Rice Compounds with Impact on Diabetes Control
Abstract
:1. Introduction
2. Rice Macronutrients
2.1. Starch
2.2. Proteins
2.3. Lipids
2.4. Dietary Fiber
3. Rice Bran Compounds
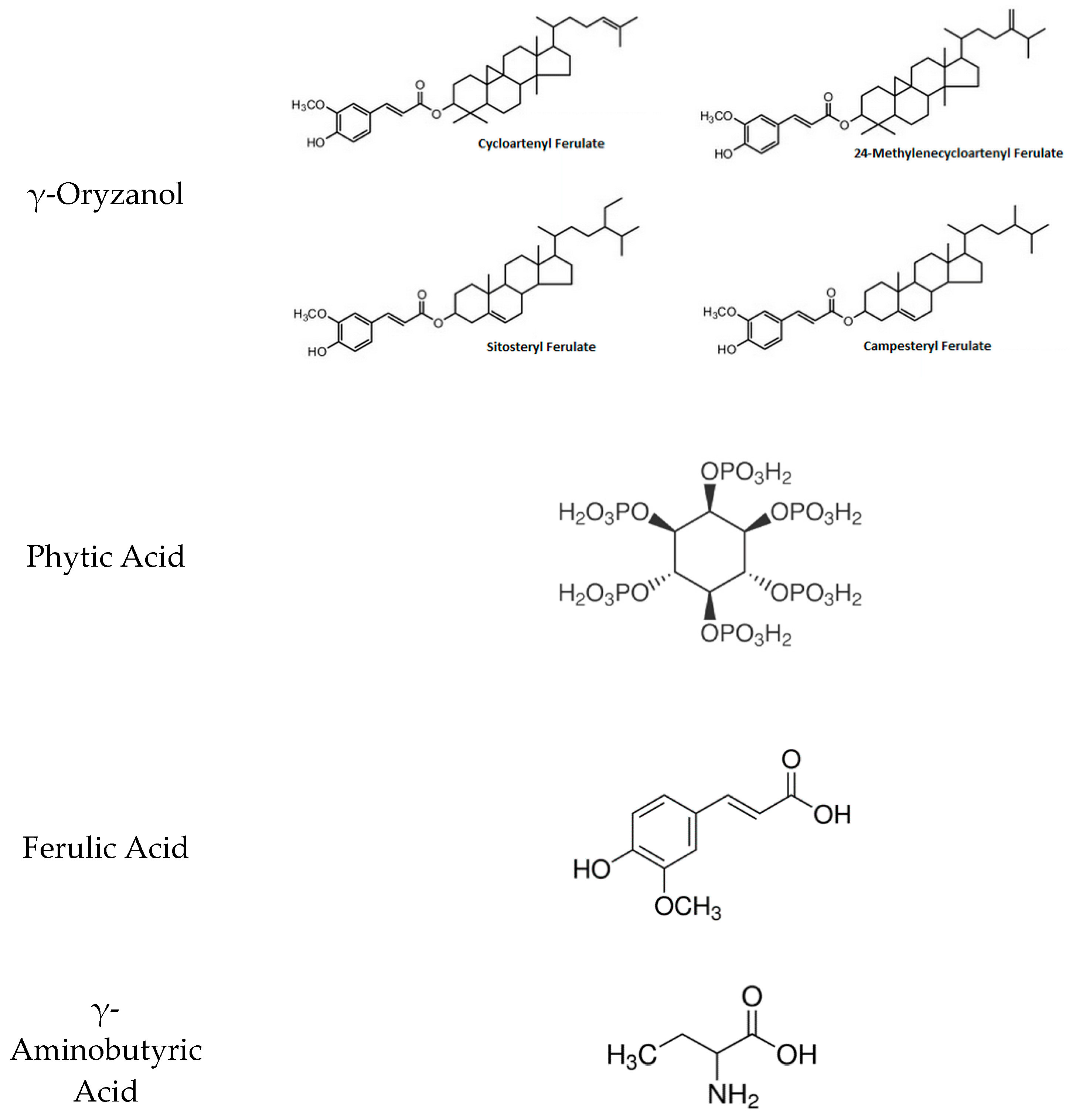
3.1. γ-Oryzanol
3.2. Phytic Acid
3.3. Ferulic Acid
3.4. γ-Aminobutyric Acid (GABA)
3.5. Tocopherols and Tocotrienols (Vitamin E)
4. Metabolic Mechanisms and Bioactive towards Diabetes
4.1. Glycemic Index of Rice and Effects in Glycemia
4.2. α-Amylase and α-Glucosidase Inhibition
4.3. Modulation of Glucose Transporters
4.4. Other Molecular Mechanisms Associated with Diabetes
4.5. Highlight on Rice Major Bioactive Compounds towards Diabetes
4.5.1. γ-Oryzanol
4.5.2. Phytic Acid
4.5.3. Ferulic Acid
4.5.4. γ-Aminobutyric Acid (GABA)
4.5.5. Tocopherols and Tocotrienols (Vitamin E)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- International Diabetes Federation (IDF). What is diabetes? In Diabetes Atlas, 9th ed.; IDF: Brussels, Belgium, 2019; Volume 1, pp. 10–22. [Google Scholar]
- Eggersdorfer, M.; Kraemer, K.; Cordaro, J.B.; Fanzo, J.; Gibney, M.; Kennedy, E.; Labrique, A.; Steffen, J. Diet and s: An urgent need for new paradigms. In Good Nutrition: Perspectives for the 21st Century; Karger: Basel, Switzerland, 2016; pp. 105–118. [Google Scholar]
- Kubota, M.; Watanabe, R.; Kabasawa, H.; Iino, N.; Saito, A.; Kumagai, T.; Kadowaki, M. Rice protein ameliorates the progression of diabetic nephropathy in Goto-Kakizaki rats with high-sucrose feeding. Br. J. Nutr. 2013, 110, 1211–1219. [Google Scholar] [CrossRef] [Green Version]
- Prakash, S. Role of Human Serum Albumin and Oxidative Stress in Diabetes. J. Appl. Biotechnol. Bioeng. 2017, 3, 281–285. [Google Scholar] [CrossRef] [Green Version]
- Cavan, D.; Fernandes, J.R.; Makarof, L.; Ogurtsova, K.; Webber, S. IDF Diabetes Atlas, 7th ed.; International Diabetes Federation: Brussels, Belgium, 2015; pp. 12–19. [Google Scholar]
- Hu, J.; La Vecchia, C.; Augustin, L.S.; Negri, E.; de Groh, M.; Morrison, H. Glycemic index, glycemic load and cancer risk. Ann. Oncol. 2013, 24, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Champagne, E.T. Rice: Chemistry and Technology, 3rd ed.; AACC International Press: Washington, DC, USA; Department of Agriculture, Agricultural Research Service, Southern Regional Research Center: New Orleans, LA, USA, 2004; pp. 77–190. [Google Scholar]
- Juliano, B.O.; Tuaño, A.P.P. Gross structure and composition of the rice grain. In Rice, Chemistry and Technology, 4th ed; AACC International Press: Washington, DC, USA, 2019; pp. 31–53. [Google Scholar]
- Faid, S.M.A.E.F. The beneficial effects of brown rice and its bran as natural antioxidants and lowering hyperglycemic in Albino rats. Middle East J. Appl. 2015, 5, 281–289. [Google Scholar]
- Mohan, V.; Spiegelman, D.; Sudha, V.; Gayathri, R.; Hong, B.; Praseena, K.; Anjana, R.M.; Wedick, N.M.; Arumugam, K.; Malik, V.; et al. Effect of brown rice, white rice, and brown rice with legumes on blood glucose and insulin responses in ovweweight Asian Indians: A randomized controlled trial. Diabetes Technol. Ther. 2014, 16, 317–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panlasigui, L.; Thompson, L. Blood glucose lowering effects of brown rice in normal and diabetic subjects. Int. J. Food Sci. Nutr. 2006, 57, 151–158. [Google Scholar] [CrossRef]
- Esa, N.M.; Ling, T.B.; Peng, L.S. By-products of rice processing: An overview of health benefits and applications. J. Rice Res. 2013, 1, 1–11. [Google Scholar]
- Mohapatra, D.; Bal, S. Effect of degree of milling on specific energy consumption, optical measurements and cooking quality of rice. J. Food Eng. 2007, 80, 119–125. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. A comprehensive review on anti-diabetic property of rice bran. Asian Pac. J. Trop. Biomed. 2018, 8, 79–84. [Google Scholar] [CrossRef]
- Bhat, F.M.; Riar, C.S.; Seesuriyachan, P.; Sommano, S.R.; Chaiyaso, T.; Prom-u-Thai, C. Status of Bioactive Compounds from Bran of Pigmented Traditional Rice Varieties and their Scope in Production of Medicinal Food with Nutraceutical Importance. Agron. J. 2020, 10, 1817. [Google Scholar] [CrossRef]
- Park, H.-Y.; Leeb, K.-W.; Choi, H.-D. Rice bran constituents: Immunomodulatory and therapeutic activities. Food Funct. 2017, 8, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ternero, C.; Sotomayor, M.A.; Herrera, M.D. Contribution of ferulic acid, γ-oryzanol and tocotrienols to the cardiometabolic protective effects of rice bran. J. Funct. Foods 2017, 32, 58–71. [Google Scholar] [CrossRef]
- Ghatak, S.B.; Panchal, S.S. Protective effect of oryzanol isolated from crude rice bran oil in experimental model of diabetic neuropathy. J. Pharmacogn. 2012, 22, 1092–1103. [Google Scholar] [CrossRef] [Green Version]
- Takemoto, K.; Doib, W.; Masuoka, N. Protective effect of vitamin E against alloxan-induced mouse hyperglycemia. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1862, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Jeng, T.L.; Shih, Y.J.; Ho, P.T.; Lai, C.C.; Lin, Y.W.; Wang, C.S.; Sung, J.M. γ-Oryzanol, tocol and mineral compositions in different grain fractions of giant embryo rice mutants. J. Sci. Food Agric. 2012, 92, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Castanho, A.; Lageiro, M.; Calhelha, R.; Ferreira, I.C.F.R.; Sokovic, M.; Cunha, L.M.; Brites, C. Exploiting the bioactive properties of γ-oryzanol from bran of different exotic rice varieties. J. Food Funct. 2019, 10, 2382–2389. [Google Scholar] [CrossRef] [PubMed]
