Functional Food for Elderly High in Antioxidant and Chicken Eggshell Calcium to Reduce the Risk of Osteoporosis—A Narrative Review
Abstract
1. Introduction
2. Bone and Osteoporosis
2.1. Role of Oxidative Stress in Osteoporosis
2.2. Effects of Antioxidants in Osteoporosis
2.2.1. Vitamin C
2.2.2. Vitamin E
2.2.3. Polyphenols
2.2.4. Lycopene
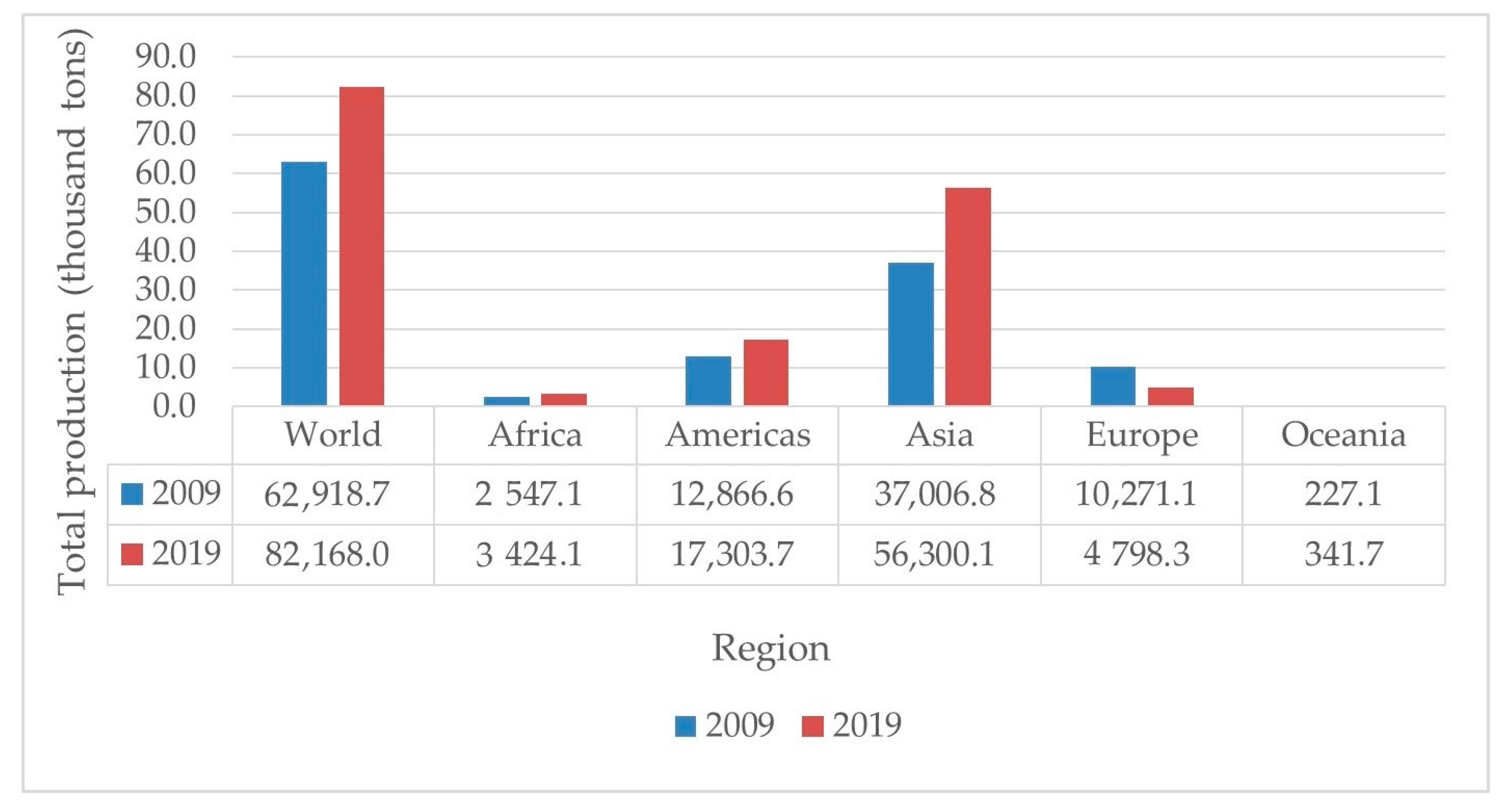
3. Chicken Eggshell as Source of Calcium and Its Application
| Specific Minerals Present in the Eggshell | Unit of Measurement | According to the Prior Research | |||
|---|---|---|---|---|---|
| [106] | [107] | [98] | [108] | ||
| Total ash content | g/100 g | 89.9–91.1 | 90.2 | N.D. 1 | N.D. |
| Calcium | mg/100 g | 35,100–35,400 | 35,080 | 38,200 | 40,100 |
| Magnesium | mg/100 g | 370–400 | 262.0 | N.D. | 450 |
| Iron | mg/100 g | N.D. | 13.06 | N.D. | 2.24 |
| Phosphorus | mg/100 g | 120 | 150.2 | N.D. | 99 |
| Zinc | mg/100 g | N.D. | 145.1 | N.D. | 0.513 |
| Sodium | mg/100 g | 150–170 | 47.9 | 510 | N.D. |
| Potassium | mg/100 g | 100–130 | 50.00 | 140 | N.D. |
| Copper | mg/100 g | N.D. | 4.1 | N.D. | 0.77 |
| Manganese | mg/100 g | N.D. | 149.9 | N.D. | N.D. |
| Strontium | μg/g | N.D. | N.D. | 140 | 372 |
| Fluorine | μg/g | N.D. | N.D. | N.D. | 3.75 |
| Selenium | ng/g | N.D. | N.D. | N.D. | 23.5 |
| Food Products | Main Findings | References | |
|---|---|---|---|
| Chicken Eggshell Powder Concentration | Notes for Recommendation | ||
| Biscuits | 6% (w/w) of wheat flour | Calcium content, texture, sensory properties, calcium bioavailability | [107] |
| Bread | 8% (w/w) of ingredients | Calcium content, specific volume of bread, sensory properties | [109] |
| Bread | 2% (w/w) of wheat flour | Increase of rheological characteristics of dough and nutritional properties, decrease of the general acceptability and odor score | [20] |
| Bread strips | 10% (w/w) substitution of wheat flour | Minor changes in sensory properties | [18] |
| Breaded fried meat, bread, pizza, spaghetti | 500 mg Ca/person | Minor changes in texture, without flavor changes | [98] |
| Chocolate cakes | 6% (w/w) of wheat flour | Calcium content, texture, sensory properties | [110] |
| Chokeberry juice, cranberry juice | 1% of chokeberry and cranberry juice | Calcium content of chokeberry and cranberry juice, no significant change in color and sediment content | [69] |
| Muffin | 8 g/500 g wheat flour | Mineral content, sensory properties | [111] |
| Nham (Thai-style fermented pork sausage) | 150 mg Ca/100 g of Nham (eggshell powder was converted to eggshell calcium lactate) | No difference in sensory scores of sour taste, flavor, and overall acceptance | [112] |
| Ser smażony (Polish bread spread) | 265 mg/100 g of ser smażony | Increased calcium contents >2.5-fold, the calcium bioavailability was higher after the addition of lysine and vitamin K | [113] |
| White bread | 1–1.5% (w/w) of ingredients | High total score in sensory evaluation | [114] |
| White bread | 2% (w/w) substitution of bread flour | Consumer acceptation in sensory evaluation | [115] |
| Yogurt | 0.15–0.30% (w/v) of milk | No significant unfavorable effects on the physicochemical, microbial, and sensory properties | [116] |
| Yogurt (from cow milk and buffalo milk) | 0.3% of yogurt (nanosized eggshell powder) | Acceptable composition, texture, and sensory attributes | [117] |
Correlation between Tannic Acid and Calcium Absorption
4. Factors Supporting Calcium Absorption
4.1. Vitamin D
4.2. Prebiotics, Probiotics, and Synbiotics
5. Eating Preference and Dietary Pattern of Elderly
6. Functional Foods for Elderly
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- United Nations. World Population Ageing 2019: Highlights; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Eurostat. Population Structure and Ageing. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Population_structure_and_ageing#The_share_of_elderly_people_continues_to_increase (accessed on 22 December 2020).
