Headspace/GC–MS Analysis and Investigation of Antibacterial, Antioxidant and Cytotoxic Activity of Essential Oils and Hydrolates from Rosmarinus officinalis L. and Lavandula angustifolia Miller
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. GC–FID and GC–MS Analysis
2.3. HS/GC–FID and HS/GC–MS Analysis
2.4. Cell Culturing
2.5. Cytotoxicity Test (MTT)
2.6. Antibacterial Activity
2.6.1. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.6.2. Agar Disk-Diffusion Method and Vapor Phase Test (VPT)
2.7. Antioxidant Activity
2.7.1. DPPH Scavenging Activity Assay
2.7.2. ABTS Radical Scavenging Assay
2.8. IC50 and Trolox Equivalents
2.9. Statistical Analysis
3. Results
3.1. Liquid and Vapor Phase EOs Chemical Volatile Composition
3.2. Vapor Phase Hys Chemical Composition
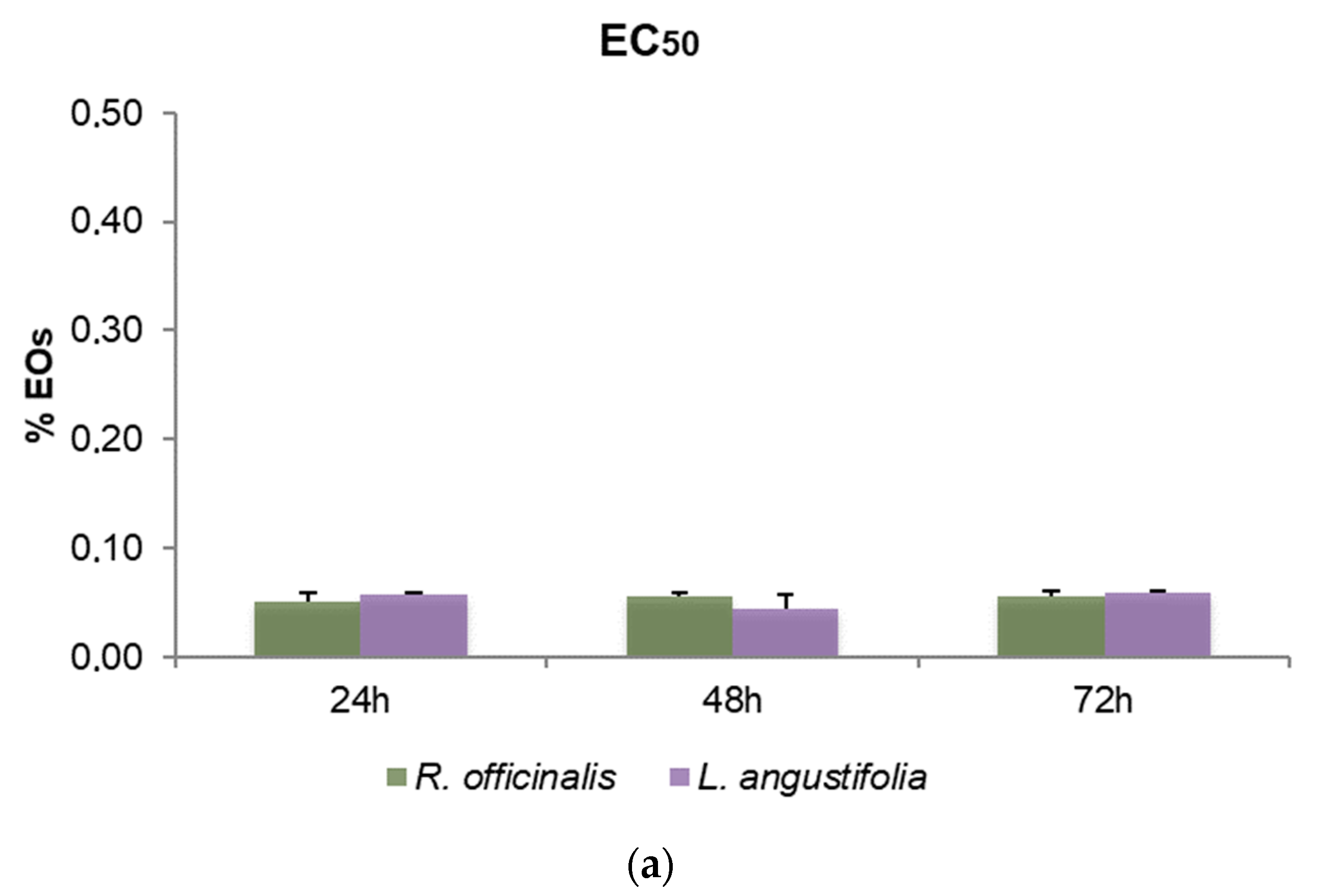
3.3. Cytotoxic Activity
3.4. Antibacterial Activity
3.5. Antioxidant Activity of R. officinalis and L. angustifolia EOs and Hys
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, T.A. Health benefits of culinary herbs and spices. J. AOAC Int. 2019, 102, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Motti, R. Wild Plants Used as Herbs and Spices in Italy: An Ethnobotanical Review. Plants 2021, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Cocan, I.; Alexa, E.; Danciu, C.; Radulov, I.; Galuscan, A.; Obistioiu, D.; Morvay, A.A.; Sumalan, R.M.; Poiana, M.; Pop, G.; et al. Phytochemical screening and biological activity of Lamiaceae family plant extracts. Exp. Ther. Med. 2018, 15, 1863–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamadalieva, N.Z.; Akramov, D.K.; Ovidi, E.; Tiezzi, A.; Nahar, L.; Azimova, S.S.; Sarker, S.D. Aromatic medicinal plants of the Lamiaceae family from Uzbekistan: Ethnopharmacology, essential oils composition, and biological activities. Medicines 2017, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- de Macedo, L.M.; Santos, É.M.D.; Militão, L.; Tundisi, L.L.; Ataide, J.A.; Souto, E.B.; Mazzola, P.G. Rosemary (Rosmarinus officinalis L., syn Salvia rosmarinus Spenn.) and Its Topical Applications: A Review. Plants 2020, 9, 651. [Google Scholar] [CrossRef]
- Ydyrys, A.; Zhaparkulova, N.; Aralbaeva, A.; Mamataeva, A.; Seilkhan, A.; Syraiyl, S.; Murzakhmetova, M. Systematic analysis of combined antioxidant and membrane-stabilizing properties of several Lamiaceae family Kazakhstani plants for potential production of tea beverages. Plants 2021, 10, 666. [Google Scholar] [CrossRef]
- González-Minero, F.J.; Bravo-Díaz, L.; Ayala-Gómez, A. Rosmarinus officinalis L. (Rosemary): An Ancient Plant with Uses in Personal Healthcare and Cosmetics. Cosmetics 2020, 7, 77. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Carvalho-Costa, D.; Cavaleiro, C.; Costa, H.S.; Albuquerque, T.G.; Castilho, M.C.; Ramos, F.; Melo, N.R.; Sanches-Silva, A. A novel insight on an ancient aromatic plant: The rosemary (Rosmarinus officinalis L.). Trends Food Sci. Tech. 2015, 45, 355–368. [Google Scholar] [CrossRef]