- Massarolo, K.C.; Ribeiro, A.C.; Furlong, E.B.; Soares, L.A.S. Effect of particle size of rice bran on gamma-oryzanol content and compound. J. Cereal Sci. 2017, 75, 54–60. [Google Scholar] [CrossRef]
- Pokkanta, P.; Sookwong, P.; Tanang, M.; Setchaiyan, S.; Boontakham, P.; Mahatheeranont, S. Simultaneous determination of tocols, γ-oryzanols, phytosterols, squalene, cholecalciferol and phylloquinone in rice bran and vegetable oil samples. Food Chem. 2019, 271, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Wanyo, P.; Kaewseejan, N.; Meeso, N.; Siriamornpun, S. Bioactive compounds and antioxidant properties of different solvent extracts derived from Thai rice by-products. Appl. Biol. Chem. 2016, 59, 373–384. [Google Scholar] [CrossRef]
- Ren, X.-L.; Liu, Q.-L.; Fu, H.-W.; Wu, D.-X.; Shu, Q.-Y. Density alteration of nutrient elements in rice grains of a lowphytate mutant. Food Chem. 2007, 102, 1400–1406. [Google Scholar] [CrossRef]
- Kalschne, D.L.; Silva-Buzanello, R.A.; Byler, A.P.I.; Scremin, F.R.; Junior, A.M.M.; Canan, C. Rice and rice bran from different cultivars: Physicochemical, spectroscopic, and thermal analysis characterization. Food Sci. 2020, 41, 3081–3092. [Google Scholar] [CrossRef]
- Wang, K.M.; Wu, J.G.; Li, G.; Zhang, D.P.; Yang, Z.W.; Shi, C.H. Distribution of phytic acid and mineral elements in threeindica rice (Oryza sativa L.) cultivars. J. Cereal Sci. 2011, 54, 116–121. [Google Scholar] [CrossRef]
- Canan, C.; Cruz, F.T.L.; Delaroza, F.; Casagrande, R.; Sarmento, C.P.M.; Shimokomaki, M.; Ida, E.I. Studies on the extraction and purification of phytic acid from rice bran. J. Food Compos. Anal. 2011, 24, 1057–1063. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.; Castanho, A.; Lageiro, M.; Brites, C. Rice phytic acid quantification and assessment of their inhibitory effect against alfa-amylase activity. In Proceedings of the Simpósio INIAV para a Segurança Alimentar “Rumo à Alimentação do Futuro”, 1ªedição, Vila do Conde, Portugal, 29 November 2019. [Google Scholar]
- Finocchiaro, F.; Ferrari, B.; Gianinetti, A.; Dall’Asta, C.; Galaverna, G.; Scazzina, F.; Pellegrini, N. Characterization of antioxidant compounds of red and white rice and changes in total antioxidant capacity during processing. Mol. Nutr. Food Res. 2007, 51, 1006–1019. [Google Scholar] [CrossRef]
- Zhou, Z.; Robards, K.; Helliwell, S.; Blanchard, C. The distribution of phenolic acids in rice. Food Chem. 2004, 87, 401–406. [Google Scholar] [CrossRef]
- Hayat, A.; Jahangir, T.M.; Khuhawar, M.Y.; Alamgir, M.; Siddiqui, A.J.; Musharraf, S.G. Simultaneous HPLC Determination of Gamma Amino Butyric Acid (GABA) and Lysine in Selected Pakistani Rice Varieties by Pre-column Derivatization with 2-Hydroxynaphthaldehyde. J. Cereal Sci. 2014, 60, 356–360. [Google Scholar] [CrossRef]
- Eamarjharn, A.; Theerakulkait, C.; Thanachasai, S. Effect of incubation time, buffer type and concentration on gamma-aminobutyric acid (GABA) production using Khao Dawk Mali 105 rice bran. Agric. Nat. Resour. 2016, 50, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Lü, Y.; Zhang, H.; Yao, H. Enzymatic Production of γ-Aminobutyric Acid Using Rice Bran. Adv. Mater. Res. 2012, 586, 85–91. [Google Scholar] [CrossRef]
- Oh, S.-J.; Kim, H.S.; Lim, S.-T.; Reddy, C.K. Enhanced accumulation of gamma-aminobutyric acid in rice bran using anaerobic incubation with various additives. Food Chem. 2019, 271, 187–192. [Google Scholar] [CrossRef]
- Rohrer, C.; Siebenmorgen, T. Nutraceutical concentrations within the bran of various rice kernel thickness fractions. Biosyst. Eng. 2004, 88, 453–460. [Google Scholar] [CrossRef]
- Schramm, R.; Abadie, A.; Hua, N.; Xu, Z.; Lima, M. Fractionation of the rice bran layer and quantification of vitamin E, oryzanol, protein, and rice bran saccharide. J. Biol. Eng. 2007, 1, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, U.J.; Hong, Y.H.; Jung, E.Y.; Suh, H.J. Rice and the glycemic index. In Wheat and Rice in Disease Prevention and Health; Academic Press: Cambridge, MA, USA, 2014; Volume 27, pp. 357–363. [Google Scholar]
- Kaur, B.; Ranawana, V.; Henry, J. The Glycemic Index of Rice and Rice Products: A Review, and Table of GI Values. Crit. Rev. Food Sci. Nutr. 2016, 56, 215–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, F.S.; Brand-Miller, J.C.; Foster-Powell, K.; Buyken, A.E.; Goletzke, J. International tables of glycemic index and glycemic load values 2021: A systemic review. Am. J. Clin. Nutr. 2021, 1–8. [Google Scholar] [CrossRef]
- Agama-Acevedo, E.; Islas-Hernández, J.J.; Pacheco-Vargas, G.; Osorio-Díaz, P.; Bello-Pérez, L.A. Starch digestibility and glycemic index of cookies partially substituted with unripe banana flour. LWT Food Sci. Technol. 2012, 46, 177–182. [Google Scholar] [CrossRef]
- Giuberti, G.; Marti, A.; Fortunati, P.; Gallo, A. Gluten free rice cookies with resistant starch ingredients from modified waxy rice starches: Nutritional aspects and textural characteristics. J. Cereal Sci. 2017, 76, 157–164. [Google Scholar] [CrossRef]