- Kim, M.H.; Lee, H.J. Osteoporosis, Vitamin C Intake, and Physical Activity in Korean Adults Aged 50 Years and Over. J. Phys. Ther. Sci. 2016, 28, 725–730. [Google Scholar] [CrossRef]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An Overview and Management of Osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A. Assessment of Osteoporosis at the Primary Health-Care Level; WHO Collaborating Centre for Metabolic Bone Diseases, University of Sheffield Medical School: Geneva, Switzerland, 2007. [Google Scholar]
- Cano, A.; Chedraui, P.; Goulis, D.G.; Lopes, P.; Mishra, G.; Mueck, A.; Senturk, L.M.; Simoncini, T.; Stevenson, J.C.; Stute, P.; et al. Calcium in the Prevention of Postmenopausal Osteoporosis: EMAS Clinical Guide. Maturitas 2018, 107, 7–12. [Google Scholar] [CrossRef]
- Jędrusek-Golińska, A.; Górecka, D.; Buchowski, M.; Wieczorowska-Tobis, K.; Gramza-Michałowska, A.; Szymandera-Buszka, K. Recent Progress in the Use of Functional Foods for Older Adults: A Narrative Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 835–856. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative Stress in Bone Remodeling: Role of Antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of Food and Agriculture: Moving Forward on Food Loss and Waste Reduction; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Lipinski, B.; Hanson, C.; Lomax, J.; Kitinoja, L.; Waite, R. Reducing Food Loss and Waste. World Resour. Inst. Work. Pap. 2013, 1–40. [Google Scholar]
- Parfitt, J.; Barthel, M.; MacNaughton, S. Food Waste within Food Supply Chains: Quantification and Potential for Change to 2050. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3065–3081. [Google Scholar] [CrossRef] [PubMed]
- Stuart, T. Waste: Uncovering the Global Food Scandal; Penguin: London, UK, 2009. [Google Scholar]
- Gustavsson, J.; Coderberg, C.; Sonesson, U.; van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste-Extent, Causes and Prevention; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011. [Google Scholar]
- Kołożyn-Krajewska, D. Jak Uniknąć Marnotrawienia Żywności-Strategie Poprawy Wydajności Łańcucha Dystrybucji w UE w Zakresie Przekazywania Darowizn Żywności Na Cele Charytatywne; Kancelaria Senatu: Warsaw, Poland, 2016. [Google Scholar]
- Śluszarczyk, B.; Machowska, E. Food Waste in the World and in Poland. АКАДЕМІЧНИЙ ОГЛЯД 2019, 2019. [Google Scholar] [CrossRef]
- Abiad, M.G.; Meho, L.I. Food Loss and Food Waste Research in the Arab World: A Systematic Review. Food Secur. 2018, 10, 311–322. [Google Scholar] [CrossRef]
- Waheed, M.; Yousaf, M.; Shehzad, A.; Inam-Ur-Raheem, M.; Khan, M.K.I.; Khan, M.R.; Ahmad, N.; Abdullah; Aadil, R.M. Channelling Eggshell Waste to Valuable and Utilizable Products: A Comprehensive Review. Trends Food Sci. Technol. 2020, 106, 78–90. [Google Scholar] [CrossRef]
- Ali, M.; Badawy, W. Utilization of Eggshells By-Product as a Mineral Source for Fortification of Bread Strips. J. Food Dairy Sci. 2017, 8, 455–459. [Google Scholar] [CrossRef]
- Malu, S.P.; Abara, A.E.; Obochi, G.O.; Ita, B.I.; Edem, C.A. Analysis of Egeria Radiata and Thais Coronata Shells as Alternative Source of Calcium for Food Industry in Nigeria. Pakistan J. Nutr. 2009, 8, 965–969. [Google Scholar] [CrossRef][Green Version]
- Alsuhaibani, A.M.A. Rheological and Nutritional Properties and Sensory Evaluation of Bread Fortified with Natural Sources of Calcium. J. Food Qual. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Benjakul, S.; Karnjanapratum, S. Characteristics and Nutritional Value of Whole Wheat Cracker Fortified with Tuna Bone Bio-Calcium Powder. Food Chem. 2018, 259, 181–187. [Google Scholar] [CrossRef]
- Quina, M.J.; Soares, M.A.R.; Quinta-Ferreira, R. Applications of Industrial Eggshell as a Valuable Anthropogenic Resource. Resour. Conserv. Recycl. 2017, 123, 176–186. [Google Scholar] [CrossRef]
- Xhelili, A.; Strube, R.; Grossi, F.; Zvěřinová, I.; Taylor, T.; Martinez-Juarez, P.; Quiroga, S.; Suárez, C.; Gjorgjev, D. A Technological Scenario for a Healthier, More Equitable and Sustainable Europe in 2040: Citizen Perceptions and Policy Implications. Int. J. Environ. Res. Public Health 2020, 17, 231. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, D.S.; Yu, J.; Rho, J. Bone Loss Triggered by the Cytokine Network in Inflammatory Autoimmune Diseases. J. Immunol. Res. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Walsh, M.C.; Kim, N.; Kadono, Y.; Rho, J.; Lee, S.Y.; Lorenzo, J.; Choi, Y. Osteoimmunology: Interplay between the Immune System and Bone Metabolism. Annu. Rev. Immunol. 2006, 24, 33–63. [Google Scholar] [CrossRef]
- Feng, X.; McDonald, J.M. Disorders of Bone Remodeling. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.G.; Kang, N.N.; Rao, A.V. Lycopene and Other Antioxidants in the Prevention and Treatment of Osteoporosis in Postmenopausal Women. In Aging: Oxidative Stress and Dietary Antioxidants, 1st ed.; Preedy, V.R., Ed.; Academic Press: Kidlington, UK, 2014; pp. 247–258. [Google Scholar]