- De Oliveira, J.R.; Camargo, S.E.A.; De Oliveira, L.D. Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. J. Biomed. Sci. 2019, 26, 1–22. [Google Scholar] [CrossRef]
- Moja, S.; Guitton, Y.; Nicolè, F.; Legendre, L.; Pasquier, B.; Upson, T.; Jullien, F. Genome size and plastid trnK-matK markers give new insights into the evolutionary history of the genus Lavandula L. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2015, 150, 1216–1224. [Google Scholar] [CrossRef]
- Hassiotis, C.N.; Ntana, F.; Lazari, D.M.; Poulios, S.; Vlachonasios, K.E. Environmental and developmental factors affect essential oil production and quality of Lavandula angustifolia during flowering period. Ind. Crop. Prod. 2014, 62, 359–366. [Google Scholar] [CrossRef]
- de Sousa, D.P.; de Almeida Soares Hocayen, P.; Andrade, L.N.; Andreatini, R. A systematic review of the anxiolytic-like effects of essential oils in animal models. Molecules 2015, 20, 18620–18660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and anti-aging potentials of essential oils from aromatic and medicinal plants. Front. Aging Neurosci. 2017, 9, 168. [Google Scholar] [CrossRef] [Green Version]
- López, V.; Nielsen, B.; Solas, M.; Ramírez, M.J.; Jäger, A.K. Exploring pharmacological mechanisms of lavender (Lavandula angustifolia) essential oil on central nervous system targets. Front. Pharmacol. 2017, 8, 280. [Google Scholar] [CrossRef]
- Nikolić, M.; Jovanović, K.K.; Marković, T.; Marković, D.; Gligorijević, N.; Radulović, S.; Soković, M. Chemical composition, antimicrobial, and cytotoxic properties of five Lamiaceae essential oils. Ind. Crop. Prod. 2014, 61, 225–232. [Google Scholar] [CrossRef]
- Gezici, S. Promising anticancer activity of lavender (Lavandula angustifolia Mill.) essential oil through induction of both apoptosis and necrosis. Ann. Phytomed. 2018, 7, 38–45. [Google Scholar] [CrossRef]
- Garzoli, S.; Turchetti, G.; Giacomello, P.; Tiezzi, A.; Laghezza Masci, V.; Ovidi, E. Liquid and vapour phase of lavandin (Lavandula × intermedia) essential oil: Chemical composition and antimicrobial activity. Molecules 2019, 24, 2701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boukhatem, M.N.; Sudha, T.; Darwish, N.H.E.; Chader, H.; Belkadi, A.; Rajabi, M.; Houche, A.; Benkebailli, F.; Oudjida, F.; Mousa, S.A. A New eucalyptol-rich lavender (Lavandula stoechas L.) essential oil: Emerging potential for therapy against inflammation and cancer. Molecules 2020, 25, 3671. [Google Scholar] [CrossRef]