- Khatun, A.; Waters, D.L.E.; Liu, L. A review of rice starch digestibility: Effect of composition and heatmoisture processing. Starch Starke 2019, 71, 9–10. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chang, D.-M.; Wu, D.-J.; Peng, H.-Y.; Chuang, L.-M. Assessment of Blood Glucose Regulation and Safety of Resistant Starch Formula-Based Diet in Healthy Normal and Subjects with Type 2 Diabetes. Medicine 2015, 94, e1332. [Google Scholar] [CrossRef]
- López-Barón, N.; Gu, Y.; Vasanthan, T.; Hoover, R. Plant proteins mitigate in vitro wheat starch digestibility. Food Hydrocoll. 2017, 69, 19–27. [Google Scholar] [CrossRef]
- Nugent, A. Health properties of resistant starch. Nutr. Bull. 2005, 30, 27–54. [Google Scholar] [CrossRef]
- Yi, D.; Maike, W.; Yi, S.; Xiaoli, S.; Dianxing, W.; Wenjian, S. Physiochemical Properties of Resistant Starch and Its Enhancement Approaches in Rice. Rice Sci. 2021, 28, 31–42. [Google Scholar] [CrossRef]
- Alcazar-Alay, S.C.; Meireles, M.A.A. Physicochemical properties, modifications and applications of starches from different botanical sources. Food Sci. Technol. 2015, 35, 215–236. [Google Scholar] [CrossRef] [Green Version]
- Deepa, G.; Singh, V.; Naidu, K.A. A comparative study on starch digestibility, glycemic index and resistant starch of pigmented (‘Njavara’ and‘Jyothi’) and a non-pigmented (‘IR 64′) rice varieties. J. Food Sci. Technol. 2010, 47, 644–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frei, M.; Siddhuraju, P.; Becker, K. Studies on the in vitro starch digestibility and the glycemic index of six different indigenous rice cultivars from the Philippines. Food Chem. 2003, 83, 395–402. [Google Scholar] [CrossRef]
- Hu, P.; Zhao, H.; Duan, Z.; Linlin, Z.; Wu, D. Starch digestibility and the estimated glycemic score of different types of rice differing in amylose contents. J. Cereal Sci. 2004, 40, 231–237. [Google Scholar] [CrossRef]
- Kumar, A.; Sahoo, U.; Baisakha, B.; Okpani, O.A.; Ngangkham, U.; Parameswaran, C.; Basak, N.; Kumar, G.; Sharma, S.G. Resistant starch could be decisive in determining the glycemic index of rice cultivars. J. Cereal Sci. 2018, 79, 348–353. [Google Scholar] [CrossRef]
- Toutounji, M.R.; Farahnaky, A.; Santhakumar, A.B.; Oli, P.; Butardo, V.M.; Blanchard, C.L. Intrinsic and extrinsic factors affecting rice starch digestibility. Trends Food Sci. Technol. 2019, 88, 10–22. [Google Scholar] [CrossRef]
- Lian, X.; Zhu, W.; Wen, Y.; Li, L.; Zhao, X. Effects of soy protein hydrolysates on maize starch retrogradation studied by IR spectra and ESI-MS analysis. Int. J. Biol. Macromol. 2013, 59, 143–150. [Google Scholar] [CrossRef]
- Ye, J.; Hu, X.; Luo, S.; McClements, D.J.; Liang, L.; Liu, C. Effect of endogenous proteins and lipids on starch digestibility in rice flour. Food Res. Int. 2018, 106, 404–409. [Google Scholar] [CrossRef]
- Anacleto, R.; Badoni, S.; Parween, S.; Butardo, V.M.; Misra, G.; Cuevas, R.P.; Kuhlmann, M.; Trinidad, T.P.; Mallillin, A.C.; Acuin, C.; et al. Integrating a genome-wide association study with a large-scale transcriptome analysis to predict genetic regions influencing the glycaemic index and texture in rice. Plant Biotechnol. J. 2019, 17, 1261–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, D.J.; Kendall, C.W.; Augustin, L.S.; Franceschi, S.; Hamidi, M.; Marchie, A. Glycemic index: Overview of implications in health and disease. Am. J. Clin. Nutr. 2002, 76, 266–273. [Google Scholar] [CrossRef]
- Augustin, L.S.; Franceschi, S.; Jenkins, D.J.A.; Kendall, C.W.C.; La Vecchia, C. Glycemic index in chronic disease: A review. Eur. J. Clin. Nutr. 2002, 56, 1049–1071. [Google Scholar] [CrossRef] [Green Version]
- Hsu, R.J.-C.; Lu, S.; Chang, Y.; Chiang, W. Effects of added water and retrogradation on starch digestibility of cooked rice flours with different amylose content. J. Cereal Sci. 2015, 61, 1–7. [Google Scholar] [CrossRef]
- Kong, X.; Chen, Y.; Zhu, P.; Sui, Z.; Corke, H.; Bao, J. On the relationships among genetic, structural and functional properties of rice starch. J. Agric. Food Chem. 2015, 63, 6241–6248. [Google Scholar] [CrossRef] [PubMed]
- Hoogenkamp, H.; Kumagai, H.; Wanasundara, J.P.D. Rice protein and rice protein products. In Sustainable Protein Sources; Academic Press: Cambridge, MA, USA, 2017; Volume 3, pp. 47–65. [Google Scholar]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. The composition, extraction, functionality and applications of rice proteins: A review. Trends Food Sci. Technol. 2017, 64, 1–12. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, N.; Wu, J.; Yin, B. Rice bran protein: Extraction, nutraceutical properties, and potential applications. In Rice bran and Rice Bran Oil; AOCS Press: Urbana, IL, USA, 2019; Volume 11, pp. 271–293. [Google Scholar]
- Adebiyi, A.P.; Adebiyi, A.O.; Hasegawa, Y.; Ogawa, T.; Muramoto, K. Isolation and characterization of protein fractions from deoiled rice bran. Eur. Food Res. Technol. 2009, 288, 391–401. [Google Scholar] [CrossRef]
- Uraipong, C.; Zhao, J. In vitro digestion of rice bran proteins produces peptides with potent inhibitory effects on alpha-glucosidase and angiotensin I converting enzyme. J. Sci. Food Agric. 2015, 98, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liu, L.; Konik-Rose, C.; Tian, L.; Singh, S.; Howitt, C.A.; Li, Z.; Liu, Q. Down-Regulation of FAD2-1 Gene Expression Alters Lysophospholipid Composition in the Endosperm of Rice Grain and Influences Starch Properties. Foods 2021, 10, 1169. [Google Scholar] [CrossRef]
