Pigmented Corn Varieties as Functional Ingredients for Gluten-Free Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
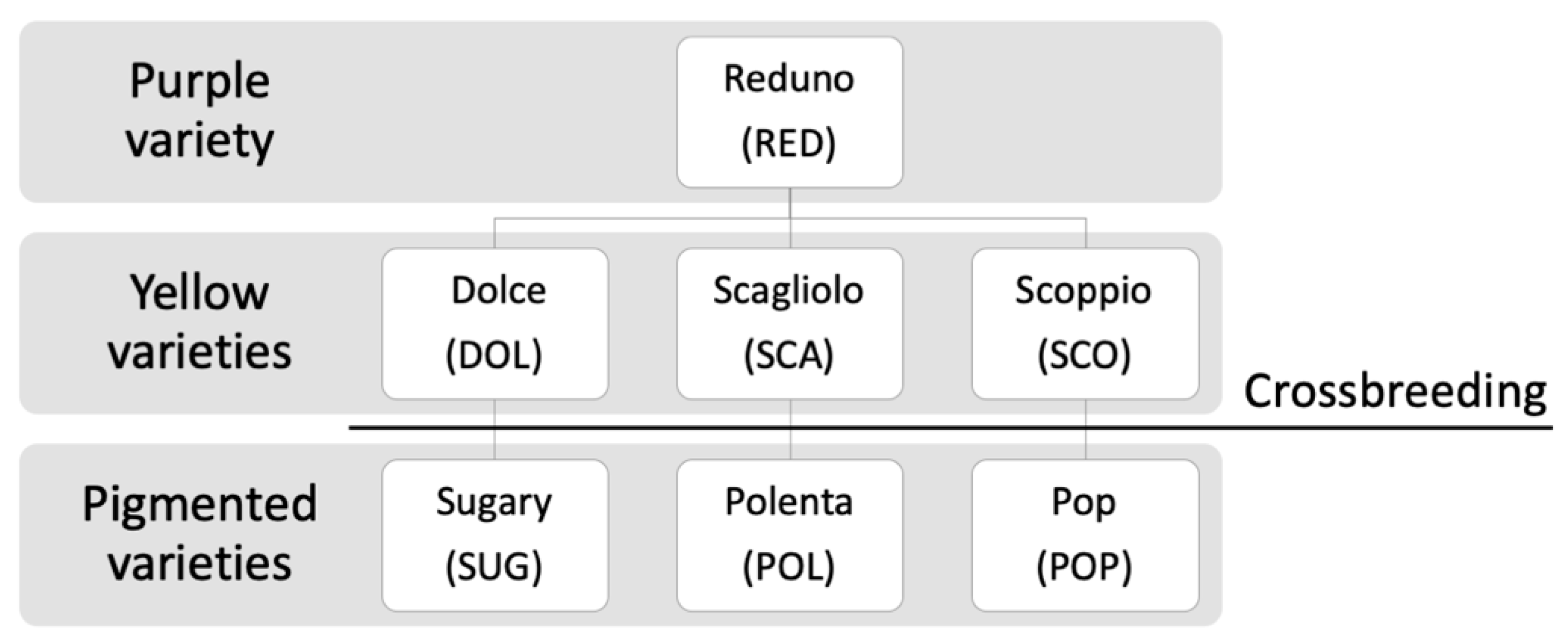
2.2. Samples
2.3. Cellular Tests as Models for the Evaluation of the Safety of Pigmented Corn Varieties in Celiac Patients
2.3.1. In Vitro Peptic-Tryptic Digestion
2.3.2. Cell Lines
2.3.3. K562(S) Cell Agglutination Test
2.3.4. Measurement of Transepithelial Electrical Resistance (TEER) across T84 Cell Monolayer
2.4. Determination of Soluble Phenolic Content
2.4.1. Extraction of Soluble Phenolic Compounds
2.4.2. Folin–Ciocalteau’s Assay
2.5. Evaluation of Antioxidant Capacity
2.5.1. DPPH Method
2.5.2. TEAC Assay
2.5.3. High Performance Thin Layer Chromatography (HPTLC)
- Sample preparation
- Soluble phenolic compounds were extracted as previously described (Section 2.4.1), but dried supernatant was suspended in 0.5 mL of methanol.
- Chromatographic conditions
- Aliquots of 5 μL of standard solutions of phenolic acids (coumaric acid and ferulic acid) at the concentration of 200 μg/mL were applied on silica-gel plates 254F (10 × 20 cm, Merck, Darmstadt, Germany), in parallel to corn samples (10 μL) using a semi-auto/sample applicator (Linomat 4, CAMAG, Muttenz, Switzerland). The chromatographic run was conducted using 10 mL of acetone:toluene:formic acid 4.5:4.5:1 (v/v/v).