- Laghezza Masci, V.; Ovidi, E.; Taddei, A.R.; Turchetti, G.; Tiezzi, A.; Giacomello, P.; Garzoli, S. Apoptotic effects on HL60 human leukaemia cells induced by lavandin essential oil treatment. Molecules 2020, 25, 538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd Rashed, A.; Rathi, D.-N.G.; Ahmad Nasir, N.A.H.; Abd Rahman, A.Z. Antifungal properties of essential oils and their compounds for application in skin fungal infections: Conventional and nonconventional approaches. Molecules 2021, 26, 1093. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Gille, E.; Trifan, A.; Luca, V.S.; Miron, A. Essential oils of Lavandula genus: A systematic review of their chemistry. Phytochem. Rev. 2017, 16, 761–799. [Google Scholar] [CrossRef]
- Lawrence, B.M. Progress in essential oils, lavender oils. Perf. Flav. 1993, 18, 58–61. [Google Scholar]
- Cseke, L.J.; Kirakosyan, A.; Kaufman, P.B.; Warber, S.L.; Duke, J.A.; Brielmann, H.L. Natural Products from Plants, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, W.; Du, Z.; Zheng, Y.; Liang, X.; Huang, G.; Zhang, Q.; Liu, Z.; Zhang, K.; Zheng, X.; Lin, L.; et al. Phytochemical composition and bioactivities of essential oils from six Lamiaceae species. Ind. Crop. Prod. 2019, 133, 357–364. [Google Scholar] [CrossRef]
- Prusinowska, R.; Śmigielski, K.B. Composition, biological properties and therapeutic effects of lavender (Lavandula angustifolia L.). A review. Herba Pol. J. 2014, 2, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Begum, A.; Sandhya, S.; Shaffath, A.S.; Vinod, K.R.; Reddy, S.; Banji, D. An in-depth review on the medicinal flora Rosmarinus officinalis (Lamiaceae). Acta Sci. Pol. Technol. Aliment. 2013, 12, 61–73. [Google Scholar] [PubMed]
- Edris, A.E. Identification and absolute quantification of the major water-soluble aroma components isolated from the hydrosols of some aromatic plants. J. Essent. Oil Bear. Plants 2009, 12, 155–161. [Google Scholar] [CrossRef]
- Hamedi, A.; Moheimani, S.M.; Sakhteman, A.; Etemadfard, H.; Moein, M. An overview on indications and chemical composition of aromatic waters (hydrosols) as functional beverages in Persian nutrition culture and folk medicine for hyperlipidemia and cardiovascular conditions. J. Evid. Based Complement. Altern. 2017, 22, 544–561. [Google Scholar] [CrossRef]
- D’Amato, S.; Serio, A.; López, C.C.; Paparella, A. Hydrosols: Biological activity and potential as antimicrobials for food applications. Food Control. 2018, 86, 126–137. [Google Scholar] [CrossRef]
- Ovidi, E.; Laghezza Masci, V.; Zambelli, M.; Tiezzi, A.; Vitalini, S.; Garzoli, S. Laurus nobilis, Salvia sclarea and Salvia officinalis Essential Oils and Hydrolates: Evaluation of Liquid and Vapor Phase Chemical Composition and Biological Activities. Plants 2021, 10, 707. [Google Scholar] [CrossRef]