- Ali, A.; Devarajan, S. Nutritional and health benefits of rice bran oil. In Brown Rice; Springer: Cham, Switzerland, 2017; Chapter 9; pp. 135–158. [Google Scholar]
- Lu, X.N.; Wang, J.; AL-Oadiri, H.M.; Ross, C.F.; Powers, J.R.; Tang, J.M.; Rasco, B.A. Determination of total phenolic content and antioxidant capacity of onion (Allium cepa) and shallot (Allium oschaninii) using infrared spectroscopy. Food Chem. 2011, 129, 637–644. [Google Scholar] [CrossRef]
- Sohail, M.; Rakha, A.; Butt, M.S.; Iqbal, M.J.; Rashid, S. Rice Bran Nutraceutics: A Comprehensive Review. Crit. Rev. Food Sci Nutr. 2017, 57, 3771–3780. [Google Scholar] [CrossRef]
- Weickert, M.O.; Pfeiffer, A.F.H. Metabolic Effects of Dietary Fiber Consumption and Prevention of Diabetes. Am. Soc. Nutr. J. Nutr. 2008, 138, 439–442. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Sami, S.A.; Khan, F.A. Effects of stabilized rice bran, its soluble and fiber fractions on blood glucose levels and serum lipid parameters in humans with diabetes mellitus Types I and II. J. Nutr. Biochem. 2002, 13, 175–187. [Google Scholar] [CrossRef]
- Ullah, U.; Khalil, A.A.; Shaukat, F.; Song, Y. Sources, Extraction and Biomedical Properties of Polysaccharides. Foods 2019, 8, 304. [Google Scholar] [CrossRef] [Green Version]
- Most, M.M.; Tulley, R.; Morales, S.; Lefevre, M. Rice bran oil, not fiber, lowers cholesterol in humans. Am. J. Clin. Nutr. 2005, 81, 64–68. [Google Scholar] [CrossRef]
- Xu, Z.; Hua, N.; Godber, J.S. Antioxidant Activity of Tocopherols, Tocotrienols, and γ-Oryzanol Components from Rice Bran against Cholesterol Oxidation Accelerated by 2,2‘-Azobis(2-methylpropionamidine) Dihydrochloride†. J. Agric. Food Chem. 2001, 49, 2077–2081. [Google Scholar] [CrossRef]
- Yu, S.; Nehus, Z.T.; Badger, T.M.; Fang, N. Quantification of Vitamin E and γ-Oryzanol Components in Rice Germ and Bran. J Agric. Food Chem. 2007, 5, 7308–7313. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, U.; Jany, K.D.; Berk, A.; Schulz, E.; Rechkemmer, G. Degradation of phytate in the gut of pigs—Pathway of gastro-intestinal inositol phosphate hydrolysis and enzymes involved. Arch. Anim. Nutr. 2001, 55, 255–280. [Google Scholar]
- Sandberg, A.S.; Andersson, H. Effect of dietary phytases on the digestion of phytate in the stomach and small intestine of humans. J. Nutr. 1988, 118, 469–473. [Google Scholar] [CrossRef]
- Kaewsorn, K.; Sirisomboon, P. Determination of the gamma-aminobutyric acid content of germinated brown rice by near infrared spectroscopy. J. Near Infrared Spectrosc. 2014, 22, 45–54. [Google Scholar] [CrossRef]
- Shelp, B.J.; Bown, A.W.; McLean, M.D. Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci. 1999, 4, 446–452. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, H.Y.; Chen, F. Accumulation of gamma-aminobutyric acid in rice germ using protease. Biosci. Biotechnol. Biochem. 2006, 70, 1160–1165. [Google Scholar] [CrossRef] [Green Version]
- Dat, L.Q.; An, N.T.; Phung, L.T.K.; Dung, N.H.; Long, N.Q. Technical assessment of gamma-amino butyric acid (GABA) production. from rice branVietnam. J. Sci. Technol. 2019, 57, 137–143. [Google Scholar]
- Dat, L.Q.; Ngan, T.T.K.; Nu, N.T.X. Gamma-amino butyric acid (GABA) synthesis of Lactobacillus in fermentation of defatted rice bran extract. In AIP Conference Proceedings; AIP Publishing LLC: New York, NY, USA, 2017; Volume 1878, p. 020045. [Google Scholar]
- Kim, H.Y.; Hwang, I.G.; Kim, T.M.; Woo, K.S.; Park, D.S.; Kim, J.H.; Kim, D.J.; Lee, J.; Lee, Y.R.; Jeong, H.S. Chemical and functional components in different parts of rough rice (Oryza sativa L.) before and after germination. Food Chem. 2012, 134, 288–293. [Google Scholar] [CrossRef]
- Chung, S.I.; Jin, X.; Kang, M.Y. Enhancement of glucose and bone metabolism in ovariectomized rats fed with germinated pigmented rice with giant embryo (Oryza sativa L. cv. Keunnunjami). Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef] [Green Version]
- Hagiwara, H.; Seki, T.; Ariga, T. The effect of pre-germinated brown rice intake on blood glucose and PAI-1 levels in streptozotocin-induced diabetic rats. Biosci. Biotechnol. Biochem. 2004, 68, 444–447. [Google Scholar] [CrossRef]
- Reboul, E. Vitamin E Bioavailability: Mechanisms of Intestinal Absorption in the Spotlight. Antioxidants 2017, 6, 95. [Google Scholar] [CrossRef] [Green Version]
- Niki, E.; Abe, K. Vitamin E: Structure, properties and functions. In Vitamin E: Chemistry and Nutritional Benefits; Royal Society of Chemistry: London, UK, 2019; Chapter 1; pp. 1–11. [Google Scholar]
- Qureshi, A.; Mo, H.; Packer, L.; Peterson, D. Isolation and identification of novel tocotrienols from rice bran with hypocholesterolemic, antioxidant, and antitumor properties. J. Agric. Food Chem. 2000, 48, 3130–3140. [Google Scholar] [CrossRef] [PubMed]
- Thilakavathy, S.; Pandeeswari, N.K. The glycemic index—Science based diet. Int. J. Pharm. Med. Bio. Sc. 2012, 1, 259–265. [Google Scholar]
- ISO 26642:2010. Food Products—Determination of the Glycaemic Index (GI) and Recommendation for Food Classification; ISO: Geneva, Switzerland, 2010. [Google Scholar]