2.6. Anthocyanins Characterization
2.6.1. Samples Preparation
2.6.2. Qualitative LC-MS Analysis
2.6.3. Spectrophotometric Analysis
2.6.4. Quantification by HPLC-DAD Method
2.7. In Vitro Gastro-Intestinal Digestion
2.7.1. Samples Preparation
2.7.2. Digestion Protocol
2.8. Statistical Analysis
3. Results and Discussion
3.1. Cellular Tests
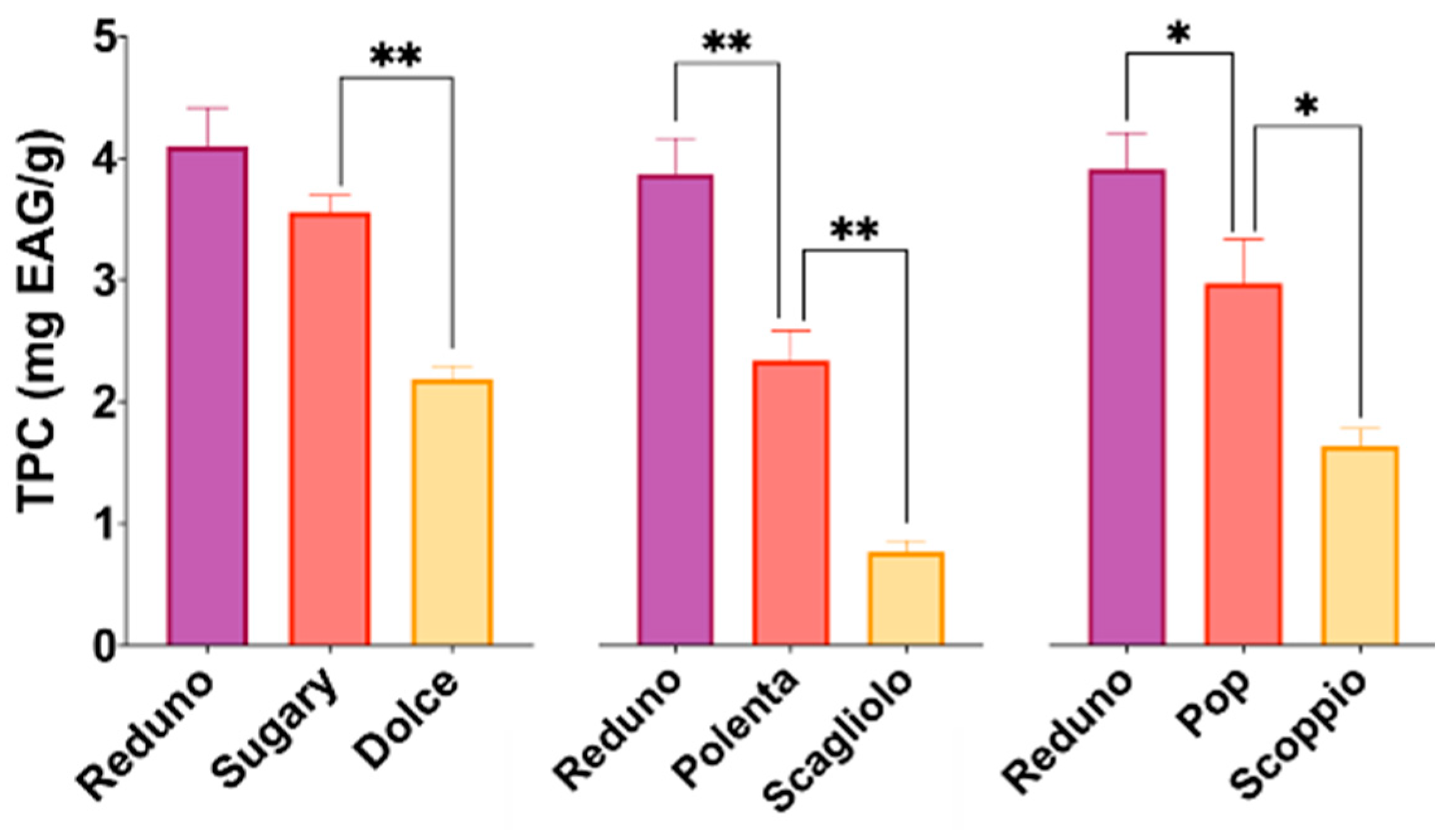
3.2. Polyphenols Content
3.3. Antioxidant Capacity
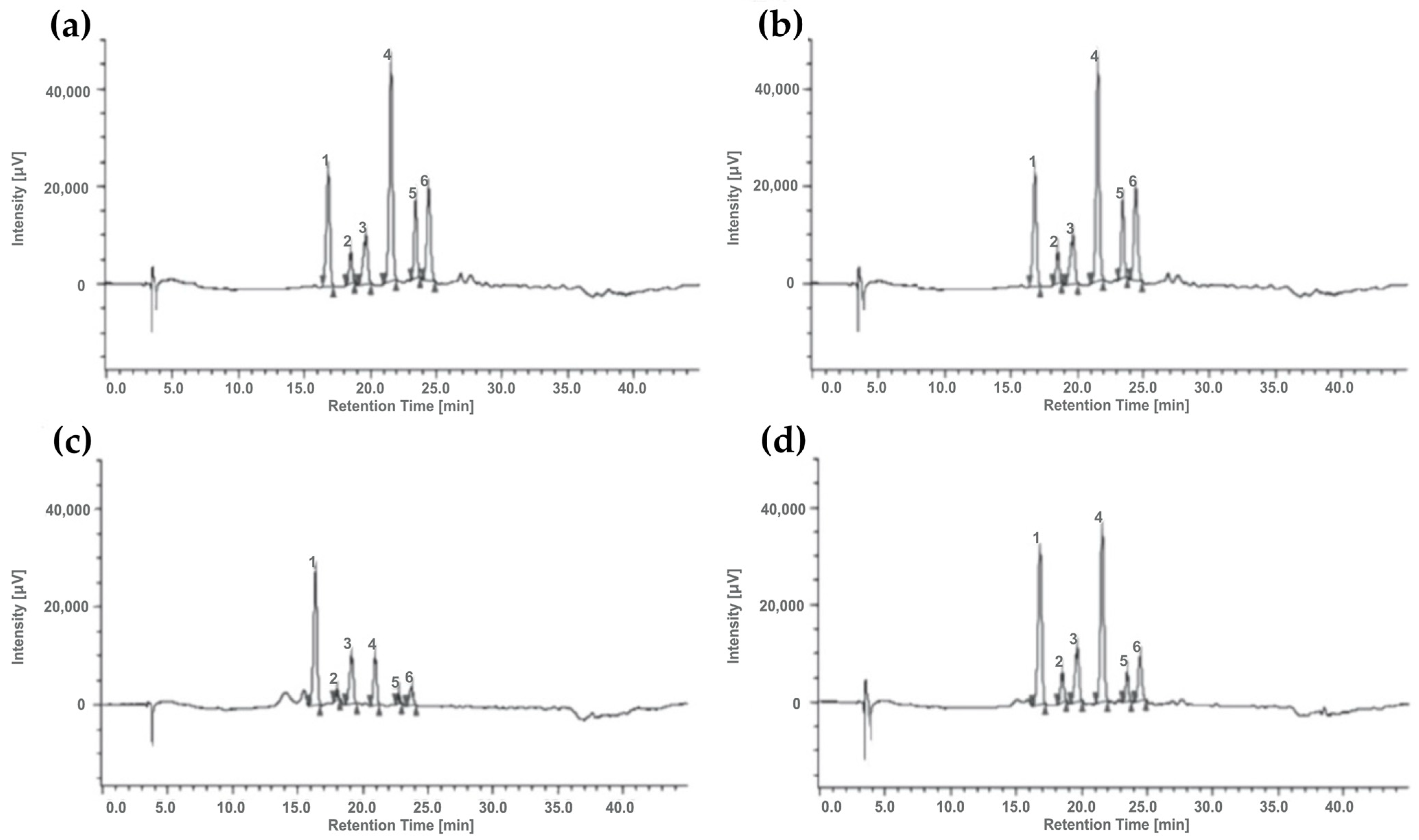
3.4. Anthocyanin Characterization
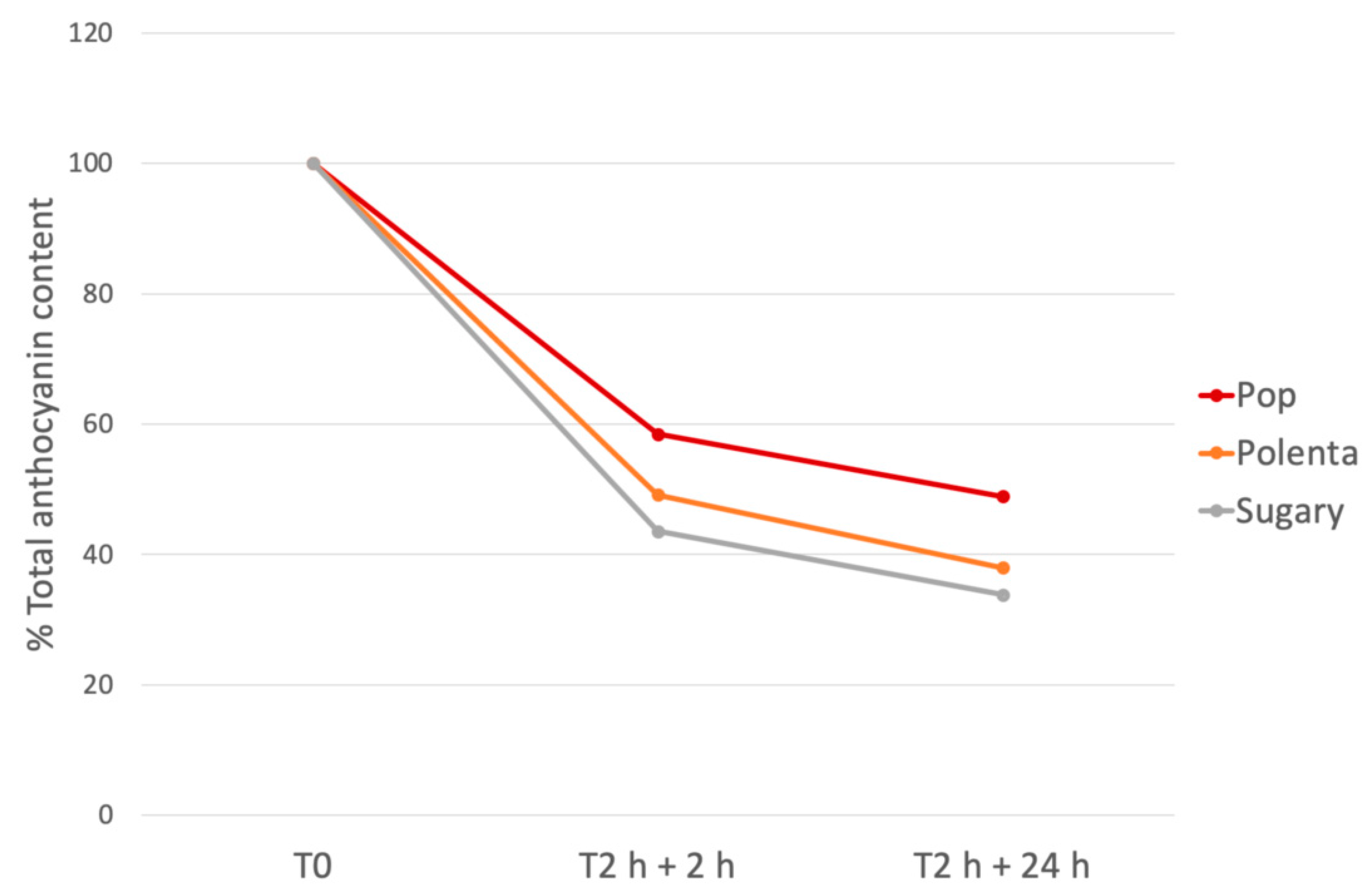
3.5. In Vitro Gastro-Intestinal Digestion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global prevalence of celiac disease: Systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef]
- Freeman, H.J. Adverse Effects of Gluten-free Diets. Int. J. Celiac Dis. 2018, 6, 71–73. [Google Scholar] [CrossRef]
- Saturni, L.; Ferretti, G.; Bacchetti, T. The gluten-free diet: Safety and nutritional quality. Nutrients 2010, 2, 16–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacchetti, T.; Saturni, L.; Turco, I.; Ferretti, G. The postprandial glucose response to some varieties of commercially available gluten-free pasta: A comparison between healthy and celiac subjects. Food Funct. 2014, 5, 3014–3017. [Google Scholar] [CrossRef] [PubMed]
- Zuccotti, G.; Fabiano, V.; Dilillo, D.; Picca, M.; Cravidi, C.; Brambilla, P. Intakes of nutrients in Italian children with celiac disease and the role of commercially available gluten-free products. J. Hum. Nutr. Diet. 2013, 26, 436–444. [Google Scholar] [CrossRef]
- Makovicky, P.; Makovicky, P.; Caja, F.; Rimarova, K.; Samasca, G.; Vannucci, L. Celiac disease and gluten-free diet: Past, present, and future. Gastroenterol. Hepatol. Bed Bench 2020, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Bacchetti, T.; Masciangelo, S.; Saturni, L. Celiac disease, inflammation and oxidative damage: A nutrigenetic approach. Nutrients 2012, 4, 243–257. [Google Scholar] [CrossRef] [Green Version]
