Influence of Durum Wheat Bran Particle Size on Phytochemical Content and on Leavened Bread Baking Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Proximate Composition
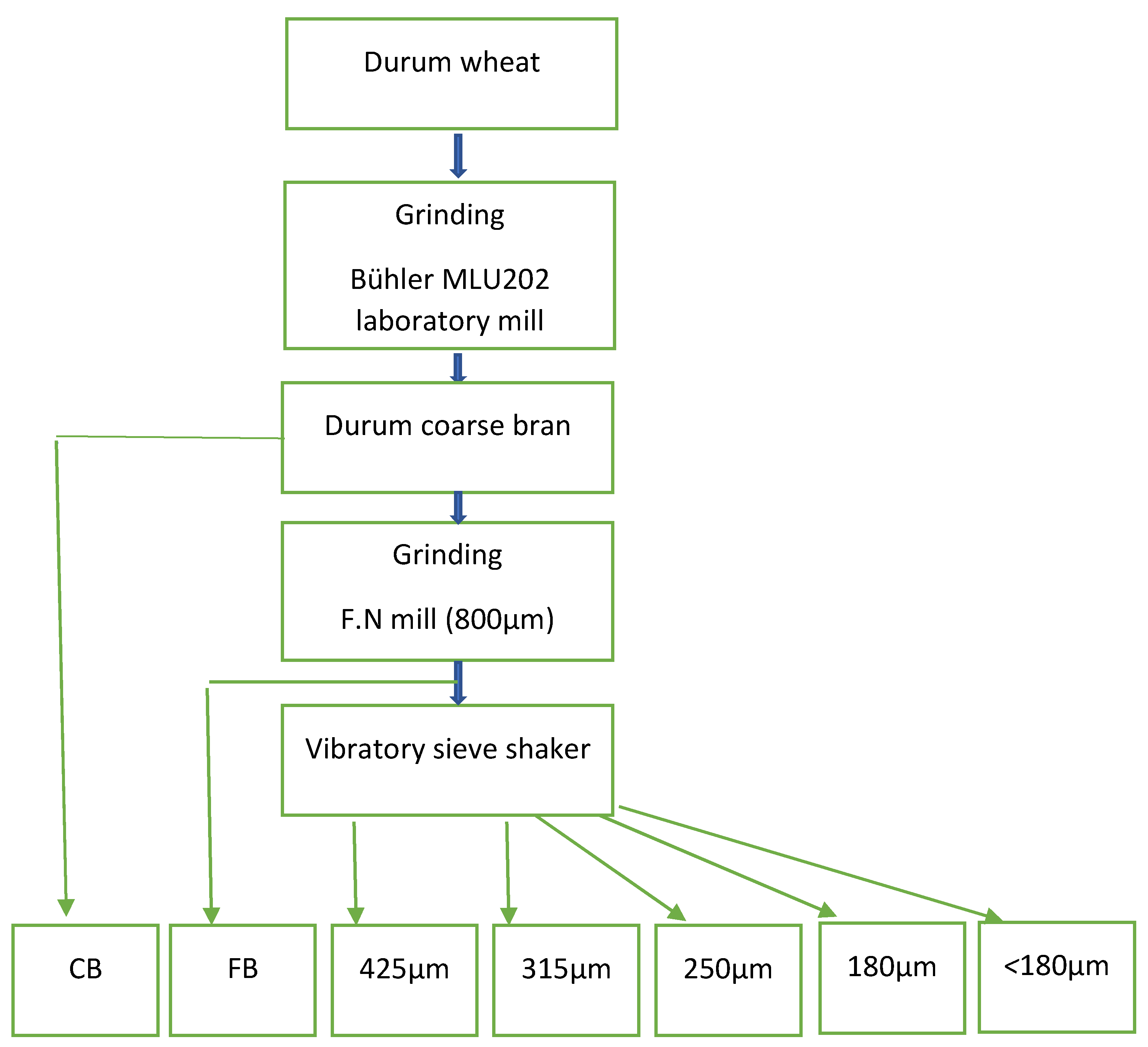
2.3. Bran Particle Size Distribution
2.4. Phytochemical Analysis
2.5. Preparation of Bread Mixtures and Analysis of Bread Quality
2.6. Statistical Analyses
3. Results
3.1. Bran Particle Size Distribution
3.2. Proximate Composition
3.3. Antioxidant Capacity
3.4. Phenolic Acids
3.5. Phytosterol Content
3.6. Effects of Bran Level and Particle Size on Bread-Making Properties
4. Discussion
4.1. Composition of Bran Fractions
4.2. Impact of Bran Fractions on Bread Quality
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prückler, M.; Siebenhandl-Ehn, S.; Apprich, S.; Höltinger, S.; Haas, C.; Schmid, E.; Kneifel, W. Wheat bran-based biorefinery 1: Composition of wheat bran and strategies of functionalization. LWT-Food Sci. Technol. 2014, 56, 211–221. [Google Scholar] [CrossRef]
- Onipe, O.O.; Jideani, A.I.; Beswa, D. Composition and functionality of wheat bran and its application in some cereal food products. Int. J. Food Sci. Technol. 2015, 50, 2509–2518. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Vodnar, D.C. Whole grains and phenolic acids: A review on bioactivity, functionality, health benefits and bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, L.; Choi, I.; Hyun, J.N.; Jeong, Y.K.; Baik, B.K. Influence of bran particle size on bread-baking quality of whole grain wheat flour and starch retrogradation. Cereal Chem. 2014, 91, 65–71. [Google Scholar] [CrossRef]
- Park, E.Y.; Fuerst, E.P.; Baik, B.K. Effects of bran prehydration on functional characteristics and bread-baking quality of bran and flour blends. Cereal Chem. 2017, 94, 834–839. [Google Scholar] [CrossRef]
- Wang, N.; Hou, G.G.; Dubat, A. Effects of flour particle size on the quality attributes of reconstituted whole-wheat flour and Chinese southern-type steamed bread. LWT-Food Sci. Technol. 2017, 82, 147–153. [Google Scholar] [CrossRef]