- Garzoli, S.; Masci, V.L.; Caradonna, V.; Tiezzi, A.; Giacomello, P.; Ovidi, E. Liquid and Vapor Phase of Four Conifer-Derived Essential Oils: Comparison of Chemical Compositions and Antimicrobial and Antioxidant Properties. Pharmaceuticals 2021, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liang, X. Headspace Gas Chromatography-Mass Spectrometry for Volatile Components Analysis in Ipomoea Cairica (L.) Sweet Leaves: Natural Deep Eutectic Solvents as Green Extraction and Dilution Matrix. Foods 2019, 8, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gören, A.C.; Topçu, A.; Bilsel, G.; Bilsel, M.; Wilkinson, J.M.; Cavanagh, H.M. Analysis of essential oil of Satureja thymbra by hydrodistillation, thermal desorber, and headspace GC/MS techniques and its antimicrobial activity. Nat. Prod. Res. 2004, 18, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Moulay Sadiki, M.; Koraichi Ibnsouda, S. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinical and Laboratory Standards Institute. M23–A3: Development of In Vitro Susceptibility Testing Criteria and Quality Control Parameters; Approved Guideline, 3rd ed.; CLSI Document; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; pp. 1–68. [Google Scholar]
- Gatsing, D.; Tchakoute, V.; Ngamga, D.; Kuiate, J.; Tamokou, J.; Nji-Nkah, B.; Tchouanguep, F.; Fodouop, S. In vitro antibacterial activity of Crinum purpurascens Herb. leaf extract against the Salmonella species causing typhoid fever and its toxicological evaluation. Iran. J. Med. Sci. 2009, 34, 126–136. [Google Scholar]
- Sanchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Hollander, M.; Douglas, A.W. Nonparametric Statistical Methods; John Wiley & Sons: New York, NY, USA, 1999; pp. 106–272. [Google Scholar]
- Al-Younis, F.; Al-Naser, Z.; Al-Hakim, W. Chemical composition of Lavandula angustifolia Miller and Rosmarinus officinalis L. essential oils and fumigant toxicity against larvae of Ephestia kuehniella Zeller. Int. J. Chem. Technol. Res. 2015, 8, 1382–1390. [Google Scholar]
- Xiaotian, C.; Lanyue, Z.; Chenyu, Q.; Zhiyun, D.; Peng, X.; Zhangmin, X. Chemical compositions of essential oil extracted from Lavandula angustifolia and its prevention of TPA-induced inflammation. Microchem. J. 2020, 153, 104458. [Google Scholar]
- Verma, R.S.; Rahman, L.U.; Chanotiya, C.S.; Verma, R.K.; Chauhan, A.; Yadav, A.; Singh, A.; Yadav, A.K. Essential oil composition of Lavandula angustifolia Mill. cultivated in the mid hills of Uttarakhand, India. J. Serb. Chem. Soc. 2010, 75, 343–348. [Google Scholar] [CrossRef]
- Jianga, Y.; Wua, N.; Fua, Y.; Wanga, W.; Luoa, M.; Zhao, C.; Zua, Y.; Liu, X. Chemical composition and antimicrobial activity of the essential oil of Rosemary. Environ. Toxicol. Pharmacol. 2011, 32, 63–68. [Google Scholar] [CrossRef]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef] [Green Version]
- Jamshidi, R.; Afzali, Z.; Afzali, D. Chemical Composition of Hydrodistillation Essential Oil of Rosemary in Different Origins in Iran and Comparison with Other Countries. Am. Eurasian J. Agric. Environ. Sci. 2009, 5, 78–81. [Google Scholar]