- Daveson, A.J.M.; Popp, A.; Taavela, J.; Goldstein, K.E.; Isola, J.; Truitt, K.E.; Mäki, M.; Anderson, R.P.; Adams, A.; Andrews, J.; et al. Baseline quantitative histology in therapeutics trials reveals villus atrophy in most patients with coeliac disease who appear well controlled on gluten-free diet. GastroHep 2020, 2, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Dotsenko, V.; Oittinen, M.; Taavela, J.; Popp, A.; Peräaho, M.; Staff, S.; Sarin, J.; Leon, F.; Isola, J.; Mäki, M.; et al. Genome-wide transcriptomic analysis of intestinal mucosa in celiac disease patients on a gluten-free diet and postgluten challenge. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 13–32. [Google Scholar] [CrossRef]
- Diaz-Castro, J.; Muriel-Neyra, C.; Martin-Masot, R.; Moreno-Fernandez, J.; Maldonado, J.; Nestares, T. Oxidative stress, DNA stability and evoked inflammatory signaling in young celiac patients consuming a gluten-free diet. Eur. J. Nutr. 2020, 59, 1577–1584. [Google Scholar] [CrossRef]
- Maluf, S.W.; Filho, D.W.; Parisotto, E.B.; de Medeiros, G.D.S.D.; Pereira, C.H.J.; Maraslis, F.T.; Schoeller, C.C.D.; da Rosa, J.S.; Fröde, T.S. DNA damage, oxidative stress, and inflammation in children with celiac disease. Genet. Mol. Biol. 2020, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moretti, S.; Mrakic-Sposta, S.; Roncoroni, L.; Vezzoli, A.; Dellanoce, C.; Monguzzi, E.; Branchi, F.; Ferretti, F.; Lombardo, V.; Doneda, L.; et al. Oxidative stress as a biomarker for monitoring treated celiac disease. Clin. Transl. Gastroenterol. 2018, 9, 157. [Google Scholar] [CrossRef] [PubMed]
- Piatek-guziewicz, A.; Zagrodzki, P.; Pasko, P.; Krosniak, M.; Ptak-belowska, A.; Przybylska-felus, M.; Mach, T.; Zwolinska-wcisło, M. Alterations in serum levels of selected markers of oxidative imbalance in adult celiac patients with extraintestinal manifestations: A pilot study. Pol. Arch. Intern. Med. 2017, 127, 532–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowicka, G.; Czaja-Bulsa, G.; Chełchowska, M.; Riahi, A.; Strucińska, M.; Weker, H.; Ambroszkiewicz, J. Oxidative and antioxidative status of children with celiac disease treated with a gluten free-diet. Oxid. Med. Cell. Longev. 2018, 2018, 1324820. [Google Scholar] [CrossRef] [Green Version]