- Abdollahi, M.; Larijani, B.; Rahimi, R.; Salari, P. Role of Oxidative Stress in Osteoporosis. Therapy 2005, 2, 787–796. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Duan, L.; Ji, Y.; Yang, S.; Zhang, Y.; Li, H.; Wang, Y.; Wang, P.; Chen, J.; et al. Effect of Low-Dose Vitamin K2 Supplementation on Bone Mineral Density in Middle-Aged and Elderly Chinese: A Randomized Controlled Study. Calcif. Tissue Int. 2020, 106, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.G.; Rao, A.V. Lycopene, Tomatoes, and Bone Health. In Lycopene and Tomatoes in Human Nutrition and Health; Rao, A.V., Young, G.L., Rao, L.G., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 89–101. [Google Scholar]
- Cao, J.J. Effects of obesity on bone metabolism. J. Orthop. Surg. Res. 2011, 6. [Google Scholar] [CrossRef]
- Curtis, J.R.; Safford, M.M. Management of Osteoporosis among the Elderly with Other Chronic Medical Conditions. Drugs Aging 2012, 29, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Faienza, M.F.; Ventura, A.; Marzano, F.; Cavallo, L. Postmenopausal osteoporosis: The role of immune system cells. Clin. Dev. 2013, 575936. [Google Scholar] [CrossRef]
- Brunetti Brunetti, G.; Storlino, G.; Oranger, A.; Colaianni, G.; Faienza, M.F.; Ingravallo, G.; Di Comite, M.; Reseland, J.E.; Celi, M.; Tarantino, U.; et al. LIGHT/TNFSF14 regulates estrogen deficiency-induced bone loss. J. Pathol. 2020, 250, 440–451. [Google Scholar] [CrossRef]
- Wade, S.W.; Strader, C.; Fitzpatrick, L.A.; Anthony, M.S.; O’Malley, C.D. Estimating Prevalence of Osteoporosis: Examples from Industrialized Countries. Arch. Osteoporos 2014, 9, 182. [Google Scholar] [CrossRef]
- Heidari, B.; Muhammadi, A.; Javadian, Y.; Bijani, A.; Hosseini, R.; Babaei, M. Associated Factors of Bone Mineral Density and Osteoporosis in Elderly Males. Int. J. Endocrinol. Metab. 2017, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lorenc, R.; Głuszko, P.; Franek, E.; Jabłoński, M.; Jaworski, M.; Kalinka-Warzocha, E.; Karczmarewicz, E.; Kostka, T.; Księżopolska-Orłowska, K.; Marcinowska-Suchowierska, E.; et al. Guidelines for the Diagnosis and Management of Osteoporosis in Poland. Update 2017. Endokrynol. Pol. 2017, 68, 604–609. [Google Scholar] [CrossRef]
- Vielma, J.R.; Picon, D.; Gutiérrez, L.V.; Lara, N.D. Pathophysiology of Osteoporosis: Genes, Oxidative Stress and Immunopathogeny. A Qualitative Systematic Review. Av. Biomed. 2018, 7, 100–111. [Google Scholar]
- Sahni, S.; Kiel, D.P.; Hannan, M.T. Vitamin C and Bone Health. In Nutritional Influences on Bone Health; Weaver, C.M., Daly, R.M., Bischoff-Ferrari, H.A., Eds.; Springer: Cham, Switzerland, 2016; pp. 87–98. [Google Scholar]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Wilson, D.W.; Nash, P.; Singh, H.; Griffiths, K.; Singh, R.; De Meester, F.; Horiuchi, R.; Takahashi, T. The Role of Food Antioxidants, Benefits of Functional Foods, and Influence of Feeding Habits on the Health of the Older Person: An Overview. Antioxidants 2017, 6, 81. [Google Scholar] [CrossRef]
- Gabbay, K.H.; Bohren, K.M.; Morello, R.; Bertin, T.; Liu, J.; Vogel, P. Ascorbate Synthesis Pathway: Dual Role of Ascorbate in Bone Homeostasis. J. Biol. Chem. 2010, 285, 19510–19520. [Google Scholar] [CrossRef]
- Arslan, A.; Orkun, S.; Aydin, G.; Keles, I.; Tosun, A.; Arslan, M.; Caglayan, O. Effects of Ovariectomy and Ascorbic Acid Supplement on Oxidative Stress Parameters and Bone Mineral Density in Rats. Libyan J. Med. 2011, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Malmir, H.; Shab-Bidar, S.; Djafarian, K. Vitamin C Intake in Relation to Bone Mineral Density and Risk of Hip Fracture and Osteoporosis: A Systematic Review and Meta-Analysis of Observational Studies. Br. J. Nutr. 2018, 119, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Finck, H.; Hart, A.R.; Jennings, A.; Welch, A.A. Is There a Role for Vitamin C in Preventing Osteoporosis and Fractures? A Review of the Potential Underlying Mechanisms and Current Epidemiological Evidence. Nutr. Res. Rev. 2014, 27, 268–283. [Google Scholar] [CrossRef]
- Morcos, S.R.; El-Shobaki, F.A.; El-Hawary, Z.; Saleh, N. Effect of Vitamin C and Carotene on the Absorption of Calcium from the Intestine. Z. Ernahrungswiss. 1976, 15, 387–390. [Google Scholar] [CrossRef]
- Chavan, S.N.; More, U.; Mulgund, S.; Saxena, V.; Sontakke, A.N. Effect of Supplementation of Vitamin C and E on Oxidative Stress in Osteoporosis. Indian J. Clin. Biochem. 2007, 22, 101–105. [Google Scholar] [CrossRef]
- Urban, K.; Höhling, H.J.; Lüttenberg, B.; Szuwart, T.; Plate, U. An in Vitro Study of Osteoblast Vitality Influenced by the Vitamins C and E. Head Face Med. 2012, 8, 1–10. [Google Scholar] [CrossRef]
- Podmore, I.D.; Griffiths, H.R.; Herbert, K.E.; Mistry, N.; Mistry, P.; Lunec, J. Vitamin C Exhibits Pro-Oxidant Properties. Nature 1998, 392, 559. [Google Scholar] [CrossRef] [PubMed]
- Brzezińska, O.; Łukasik, Z.; Makowska, J.; Walczak, K. Role of Vitamin C in Osteoporosis Development and Treatment-a Literature Review. Nutrients 2020, 12, 2394. [Google Scholar] [CrossRef] [PubMed]
- Le Nihouannen, D.; Barralet, J.E.; Fong, J.E.; Komarova, S.V. Ascorbic Acid Accelerates Osteoclast Formation and Death. Bone 2010, 46, 1336–1343. [Google Scholar] [CrossRef]