- Zhang, D.; Moore, W.R. Wheat bran particle size effects on bread baking performance and quality. J. Sci. Food Agric. 1999, 79, 805–809. [Google Scholar] [CrossRef]
- Kim, B.K.; Cho, A.R.; Chun, Y.G.; Park, D.J. Effect of microparticulated wheat bran on the physical properties of bread. Int. J. Food Sci. Nutr. 2013, 64, 122–129. [Google Scholar] [CrossRef]
- Brewer, L.R.; Kubola, J.; Siriamornpun, S.; Herald, T.J.; Shi, Y.C. Wheat bran particle size influence on phytochemical extractability and antioxidant properties. Food Chem. 2014, 152, 483–490. [Google Scholar] [CrossRef]
- Pasqualone, A.; Laddomada, B.; Centomani, I.; Paradiso, V.M.; Minervini, D.; Caponio, F.; Summo, C. Bread making aptitude of mixtures of re-milled semolina and selected durum wheat milling by-products. LWT-Food Sci. Technol. 2017, 78, 151–159. [Google Scholar] [CrossRef]
- Noort, M.W.; van Haaster, D.; Hemery, Y.; Schols, H.A.; Hamer, R.J. The effect of particle size of wheat bran fractions on bread quality–evidence for fibre–protein interactions. J. Cereal Sci. 2010, 52, 59–64. [Google Scholar] [CrossRef]
- Alzuwaid, N.; Fellows, C.; Laddomada, B.; Sissons, M. Impact of wheat bran particle size on the technological and phytochemical properties of durum wheat pasta. J. Cereal Sci. 2020, 95, 103033. [Google Scholar] [CrossRef]
- Alzuwaid, N.; Sissons, M.; Laddomada, B.; Fellows, C. Nutritional and functional properties of durum wheat bran protein concentrate. Cereal Chem. 2020, 97, 304–315. [Google Scholar] [CrossRef]
- Aravind, N.; Sissons, M.J.; Egan, N.E.; Fellows, C.M. Effect of insoluble dietary fibre addition on technological, sensory, and structural properties of durum wheat spaghetti. Food Chem. 2012, 130, 299–309. [Google Scholar] [CrossRef]
- Royal Australian Chemical Institute, Cereal Chemistry Division. CCD Official Method 07-03, Supplement. In Official Testing Methods of the Cereal Chemistry Division, 4th ed.; The Royal Australian Chemical Institute Inc.: North Melbourne, Australia, 2010. [Google Scholar]
- Overview of Texture Profile Analysis. Available online: https://texturetechnologies.com/resources/texture-profile-analysis (accessed on 9 February 2021).