- Luciani, A.; Rachela Villella, V.; Vasaturo, A.; Giardino, I.; Pettoello-Mantovani, M.; Guido, S.; Cexus, O.N.; Peake, N.; Londei, M.; Quaratino, S.; et al. Lysosomal accumulation of gliadin p31e43 peptide induces oxidative stress and tissue transglutaminase-mediated PPARγ downregulation in intestinal epithelial cells and coeliac mucosa. Gut 2010, 59, 311–319. [Google Scholar] [CrossRef]
- Dias, R.; Pereira, C.B.; Pérez-Gregorio, R.; Mateus, N.; Freitas, V. Recent advances on dietary polyphenol’s potential roles in Celiac Disease. Trends Food Sci. Technol. 2021, 107, 213–225. [Google Scholar] [CrossRef]
- Zhirkova, Y.V.; Skorokhodova, M.V.; Martirosyan, V.V.; Sotchenko, E.F.; Malkina, V.D.; Shatalova, T.A. Chemical composition and antioxidant activity of corn hybrids grain of different pigmentation. Foods Raw Mater. 2016, 4, 85–91. [Google Scholar] [CrossRef]
- Petroni, K.; Pilu, R.; Tonelli, C. Anthocyanins in corn: A wealth of genes for human health. Planta 2014, 240, 901–911. [Google Scholar] [CrossRef]
- Ortiz-Sánchez, J.P.; Cabrera-Chávez, F.; Calderón Barca, A.M. Maize prolamins could induce a gluten-like cellular immune response in some celiac disease patients. Nutrients 2013, 5, 4174–4183. [Google Scholar] [CrossRef]
- Zhu, F. Anthocyanins in cereals: Composition and health effects. Food Res. Int. 2018, 109, 232–249. [Google Scholar] [CrossRef]
- Silano, M.; Penas Pozo, E.; Uberti, F.; Manferdelli, S.; Del Pinto, T.; Felli, C.; Budelli, A.; Vincentini, O.; Restani, P. Diversity of oat varieties in eliciting the early inflammatory events in celiac disease. Eur. J. Nutr. 2014, 53, 1177–1186. [Google Scholar] [CrossRef] [Green Version]