- Goni, I.; Garcia-AIonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Shi, M.; Zhanga, Z.; Yua, S.; Wang, K.; Gilbert, R.; Gao, Q. Pea starch (Pisumsativum, L.) with slow digestion property produced using b-amylase and transglucosidase. Food Chem. 2014, 164, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Ina, S.; Ninomiya, K.; Mogi, T.; Hase, A.; Ando, T.; Matsukaze, N.; Ogihara, J.; Akao, M.; Kumagai, H.; Kumagai, H. Rice (Oryza sativa japonica) Albumin Suppresses the Elevation of Blood Glucose and Plasma Insulin Levels after Oral Glucose Loading. J. Agric. Food Chem. 2016, 64, 4882–4890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, S.-Y.; Siow, P.C.; Peh, E.; Henry, C.J. Influence of rice, pea and oat proteins in attenuating glycemic response of sugar-sweetened beverages. Eur. J. Nutr. 2017, 57, 2795–2803. [Google Scholar] [CrossRef]
- Dilworth, L.; Omoruyi, F.; Simon, O.; Morrison, E.S.A.; Asemota, H. The effect of phytic acid on the levels of blood glucose and some enzymes of carbohydrate and lipid metabolism. West Indian Med. J. 2005, 54, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, A.; Muthusamy, U.; Andichetiar, T.S.; Varadharajan, S.; Ramasamy, K.; Ramanathan, S. In vitro (α-Glucosidase and α-Amylase Inhibition) and in vivo Antidiabetic Property of Phytic Acid (IP6) in Streptozotocin-Nicotinamide-Induced Type 2 Diabetes Mellitus (NIDDM) in Rats. J. Complementary Integr. Med. 2011, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Rico, C.W.; Lee, S.C.; Kang, M.Y. Modulatory Effect of Rice Bran and Phytic Acid on Glucose Metabolism in High Fat-Fed C57BL/6N Mice. J. Clin. Biochem. Nutr. 2010, 47, 12–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-H.; Park, H.-J.; Chun, H.-K.; Cho, S.-Y.; Cho, S.-M.; Lillehoj, H.S. Dietary phytic acid lowers the blood glucose level in diabetic KK mice. Nutr. Res. 2006, 26, 474–479. [Google Scholar] [CrossRef]
- Jung, E.H.; Kim, S.R.; Hwang, I.K.; Ha, T.Y. Hypoglycemic effects of a phenolic acid fraction of rice bran and ferulic acid in C57BL/KsJ-db/db mice. J. Agric. FoodChem. 2007, 55, 9800–9804. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Dang, H.N.; Yong, J.; Chui, W.S.; Dizon, M.P.; Yaw, C.K.; Kaufman, D.L. Oral treatment with ϒ-aminobutyric acid improves glucose tolerance and insulin sensitivity by inhibiting inflammation in high fat diet-fed mice. PLoS ONE 2011, 6, e25338. [Google Scholar] [CrossRef] [Green Version]
- Manning, P.J.; Sutherland, W.H.F.; Walker, R.J.; Williams, S.M.; Jong, S.A.; Ryalls, A.R.; Berry, E.A. Effect of high-dose vitamin E on insulin resistance and associated parameters in overweight subjects. Diabetes Care 2004, 27, 2166–2171. [Google Scholar] [CrossRef] [Green Version]
- Nazaimoon, W.M.W.; Khalid, B.A.K. Tocotrienols-rich diet decreases advanced glycosylation end-products in non-diabetic rats and improves glycemic control in streptozotocin-induced diabetic rats. Malays J. Pathol. 2002, 24, 77–82. [Google Scholar]
- Scheen, A.J. Is there a role for alpha-glucosidase inhibitors in the prevention of type 2 diabetes mellitus. Drugs 2003, 63, 933–951. [Google Scholar] [CrossRef]
- Kellett, G.L.; Brot-Laroche, E. Apical GLUT2: A Major Pathway of Intestinal Sugar Absorption. Diabetes 2005, 54, 3056–3062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitcomb, D.C.; Lowe, M.E. Human pancreatic digestive enzymes. Dig. Dis. Sci. 2007, 52, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sobhy, R.; Eid, M.; Zhan, F.; Liang, H.; Li, B. Toward understanding the in vitro anti-amylolytic effects of three structurally different phytosterols in an aqueous medium using multispectral and molecular docking studies. J. Mol. Liq. 2019, 283, 225–234. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Chantarasinlapin, P.; Thammarat, H.; Yibchok-Anun, S. A series of cinnamic acid derivatives and their inhibitory activity on intestinal alpha-glucosidase. J. Enzym. Inhib. Med. Chem. 2009, 24, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-M.; Lee, J.-S. Production and Its Anti-hyperglycemic Effects of γ-Aminobutyric Acid from the Wild Yeast Strain Pichia silvicola UL6-1 and Sporobolomyces carnicolor 402-JB-1. Mycobiology 2017, 45, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Wright, E.M.; Martín, M.G.; Turk, E. Intestinal absorption in health and disease—Sugar. Best Pr. Res Clin Gastroenterol. 2003, 17, 943–956. [Google Scholar] [CrossRef]
- Hussain, T. The role of intestinal sweet taste receptors (STRS) in the regulation of glucose absorption: Effects of short term high sucrose diet (HSD). In HIM 1990-2015; University of Central Florida: Orlando, FL, USA, 2014; Volume 1839, pp. 1–51. [Google Scholar]
- Poulsen, S.B.; Fenton, R.A.; Riegb, T. Sodium-glucose cotransport. Curr. Opin. Nephrol. Hypertens. 2015, 24, 463–469. [Google Scholar] [CrossRef] [Green Version]
- Tahrani, A.A.; Barnett, A.H.; Bailey, C.J. SGLT inhibitors in management of diabetes. Lancet Diabetes Endocrinol. 2013, 1, 140–151. [Google Scholar] [CrossRef]
- Ina, S.; Hamada, A.; Nakamura, H.; Yamaguchi, Y.; Kumagai, H.; Kumagai, H. Rice (Oryza sativa japonica) albumin hydrolysates suppress postprandial blood glucose elevation by adsorbing glucose and inhibiting Na+-d-glucose cotransporter SGLT1 expression. J. Funct. Foods 2020, 64, 103603. [Google Scholar] [CrossRef]
- Woyengo, T.A.; Rodriguez-Lecompte, J.C.; Adeola, O.; Nyachoti, C.M. Histomorphology and small intestinal sodium-dependent glucose transporter 1 gene expression in piglets fed phytic acid and phytase-supplemented diets. J. Anim. Sci. 2011, 89, 2485–2490. [Google Scholar] [CrossRef] [Green Version]
