalleh, W. Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower. J. Med. Plants Res. 2012, 6, 4724–4730. [Google Scholar] [CrossRef]
- Cadahía, E.; Varea, S.; Muñoz, L.; de Simón, B.F.; García-Vallejo, M.C. Evolution of Ellagitannins in Spanish, French, and American Oak Woods during Natural Seasoning and Toasting. J. Agric. Food Chem. 2001, 49, 3677–3684. [Google Scholar] [CrossRef]
- De Simón, B.F.; Cadahía, E.; del Álamo, M.; Nevares, I. Effect of size, seasoning and toasting in the volatile compounds in toasted oak wood and in a red wine treated with them. Anal. Chim. Acta 2010, 660, 211–220. [Google Scholar] [CrossRef]
- Tian, F.; Li, B.; Ji, B.; Yang, J.; Zhang, G.; Chen, Y.; Luo, Y. Antioxidant and antimicrobial activities of consecutive extracts from Galla chinensis:The polarity affects the bioactivities. Food Chem. 2009, 113, 173–179. [Google Scholar] [CrossRef]
- Tohma, H.; Altay, A.; Köksal, E.; Gören, A.C.; Gülçin, İ. Measurement of anticancer, antidiabetic and anticholinergic properties of sumac (Rhus coriaria): Analysis of its phenolic compounds by LC–MS/MS. J. Food Meas. Charact. 2019, 13, 1607–1619. [Google Scholar] [CrossRef]
- Saraiva, A.M.; Castro, R.H.A.; Cordeiro, R.P.; Peixoto Sobrinho, T.J.S.; Castro, V.T.N.A.; Amorim, E.L.C.; Xavier, H.S.; Pisciottano, M.N.C. In vitro evaluation of antioxidant, antimicrobial and toxicity properties of extracts of schinopsis brasiliensis engl. (Anacardiaceae). Afr. J. Pharm. Pharmacol. 2011, 5, 1724–1731. [Google Scholar] [CrossRef] [Green Version]
- Travaglia, F.; Bordiga, M.; Locatelli, M.; Coïsson, J.D.; Arlorio, M. Polymeric Proanthocyanidins in Skins and Seeds of 37 Vitis vinifera L. Cultivars: A Methodological Comparative Study. J. Food Sci. 2011, 76, 742–749. [Google Scholar] [CrossRef]
- Schofield, P.; Mbugua, D.; Pell, A. Analysis of condensed tannins: A review. Anim. Feed Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]
- Giner-Chavez, B.I.; Van Soest, P.J.; Robertson, J.B.; Lascano, C.; Pell, A.N. Comparison of the Precipitation of Alfalfa Leaf Protein and Bovine Serum Albumin by Tannins in the Radial Diffusion Method. J. Sci. Food Agric. 1997, 74, 513–523. [Google Scholar] [CrossRef]
- Fagundes, G.M.; Benetel, G.; Santos, K.C.; Welter, K.C.; Melo, F.A.; Muir, J.P.; Bueno, I.C.S. Tannin-rich plants as natural manipulators of rumen fermentation in the livestock industry. Molecules 2020, 25, 2943. [Google Scholar] [CrossRef]
- McAllister, T.A.; Martinez, T.; Bae, H.D.; Muir, A.D.; Yanke, L.J.; Jones, G.A. Characterization of Condensed Tannins Purified From Legume Forages: Chromophore Production, Protein Precipitation, and Inhibitory Effects on Cellulose Digestion. J. Chem. Ecol. 2005, 31, 2049–2068. [Google Scholar] [CrossRef]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Martinez, K.B.; Mackert, J.D.; McIntosh, M.K. Chapter 18—Polyphenols and Intestinal Health. In Nutrition and Functional Foods for Healthy Aging; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 191–210. ISBN 978-0-12-805376-8. [Google Scholar]
- Arnott, J.A.; Planey, S.L. The influence of lipophilicity in drug discovery and design. Expert Opin. Drug Discov. 2012, 7, 863–875. [Google Scholar] [CrossRef]
- Appeldoorn, M.M.; Vincken, J.-P.; Gruppen, H.; Hollman, P.C.H. Procyanidin dimers A1, A2, and B2 are absorbed without conjugation or methylation from the small intestine of rats. J. Nutr. 2009, 139, 1469–1473. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Li, J.; Sun, Y.; Huang, Y.; He, J.; Zhu, Z. Transport of Flavanolic Monomers and Procyanidin Dimer A2 across Human Adenocarcinoma Stomach Cells (MKN-28). J. Agric. Food Chem. 2019, 67, 3354–3362. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; González-Barrio, R.; Cerdá, B.; López-Bote, C.; Rey, A.I.; Tomás-Barberán, F.A. Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J. Agric. Food Chem. 2007, 55, 10476–10485. [Google Scholar] [CrossRef] [PubMed]