- Silano, M.; Vincentini, O.; Luciani, A.; Felli, C.; Caserta, S.; Esposito, S.; Villella, V.R.; Pettoello-Mantovani, M.; Guido, S.; Maiuri, L. Early tissue transglutaminase-mediated response underlies K562(S)-cell gliadin-dependent agglutination. Pediatr. Res. 2012, 71, 532–538. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Martinez, L.X.; Oliart-Ros, R.M.; Valerio-Alfaro, G.; Lee, C.H.; Parkin, K.L.; Garcia, H.S. Antioxidant activity, phenolic compounds and anthocyanins content of eighteen strains of Mexican maize. LWT Food Sci. Technol. 2009, 42, 1187–1192. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry to total phenolics with phosphomolybdic acid reagents. Am. J. Enol. Vinic. 1965, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Reich, E.; Schibli, A. High Performance Thin Layer Chromatography for the Analysis of Medicinal Plants; Schibli, A., Ed.; Thieme: New York, NY, USA, 2007. [Google Scholar]
- Di Lorenzo, C.; Badea, M.; Colombo, F.; Orgiu, F.; Frigerio, G.; Pastor, R.F.; Restani, P. Antioxidant activity of wine assessed by different in vitro methods. BIO Web Conf. 2017, 9, 04008. [Google Scholar] [CrossRef]
- Shipp, J.; Abdel-Aal, E.-S.M. Food applications and physiological effects of anthocyanins as functional food ingredients. Open Food Sci. J. 2010, 4, 7–22. [Google Scholar] [CrossRef]
- Yang, Z.; Zhai, W. Optimization of microwave-assisted extraction of anthocyanins from purple corn (Zea mays L.) cob and identification with HPLC-MS. Innov. Food Sci. Emerg. Technol. 2010, 11, 470–476. [Google Scholar] [CrossRef]
- Cuevas Montilla, E.; Hillebrand, S.; Antezana, A.; Winterhalter, P. Soluble and bound phenolic compounds in different Bolivian purple corn (Zea mays L.) cultivars. J. Agric. Food Chem. 2011, 59, 7068–7074. [Google Scholar] [CrossRef]
- Žilić, S.; Serpen, A.; Akillioǧlu, G.; Gökmen, V.; Vančetović, J. Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of colored maize (Zea mays L.) kernels. J. Agric. Food Chem. 2012, 60, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines. In Official Methods of Analysis; AOAC Official Method: Washington, DC, USA, 2005; ISBN 0-935584-77-3.