- Malunga, L.N.; Eck, P.; Beta, T. Inhibition of Intestinal α-Glucosidase and Glucose Absorption by Feruloylated Arabinoxylan Mono- and Oligosaccharides from Corn Bran and Wheat Aleurone. J. Nutr. Metab. 2016, 1932532, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Soltani, N.; Qiu, H.; Aleksic, M.; Glinka, Y.; Zhao, F.; Liu, R.; Li, Y.; Zhang, N.; Chakrabarti, R.; Ng, T.; et al. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc. Natl. Acad. Sci. USA 2011, 108, 11692–11697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Untereiner, A.; Abdo, S.; Bhattacharjee, A.; Gohil, H.; Pourasgari, F.; Ibeh, N.; Lai, M.; Batchuluun, B.; Wong, A.; Khuu, N.; et al. GABA promotes β-cell proliferation, but does not overcome impaired glucose homeostasis associated with diet-induced obesity. FASEB J. 2019, 33, 3968–3984. [Google Scholar] [CrossRef] [PubMed]
- Kozuka, C.; Sunagawa, S.; Ueda, R.; Higa, M.; Tanaka, H.; Shimizu-Okabe, C.; Takayama, S.I.C.; Matsushita, M.; Tsutsui, M.; Miyazaki, J.; et al. ϒ-Oryzanol Protects Pancreatic β-Cells Against Endoplasmic Reticulum Stress in Male Mice. Endocrinology 2015, 156, 1242–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, A.; Morales-Scheihing, D.; Salvadores, N.; Moreno-Gonzalez, I.; Gonzalez, C.; Taylor-Presse, K.; Mendez, N.; Shahnawaz, M.; Gaber, A.O.; Sabek, O.M.; et al. Induction of IAPP amyloid deposition and associated diabetic abnormalities by a prion-like mechanism. J. Exp. Med. 2017, 214, 2591–2610. [Google Scholar] [CrossRef] [PubMed]
- Akter, R.; Cao, P.; Noor, H.; Ridgway, Z.; Tu, L.-H.; Wang, H.; Wong, A.G.; Zhang, X.; Abedini, A.; Schmidt, A.M.; et al. Islet amyloid polypeptide: Structure, function, and pathophysiology. J. Diabetes Res. 2016, 33, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Reddy, N.; Brender, J.R.; Vivekanandan, S.; Ramamoorthy, A. Structure and membrane orientation of IAPP in its natively amidated form at pH in a membrane environment. Biochim. Biophys. Acta. 2011, 1808, 2337–2342. [Google Scholar]
- Kanatsuka, A.; Kou, S.; Makino, H. IAPP/amylin and betacell failure: Implication of the risk factors of type 2 diabetes. Diabetol. Int. 2018, 9, 143–157. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Pan, Y.-H.; Huang, Y.-M.; Zhao, H.-L. Neuroendocrine hormone amylin in diabetes. World J. Diabetes 2016, 7, 189–197. [Google Scholar] [CrossRef]
- Clark, A.; Nilsson, M.R. Islet amyloid: A complication of islet dysfunction or an a etiological factor in type 2 diabetes? Diabetologia 2004, 47, 157–169. [Google Scholar] [CrossRef]
- Asthana, S.; Mallick, B.; Alexandrescu, A.T.; Jha, S. IAPP in type II diabetes: Basic research on structure, molecular interactions, and disease mechanisms suggests potential intervention strategies. BBA Biomembr. 2018, 1860, 1765–1782. [Google Scholar] [CrossRef] [PubMed]
- Rungratanawanich, W.; Abate, G.; Uberti, D. Pharmacological profile of γ-oryzanol: Its antioxidant mechanisms and its effects in age-related diseases. In Aging; Academic Press: Cambridge, MA, USA, 2020; pp. 201–208. [Google Scholar]
- Minatel, I.O.; Francisqueti, F.V.; C. Corrêa, C.R.; Lima, G.P.P. Antioxidant Activity of ϒ-Oryzanol: A Complex Network of Interactions. Int. J. Mol. Sci. 2016, 17, 1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Deemer, E. Development and validation of oxygen radical absorbance capacity assay for lipophilic antioxidants using randomly methylated β-cyclodextrin as the solubility enhancer. J. Agric. Food Chem. 2002, 50, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.J. Potential functionality and digestibility of oryzanol as determined using in vitro cell culture models. Ph.D. Dissertation, Louisiana State University and Agricultural and Mechanical College, Baton Rouge, LA, USA, 2003; pp. 1–159. [Google Scholar]
- Chotimarkorn, C.; Ushio, H. The effect of trans-ferulic acid and gamma-oryzanol on ethanol-induced liver injury in C57BL mouse. Phytomedicine 2008, 15, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Al-Naqeeb, A.; Mamat, W.A.A.; Ahmad, Z. Gamma-oryzanol rich fraction regulates the expression of antioxidant and oxidative stress related genes in stressed rat’s liver. Nutr. Metab. 2010, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Jin Son, M.W.; Rico, C.; Hyun Nam, S.; Young Kang, M. Influence of Oryzanol and Ferulic Acid on the Lipid Metabolism and Antioxidative Status in High Fat-Fed Mice. J. Clin. Biochem. Nutr. 2010, 46, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Spiazzi, C.C.; Manfredini, V.; Silva, F.E.; Flores, E.M.M.; Izaguirry, A.P.; Vargas, L.M.; Soares, M.B.; Santos, F.W. γ-Oryzanol protects against acute cadmium-induced oxidative damage in mice testes. Food Chem. Toxicol. 2013, 55, 526–532. [Google Scholar] [CrossRef]
- Mirhashemi, S.M. To evaluate likely antiamyloidogenic property of ferulic acid and baicalein against human islet amyloid polypeptide aggregation, in vitro study. Afr. J. Pharm. Pharmacol. 2012, 6, 671–676. [Google Scholar]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.S.; Becker, K. Dietary roles of phytate and phytase in human nutrition: A review. Food Chem. 2010, 120, 945–959. [Google Scholar] [CrossRef]
- Nissar, J.; Ahad, T.; Naik, H.R.; Hussain, S.Z. A review phytic acid: As antinutrient or nutraceutical. J. Pharmacogn. Phytochem. 2017, 6, 1554–1560. [Google Scholar]