- González-Barrio, R.; Borges, G.; Mullen, W.; Crozier, A. Bioavailability of anthocyanins and ellagitannins following consumption of raspberries by healthy humans and subjects with an ileostomy. J. Agric. Food Chem. 2010, 58, 3933–3939. [Google Scholar] [CrossRef] [PubMed]
- Cerdá, B.; Periago, P.; Espín, J.C.; Tomás-Barberán, F.A. Identification of urolithin A as a metabolite produced by human colon microflora from ellagic acid and related compounds. J. Agric. Food Chem. 2005, 53, 5571–5576. [Google Scholar] [CrossRef]
- Borges, G.; Ottaviani, J.I.; van der Hooft, J.J.J.; Schroeter, H.; Crozier, A. Absorption, metabolism, distribution and excretion of (−)-epicatechin: A review of recent findings. Mol. Asp. Med. 2018, 61, 18–30. [Google Scholar] [CrossRef]
- Huang, W.; Niu, H.; Xue, X.; Li, J.; Li, C. Robinetinidol-(4β→8)-epigallocatechin 3-O-gallate, A Galloyl Dimer Prorobinetinidin From Acacia Mearnsii De Wild, Effectively Protects Human Neuroblastoma SH-SY5Y Cells Against Acrolein-Induced Oxidative Damage. J. Alzheimer Dis. 2010, 21, 493–506. [Google Scholar] [CrossRef]
- Dudzinska, D.; Bednarska, K.; Boncler, M.; Luzak, B.; Watala, C. The influence of Rubus idaeus and Rubus caesius leaf extracts on platelet aggregation in whole blood. Cross-talk of platelets and neutrophils. Platelets 2016, 27, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Mirazi, N.; Hosseini, A. Attenuating properties of Rubus fruticosus L. on oxidative damage and inflammatory response following streptozotocin-induced diabetes in the male Wistar rats. J. Diabetes Metab. Disord. 2020. [Google Scholar] [CrossRef]
- Tonin, T.D.; Thiesen, L.C.; de Oliveira Nunes, M.L.; Broering, M.F.; Donato, M.P.; Goss, M.J.; Petreanu, M.; Niero, R.; Machado, I.D.; Santin, J.R. Rubus imperialis (Rosaceae) extract and pure compound niga-ichigoside F1: Wound healing and anti-inflammatory effects. Naunyn. Schmiedebergs. Arch. Pharmacol. 2016, 389, 1235–1244. [Google Scholar] [CrossRef]
- Martini, S.; D’Addario, C.; Colacevich, A.; Focardi, S.; Borghini, F.; Santucci, A.; Figura, N.; Rossi, C. Antimicrobial activity against Helicobacter pylori strains and antioxidant properties of blackberry leaves (Rubus ulmifolius) and isolated compounds. Int. J. Antimicrob. Agents 2009, 34, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Hajaji, S.; Jabri, M.-A.; Sifaoui, I.; López-Arencibia, A.; Reyes-Batlle, M.; B’chir, F.; Valladares, B.; Pinero, J.E.; Lorenzo-Morales, J.; Akkari, H. Amoebicidal, antimicrobial and in vitro ROS scavenging activities of Tunisian Rubus ulmifolius Schott, methanolic extract. Exp. Parasitol. 2017, 183, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Sisti, M.; De Santi, M.; Fraternale, D.; Ninfali, P.; Scoccianti, V.; Brandi, G. Antifungal activity of Rubus ulmifolius Schott standardized in vitro culture. LWT Food Sci. Technol. 2008, 41, 946–950. [Google Scholar] [CrossRef]
- Gabr, S.A.; Alghadir, A.H. Evaluation of the Biological Effects of Lyophilized Hydrophilic Extract of Rhus coriaria on Myeloperoxidase (MPO) Activity, Wound Healing, and Microbial Infections of Skin Wound Tissues. Evid.-Based Complement. Altern. Med. 2019, 2019, 5861537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; Adeyemi, O.S.; Nadwa, E.H.; kadry Mohamed Rashwan, E.; Alkazmi, L.M.; Elkelish, A.A.; Igarashi, I. Phytochemical Screening and Antiprotozoal Effects of the Methanolic Berberis Vulgaris and Acetonic Rhus Coriaria Extracts. Molecules 2020, 25, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, K.; Yokoyama, S.I. Induction of uncoupling protein-1 and -3 in brown adipose tissue by kaki-tannin in type 2 diabetic NSY/Hos mice. Food Chem. Toxicol. 2012, 50, 184–190. [Google Scholar] [CrossRef]