- Sangiovanni, E.; Di Lorenzo, C.; Colombo, E.; Colombo, F.; Fumagalli, M.; Frigerio, G.; Restani, P.; Dell’Agli, M. The effect of in vitro gastrointestinal digestion on the anti-inflammatory activity of Vitis vinifera L. leaves. Food Funct. 2015, 6, 2453–2463. [Google Scholar] [CrossRef] [PubMed]
- FDA Bioanalytical Method Validation Guidance. Available online: https://www.fda.gov/media/70858/download (accessed on 1 April 2020).
- Oomen, A.G.; Rompelberg, C.J.M.; Bruil, M.A.; Dobbe, C.J.G.; Pereboom, D.P.K.H.; Sips, A.J.A.M. Development of an in vitro digestion model for estimating the bioaccessibility of soil contaminants. Arch. Environ. Contam. Toxicol. 2003, 44, 281–287. [Google Scholar] [CrossRef]
- Versantvoort, C.H.M.; Oomen, A.G.; Van De Kamp, E.; Rompelberg, C.J.M.; Sips, A.J.A.M. Applicability of an in vitro digestion model in assessing the bioaccessibility of mycotoxins from food. Food Chem. Toxicol. 2005, 43, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Cheryan, M. Zein: The industrial protein from corn. Ind. Crops Prod. 2001, 13, 171–192. [Google Scholar] [CrossRef]
- Tschiersch, C.; Nikfardjam, M.P.; Schmidt, O.; Schwack, W. Degree of hydrolysis of some vegetable proteins used as fining agents and its influence on polyphenol removal from red wine. Eur. Food Res. Technol. 2010, 231, 65–74. [Google Scholar] [CrossRef]
- Cabrera-CháVez, F.; Rouzaud-Sández, O.; Sotelo-Cruz, N.; Barca, A.M.C.D. La Bovine milk caseins and transglutaminase-treated cereal prolamins are differentially recognized by iga of celiac disease patients according to their age. J. Agric. Food Chem. 2009, 57, 3754–3759. [Google Scholar] [CrossRef]
- Bergamo, P.; Maurano, F.; Mazzarella, G.; Iaquinto, G.; Vocca, I.; Rivelli, A.R.; De Falco, E.; Gianfrani, C.; Rossi, M. Immunological evaluation of the alcohol-soluble protein fraction from gluten-free grains in relation to celiac disease. Mol. Nutr. Food Res. 2011, 55, 1266–1270. [Google Scholar] [CrossRef] [PubMed]
- Monguzzi, E.; Marabini, L.; Elli, L.; Vaira, V.; Ferrero, S.; Ferretti, F.; Branchi, F.; Gaudioso, G.; Scricciolo, A.; Lombardo, V.; et al. Gliadin effect on the oxidative balance and DNA damage: An in-vitro, ex-vivo study. Dig. Liver Dis. 2019, 51, 47–54. [Google Scholar] [CrossRef]
- Maiuri, L.; Ciacci, C.; Ricciardelli, I.; Vacca, L.; Raia, V.; Rispo, A.; Griffin, M.; Issekutz, T.; Quaratino, S.; Londei, M. Unexpected role of surface transglutaminase type II in celiac disease. Gastroenterology 2005, 129, 1400–1413. [Google Scholar] [CrossRef]
- Cömert, E.D.; Gökmen, V. Antioxidants bound to an insoluble food matrix: Their analysis, regeneration behavior, and physiological importance. Compr. Rev. Food Sci. Food Saf. 2017, 16, 382–399. [Google Scholar] [CrossRef] [Green Version]
- Suriano, S.; Balconi, C.; Valoti, P.; Redaelli, R. Comparison of total polyphenols, profile anthocyanins, color analysis, carotenoids and tocols in pigmented maize. LWT 2021, 144, 111257. [Google Scholar] [CrossRef]
- Rodríguez-Salinas, P.A.; Zavala-García, F.; Urías-Orona, V.; Muy-Rangel, D.; Heredia, J.B.; Niño-Medina, G. Chromatic, nutritional and nutraceutical properties of pigmented native maize (Zea mays L.) genotypes from the northeast of Mexico. Arab. J. Sci. Eng. 2020, 45, 95–112. [Google Scholar] [CrossRef]
- Trehan, S.; Singh, N.; Kaur, A. Characteristics of white, yellow, purple corn accessions: Phenolic profile, textural, rheological properties and muffin making potential. J. Food Sci. Technol. 2018, 55, 2334–2343. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Kaur, A.; Shevkani, K. Maize: Grain structure, composition, milling, and starch characteristics. In Maize: Nutrition Dynamics and Novel Uses; Springer: New Delhi, India, 2014; pp. 65–76. ISBN 9788132216230. [Google Scholar]