- Rowe, S.; Carr, A.C. Global Vitamin C Status and Prevalence of Deficiency: A Cause for Concern? Nutrients 2020, 12, 2008. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, X.; Zhang, J. Associations between Serum Vitamin E Concentration and Bone Mineral Density in the US Elderly Population. Osteoporos Int. 2017, 28, 1245–1253. [Google Scholar] [CrossRef]
- Yang, T.C.; Macdonald, H.M. Vitamin E Homologues: Current Evidence. In Nutritional Influences on Bone Health; Weaver, C.M., Daly, R.M., Bischoff-Ferrari, H.A., Eds.; Springer: Cham, Switzerland, 2016; pp. 107–120. [Google Scholar]
- Shi, W.; Liu, J.; Cao, Y.; Zhu, Y.; Guan, K.; Chen, Y. Association of Dietary and Serum Vitamin E with Bone Mineral Density in Middle-Aged and Elderly Chinese Adults: A Cross-Sectional Study. Br. J. Nutr. 2016, 115, 113–120. [Google Scholar] [CrossRef]
- Östman, B.; Michaëlsson, K.; Helmersson, J.; Byberg, L.; Gedeborg, R.; Melhus, H.; Basu, S. Oxidative Stress and Bone Mineral Density in Elderly Men: Antioxidant Activity of Alpha-Tocopherol. Free Radic. Biol. Med. 2009, 47, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsao, R. Dietary Polyphenols, Oxidative Stress and Antioxidant and Anti-Inflammatory Effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Jelena, C.H.; Giorgio, R.; Justyna, G.; Neda, M.D.; Natasa, S.; Artur, B.; Giuseppe, G. Beneficial Effects of Polyphenols on Chronic Diseases and Ageing. In Polyphenols: Properties, Recovery and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Duxford, UK, 2018; pp. 69–102. [Google Scholar]
- Sun, K.; Wang, L.; Ma, Q.; Cui, Q.; Lv, Q.; Zhang, W.; Li, X. Association between Tea Consumption and Osteoporosis: A Meta-Analysis. Medicine (Baltimore) 2017, 96, 1–9. [Google Scholar] [CrossRef]
- Passali, C.; Patsaki, A.; Lelovas, P.; Aligiannis, N.; Makropoulou, M.; Kourkoulis, S.; Papaioannou, N.; Mitakou, S.; Skaltsounis, A.L.; Dontas, I. Red Wine Polyphenols Modulate Bone Loss in the Ovariectomized Rat Model of Postmenopausal Osteoporosis. J. Hell. Vet. Med. Soc. 2019, 70, 1541–1550. [Google Scholar] [CrossRef]
- Shen, C.L.; von Bergen, V.; Chyu, M.C.; Jenkins, M.R.; Mo, H.; Chen, C.H.; Kwun, I.S. Fruits and Dietary Phytochemicals in Bone Protection. Nutr. Res. 2012, 32, 897–910. [Google Scholar] [CrossRef]
- Hubert, P.A.; Lee, S.G.; Lee, S.K.; Chun, O.K. Dietary Polyphenols, Berries, and Age-Related Bone Loss: A Review Based on Human, Animal, and Cell Studies. Antioxidants 2014, 3, 144–158. [Google Scholar] [CrossRef]
- Zhang, J.; Lazarenko, O.P.; Blackburn, M.L.; Shankar, K.; Badger, T.M.; Ronis, M.J.J.; Chen, J.R. Feeding Blueberry Diets in Early Life Prevent Senescence of Osteoblasts and Bone Loss in Ovariectomized Adult Female Rats. PLoS ONE 2011, 6, 1–13. [Google Scholar] [CrossRef]
- Chen, J.R.; Lazarenko, O.P.; Wu, X.; Kang, J.; Blackburn, M.L.; Shankar, K.; Badger, T.M.; Ronis, M.J. Dietary-Induced Serum Phenolic Acids Promote Bone Growth via P38 MAPK/β-Catenin Canonical Wnt Signaling. J. Bone Miner. Res. 2010, 25, 2399–2411. [Google Scholar] [CrossRef]
- Devareddy, L.; Hooshmand, S.; Collins, J.K.; Lucas, E.A.; Chai, S.C.; Arjmandi, B.H. Blueberry Prevents Bone Loss in Ovariectomized Rat Model of Postmenopausal Osteoporosis. J. Nutr. Biochem. 2008, 19, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Borowska, S.; Brzóska, M.M. Chokeberries (Aronia Melanocarpa) and Their Products as a Possible Means for the Prevention and Treatment of Noncommunicable Diseases and Unfavorable Health Effects Due to Exposure to Xenobiotics. Compr. Rev. Food Sci. Food Saf. 2016, 15, 982–1017. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, M.M.; Roszczenko, A.; Rogalska, J.; Gałażyn-Sidorczuk, M.; Mężyńska, M. Protective Effect of Chokeberry (Aronia Melanocarpa L.) Extract against Cadmium Impact on the Biomechanical Properties of the Femur: A Study in a Rat Model of Low and Moderate Lifetime Women Exposure to This Heavy Metal. Nutrients 2017, 9, 8–10. [Google Scholar]
- Marchev, S.; Temelkova, K.; Todorova, M.; Eftimov, M.; Georgieva, A.; Kuzmanova, V.; Kuzmanov, A.; Bankova, V.; Surcheva, S.; Vlaskovska, M.; et al. Effects of Antioxidants from Aronia Melanocarpa and Apium Graveolens on Experimental Model of Osteoporosis. In Osteoporosis International; Kanis, J.A., Cosman, F., Eds.; Springer: Cham, Switzerland, 2018; Volume 29. [Google Scholar]
- Lachowicz, S.; Oszmiański, J.; Wilczyńska, M.; Zaguła, G.; Saletnik, B.; Puchalski, C. Impact Mineralization of Chokeberry and Cranberry Fruit Juices Using a New Functional Additive on the Protection of Bioactive Compounds and Antioxidative Properties. Molecules 2020, 25, 659. [Google Scholar] [CrossRef]
- Tanabe, S.; Santos, J.; La, V.D.; Howell, A.B.; Grenier, D. A-Type Cranberry Proanthocyanidins Inhibit the RANKL-Dependent Differentiation and Function of Human Osteoclasts. Molecules 2011, 16, 2365–2374. [Google Scholar] [CrossRef]
- Villarreal, A.; Stoecker, B.J.; Garcia, C.; Garcia, K.; Rios, R.; Gonzales, C.; Mandadi, K.; Faraji, B.; Patil, B.S.; Deyhim, F. Cranberry Juice Improved Antioxidant Status without Affecting Bone Quality in Orchidectomized Male Rats. Phytomedicine 2007, 14, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Huang, Q.; Zhao, K.; Shang, P. Biological Activities and Potential Health Benefit Effects of Polysaccharides Isolated from Lycium Barbarum L. Int. J. Biol. Macromol. 2013, 54, 16–23. [Google Scholar] [CrossRef]