- Chandrasekhar, Y.; Phani Kumar, G.; Navya, K.; Ramya, E.M.; Anilakumar, K.R. Tannins from Terminalia chebula fruits attenuates GABA antagonist-induced anxiety-like behaviour via modulation of neurotransmitters. J. Pharm. Pharmacol. 2018, 70, 1662–1674. [Google Scholar] [CrossRef]


| Traditional Use of Plants and Macroalgae Rich in Tannins | ||||
|---|---|---|---|---|
| Plant | Admin. | Treatment, Remedy, Uses | Mechanism of Action | Ref. |
| PLANTS | ||||
| Acacia | ||||
| A. nilotica | O, T | Gastrointestinal, respiratory, inflammatory, parasitic, neurological diseases, sexual disorders, skin issues, diabetes. Aphrodisiac, chemo-preventive, antimutagenic | Antioxidant, anti-inflammatory, anti-nociceptive, and antipyretic | [29,30,31,32,33] |
| A. arabica | O (G, S) | Used for sweetmeats (G) or roasted (S, India) | ||
| A. tortilis | O, T | Gastrointestinal disorders in camelids, skin issues (edema, allergic dermatitis, wound/burns healing) | Antiparasitic and anti-inflammatory | [34] |
| Betula | ||||
| B. pendula | O (B in I/D) | Urinary, respiratory affections. Systematic diseases | Anti-viral | [35,36] |
| Juglans | ||||
| J. regia | O (N), T | Hemorrhoids, rheumatism, varicose veins, skin wounds, fever, cough, toothache, infecundity. Local analgesic. Hypercholesterolemic, antidiabetic, cardiotonic, vasodilator. Aromatizer. Antiparasitic | Anti-platelet, cardioprotective, antiatherogenic and anti-inflammatory | [37,38,39,40,41,42] |
| Picea | ||||
| P. abies | O (Sp/L/F/R/B) | Food ingredients or supplements (Sp, L, F, R). Bread-preparing flour or thicker in soups (B) | Antioxidant, antimicrobial, preservative | [43] |
| Pistacia | ||||
| P. lentiscus | O, T (St, FR-oil) | Improvement of gastrointestinal function. Infected wounds, scabies, bloat, constipation | Antiparasitic, anti-inflammatory | [44,45,46,47] |
| Phyllanthus | ||||
| P. niruri | O (L and FR) | Liver diseases (jaundice), urinary infections, inflammatory processes and malaria | Anti-inflammatory, antioxidant, hypoglycemic, hypolipidemic, hepatoprotective | [48,49] |
| Quercus | ||||
| Quercus sp. | O, T (R/S in D/FR) | Skin injuries (burn, boil wound). Respiratory affections (cold and flu). Diabetes | Antioxidant, antidiabetic | [37,42,50,51,52] |
| Rhus | ||||
| Rhus sp. | O | Gastrointestinal diseases (diarrhea, ulcers, hemorrhoids), dysentery, or stroke | Antimicrobial, anti-inflammatory, antiapoptotic, immunomodulatory, healing | [53,54] |
| Schinopsis | ||||
| Schinopsis sp. | O, T (I/D of L/B/Rs/FR/Br/C/W/S) | Anti-inflammatory, antimicrobial, antipyretic, astringent and cicatrizing. Respiration affections (cold, cough, asthma), stomachache, headache, dysentery or fractures | Antioxidant, antimicrobial, anthelmintic | [1,55,56,57,58,59,60] |
| Smilax | ||||
| S. aspera | O, T (D) | Urinary retention, antiseptic in cows, enhancing health state of rabbits, treatment of purulent vesicles | Antioxidant, anti-inflammatory, diuretic | [47,61] |
| Umbilicus | ||||
| U. rupestris | O, T (minced L) | Infected wounds, diarrhea, fever, intoxications, antiparasitic in hens | Anti-inflammatory, antiparasitic | [47,61,62] |
| Urtica | ||||