- Özcan, M.M.; Chalchat, J.-C. Chemical composition and antifungal activity of rosemary (Rosmarinus officinalis L.) oil from Turkey. Int. J. Food Sci. Nutr. 2008, 59, 691–698. [Google Scholar] [CrossRef]
- Tomi, K.; Kitao, M.; Konishi, N.; Murakami, H.; Matsumura, Y.; Hayashi, T. Enantioselective GC–MS analysis of volatile components from rosemary (Rosmarinus officinalis L.) essential oils and hydrosols. Biosci. Biotechnol. Biochem. 2016, 80, 840–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hay, Y.-O.; Abril-Sierra, M.A.; Sequeda-Castañeda, L.G.; Bonnafous, C.; Raynaud, C. Evaluation of combinations of essential oils and essential oils with hydrosols on antimicrobial and antioxidant activities. J. Pharm. Pharmacogn. Res. 2018, 6, 216–230. [Google Scholar]
- Hyeon Jeon, D.; Yong Moon, J.; Bong Hyun, H.; Kim Cho, S. Composition Analysis and Antioxidant Activities of the Essential Oil and the Hydrosol Extracted from Rosmarinus officinalis L. and Lavandula angustifolia Mill. Produced in Jeju. J. Appl. Biol. Chem. 2013, 56, 141–146. [Google Scholar] [CrossRef]
- Śmigielski, K.B.; Prusinowska, R.; Krosowiak, K.; Sikora, M. Comparison of qualitative and quantitative chemical composition of hydrolate and essential oils of lavender (Lavandula angustifolia). J. Essent. Oil Res. 2013, 25, 291–299. [Google Scholar] [CrossRef]
- Šilha, D.; Švarcová, K.; Bajer, T.; Královec, K.; Tesařová, E.; Moučková, K.; Pejchalová, M.; Bajerová, P. Chemical Composition of Natural Hydrolates and Their Antimicrobial Activity on Arcobacter-Like Cells in Comparison with Other Microorganisms. Molecules 2020, 25, 5654. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, M.G.; Tešević, V.V.; Smiljanić, K.T.; Cvetković, M.T.; Stanković, J.M.; Kiprovski, B.M.; Sikora, V.S. Hydrolates—by-products of essential oil distillation: Chemical composition, biological activity and potential uses. Adv. Mater. Technol. 2020, 9, 54–70. [Google Scholar]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–56. [Google Scholar] [CrossRef]
- Benny, A.; Thomas, J. Essential oils as treatment strategy for Alzheimer’s disease: Current and future perspectives. Planta Med. 2019, 85, 239–248. [Google Scholar] [PubMed] [Green Version]
- Elshafie, H.S.; Camele, I. An Overview of the biological effects of some mediterranean essential oils on human health. Biomed. Res. Int. 2017, 2017, 9268468. [Google Scholar] [CrossRef] [PubMed]
- Şimşek Sezer, E.; Bozkurt, M.; Tulukcu, E.; Uysal, T. Comparative Cytotoxic Effects of the Hydrosols of Some Ethnobotanic Plants. Curr. Perspect. Med. Aromat. Plants (CUPMAP) 2020, 3, 97–103. [Google Scholar] [CrossRef]