- AACC International. Approved Methods of Analysis, 11th ed.; AACC International: St. Paul, MN, USA, 2010. [Google Scholar]
- Curti, E.; Carini, E.; Bonacini, G.; Tribuzio, G.; Vittadini, E. Effect of the addition of bran fractions on bread properties. J. Cereal Sci. 2013, 57, 325–332. [Google Scholar] [CrossRef]
- Marangoni, F.; Poli, A. Phytosterols and cardiovascular health. Pharm. Res. 2010, 61, 193–199. [Google Scholar] [CrossRef]
- Ahmad, F.; Pasha, I.; Saeed, M.; Asgher, M. Antioxidant profiling of native and modified cereal brans. Int. J. Food Sci. Technol. 2019, 54, 1206–1214. [Google Scholar] [CrossRef]
- Cardenia, V.; Sgarzi, F.; Mandrioli, M.; Tribuzio, G.; Rodriguez-Estrada, M.T.; Toschi, T.G. Durum wheat bran by-products: Oil and phenolic acids to be valorized by industrial symbiosis. Eur. J. Lipid Sci. Technol. 2018, 120, 1–13. [Google Scholar] [CrossRef]
- Lineback, D.R.; Rasper, V.F. Wheat carbohydrates. In Wheat: Chemistry and Technology; Pomeranz, Y., Ed.; AACC International: St. Paul, MN, USA, 1988; pp. 277–372. [Google Scholar]
- Ktenioudaki, A.; Gallagher, E. Recent advances in the development of high-fibre baked products. Trends Food Sci. Technol. 2012, 28, 4–14. [Google Scholar] [CrossRef]
- Schmiele, M.; Jaekel, L.; Patricio, S.; Steel, C.; Chang, Y. Rheological properties of wheat flour and quality characteristics of pan bread as modified by partial additions of wheat bran or whole grain wheat flour. Int. J. Food Sci. Technol. 2012, 47, 2141–2150. [Google Scholar] [CrossRef]
- Chinma, C.E.; Ramakrishnan, Y.; Ilowefah, M.; Hanis-Syazwani, M.; Muhammad, K. Properties of cereal brans: A review. Cereal Chem. 2015, 92, 1–7. [Google Scholar] [CrossRef]
- Tebben, L.; Shen, Y.; Li, Y. Improvers and functional ingredients in whole wheat bread: A review of their effects on dough properties and bread quality. Trends Food Sci. Technol. 2018, 81, 10–24. [Google Scholar] [CrossRef]
- Hemdane, S.; Jacobs, P.J.; Bosmans, G.M.; Verspreet, J.; Delcour, J.A.; Courtin, C.M. Study on the effects of wheat bran incorporation on water mobility and biopolymer behavior during bread making and storage using time-domain 1H NMR relaxometry. Food Chem. 2017, 236, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.W.; van Vliet, T.; Hamer, R.J. How gluten properties are affected by pentosans. J. Cereal Sci. 2004, 39, 395–402. [Google Scholar] [CrossRef]
- Finney, P.L.; Henry, S.; Jeffers, H. Effect of wheat variety, flour grinding, and egg yolk on whole wheat bread quality. Cereal Chem. 1985, 62, 170–173. [Google Scholar]
- Hemdane, S.; Jacobs, P.J.; Dornez, E.; Verspreet, J.; Delcour, J.A.; Courtin, C.M. Wheat (Triticum aestivum L.) bran in bread making: A Critical Review. Comp. Rev. Food Sci. Food Saf. 2016, 15, 28–42. [Google Scholar] [CrossRef] [Green Version]
- Ou, J.; Wang, M.; Zheng, J.; Ou, S. Positive and negative effects of polyphenol incorporation in baked foods. Food Chem. 2019, 284, 90–99. [Google Scholar] [CrossRef]
- Taranto, F.; Delvecchio, L.N.; Mangini, G.; Del Faro, L.; Blanco, A.; Pasqualone, A. Molecular and physico-chemical evaluation of enzymatic browning of whole meal and dough in a collection of tetraploid wheats. J. Cereal Sci. 2012, 55, 405–414. [Google Scholar] [CrossRef]
- Sanz-Penella, J.M.; Laparra, J.M.; Sanz, Y.; Haros, M. Influence of added enzymes and bran particle size on bread quality and iron availability. Cereal Chem. 2012, 89, 223–229. [Google Scholar] [CrossRef]
- Lin, S.; Gao, J.; Jin, X.; Wang, Y.; Dong, Z. Whole-wheat flour particle size influences dough properties, bread structure and in vitro starch digestibility. Food Funct. 2020, 11, 3610–3620. [Google Scholar] [CrossRef]