| U. dioica | O, T (I, direct application) | Arthritis, lumbago, rheumatism, muscular or limb paralysis. Rubefacient, blood circulation stimulant. Relief allergic rhinitis symptoms. Revitalizing. In animal promotes weight gain, growth and increases galactagogue production (ruminants) | Antioxidant, anti-inflammatory, antimicrobial, analgesic, anti-diabetic, antimutagenic. Emulsifier, gelling agent | [63,64,65,66,67,68] |
| Vitis | ||||
| V. vinifera | O (raw sp, vinegar) | Gastrointestinal diseases, headaches, and colds. Thirst-quenching, revitalizing and anti-inflammatory | Antioxidant, anti-obesity, anti-inflammatory | [42,69] |
| Combination of plants | ||||
| “Triphala” | Oral | Restorative, revitalizing, boosting of the immune system, treatment for chronic gastrointestinal diseases | - | [48,70] |
| MACROALGAE | ||||
| Sargassum | ||||
| Sargassum sp. | O, T | Nutritional value. Treatment for inflammations, goiter, dropsy, edema, dysuria, respiratory affections, angina pectoris, high blood pressure, skin diseases, neurosis, pregnancy-related depression and diabetes mellitus | Antioxidant, antibacterial, antiproliferative, anti-inflammatory. Gelling hydrocolloid, emulsifier | [71,72,73,74] |
| Ecklonia | ||||
| E. cava | Oral | Common food ingredient, attenuation of goiter, treatment for mammary hyperplasia and diuretic | Antioxidant, anti-inflammatory | [71,75,76] |
| Genera | Species | Representative Tannins and Relevant Related Molecules | Ref. |
|---|---|---|---|
| Acacia sp. | A. catechu | CT monomers, dimers and trimers: trihydroxiflavan, profisetidin, gallocatechin, prorobinetidin, 3,5,7,4′-tetrahydroxyflavan | [110] |
| A. mearnsii | CT monomers, dimers and trimers: fisetinidol, quercetin, myricetin, prodelphinidin and gallocatechin | [111,112] | |
| Relevant dimers: | |||
| robinetinidol-(4α-8)-gallocatechin | |||
| fisetinidol-(4α-8)-catechin and robinetinidol-(4α-8)-catechin | |||
| robinetinidol-(4α-8”)-robinetinidol (4′α-6”)-catechin | |||
| A. nilotica | Phenolic acids GA and EA | [30,113] | |
| GT: methyl gallate | |||
| Polygalloyl units: | |||
| ethyl gallate-1-galloyl-β-D-glucose, diGA and dicatechin | |||
| 1,6-di-galloyl-β-D-glucose and gallocatechin-5-gallate | |||
| epigallocatechin-7-gallate and -5,7-digallate | |||
| Betula sp. | B. pendula | Phenolic acids: glycosylated flavonoids and salicylates | [114,115] |
| CT: oligomeric and polymeric flavan-3-ols | |||
| Flavonoid-aglycones: apigenin, luteolin, chrysoeriol derivatives | |||
| Castanea sp. | C. sativa | Phenolic acids: EA | [101,116,117,118] |
| GT and ET: chestanin, chesnatin, isochesnatin, chebulagic acid, pedunculagin, tellimagrandin I, castalagin/vescalagin, stachyurin or casuarinin, deoxyhexoside | |||
| Other molecules: cocciferin d2, castacrenin A-C isomers, trimethyl-ellagic acid hexoside, cretanin, methylvescalagin, vescavaloninic acid | |||
| Ecklonia sp. | E. cava | PT: phloroglucinol, eckol, dieckol, 7-phloroeckol, 2,7-phloroglucinol-6,6-bieckol, phlorofucofuroeckol-A, pyrogallol-phloroglucinol-6,6-bieckol | [104,119] |
| Juglans sp. | J. regia | HT: pedunculagin, casuariin, valoneoyl, sanguisorboyl, tergalloyltellimagrandin I, praecoxin A, platycariin, glansrin C, alnusnin Bpterocarinin, breginin A and alienanin B, flavogallonic acid dilactone | [39] |
| Lotus sp. | L. corniculatus | Tannin heteropolymers: units of catechin/epicatechin and gallocatechin/epigallocatechin | [120] |
| Picea sp. | P. abies | CT monomers: catechin, epicatechin, gallocatechin, delphinidin | [121,122] |
| Tannin-related molecules: myricetin; astringin, piceid, isorhapontin | |||
| Quercus sp. | Q. robur | Phenolic acids: EA and GA | [50] |
| Ellagitannins: castalagin, grandinin, castalin, vescalagin, vescalin | |||