- Zhu, M.; Jinggang, M.; ChangSheng, H.; Haiping, X.; Ning, M.; Caijiao, W. Extraction, Characterization of Polysaccharides from Lycium Barbarum and Its Effect on Bone Gene Expression in Rats. Carbohydr. Polym. 2010, 80, 672–676. [Google Scholar] [CrossRef]
- Faienza, M.; Corbo, F.; Carocci, A.; Catalano, A.; Clodoveo, M.; Grano, M.; Wang, D.; D’Amato, G.; Muraglia, M.; Franchini, C.; et al. Novel insights in health-promoting properties of sweet cherries. J. Funct. Foods 2020, 69, 103945. [Google Scholar] [CrossRef]
- Corbo, F.; Brunetti, G.; Crupi, P.; Bortolotti, S.; Storlino, G.; Piacente, L.; Carocci, A.; Catalano, A.; Milani, G.; Colaianni, G.; et al. Effects of Sweet Cherry Polyphenols on Enhanced Osteoclastogenesis Associated With Childhood Obesity. Front. Immunol. 2019, 10, 1001. [Google Scholar] [CrossRef] [PubMed]
- Gramza-Michałowska, A. Caffeine in Tea Camellia Sinensis-Content, Absorption, Benefits and Risks of Consumption. J. Nutr. Health Aging 2014, 18, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Tang, R. Tea Drinking Habits and Osteoporotic Hip/Femur Fractures: A Case-Control Study. Pakistan J. Med. Sci. 2016, 32, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Kochman, J.; Kwiatkowska, A.; Kałdunska, J.; Dec, K.; Kawczuga, D.; Janda, K. Antioxidant Properties and Nutritional Composition of Matcha Green Tea. Foods 2020, 9, 483. [Google Scholar] [CrossRef] [PubMed]
- Muraki, S.; Yamamoto, S.; Ishibashi, H.; Oka, H.; Yoshimura, N.; Kawaguchi, H.; Nakamura, K. Diet and Lifestyle Associated with Increased Bone Mineral Density: Cross-Sectional Study of Japanese Elderly Women at an Osteoporosis Outpatient Clinic. J. Orthop. Sci. 2007, 12, 317–320. [Google Scholar] [CrossRef]
- Shen, C.L.; Wang, P.; Guerrieri, J.; Yeh, J.K.; Wang, J.S. Protective Effect of Green Tea Polyphenols on Bone Loss in Middle-Aged Female Rats. Osteoporos Int. 2008, 19, 979–990. [Google Scholar] [CrossRef]
- Rapuri, P.B.; Gallagher, J.C.; Kinyamu, H.K.; Ryschon, K.L. Caffeine Intake Increases the Rate of Bone Loss in Elderly Women and Interacts with Vitamin D Receptor Genotypes. Am. J. Clin. Nutr. 2001, 74, 694–700. [Google Scholar] [CrossRef]
- Heaney, R.P. Effects of Caffeine on Bone and the Calcium Economy. Food Chem. Toxicol. 2002, 40, 1263–1270. [Google Scholar] [CrossRef]
- Namkung, W.; Thiagarajah, J.R.; Phuan, P.; Verkman, A.S. Inhibition of Ca 2+ -activated Cl—Channels by Gallotannins as a Possible Molecular Basis for Health Benefits of Red Wine and Green Tea. FASEB J. 2010, 24, 4178–4186. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Rao, A.V. Tomato Lycopene and Its Role in Human Health and Chronic Diseases. CMAJ 2000, 163, 739–744. [Google Scholar] [PubMed]
- Russo, C.; Ferro, Y.; Maurotti, S.; Salvati, M.A.; Mazza, E.; Pujia, R.; Terracciano, R.; Maggisano, G.; Mare, R.; Giannini, S.; et al. Lycopene and Bone: An in Vitro Investigation and a Pilot Prospective Clinical Study. J. Transl. Med. 2020, 18, 43. [Google Scholar] [CrossRef]
- Böhm, V. Lycopene, Tomatoes, and Cardiovascular Diseases. In Lycopene and Tomatoes in Human Nutrition and Health; Rao, A.V., Young, G.L., Rao, L.G., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 51–68. [Google Scholar]
- Rao, L.G.; Krishnadev, N.; Banasikowska, K.; Rao, A.V. Lycopene I-Effect on Osteoclasts: Lycopene Inhibits Basal and Parathyroid Hormone-Stimulated Osteoclast Formation and Mineral Resorption Mediated by Reactive Oxygen Species in Rat Bone Marrow Cultures. J. Med. Food 2003, 6, 69–78. [Google Scholar] [CrossRef]
- Ishimi, Y.; Ohmura, M.; Yamaguchi, X.M.; Ikegami, S. Inhibition by Carotenoids and Retinoic Acid of Osteoclast-like Cell Formation Induced by Bone Resorbing Agents in Vitro. J. Clin. Biochem. Nutr. 1999, 27, 113–122. [Google Scholar] [CrossRef]
- Ardawi, M.S.M.; Badawoud, M.H.; Hassan, S.M.; Rouzi, A.A.; Ardawi, J.M.S.; AlNosani, N.M.; Qari, M.H.; Mousa, S.A. Lycopene Treatment against Loss of Bone Mass, Microarchitecture and Strength in Relation to Regulatory Mechanisms in a Postmenopausal Osteoporosis Model. Bone 2016, 83, 127–140. [Google Scholar] [CrossRef]
- Oliveira, G.R.; Vargas-Sanchez, P.K.; Fernandes, R.R.; Ricoldi, M.S.T.; Semeghini, M.S.; Pitol, D.L.; de Sousa, L.G.; Siessere, S.; Bombonato-Prado, K.F. Lycopene Influences Osteoblast Functional Activity and Prevents Femur Bone Loss in Female Rats Submitted to an Experimental Model of Osteoporosis. J. Bone Miner. Metab. 2019, 37, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Hannan, M.T.; Blumberg, J.; Cupples, L.A.; Kiel, D.P.; Tucker, K.L. Protective Effect of Total Carotenoid and Lycopene Intake on the Risk of Hip Fracture: A 17-Year Follow-up from the Framingham Osteoporosis Study. J. Bone Miner. Res. 2009, 24, 1086–1094. [Google Scholar] [CrossRef]
- Rao, L.G.; Mackinnon, E.S.; Josse, R.G.; Murray, T.M.; Strauss, A.; Rao, A.V. Lycopene Consumption Decreases Oxidative Stress and Bone Resorption Markers in Postmenopausal Women. Osteoporos. Int. 2007, 18, 109–115. [Google Scholar] [CrossRef]
- FAO. Livestock Primary. Available online: http://www.fao.org/faostat/en/?#data/QL (accessed on 27 December 2020).