- Pareyt, B.; Finnie, S.M.; Putseys, J.A.; Delcour, J.A. Lipids in breadmaking: Sources, interactions, and impact on bread quality. J. Cereal Sci. 2011, 54, 266–279. [Google Scholar] [CrossRef]




| Sample | D50 (µm) | Moisture | * Protein | Ash | Total Starch |
|---|---|---|---|---|---|
| CB | 1497 | 13.1 ± 0.32 | 15.6 | 3.2 ± 0.04 | 9.0 ± 0.17 |
| FB | 350 | 10.6 ± 0.14 | 17.2 | 3.1 ± 0.03 | 9.4 ± 0.04 |
| 425 | 641 | 10.8 ± 0.16 | 11.9 | 4.2 ± 0.02 | 7.3 ± 0.74 |
| 315 | 481 | 10.8 ± 0.21 | 13.3 | 3.6 ± 0.01 | 15.9 ± 0.42 |
| 250 | 357 | 11.2 ± 0.15 | 15.2 | 3.5 ± 0.18 | 26.0 ± 1.35 |
| 180 | 247 | 11.2 ± 0.21 | 16.6 | 4.1 ± 0.77 | 28.2 ± 0.66 |
| <180 | 115 | 11.2 ± 0.10 | 19.2 | 3.9 ± 0.01 | 29.7 ± 1.47 |
| Sample | D50 (µm) | Campesterol | Campestanol | Stigmasterol | β-Sitosterol | Sitostanol | ∆5-Avenasterol | Total |
|---|---|---|---|---|---|---|---|---|
| CB | 1497 | 0.204 a | 0.187 a | 0.027 a | 0.502 a | 0.185 a | 0.079 a | 1.184 ac |
| FB | 350 | 0.337 b | 0.323 b | 0.048 a | 0.913 b | 0.310 b | 0.125 b | 2.055 b |
| 425 | 641 | 0.164 a | 0.287 b | 0.042 a | 0.414 a | 0.202 a | 0.055 c | 1.164 ac |
| 315 | 481 | 0.167 a | 0.201 a | 0.034 a | 0.403 a | 0.152 ac | 0.056 cd | 1.014 ac |
| 250 | 357 | 0.210 a | 0.219 a | 0.036 a | 0.558 a | 0.168 ac | 0.073 ad | 1.263 a |
| 180 | 247 | 0.342 b | 0.318 b | 0.119 b | 0.927 b | 0.272 b | 0.127 b | 2.107 b |
| <180 | 115 | 0.153 a | 0.147 c | 0.046 a | 0.431 a | 0.128 c | 0.055 c | 0.958 c |
| Samples | Loaf Volume (cm3) | Specific Volume (cm3/g) | % Oven Spring |
|---|---|---|---|
| BF (control) | 752 a | 4.89 a | 20.20 a |
| CB | 666 b | 4.30 b | 11.70 b |
| FB | 629 ce | 4.03 c | 8.45 c |
| 425 | 604 d | 3.83 df | 6.20 cd |
| 315 | 635 ce | 4.06 c | 7.40 c |
| 250 | 642 c | 4.10 c | 8.15 c |
| 180 | 621 e | 3.91 d | 4.05 de |
| <180 | 598 d | 3.76 f | 3.65 e |
| Samples | Colour | Texture | ||||||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | Firmness | Springiness | Resilience | Cohesiveness | Chewiness | |
| BF | 78.9 a | −0.1 a | 14.7 a | 586 a | 0.94 a | 0.34 a | 0.69 a | 381.0 a |
| CB | 73.2 b | 2.05 b | 16.5 b | 750 bd | 0.93 a | 0.31 b | 0.67 b | 463.3 b |
| FB | 73.7 c | 1.95 be | 17.7 c | 924 cd | 0.92 ab | 0.28 c | 0.64 c | 538.3 bc |
| 425 | 70.8 d | 3.05 c | 17.9 cd | 931 cd | 0.89 b | 0.28 c | 0.65 d | 538.3 bc |
| 315 | 71.9 e | 2.55 d | 17.7 c | 874 d | 0.91 b | 0.29 c | 0.65 d | 519.0 bc |
| 250 | 73.4 b | 1.95 b | 17.8 c | 905 cd | 0.92 ba | 0.28 c | 0.64 c | 534.5 bc |
| 180 | 74.0 f | 1.8 e | 17.9 cd | 1055 c | 0.91 b | 0.26 d | 0.63 e | 594.5 c |
| <180 | 75.1 g | 1.45 f | 18.1 d | 1272 e | 0.89 b | 0.26 d | 0.62 f | 706.3 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzuwaid, N.T.; Pleming, D.; Fellows, C.M.; Laddomada, B.; Sissons, M. Influence of Durum Wheat Bran Particle Size on Phytochemical Content and on Leavened Bread Baking Quality. Foods 2021, 10, 489. https://doi.org/10.3390/foods10030489
Alzuwaid NT, Pleming D, Fellows CM, Laddomada B, Sissons M. Influence of Durum Wheat Bran Particle Size on Phytochemical Content and on Leavened Bread Baking Quality. Foods. 2021; 10(3):489. https://doi.org/10.3390/foods10030489
Chicago/Turabian StyleAlzuwaid, Nabeel T., Denise Pleming, Christopher M. Fellows, Barbara Laddomada, and Mike Sissons. 2021. "Influence of Durum Wheat Bran Particle Size on Phytochemical Content and on Leavened Bread Baking Quality" Foods 10, no. 3: 489. https://doi.org/10.3390/foods10030489