- Aoki, H.; Kuze, N.; Kato, Y. Anthocyanins isolated from purple corn (Zea mays L.). Foods Food Ingred. J. Japan 2002, 199, 41–45. [Google Scholar]
- Abdel-Aal, E.S.M.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B.; Self, R. Malonated cyanidin 3-glucosides in Zea mays and other grasses. Phytochemistry 1987, 26, 2417–2418. [Google Scholar] [CrossRef]
- Fossen, T.; Slimestad, R.; Andersen, O.M. Anthocyanins from maize (Zea mays) and reed canarygrass (Phalaris arundinacea). J. Agric. Food Chem. 2001, 49, 2318–2321. [Google Scholar] [CrossRef]
- De Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. LC-MS analysis of anthocyanins from purple corn cob. J. Sci. Food Agric. 2002, 82, 1003–1006. [Google Scholar] [CrossRef]
- van Dooren-Flipsen, M.M.H.; van Klaveren, J.D.; van Donkersgoed, G. Theoretical Maximum Daily Intake of Pesticide Residues in the Netherlands-a Model for Risk Assessment; RIKILT: Wageningen, The Netherlands, 1996. [Google Scholar]
- De Nijs, M.; Van Egmond, H.P.; Nauta, M.; Rombouts, F.M.; Notermans, S.H.W. Assessment of human exposure to fumonisin B1. J. Food Prot. 1998, 61, 879–884. [Google Scholar] [CrossRef]
- Smith, J.E.; Solomons, G.L. Mycotoxins in Human Nutrition and Health; Directorate-General XII, Science, Research and Development, European Commission: Brussels, Belgium, 1994. [Google Scholar]
- Morais, C.A.; de Rosso, V.V.; Estadella, D.; Pisani, L.P. Anthocyanins as inflammatory modulators and the role of the gut microbiota. J. Nutr. Biochem. 2016, 33, 1–7. [Google Scholar] [CrossRef]
- Edirisinghe, I.; Banaszewski, K.; Cappozzo, J.; Sandhya, K.; Ellis, C.L.; Tadapaneni, R.; Kappagoda, C.T.; Burton-Freeman, B.M. Strawberry anthocyanin and its association with postprandial inflammation and insulin. Br. J. Nutr. 2011, 106, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.L.; Edirisinghe, I.; Kappagoda, T.; Burton-Freeman, B. Attenuation of meal-induced inflammatory and thrombotic responses in overweight men and women after 6-week daily strawberry (Fragaria) intake. A randomized placebo-controlled trial. J. Atheroscler. Thromb. 2011, 18, 318–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruel, G.; Pomerleau, S.; Couture, P.; Lemieux, S.; Lamarche, B.; Couillard, C. Low-calorie cranberry juice supplementation reduces plasma oxidized LDL and cell adhesion molecule concentrations in men. Br. J. Nutr. 2008, 99, 352–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruel, G.; Pomerleau, S.; Couture, P.; Lemieux, S.; Lamarche, B.; Couillard, C. Plasma matrix metalloproteinase (MMP)-9 levels are reduced following low-calorie cranberry juice supplementation in men. J. Am. Coll. Nutr. 2009, 28, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, B.; Gul, K.; Wani, A.A.; Singh, P. Health benefits of anthocyanins and their encapsulation for potential use in food systems: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2223–2230. [Google Scholar] [CrossRef]
- Pérez-Vicente, A.; Gil-Izquierdo, A.; García-Viguera, C. In vitro gastrointestinal digestion study of pomegranate juice phenolic compounds, anthocyanins, and vitamin C. J. Agric. Food Chem. 2002, 50, 2308–2312. [Google Scholar] [CrossRef]
- McDougall, G.J.; Fyffe, S.; Dobson, P.; Stewart, D. Anthocyanins from red wine-Their stability under simulated gastrointestinal digestion. Phytochemistry 2005, 66, 2540–2548. [Google Scholar] [CrossRef]
- Aura, A.M.; Martin-Lopez, P.; O’Leary, K.A.; Williamson, G.; Oksman-Caldentey, K.M.; Poutanen, K.; Santos-Buelga, C. In vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005, 44, 133–142. [Google Scholar] [CrossRef]