- Magdelaine, P. Egg and Egg Product Production and Consumption in Europe and the Rest of the World. In Improving the Safety and Quality of Eggs and Egg Products: Egg Chemistry, Production and Consumption; Nys, Y., Bain, M., Immerseel, F.V., Eds.; Woodhead Publishing Limited: Sawston, UK, 2011; pp. 3–16. [Google Scholar]
- Piskorska-Pliszczynska, J.; Mikolajczyk, S.; Warenik-Bany, M.; Maszewski, S.; Strucinski, P. Soil as a Source of Dioxin Contamination in Eggs from Free-Range Hens on a Polish Farm. Sci. Total Environ. 2014, 466–467, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; De, S.D. Eggshell: An Alternative, Cheap, Bioavailable Source of Calcium in Human Diet. Res. Rev. J. Dairy Sci. Technol. 2019, 8, 25–33. [Google Scholar]
- Waheed, M.; Butt, M.S.; Shehzad, A.; Adzahan, N.M.; Shabbir, M.A.; Rasul Suleria, H.A.; Aadil, R.M. Eggshell Calcium: A Cheap Alternative to Expensive Supplements. Trends Food Sci. Technol. 2019, 91, 219–230. [Google Scholar] [CrossRef]
- Brun, L.R.; Lupo, M.; Delorenzi, D.A.; Di Loreto, V.E.; Rigalli, A. Chicken Eggshell as Suitable Calcium Source at Home. Int. J. Food Sci. Nutr. 2013, 64, 740–743. [Google Scholar] [CrossRef]
- Bartter, J.; Diffey, H.; Yeung, Y.H.; O’Leary, F.; Häsler, B.; Maulaga, W.; Alders, R. Use of Chicken Eggshell to Improve Dietary Calcium Intake in Rural Sub-Saharan Africa. Matern. Child Nutr. 2018, 14, 1–10. [Google Scholar] [CrossRef]
- Lesnierowski, G.; Stangierski, J. What’s New in Chicken Egg Research and Technology for Human Health Promotion?-A Review. Trends Food Sci. Technol. 2018, 71, 46–51. [Google Scholar] [CrossRef]
- Roberts, J.R. Factors affecting egg internal quality and egg shell quality in laying hens. J. Poult. Sci. 2004, 41, 161–177. [Google Scholar] [CrossRef]
- Basri, H.; Sulastri, M. Physical Quality of the First Egg of Japanese Quail (Coturnix japonica L.) after Given Liquid Herbal Concoction. Mangifera Edu. 2021, 5, 121–130. [Google Scholar] [CrossRef]
- Kowalska, E.; Kucharska-Gaca, J.; Kuźniacka, J.; Lewko, L.; Gornowicz, E.; Biesek, J.; Adamski, M. Egg quality depending on the diet with different sources of protein and age of the hens. Sci. Rep. 2021, 11, 2638. [Google Scholar] [CrossRef] [PubMed]
- Susanna, L.; Grilli, G.; Ferrari, L.; Battelli, G.; Pozzo, S.; Galasso, I.; Russo, R.; Brasca, M.; Reggiani, R.; Ferrante, V. Effect of Different Percentage of Camelina sativa Cake in Laying Hens Diet: Performance, Welfare, and Eggshell Quality. Animals 2020, 10, 1396. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Wang, J.P.; Ding, X.M.; Bai, S.P.; Qi, S.R.N.; Zeng, Q.F.; Xuan, Y.; Su, Z.W.; Zhang, K.Y. Effect of different tea polyphenol products on egg production performance, egg quality and antioxidative status of laying hens. Anim. Feed Sci. Technol. 2020, 267, 114544. [Google Scholar] [CrossRef]
- Al-awwal, N.Y.; Ali, U.L. Proximate Analyses of Different Samples of Egg Shells Obtained from Sokoto Market in Nigeria. Int. J. Sci. Res. 2015, 4, 564–566. [Google Scholar]
- Hassan, N.M.M. Chicken Eggshell Powder as Dietary Calcium Source in Biscuits. World J. Dairy Food Sci. 2015, 10, 199–206. [Google Scholar]
- Schaafsma, A.; Pakan, I.; Hofstede, G.J.H.; Muskiet, F.A.J.; Van Der Veer, E.; De Vries, P.J.F. Mineral, Amino Acid, and Hormonal Composition of Chicken Eggshell Powder and the Evaluation of Its Use in Human Nutrition. Poult. Sci. 2000, 79, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.S.; Hasan, S.T.; Alssiraj, M.A. Effect of Chicken Eggshell Powder Fortification on the Chemical Physical and Rheologicalcharacteristics of the Bread. Biochem. Cell. Arch. 2019, 19, 543–547. [Google Scholar]
- Ray, S.; Barman, A.K.; Roy, P.K.; Singh, B.K. Chicken Eggshell Powder as Dietary Calcium Source in Chocolate Cakes. Pharma Innov. J. 2017, 6, 1–4. [Google Scholar]
- Afzal, F.; Mueen-ud-Din, G.; Nadeem, M.; Murtaza, M.A.; Mahmood, S. Effect of Eggshell Powder Fortification on the Physicochemical and Organoleptic Characteristics of Muffins. Pure Appl. Biol. 2020, 9, 1488–1496. [Google Scholar] [CrossRef]
- Daengprok, W.; Garnjanagoonchorn, W.; Mine, Y. Fermented Pork Sausage Fortified with Commercial or Hen Eggshell Calcium Lactate. Meat Sci. 2002, 62, 199–204. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Szymanowska-Powałowska, D.; Szymandera-Buszka, K.; Rezler, R.; Jarzębski, M.; Szczepaniak, O.; Marciniak, G.; Jędrusek-Golińska, A.; Kobus-Moryson, M. Effect of Fortification with Calcium from Eggshells on Bioavailability, Quality, and Rheological Characteristics of Traditional Polish Bread Spread. J. Dairy Sci. 2020, 103, 6918–6929. [Google Scholar] [CrossRef]
- Platon, N.; Arus, V.A.; Georgescu, A.M.; Nistor, I.D.; Barsan, N. White Bread Fortified with Calcium from Eggshell Powder. Rev. Chim. 2020, 71, 299–306. [Google Scholar] [CrossRef]
- Chilek, T.Z.T.; Kairuaman, N.A.; Ahmad, F.; Wahab, R.A.; Zamri, A.I.; Mahmood, A. Development of White Bread Fortified with Calcium Derived from Eggshell Powder. Malays. Appl. Biol. 2018, 47, 29–39. [Google Scholar]
- Al Mijan, M.; Choi, K.H.; Kwak, H.S. Physicochemical, Microbial, and Sensory Properties of Nanopowdered Eggshell-Supplemented Yogurt during Storage. J. Dairy Sci. 2014, 97, 3273–3280. [Google Scholar] [CrossRef]
- El-Shibiny, S.; Abd El-Gawad, M.A.E.K.M.; Assem, F.M.; El-Sayed, S.M. The Use of Nano-Sized Eggshell Powder for Calcium Fortification of Cow’s and Buffalo’s Milk Yogurts. Acta Sci. Pol. Technol. Aliment. 2018, 17, 37–49. [Google Scholar]
- Amalraj, A.; Pius, A. Relative Contribution of Oxalic Acid, Phytate and Tannic Acid on the Bioavailability of Calcium from Various Calcium Salts-An in Vitro Study. Int. Food Res. J. 2017, 24, 1278–1285. [Google Scholar]
- Proulx, W.R.; Weaver, C.M.; Bock, M.A. Trypsin Inhibitor Activity and Tannin Content Do Not Affect Calcium Bioavailability of Three Commonly Consumed Legumes. J. Food Sci. 1993, 58, 382–384. [Google Scholar] [CrossRef]
- Gupta, S.; Lakshmi, A.J.; Prakash, J. In Vitro Bioavailability of Calcium and Iron from Selected Green Leafy Vegetables. J. Sci. Food Agric. 2006, 86, 2147–2152. [Google Scholar] [CrossRef]