- Omoruyi, F.O.; Budiaman, A.; Eng, Y.; Olumese, F.E.; Hoesel, J.L.; Ejilemele, A.; Okorodudu, A.O. The Potential Benefits and Adverse Effects of Phytic Acid Supplement in Streptozotocin-Induced Diabetic Rats. Adv. Pharmacol. Sci. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchis, P.; Rivera, R.; Berga, F.; Fortuny, R.; Adrover, M.; Costa-Bauza, A.; Grases, F.; Masmiquel, L. Phytate Decreases Formation of Advanced Glycation End-Products in Patients with Type II Diabetes: Randomized Crossover Trial. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, M.; Sdheer, A.R.; Menon, V.P. Ferulic Acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gohil, K.J.; Kshirsagar, S.B.; Sahane, R.S. Ferulic acid—A comprehensive 445 pharmacology of an important bioflavonoid. Int. J. Pharm. Sci. Res. 2012, 3, 700–710. [Google Scholar]
- Prabhakar, P.K.; Prasad, R.; Ali, S.; Doble, M. Synergistic interaction of ferulic acid with commercial hypoglycemic drugs in streptozotocin induced diabetic rats. Phytomedicine 2013, 20, 488–494. [Google Scholar] [CrossRef]
- Nomura, E.; Kashiwada, A.; Hosoda, A.; Nakamura, K.; Morishita, H.; Tsuno, T.; Taniguchi, H. Synthesis of amide compounds of ferulic acid, and their stimulatory effects on 518 insulin secretion in vitro. Bioorg. Med. Chem. 2003, 11, 3807–3813. [Google Scholar] [CrossRef]
- Shoichi, I.; Yukihiro, I. Marketing of Value-Added Rice Products in Japan: Germinated Brown Rice and Rice Bread. In Proceeding of FAO Rice Conference, Rome Italy, 12–13 February 2004; pp. 1–10. [Google Scholar]
- Akama, S.; Kanetue, J.; Shimasaki, S.; Kawakami, K.; Tsuchikura, S.; Takaiwa, F. Seed-specific expression of truncated OsGAD2 produces GABA-enriched rice grains that influence a decrease in blood pressure in spontaneously hypertensive rats. Transgenic Res. 2009, 18, 865–876. [Google Scholar] [CrossRef] [Green Version]
- Adeghate, E.; Ponery, A.S. GABA in the endocrine pancreas: Cellular localization and function in normal and diabetic rats. Tissue Cell 2002, 34, 1–6. [Google Scholar] [CrossRef]
- Napolitano, T.; Avolio, F.; Vieira, A.; Ben-Othman, N.; Courtney, M.; Gjernes, E.; Hadzic, B.; Druelle, N.; Sanz, S.N.; Silvano, S.; et al. GABA signaling stimulates α-cell-mediated β-like cell neogenesis. Commun. Integr. Biol. 2017, 10, e1300215. [Google Scholar] [CrossRef]
- Jain, A.B.; Jain, V.A. Vitamin E, Its Beneficial Role in Diabetes Mellitus (DM) and Its Complications. J. Clin. Diagn. Res. 2012, 6, 1624–1628. [Google Scholar] [CrossRef]
- Kneckt, P.; Reunanen, A.; Marniemi, J.; Leino, A.; Aromaa, A. Low vitamin E status is a potential risk factor for insulin-dependent diabetes mellitus. J. Intern. Med. 1999, 245, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Pazdro, R.; Burgess, J.R. The role of vitamin E and oxidative stress in diabetes complications. Mech. Ageing Dev. 2010, 131, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Kang, Z.; Wong, C. Vitamin E tocotrienols improve insulin sensitivity through activating peroxisome proliferator-activated receptors. Mol. Nutr. Food Res. 2010, 54, 345–352. [Google Scholar] [CrossRef] [PubMed]
| Nutrients | White Rice | Brown Rice | Rice Bran |
|---|---|---|---|
| Carbohydrates Starch | 77.0–89.0 77.6 | 73.0–87.0 66.4 | 34.0–62.0 13.8 |
| Lipids | 0.3–0.5 | 1.6–2.8 | 15.0–19.7 |
| Protein | 4.5–10.5 | 4.3–18.2 | 11.3–14.9 |
| Crude Fiber Dietary Fiber | 0.2–0.5 0.7–2.7 | 0.6–1.0 2.9–4.4 * | 7.0–11.4 19.0–29.0 * |
| Ash | 0.3–0.8 | 1.0–1.5 | 6.6–9.9 |
| Compounds | White Rice | References | Rice Bran | References |
|---|---|---|---|---|
| γ-oryzanol | 5.0–7.2 | [20] | 59.4–912.0 | [21,22,23,24] |
| Phytic acid | 13.0–1104.0 | [25,26,27] | 4000.0–22,500.0 | [25,26,28,29] |
| Ferulic acid | 0.5–8.4 | [30,31] | 1.4–225.4 | [31] |
| γ-Aminobutyric acid | 0.3–0.7 | [32] | 10.7–58.0 (before grain germination) 90.0–350.0 (after grain germination) | [33,34,35] |
| Tocopherols and tocotrienols (vitamin E) | 0.6–1.8 | [20] | 17.0–22.9 | [20,36,37] |
| Type of Rice | GI |
|---|---|
| Glutinous white rice (unique study) | 98 |
| Glutinous white rice (unique study) | 94 |
| Sticky rice, Thai glutinous rice (unique study) | 92 |
| Jasmine white rice (mean of 18 studies) | 89 |
| Japanese Style Sushi white rice (unique study) | 85 |
| Arborio risotto rice (unique study) | 69 |
| Carnaroli white rice (unique study) | 64 |
| Parboiled rice (mean of 10 studies) | 64 |
| Rice Long grain (mean of 6 studies) | 62 |
| Basmati white rice boiled (mean of 10 studies) | 60 |
| Taiken brown rice (japonica rice) (unique study) | 58 |
| Bapatla brown rice (indian rice) (unique study) | 58 |
| Compound | Model/Dose | Effect * | Reference |
|---|---|---|---|
| Rice Proteins | Wistar rats, diet supplemented with 50–200 mg rice albumin/Kg weight after 15 min of oral starch or glucose administration (1 g/Kg weight) | ↓ Blood glucose | [93] |
| In vitro digestion: native rice protein (12%, pressure cooking 95 °C 30 min) + wheat starch (70%) + digestive enzymes, during 30 min | ↓ RDS content after pressure cooking Native rice protein promotes starch-protein interaction and restricts starch hydration and enzymatic cleavage | [45] | |
| Chinese males, diet: drink rich in carbohydrates (50 g) + rice protein (24 g) during 15, 30, 45, 60, 90, 120, 150, and 180 min | ↓ Post-prandial blood glucose | [94] | |
| In vitro digestion: rice flour with and without endogenous protein (8.4%) + starch enzymes, during digestion | ↓ Starch digestibility in rice flour with endogenous protein (GI = 92.3 to GI = 88.9) | [55] | |