- Karampoula, F.; Giaouris, E.; Deschamps, J.; Doulgeraki, A.; Nychas, G.J.E.; Dubois-Brissonnet, F. Hydrosol of Thymbra capitata is a highly efficient biocide against Salmonella enterica serovar typhimurium biofilm. Appl. Environ. Microbiol. 2016, 82, 5309–5319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chorianopoulos, N.G.; Giaouris, E.D.; Skandamis, P.N.; Haroutounian, S.A.; Nychas, G.J.E. Disinfectant test agains monoculture and mixed-culture biofilms composed of technological, spoilage and pathogenic bacteria: Bactericidal effect of essential oil and hydrosol of Satureja thymbra and comparison with standard acid-base sanitizers. J. Appl. Microbiol. 2008, 104, 1586–1596. [Google Scholar] [CrossRef]
- Garzoli, S.; Petralito, S.; Ovidi, E.; Turchetti, G.; Masci, V.L.; Tiezzi, A.; Trilli, J.; Cesa, S.; Casadei, M.A.; Giacomello, P.; et al. Lavandula x intermedia essential oil and hydrolate: Evaluation of chemical composition and antibacterial activity before and after formulation in nanoemulsion. Ind. Crop. Prod. 2020, 145, 112068. [Google Scholar] [CrossRef]
- Inouye, S. A comparative study on the composition of forty-four hydrosols and their essential oils. Int. J. Essent. Oil Ther. 2008, 2, 89–104. [Google Scholar]
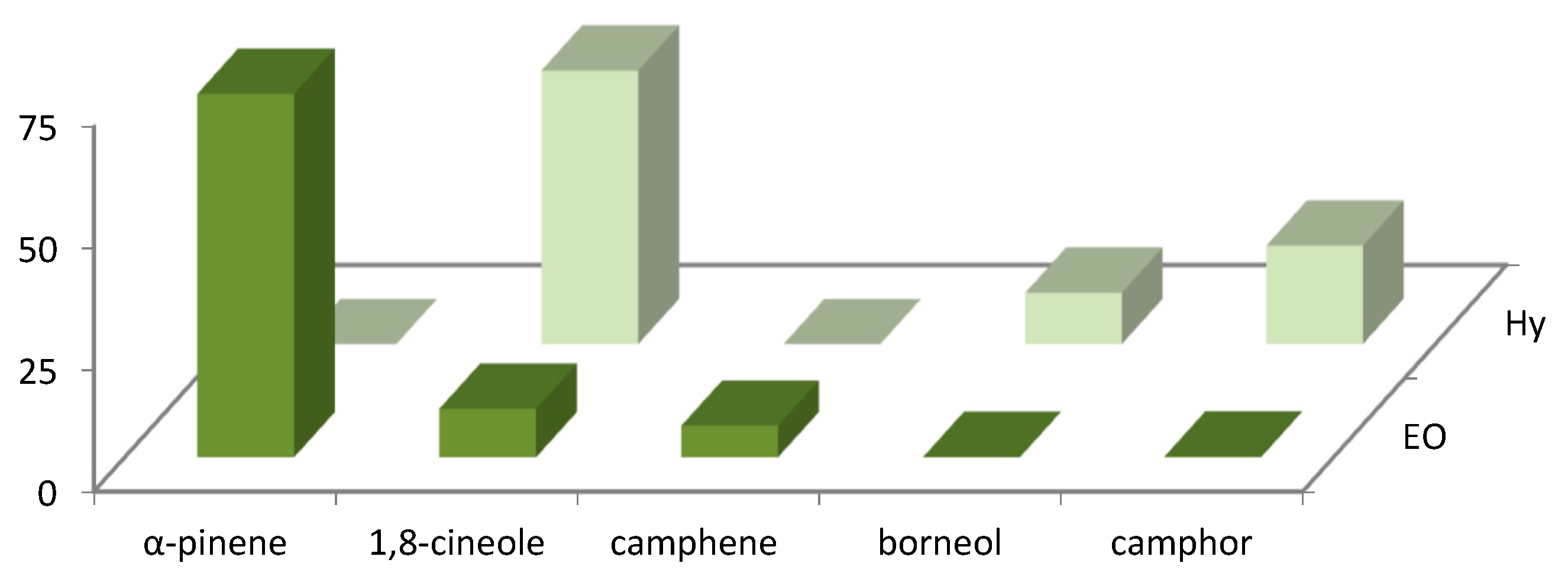
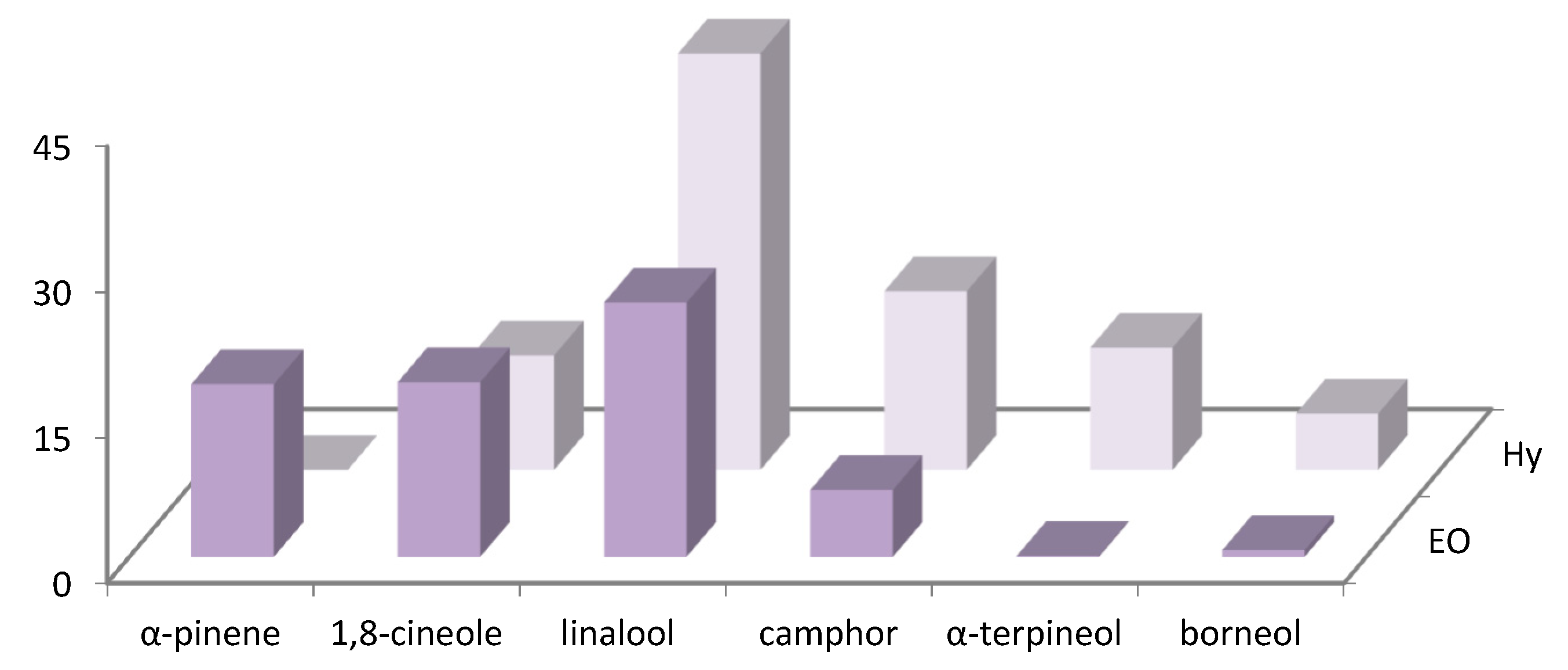

| N° | COMPONENT 1 | LRI 2 | LRI 3 | R. officinalis (%) 4 | R. officinalis (%) 5 |
|---|---|---|---|---|---|
| 1 | α-pinene | 945 | 943 | 51.2 ± 0.02 | 74.7 ± 0.1 |
| 2 | camphene | 948 | 946 | 5.6 ± 0.04 | 6.5 ± 0.02 |
| 3 | dehydrosabinene | 961 | 958 | 1.1 ± 0.04 | 1.1 ± 0.02 |
| 4 | β-myrcene | 982 | 983 | 2.0 ± 0.05 | 1.1 ± 0.02 |
| 5 | β-pinene | 990 | 986 | 3.4 ± 0.03 | 2.8 ± 0.02 |
| 6 | α-phellandrene | 1000 | 1006 * | 0.2 ± 0.02 | - |
| 7 | α-terpinene | 1010 | 1008 | 0.4 ± 0.02 | 0.2 ± 0.02 |
| 8 | p-cymene | 1020 | 1016 | 2.0 ± 0.05 | 0.9 ± 0.02 |
| 9 | limonene | 1022 | 1023 | 4.8 ± 0.03 | 1.7 ± 0.02 |
| 10 | 1,8-cineole | 1025 | 1027 | 20.1 ± 0.15 | 10.0 ± 0.05 |
| 11 | γ-terpinene | 1051 | 1054 | 0.9 ± 0.04 | 0.3 ± 0.02 |
| 12 | terpinolene | 1080 | 1078 | 0.7 ± 0.02 | 0.2 ± 0.02 |
| 13 | p-cymenene | 1085 | 1083.4 | 0.1 ± 0.01 | - |
| 14 | linalool | 1096 | 1092 | 1.0 ± 0.05 | 0.2 ± 0.02 |
| 15 | camphor | 1125 | 1126 | 0.8 ± 0.06 | 0.2 ± 0.04 |
| 16 | borneol | 1155 | 1152 | 0.2 ± 0.02 | 0.1 ± 0.01 |
| 17 | endo-borneol | 1158 | 1155 | 0.9 ± 0.03 | - |
| 18 | terpinen-4-ol | 1161 | 1160 | 0.3 ± 0.02 | - |
| 19 | α-terpineol | 1182 | 1183 | 0.4 ± 0.01 | - |
| 20 | verbenone | 1192 | 1196 | 0.6 ± 0.02 | - |
| 21 | geraniol | 1234 | 1237 | 0.4 ± 0.02 | - |
| 22 | bornyl acetate | 1262 | 1268 | 1.0 ± 0.07 | - |
| 23 | nerol acetate | 1362 | 1363 | 0.3 ± 0.02 | - |
| 24 | β-caryophyllene | 1424 | 1426 | 1.4 ± 0.03 | - |
| 25 | α-curcumene | 1478 | 1475 | 0.1 ± 0.02 | - |
| 26 | caryophyllene oxide | 1586 | 1583 | 0.1 | - |
| SUM (%) | 100.0 | 100.0 | |||
| Monoterpene hydrocarbons | 71.5 | 89.5 | |||
| Oxygenated monoterpenes | 26.0 | 10.5 | |||
| Sesquiterpene hydrocarbons | 1.5 | - | |||
| Oxygenated sesquiterpene | 0.1 | - | |||
| Others | - | - |
| N° | COMPONENT 1 | LRI 2 | LRI 3 | L. angustifolia (%) 4 | L. angustifolia (%) 5 |