- McGhie, T.K.; Walton, M.C. The bioavailability and absorption of anthocyanins: Towards a better understanding. Mol. Nutr. Food Res. 2007, 51, 702–713. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Danciu, V.; Moldovan, B.; Filip, A. Effects of in vitro gastrointestinal digestion on the antioxidant capacity and anthocyanin content of cornelian cherry fruit extract. Antioxidants 2019, 8, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emilsson, L.; Lebwohl, B.; Green, P.H.R.; Murray, J.A.; Mårild, K.; Ludvigsson, J.F. Mucosal healing and the risk of serious infections in patients with celiac disease. United Eur. Gastroenterol. J. 2018, 6, 55–62. [Google Scholar] [CrossRef] [PubMed]



| Digested Sample | % of Agglutination | TEER (TEERsample/TEERcontrol) | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | |
| Wheat | 90 | 78 | 94 | 3.3 | 4 |
| Rice | 0 | 0 | 0 | 1 | - |
| Reduno | 0 | 0 | 0 | 1 | 0.9 |
| Polenta | 0 | 0 | 0 | 1 | 1 |
| m/z | Samples | ||||
|---|---|---|---|---|---|
| RED | SUG | POL | POP | ||
| Cyanidin-3-O-glucoside | 449 | + | + | + | + |
| Pelargonidin-3-O-glucoside | 493 | + | + | + | + |
| Peonidin-3-O-glucoside | 463 | + | + | + | + |
| Cyanidin-3-O-(6′′-malonil-glucoside) * | 535 | + | + | + | + |
| Pelargonidin-3-O-(6′′-malonil-glucoside) * | 519 | + | + | - | + |
| Peonidin-3-O-(6′′-malonil-glucoside) * | 549 | + | + | - | + |
| Precision a | Linearity | Sensitivity | Recovery # | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Intraday (CV%) | Interday (CV%) | Linear Range (μg/mL) | R2 | LOD | LOQ | % (mean ± SD) | |||
| (μg/mL) | (μg/g) | (μg/mL) | (μg/g) | ||||||
| CY | 2.44 | 5.79 | 0.5–10 | 0.9987 | 0.04 | 0.86 | 0.15 | 3.00 | 92.2 ± 3.6 |
| PL | 2.06 | 4.77 | 0.5–10 | 0.9951 | 0.07 | 1.49 | 0.25 | 5.00 | 88.4 ± 7.0 |
| PE | 3.25 | 4.17 | 0.5–10 | 0.9972 | 0.14 | 2.79 | 0.50 | 10.00 | 96.3 ± 6.4 |
| TA (CY mg/g) (mean ± SD) | HPLC-DAD Analysis (μg/g) (mean ± SD) | |||||||
|---|---|---|---|---|---|---|---|---|
| CY | PL | PE | CYm | PLm | PEm | Total | ||
| RED | 0.98 ± 0.03 | 176.0 ± 11.4 | 24.5 ± 1.9 | 155.8 ± 14.8 | 100.4 ± 8.2 | 14.7 ± 1.4 | 97.4 ± 10.3 | 568.8 ± 46.1 |
| POL | 0.48 ± 005 | 142.6 ± 11.0 | 9.6 ± 1.2 | 74.2 ± 4.9 | 37.8 ± 3.5 | NQ | 21.8 ± 2.4 | 286.0 ± 22.3 |
| POP | 0.69 ± 0.05 | 75.9 ± 5.4 | 16.4 ± 0.1 | 56.7 ± 3.4 | 132.4 ± 11.7 | 39.5 ± 3.7 | 90.3 ± 6.3 | 411.1 ± 29.8 |
| SUG | 0.57 ± 0.03 | 114.7 ± 5.3 | 17.3 ± 2.1 | 64.5 ± 2.8 | 112.7 ± 3.9 | 13.7 ± 0.8 | 46.6 ± 1.9 | 369.5 ± 15.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, F.; Di Lorenzo, C.; Petroni, K.; Silano, M.; Pilu, R.; Falletta, E.; Biella, S.; Restani, P. Pigmented Corn Varieties as Functional Ingredients for Gluten-Free Products. Foods 2021, 10, 1770. https://doi.org/10.3390/foods10081770
Colombo F, Di Lorenzo C, Petroni K, Silano M, Pilu R, Falletta E, Biella S, Restani P. Pigmented Corn Varieties as Functional Ingredients for Gluten-Free Products. Foods. 2021; 10(8):1770. https://doi.org/10.3390/foods10081770
Chicago/Turabian StyleColombo, Francesca, Chiara Di Lorenzo, Katia Petroni, Marco Silano, Roberto Pilu, Ermelinda Falletta, Simone Biella, and Patrizia Restani. 2021. "Pigmented Corn Varieties as Functional Ingredients for Gluten-Free Products" Foods 10, no. 8: 1770. https://doi.org/10.3390/foods10081770
APA StyleColombo, F., Di Lorenzo, C., Petroni, K., Silano, M., Pilu, R., Falletta, E., Biella, S., & Restani, P. (2021). Pigmented Corn Varieties as Functional Ingredients for Gluten-Free Products. Foods, 10(8), 1770. https://doi.org/10.3390/foods10081770