| Resistant Starch | In vitro digestion: cookies with 50% rice flour + 50% RS from de-branched (RSa) or from acid and heat-moisture (RSc) | ↓ Starch digestibility reducing cookies estimated GI | [42] |
| In vivo: healthy and T2DM individuals: meals contained PPB-R-203-derived rice/noodles enriched with RS (10% of starch),3 days | ↓ Blood glucose and insulin ↓ Postprandial hyperglycemia in T2DM individuals | [44] | |
| γ-oryzanol | STZ treated Wistar rats; diet supplemented with oryzanol from rice bran 50 and 100 mg/Kg/day, 8 weeks | ↓ Serum glucose ↓ Oxidative stress | [18] |
| Phytic acid (PA) | Wistar rat diet supplemented with PA at 10 to 13 g diet/day, 3 weeks | ↓ Blood glucose | [95] |
| Diabetic Wistar albino rats supplemented with 650 mg PA/kg, 28 days | ↓ Blood glucose | [96] | |
| C57BL/6N mice; 3 g diet/day contained 0.5% PA, 7 weeks | ↓ Blood glucose | [97] | |
| Diabetic KK mice, diet with 0.5–1.0% sodium phytate, 8 weeks | ↓ Blood glucose | [98] | |
| Ferulic Acid (FA) | C57BL/KsJ-db/db diabetic mice; diet supplemented with FA from rice, 0.05 g/kg/day, 17 days | ↓ Blood glucose ↑ Plasma insulin | [99] |
| γ-Aminobutyric acid (GABA) | C57BL/6 mouse diet supplemented with 2 mg/mL GABA, 20 weeks | ↓ Glucose intolerance ↓ Fasting blood glucose | [100] |
| Tocopherols and tocotrienols (vitamin E) | Overweight individuals group supplemented with 800–1200 IU vitamin E/day, 3 months | ↓ Fasting plasma glucose and insulin | [101] |
| Sprague–Dawley diabetic rats; diet with vitamin E extract (1 g/kg weight), 12 weeks | ↓ Blood glucose | [102] |
| Compound | Model/Dose | Effect | Reference |
|---|---|---|---|
| Rice Proteins | Intestinal STC-1 cells with 100 μg of tripsin-digested rice albumin/mL, 48 h | Suppressed SGLT1 | [113] |
| Phytic acid (PA) | Piglets (Yorkshire-Landracex Duroc) supplemented with 2 g PA or Na phytate/1 kg diet, 10 days | ↓ Crypt depth in the jejunum ↓ SGLT1 expression in the duodenum, jejunum, and ileum ↓ Nutrient utilization in pigs, which is involved in glucose and Na absorption | [114] |
| Ferulic acid (FA) | Caco-2 cells, 0–0.1 mg/mL FA, 15 min; Xenopus laevis oocytes, 100–300 µM FA, 30 min | ↓ Glucose uptake in Caco-2 cells Block glucose uptake in oocytes ≥100 μM ↓ GLUT2 in oocytes | [115] |
| Source | Model/Dose | Effect | Reference |
|---|---|---|---|
| γ-oryzanol | C57BL/6J mouse, diet supplemented with oryzanol 320 μg/g weight/day, 13 weeks | ↓ ER stress in pancreatic β-cells ↓ Pancreatic islet dysfunction ↑ Protection of β-cells against apoptosis | [118] |
| Ferulic acid (FA) | Cell-free, human amylin peptide + 10–40 µM FA, 192 h | ↓ IAPP amyloid formation by 27.7% to 22.6% | [134] |
| γ-Aminobutyric acid (GABA) | CD1 mice + 2 injections of 20 μmol GABA/mouse during 48 h; INS-1 cells challenged with streptozotocin (STZ) (15 mM, 24 h) + 1, 10, 100 μM of GABA | ↑ Islet cell function ↑ Protection of β-cell from apoptosis | [116] |
| CD1 or C57 mouse, supplemented with 6 mg GABA/mL, 10 weeks | ↑ β-cell proliferation ↑ Insulin secretion | [117] | |
| Tocopherols and tocotrienols (vitamin E) | C3H/AnLCSaCSa mouse induced with alloxan and supplemented with 50 mg α-tocopherol/100 g diet, 14 weeks | ↑ Insulin secretion ↓ Apoptosis caused by oxidative stress | [19] |
| Compound | Model/Dose | Effect | Reference |
|---|---|---|---|
| Rice Proteins | Protein hydrolysates from rice bran (cultivar Reiziq); 10 mg/mL + in vitro enzyme preparations + 1% starch solution, 240 min | High inhibition of starch enzymes activities by albumin and glutelin | [65] |
| γ-oryzanol | 40 µL α-amylase 50 U/mg + 0.5 mL γ-oryzanol + 40 µL of starch 1%; 100 µL α-glucosidase 50 U/mg + 5 mg γ-oryzanol, 30 min | Inhibition of α-amylase (IC50 = 0.78 mg/mL) and α-glucosidase (IC50 = 0.81 mg/mL) * | [106] |
| Phytic acid (PA) | 1 U/mL α-amylase + 0.25–8 µg PA/mL, 3 min; 5–100 µ g PA/mL + ~0.1 mL α-glucosidase + starch solution 0.5%, 60 min | Inhibition of α-amylase (IC50 = 1.2 µg/mL) and α-glucosidase (IC50 =3.2 µg/mL) * | [96] |
| Ferulic Acid (FA) | In vitro: 100 mg Rat intestinal acetone powder + 10 µL maltase or 40 µL sucrase + 0–1 mM FA, 30–60 min | Inhibition of α-glucosidase (IC50 = 0.79 mM) and intestinal maltase and sucrase (IC50 = 0.45 mM) * | [107] |
| 20 µL α-glucosidase from baker’s yeast + 20 µL FA, 30 min | Inhibition of α-glucosidase (IC50 = 0.8 mg/mL) * | [99] | |
| γ-Aminobutyric acid (GABA) | 50 µL α-glucosidase 0.2 U/mL + 50 µL cell-free extract from yeasts containing GABA (86.2–179.2 µL/mL), 30 min | Anti-hyperglycemic effect High inhibition of α-glucosidase (up to 72.3%) | [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, C.; Lourenço, V.M.; Menezes, R.; Brites, C. Rice Compounds with Impact on Diabetes Control. Foods 2021, 10, 1992. https://doi.org/10.3390/foods10091992
Pereira C, Lourenço VM, Menezes R, Brites C. Rice Compounds with Impact on Diabetes Control. Foods. 2021; 10(9):1992. https://doi.org/10.3390/foods10091992
Chicago/Turabian StylePereira, Cristiana, Vanda M. Lourenço, Regina Menezes, and Carla Brites. 2021. "Rice Compounds with Impact on Diabetes Control" Foods 10, no. 9: 1992. https://doi.org/10.3390/foods10091992
APA StylePereira, C., Lourenço, V. M., Menezes, R., & Brites, C. (2021). Rice Compounds with Impact on Diabetes Control. Foods, 10(9), 1992. https://doi.org/10.3390/foods10091992