|---|---|---|---|---|---|
| 1 | α-pinene | 945 | 943 | 1.6 ± 0.02 | 17.8 ± 0.02 |
| 2 | camphene | 948 | 946 | 0.8 ± 0.02 | 8.9 ± 0.05 |
| 3 | β-pinene | 990 | 986 | 2.3 ± 0.02 | 1.2 ± 0.02 |
| 4 | α-phellandrene | 1000 | 1006 * | 0.1 ± 0.02 | 0.5 ± 0.05 |
| 5 | α-terpinene | 1010 | 1008 | 0.2 ± 0.02 | 0.5 ± 0.03 |
| 6 | p-cymene | 1020 | 1016 | 0.1 ± 0.02 | 1.0 ± 0.02 |
| 7 | limonene | 1022 | 1023 | 1.2 ± 0.02 | 6.2 ± 0.02 |
| 8 | 1,8-cineole | 1025 | 1027 | 5.7 ± 0.02 | 18.0 ± 0.03 |
| 9 | cis-β-ocimene | 1033 | 1032 | 0.1 ± 0.02 | 3.2 ± 0.05 |
| 10 | trans-β-ocimene | 1041 | 1043 | 1.4 ± 0.02 | 3.5 ± 0.03 |
| 11 | γ-terpinene | 1051 | 1054 | 0.6 ± 0.02 | 2.3 ± 0.03 |
| 12 | linalol oxide | 1073 | 1073 | 0.5 ± 0.02 | 0.2 ± 0.03 |
| 13 | terpinolene | 1080 | 1078 | 0.6 ± 0.01 | 1.1 ± 0.03 |
| 14 | linalool | 1096 | 1092 | 49.9 ± 0.14 | 26.2 ± 0.05 |
| 15 | camphor | 1125 | 1126 | 3.2 ± 0.04 | 6.9 ± 0.05 |
| 16 | borneol | 1155 | 1152 | 3.9 ± 0.02 | 0.7 ± 0.02 |
| 17 | terpinen-4-ol | 1161 | 1160 | 5.0 ± 0.05 | 1.4 ± 0.02 |
| 18 | α-terpineol | 1182 | 1183 | 0.8 ± 0.04 | 0.1 ± 0.02 |
| 19 | linalyl acetate | 1251 | 1252 | 17.9 ± 0.02 | 0.2 ± 0.02 |
| 20 | β-caryophyllene | 1424 | 1426 | 1.5 ± 0.02 | - |
| 21 | cis-β-farnesene | 1444 | 1441 | 0.8 ± 0.01 | - |
| 22 | β-bisabolene | 1500 | 1501 | 0.3 ± 0.02 | - |
| 23 | α-farnesene | 1505 | 1506 | 1.2 ± 0.02 | - |
| 24 | caryophyllene oxide | 1586 | 1583 | tr | - |
| 25 | α-bisabolol | 1662 | 1665 | 0.3 ± 0.02 | - |
| SUM (%) | 100.0 | 99.9 | |||
| Monoterpene hydrocarbons | 9.0 | 46.2 | |||
| Oxygenated monoterpenes | 86.9 | 53.7 | |||
| Sesquiterpene hydrocarbons | 3.8 | - | |||
| Oxygenated sesquiterpene | 0.3 | - | |||
| Others | - | - |
| N° | COMPONENT 1 | LRI 2 | LRI 3 | R. officinalis (%) 4 | L. angustifolia (%) 5 |
|---|---|---|---|---|---|
| 1 | 1,8-cineole | 1025 | 1027 | 56.2 ± 0.04 | 11.8 ± 0.03 |
| 2 | linalol oxide | 1073 | 1073 | - | 0.1 ± 0.01 |
| 3 | linalool | 1096 | 1092 | 4.2 ± 0.02 | 42.9 ± 0.05 |
| 4 | camphor | 1125 | 1126 | 20.3 ± 0.02 | 18.4 ± 0.02 |
| 5 | borneol | 1155 | 1152 | 10.6 ± 0.02 | 5.8 ± 0.02 |
| 6 | terpinen-4-ol | 1161 | 1160 | 1.6 ± 0.02 | 8.4 ± 0.02 |
| 7 | α-terpineol | 1182 | 1183 | 2.0 ± 0.04 | 12.6 ± 0.02 |
| 8 | verbenone | 1192 | 1196 | 5.1 ± 0.09 | - |
| SUM (%) | 100.0 | 100.0 | |||
| Monoterpene hydrocarbons | - | - | |||
| Oxygenated monoterpenes | 100.0 | 100.0 | |||
| Sesquiterpene hydrocarbons | - | - | |||
| Oxygenated sesquiterpene | - | - | |||
| Others | - | - |
| Sample | p-Value |
|---|---|
| R. officinalis EOs | 0.717 |
| L. angustifolia EOs | 0.097 |
| R. officinalis Hys | 0.050 |
| L. angustifolia Hys | 0.050 |
| R. officinalis EO | R. officinalis Hy | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC 1 | MBC 2 | MBC/MIC Ratio | IZ 3 | VIZ 4 | MIC 1 | MBC 2 | MBC/MIC Ratio | IZ 3 | VIZ 4 | |