| Triterpenoid glycosides: polygalloylquinic acid derivatives | |||
| Rhus sp. | R. coriaria | Phenolic acid: GA | [54,123] |
| QUERG | |||
| CYANG derivatives: | |||
| cyanidin-3-(2″galloyl)-galactoside and methyl delphinidin aglycone | |||
| 7-methyl-delphinidin-3-(2″galloyl)-galactoside | |||
| 7-methyl-cyanidin-3-(2″galloyl)-galactoside) | |||
| Other bioactive compounds: | |||
| galloylhexose, benzoic acid, galloylquinic acid and quinic acid | |||
| 3,4, 5-trihydroxy-, 2-oxo-1,3-propanediyl ester | |||
| myricetin galloylhexoside, triGA | |||
| Rubus sp. | R. fruticosus | Phenolic acid: GA | [124] |
| CYANG and vanillic acid | |||
| Flavonoids: flavanone naringenin | |||
| Anthocyanins and anthocyanidins: malvidin-3-galactoside, cyanidin-3-galactoside and delphinidin-3-galactoside | |||
| Sapium sp. | S. baccatum, | Flavonoid quercetin 3-α-L-arabinopyranoside tannins: | [125] |
| methyl gallate, corilagin and tercatain | |||
| chebulagic acid and chebulinic acid | |||
| Sargassum sp. | S. fusiforme | PT: fuhalols, fucols, ethols, carmalol derivatives | [73] |
| S. muticum | PT: fuhalols (hydroxytrifuhalol B, hydroxypentafuhalol A, hydroxyheptafuhalol B and hydroxynonafuhalol A) | [126] | |
| Schinopsis sp. | S. lorentzii | GT: TGG, PGG, FIS-catechin polymers and quinic acid-GA esters. | [59] |
| CT: catechin-fisetinidol polymers | |||
| S. balansae | CT: fisetinidol and robinetinidol polymers | [127] | |
| Terminalia sp. | T. chebula | Phenolic acids: EA, GA, chebulinic acid, chebulic acid. | [95,128] |
| GT: TGG, methyl gallate | |||
| ET: chebulanin, methyl neo-chebulanin, corilagin, punicalagin, terflavin, flavogallonic acid, gallagic acid, methyl flavogallonate. | |||
| Simple gallate esters: | |||
| 1,6-di-galloyl-β-ᴅ-glucose and 3,4,6-tri-galloyl-β-ᴅ-glucose | |||
| 1,3,4,6-tetra-galloyl-β-ᴅ-glucose and 1,2,3,4,6-penta-galloyl-β-ᴅ-glucose | |||
| Vitis sp. | V. vinifera | PC, PD, galloylated-PC and flavan-3-ols | [129,130] |
| Epicatechins: epigallocatechin, epicatechin-3-gallate and procyanidins B, catechin-gallocatechin dimers and fisetinidin dimers |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraga-Corral, M.; Otero, P.; Cassani, L.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Chamorro, F.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Traditional Applications of Tannin Rich Extracts Supported by Scientific Data: Chemical Composition, Bioavailability and Bioaccessibility. Foods 2021, 10, 251. https://doi.org/10.3390/foods10020251
Fraga-Corral M, Otero P, Cassani L, Echave J, Garcia-Oliveira P, Carpena M, Chamorro F, Lourenço-Lopes C, Prieto MA, Simal-Gandara J. Traditional Applications of Tannin Rich Extracts Supported by Scientific Data: Chemical Composition, Bioavailability and Bioaccessibility. Foods. 2021; 10(2):251. https://doi.org/10.3390/foods10020251
Chicago/Turabian StyleFraga-Corral, Maria, Paz Otero, Lucia Cassani, Javier Echave, Paula Garcia-Oliveira, Maria Carpena, Franklin Chamorro, Catarina Lourenço-Lopes, Miguel A. Prieto, and Jesus Simal-Gandara. 2021. "Traditional Applications of Tannin Rich Extracts Supported by Scientific Data: Chemical Composition, Bioavailability and Bioaccessibility" Foods 10, no. 2: 251. https://doi.org/10.3390/foods10020251
APA StyleFraga-Corral, M., Otero, P., Cassani, L., Echave, J., Garcia-Oliveira, P., Carpena, M., Chamorro, F., Lourenço-Lopes, C., Prieto, M. A., & Simal-Gandara, J. (2021). Traditional Applications of Tannin Rich Extracts Supported by Scientific Data: Chemical Composition, Bioavailability and Bioaccessibility. Foods, 10(2), 251. https://doi.org/10.3390/foods10020251