- Glibowski, P. Dietary Factors Affecting Osteoporosis and Bone Health in the Elderly. In Molecular Basis of Nutrition and Aging: A Volume in the Molecular Nutrition Series; Malavolta, M., Young, G.L., Mocchegiani, E., Eds.; Academic Press: Kidlington, UK, 2016; pp. 345–354. [Google Scholar]
- Houtkooper, L.; Farrell, V.A.; Mullins, V. Calcium Supplement Guidelines; The University of Arizona Cooperative Extension: Tucson, AZ, USA, 2017. [Google Scholar]
- Dubey, M.R.; Patel, V.P. Probiotics: A Promising Tool for Calcium Absorption. Open Nutr. J. 2018, 12, 59–69. [Google Scholar] [CrossRef]
- Schaafsma, A.; Pakan, I. Short-term effects of a chicken egg shell powder enriched dairy-based products on bone mineral density in persons with osteoporosis or osteopenia. Bratisl Lek Listy 1999, 100, 651–656. [Google Scholar] [PubMed]
- Schaafsma, A.; van Doormaal, J.J.; Muskiet, F.A.; Hofstede, G.J.; Pakan, I.; van der Veer, E. Positive effects of a chicken eggshell powder-enriched vitamin-mineral supplement on femoral neck bone mineral density in healthy late post-menopausal Dutch women. Br. J. Nutr. 2002, 87, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Polzonetti, V.; Pucciarelli, S.; Vincenzetti, S.; Polidori, P. Dietary Intake of Vitamin d from Dairy Products Reduces the Risk of Osteoporosis. Nutrients 2020, 12, 1743. [Google Scholar] [CrossRef] [PubMed]
- Veldurthy, V.; Wei, R.; Oz, L.; Dhawan, P.; Jeon, Y.H.; Christakos, S. Vitamin D, Calcium Homeostasis and Aging. Bone Res. 2016, 4, 16041. [Google Scholar] [CrossRef] [PubMed]
- Zhen, D.; Liu, L.; Guan, C.; Zhao, N.; Tang, X. High Prevalence of Vitamin D Deficiency among Middle-Aged and Elderly Individuals in Northwestern China: Its Relationship to Osteoporosis and Lifestyle Factors. Bone 2015, 71, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Cedillo, T. Changes in Nutritional Needs with Aging. In Nutrition and Functional Foods for Healthy Aging; Watson, R.R., Ed.; Academic Press: Kidlington, UK, 2017; pp. 17–22. [Google Scholar]
- ten Haaf, D.S.M.; Balvers, M.G.J.; Timmers, S.; Eijsvogels, T.M.H.; Hopman, M.T.E.; Klein Gunnewiek, J.M.T. Determinants of Vitamin D Status in Physically Active Elderly in the Netherlands. Eur. J. Nutr. 2019, 58, 3121–3128. [Google Scholar] [CrossRef]
- Rahme, M.; Sharara, S.L.; Baddoura, R.; Habib, R.H.; Halaby, G.; Arabi, A.; Singh, R.J.; Kassem, M.; Mahfoud, Z.; Hoteit, M.; et al. Impact of Calcium and Two Doses of Vitamin D on Bone Metabolism in the Elderly: A Randomized Controlled Trial. J. Bone Miner. Res. 2017, 32, 1486–1495. [Google Scholar] [CrossRef]
- Vitezova, A.; Muka, T.; Zillikens, M.C.; Voortman, T.; Uitterlinden, A.G.; Hofman, A.; Rivadeneira, F.; Kiefte-de Jong, J.C.; Franco, O.H. Vitamin D and Body Composition in the Elderly. Clin. Nutr. 2017, 36, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Verhaar, H.J.J. Medical Treatment of Osteoporosis in the Elderly. Aging Clin. Exp. Res. 2009, 21, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Lips, P. Vitamin D Deficiency and Secondary Hyperparathyroidism in the Elderly: Consequences for Bone Loss and Fractures and Therapeutic Implications. Endocr. Rev. 2001, 22, 477–501. [Google Scholar] [CrossRef] [PubMed]
- Nowson, C.A. Prevention of Fractures in Older People with Calcium and Vitamin D. Nutrients 2010, 2, 975–984. [Google Scholar] [CrossRef]
- Taylor, R. Older People and Functional Foods: A Role to Play, but No Silver Bullet. Nutr. Bull. 2011, 36, 403–407. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion in Relation to the Authorisation Procedure for Health Claims on Calcium and Vitamin D and the Reduction of the Risk of Osteoporotic Fractures by Reducing Bone Loss Pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1609. [Google Scholar] [CrossRef]
- Weaver, C.M.; Jakeman, S. Prebiotics, Calcium Absorption, and Bone Health. In Nutritional Influences on Bone Health; Weaver, C.M., Daly, R.M., Bischoff-Ferrari, H.A., Eds.; Springer: Cham, Switzerland, 2016; pp. 145–152. [Google Scholar]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, Prebiotics and Synbiotics-a Review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Świątecka, D.; Bączek, N.; Brzóska, M.M. Inulin and Fructooligosaccharide Affect: In Vitro Calcium Uptake and Absorption from Calcium-Enriched Gluten-Free Bread. Food Funct. 2016, 7, 1950–1958. [Google Scholar] [CrossRef]
- Jakeman, S.A.; Henry, C.N.; Martin, B.R.; McCabe, G.P.; McCabe, L.D.; Jackson, G.S.; Peacock, M.; Weaver, C.M. Soluble Corn Fiber Increases Bone Calcium Retention in Postmenopausal Women in a Dose-Dependent Manner: A Randomized Crossover Trial. Am. J. Clin. Nutr. 2016, 104, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.K.; Patel, A.A. In Vivo Effect of Two Different Dietary Fiber Blends on the Milk Calcium Bioavailability. J. Food Sci. Technol. 2019, 56, 2126–2133. [Google Scholar] [CrossRef] [PubMed]
- Parvaneh, K.; Jamaluddin, R.; Karimi, G.; Erfani, R. Effect of Probiotics Supplementation on Bone Mineral Content and Bone Mass Density. Sci. World J. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gilman, J.; Cashman, K.D. The Effect of Probiotic Bacteria on Transepithelial Calcium Transport and Calcium Uptake in Human Intestinal-like Caco-2 Cells. Curr. Issues Intest. Microbiol. 2006, 7, 1–6. [Google Scholar] [PubMed]
- Jansson, P.A.; Curiac, D.; Ahrén, I.L.; Hansson, F.; Niskanen, T.M.; Sjögren, K.; Ohlsson, C. Probiotic Treatment Using a Mix of Three Lactobacillus Strains for Lumbar Spine Bone Loss in Postmenopausal Women: A Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial. Lancet Rheumatol. 2019, 1, 4–5. [Google Scholar] [CrossRef]
- Scholz-Ahrens, K.E.; Adolphi, B.; Rochat, F.; Barclay, D.V.; de Vrese, M.; Açil, Y.; Schrezenmeir, J. Effects of Probiotics, Prebiotics, and Synbiotics on Mineral Metabolism in Ovariectomized Rats-Impact of Bacterial Mass, Intestinal Absorptive Area and Reduction of Bone Turn-Over. NFS J. 2016, 3, 41–50. [Google Scholar] [CrossRef]
- Mohanty, D.; Misra, S.; Mohapatra, S.; Sahu, P.S. Prebiotics and Synbiotics: Recent Concepts in Nutrition. Food Biosci. 2018, 26, 152–160. [Google Scholar] [CrossRef]