| E. coli | 3.13 | 3.13 | 1.00 | 7.00 ± 0.00 | - | na | na | - | - | - |
| P. fluorescens | 3.13 | 6.25 | 0.50 | - | - | na | na | - | - | - |
| A. bohemicus | 0.19 | 0.39 | 0.50 | 9.17 ± 0.76 | 80 ± 00 | na | na | - | - | - |
| K. marina | 1.56 | 3.13 | 0.50 | 7.33 ± 0.58 | 80 ± 00 | na | na | - | - | - |
| B. cereus | 0.39 | 0.39 | 1.00 | 8.33 ± 1.52 | 80 ± 00 | na | na | - | - | - |
| L. angustifolia EO | L. angustifolia Hy | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC 1 | MBC 2 | MBC/MIC Ratio | IZ 3 | VIZ 4 | MIC 1 | MBC 2 | MBC/MIC Ratio | IZ 3 | VIZ 4 | |
| E. coli | 0.39 | 0.39 | 1.00 | 11.00 ± 1.00 | - | na | na | - | - | - |
| P. fluorescens | 1.56 | 3.13 | 0.50 | 7.17 ± 0.76 | - | na | na | - | - | - |
| A. bohemicus | 0.19 | 0.39 | 0.50 | 11.67 ± 1.15 | 6.17 ± 1.04 | na | na | - | - | - |
| K. marina | 0.78 | 0.78 | 1.00 | 11.33 ± 1.53 | - | na | na | - | - | - |
| B. cereus | 0.19 | 0.19 | 1.00 | 10.67 ± 0.58 | 0.67 ± 1.15 | na | na | - | - | - |
| R. officinalis EO | R. officinalis Hy | L. angustifolia EO | L. angustifolia Hy | ||
|---|---|---|---|---|---|
| DPPH | IC50 * | 13.48 ± 1.59 | 136.30 ± 3.85 | 7.75 ± 0.10 | 240.02 ± 13.65 |
| TEAC ** | 1.90 ± 0.15 | 0.22 ± 0.03 | 3.30 ± 0.10 | 0.12 ± 0.01 | |
| ABTS•+ | IC50 * | 20.20 ± 2.72 | 349.42 ± 19.32 | 18.71 ± 2.16 | 181.24 ±15.71 |
| TEAC ** | 23.53 ± 2.43 | 1.35 ± 0.03 | 25.45 ± 3.73 | 2.62 ± 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garzoli, S.; Laghezza Masci, V.; Franceschi, S.; Tiezzi, A.; Giacomello, P.; Ovidi, E. Headspace/GC–MS Analysis and Investigation of Antibacterial, Antioxidant and Cytotoxic Activity of Essential Oils and Hydrolates from Rosmarinus officinalis L. and Lavandula angustifolia Miller. Foods 2021, 10, 1768. https://doi.org/10.3390/foods10081768
Garzoli S, Laghezza Masci V, Franceschi S, Tiezzi A, Giacomello P, Ovidi E. Headspace/GC–MS Analysis and Investigation of Antibacterial, Antioxidant and Cytotoxic Activity of Essential Oils and Hydrolates from Rosmarinus officinalis L. and Lavandula angustifolia Miller. Foods. 2021; 10(8):1768. https://doi.org/10.3390/foods10081768
Chicago/Turabian StyleGarzoli, Stefania, Valentina Laghezza Masci, Sara Franceschi, Antonio Tiezzi, Pierluigi Giacomello, and Elisa Ovidi. 2021. "Headspace/GC–MS Analysis and Investigation of Antibacterial, Antioxidant and Cytotoxic Activity of Essential Oils and Hydrolates from Rosmarinus officinalis L. and Lavandula angustifolia Miller" Foods 10, no. 8: 1768. https://doi.org/10.3390/foods10081768
APA StyleGarzoli, S., Laghezza Masci, V., Franceschi, S., Tiezzi, A., Giacomello, P., & Ovidi, E. (2021). Headspace/GC–MS Analysis and Investigation of Antibacterial, Antioxidant and Cytotoxic Activity of Essential Oils and Hydrolates from Rosmarinus officinalis L. and Lavandula angustifolia Miller. Foods, 10(8), 1768. https://doi.org/10.3390/foods10081768