- Scholz-Ahrens, K.E. Prebiotics, Probiotics, Synbiotics and Foods with Regard to Bone Metabolism. In Nutritional Influences on Bone Health; Weaver, C.M., Daly, R.M., Bischoff-Ferrari, H.A., Eds.; Springer: Cham, Switzerland, 2016; pp. 153–167. [Google Scholar]
- Klobukowski, J.; Modzelewska-Kapitula, M.; Kornacki, K. Calcium Bioavailability from Diets Based on White Cheese Containing Probiotics or Synbiotics in Short-Time Study in Rats. Pakistan J. Nutr. 2009, 8, 933–936. [Google Scholar] [CrossRef]
- Schwartz, C.; Vandenberghe-Descamps, M.; Sulmont-Rossé, C.; Tournier, C.; Feron, G. Behavioral and Physiological Determinants of Food Choice and Consumption at Sensitive Periods of the Life Span, a Focus on Infants and Elderly. Innov. Food Sci. Emerg. Technol. 2018, 46, 91–106. [Google Scholar] [CrossRef]
- Vandenberghe-Descamps, M.; Labouré, H.; Septier, C.; Feron, G.; Sulmont-Rossé, C. Oral Comfort: A New Concept to Understand Elderly People’s Expectations in Terms of Food Sensory Characteristics. Food Qual. Prefer. 2018, 70, 57–67. [Google Scholar] [CrossRef]
- Briley, M.E. Food Preferences of the Elderly. Nutr. Rev. 1994, 52, S21–S23. [Google Scholar] [CrossRef]
- Laureati, M.; Pagliarini, E.; Calcinoni, O.; Bidoglio, M. Sensory Acceptability of Traditional Food Preparations by Elderly People. Food Qual. Prefer. 2006, 17, 43–52. [Google Scholar] [CrossRef]
- De Almeida, M.D.V.; Graça, P.; Afonso, C.; Kearney, J.M.; Gibney, M.J. Healthy Eating in European Elderly: Concepts, Barriers and Benefits. J. Nutr. Health Aging 2001, 5, 217–219. [Google Scholar]
- Hsiao, P.Y.; Mitchell, D.C.; Coffman, D.L.; Allman, R.M.; Locher, J.L.; Sawyer, P.; Jensen, G.L.; Hartman, T.J. Dietary Patterns and Diet Quality among Diverse Older Adults: The University of Alabama at Birmingham Study of Aging. J. Nutr. Health Aging 2013, 17, 19–25. [Google Scholar] [CrossRef]
- Ferreira, M.P. do N.; Previdelli, Á.N.; Freitas, T.I. de; Marques, K.M.; Goulart, R.M.M.; Aquino, R. de C. de. Dietary Patterns and Associated Factors among the Elderly. Rev. Bras. Geriatr. Gerontol. 2017, 20, 534–544. [Google Scholar] [CrossRef]
- Capurso, A.; Capurso, C. The Mediterranean Way: Why Elderly People Should Eat Wholewheat Sourdough Bread—a Little Known Component of the Mediterranean Diet and Healthy Food for Elderly Adults. Aging Clin. Exp. Res. 2020, 32, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mingioni, M.; Mehinagic, E.; Laguna, L.; Sarkar, A.; Pirttijärvi, T.; Van Wymelbeke, V.; Artigas, G.; Chen, J.; Kautola, H.; Järvenpää, E.; et al. Fruit and Vegetables Liking among European Elderly According to Food Preferences, Attitudes towards Food and Dependency. Food Qual. Prefer. 2016, 50, 27–37. [Google Scholar] [CrossRef]
- Maruapula, S.; Chapman-Novakofski, K. Health and Dietary Patterns of the Elderly in Botswana. J. Nutr. Educ. Behav. 2007, 39, 311–319. [Google Scholar] [CrossRef]
- Shin, K.J.; Lee, E.J.; Lee, S.J. Study on Demand Elderly Foods and Food Preferences among Elderly People at Senior Welfare Centers in Seoul. J. East Asian Soc. Diet. Life 2016, 26, 1–10. [Google Scholar] [CrossRef]
- Australia New Zealand Food Standards. Standard 2.1.1–Cereal and Cereal Products. Available online: https://www.legislation.gov.au/Details/F2015L00420 (accessed on 22 December 2020).
- Brown, R.D.; Langshaw, M.R.; Uhr, E.J.; Gibson, J.N.; Joshua, D.E. The Impact of Mandatory Fortification of Flour with Folic Acid on the Blood Folate Levels of an Australian Population. Med. J. Aust. 2011, 194, 65–67. [Google Scholar] [CrossRef]
- Beckett, E.L.; Martin, C.; Boyd, L.; Porter, T.; King, K.; Niblett, S.; Yates, Z.; Veysey, M.; Lucock, M. Reduced Plasma Homocysteine Levels in Elderly Australians Following Mandatory Folic Acid Fortification–a Comparison of Two Cross-Sectional Cohorts. J. Nutr. Intermed. Metab. 2017, 8, 14–20. [Google Scholar] [CrossRef]
- Poli, A. The Food Pyramid and the Environmental Pyramid. Available online: http://www.fao.org/ag/humannutrition/25396-02b25569cfe3b55b6da39c3dacc6a26.pdf (accessed on 25 December 2020).
- Chiara, F.; Salvatore, F.P.; Colantuono, F.; Fiore, M. Functional Foods for Elderly People: New Paths for Multi “Functional” Agriculture. Open Agric. 2019, 4, 530–543. [Google Scholar] [CrossRef]
- van der Zanden, L.D.T.; van Kleef, E.; de Wijk, R.A.; van Trijp, H.C.M. Knowledge, Perceptions and Preferences of Elderly Regarding Protein-Enriched Functional Food. Appetite 2014, 80, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Meydani, M. Effect of Functional Food Ingredients: Vitamin E Modulation of Cardiovascular Diseases and Immune Status in the Elderly. Am. J. Clin. Nutr. 2000, 71, 53–56. [Google Scholar] [CrossRef]
- Saunier, K.; Doré, J. Gastrointestinal Tract and the Elderly: Functional Foods, Gut Microflora and Healthy Ageing. Dig. Liver Dis. 2002, 34, S19–S24. [Google Scholar] [CrossRef]
- McCabe, L.R.; Irwin, R.; Schaefer, L.; Britton, R.A. Probiotic Use Decreases Intestinal Inflammation and Increases Bone Density in Healthy Male but Not Female Mice. J. Cell. Physiol. 2013, 228, 1793–1798. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnold, M.; Rajagukguk, Y.V.; Gramza-Michałowska, A. Functional Food for Elderly High in Antioxidant and Chicken Eggshell Calcium to Reduce the Risk of Osteoporosis—A Narrative Review. Foods 2021, 10, 656. https://doi.org/10.3390/foods10030656
Arnold M, Rajagukguk YV, Gramza-Michałowska A. Functional Food for Elderly High in Antioxidant and Chicken Eggshell Calcium to Reduce the Risk of Osteoporosis—A Narrative Review. Foods. 2021; 10(3):656. https://doi.org/10.3390/foods10030656
Chicago/Turabian StyleArnold, Marcellus, Yolanda Victoria Rajagukguk, and Anna Gramza-Michałowska. 2021. "Functional Food for Elderly High in Antioxidant and Chicken Eggshell Calcium to Reduce the Risk of Osteoporosis—A Narrative Review" Foods 10, no. 3: 656. https://doi.org/10.3390/foods10030656
APA StyleArnold, M., Rajagukguk, Y. V., & Gramza-Michałowska, A. (2021). Functional Food for Elderly High in Antioxidant and Chicken Eggshell Calcium to Reduce the Risk of Osteoporosis—A Narrative Review. Foods, 10(3), 656. https://doi.org/10.3390/foods10030656









