Changes in Phenolics during Cooking Extrusion: A Review
Abstract
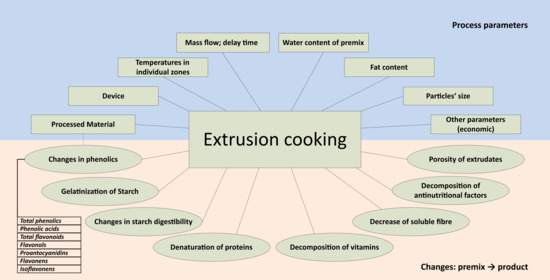
:1. Introduction
2. Total Phenolic Content (TPC) in Raw Materials and Extrudates
3. Phenolic Acids
4. Flavonoids, Flavonols, Proanthocyanidins, Flavanones, Flavones, Isoflavons, and 3-Deoxyanthocyanidins
5. Conclusions
Funding
Conflicts of Interest
References
- Alam, M.S.; Kaur, J.; Khaira, H.; Gupta, K. Extrusion and extruded products: Changes in quality attributes as affected by extrusion process parameters: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 445–473. [Google Scholar] [CrossRef] [PubMed]
- Ying, D.Y.; Hlaing, M.M.; Lerisson, J.; Pitts, K.; Chenga, L.; Sanguansri, L.; Augustin, M.A. Physical properties and FTIR analysis of rice-oat flour and maize-oat flour based extruded food products containing olive pomace. Food Res. Int. 2017, 100, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Özer, E.A.; Herken, E.N.; Güzel, S.; Ainsworth, P.; Ibanoglu, S. Effect of extrusion process on the antioxidant activity and total phenolics in a nutritious snack food. Int. J. Food Sci. Technol. 2006, 41, 289–293. [Google Scholar] [CrossRef]
- Kosinska-Cagnazzo, A.; Bocquel, D.; Marmillod, I.; Andlauer, W. Stability of goji bioactives during extrusion cooking process. Food Chem. 2017, 230, 250–256. [Google Scholar] [CrossRef]
- Marti, A.; Pagani, M.A. What can play the role of gluten in gluten free pasta? Trends Food Sci. Technol. 2013, 31, 63–71. [Google Scholar] [CrossRef]
- Wójtowicz, A.; Moscicki, L. Influence of legume type and addition level on quality characteristics, texture and microstructure of enriched precooked pasta. LWT Food Sci. Technol. 2014, 59, 1175–1185. [Google Scholar] [CrossRef]
- Palanisamy, M.; Töpfl, S.; Berger, R.G.; Hertel, C. Physico-chemical and nutritional properties of meat analogues based on Spirulina/lupin protein mixtures. Eur. Food Res. Technol. 2019, 245, 1889–1898. [Google Scholar] [CrossRef]
- Sarka, E.; Smrckova, P.; Aldao, D.A.C.; Saglamtas, M.; Kolacek, J.; Pour, V. Influence of process parameters and added starches on resistant starch content and sensory properties of maize extrudates. Starch Stärke 2015, 67, 737–744. [Google Scholar] [CrossRef]
- Nikmaram, N.; Kamani, M.H.; Ghalavand, R. The effects of extrusion cooking on antinutritional factors, chemical properties and contaminating microorganisms of food. Int. J. Farm. Allied Sci. 2015, 4, 352–354. Available online: http://ijfas.com/wp-content/uploads/2015/05/352-354.pdf (accessed on 25 June 2021).
- Shahidi, F.; Yeo, J.D. Insoluble-bound phenolics in food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef]
- Eskin, N.A.M.; Hoy, C.-T.; Shahidi, F. Browning reactions in foods. In Biochemistry of Foods, 3rd ed.; Eskin, N.A.M., Shahidi, F., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 245–289. [Google Scholar]
- Osakabe, N.; Yasuda, A.; Natsume, N.; Yoshikawa, T. Rosmarinic acid inhibits epidermal inflammatory responses: Anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogen 2004, 25, 549–557. [Google Scholar] [CrossRef]
- Liu, R.H. Whole grain phytochemicals and health. J. Cereal Sci. 2007, 46, 207–219. [Google Scholar] [CrossRef]
- Wang, T.; He, F.; Chen, G. Improving bioaccessibility and bioavailability of phenolic compounds in cereal grains through processing technologies: A concise review. J. Funct. Foods 2014, 7, 101–111. [Google Scholar] [CrossRef]
- Vaculová, K.; Sedláčková, I.; Jirsa, O.; Sluková, M.; Skřivan, P. Changes in chemical composition and nutritional properties of wholemeal flours from different barley materials due to controlled germination. In Proceedings of the 13th International Conference on Polysaccharides-Glycoscience, Prague, Czech Republic, 8–10 November 2017; Řápková, R., Čopíková, J., Šárka, E., Eds.; Czech Chemical Society: Prague, Czech Republic, 2017; pp. 302–306. [Google Scholar]
- Sarawong, C.; Schoenlechner, R.; Sekiguch, K.; Berghofer, E.; Ng, P.K.W. Effect of extrusion cooking on the physicochemical properties, resistant starch, phenolic content and antioxidant capacities of green banana flour. Food Chem. 2014, 143, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Hirth, M.; Leiter, A.; Beck, S.M.; Schuchmann, H.P. Effect of extrusion cooking process parameters on the retention of bilberry anthocyanins in starch based food. J. Food Eng. 2014, 125, 139–146. [Google Scholar] [CrossRef]
- Xu, E.; Wu, Z.; Jiao, A.; Long, J.; Liab, J.; Jin, Z. Dynamics of rapid starch gelatinization and total phenolic thermomechanical destruction moderated via rice bio-extrusion with alpha-amylase activation. RSC Adv. 2017, 7, 19464–19478. [Google Scholar] [CrossRef] [Green Version]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Zhu, F. Interactions between starch and phenolic compound. Trends Food Sci. Technol. 2015, 43, 129–143. [Google Scholar] [CrossRef]
- Barrett, A.; Ndou, T.; Hughey, C.A.; Straut, C.; Howell, A.; Dai, Z.; Kaletunc, G. Inhibition of α-amylase and glucoamylase by tannins extracted from cocoa, pomegranates, cranberries, and grapes. J. Agric. Food Chem. 2013, 61, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Smrckova, P.; Saglamtas, M.; Hofmanova, T.; Kolacek, J.; Chena, D.; Sarka, E. Effect of process parameters on slowly digestible and resistant starch content in extrudates. Czech J. Food Sci. 2014, 32, 503–508. [Google Scholar] [CrossRef] [Green Version]
- Aguayo-Rojas, J.; Mora-Rochin, S.; Cuevas-Rodriguez, E.O.; Serna-Saldivar, S.O.; Gutierrez-Uribe, J.A.; Reyes-Moreno, C.; Milan-Carrillo, J. Phytochemicals and antioxidant capacity of tortillas obtained after lime-cooking extrusion process of whole pigmented Mexican maize. Plant Foods Human Nutr. 2012, 67, 178–185. [Google Scholar] [CrossRef]
- Gaxiola-Cuevas, N.; Mora-Rochín, S.; Cuevas-Rodriguez, E.O.; León-López, L.; Reyes-Moreno, C.; Montoya-Rodríguez, A.; Milan-Carrillo, J. Phenolic acids profiles and cellular antioxidant activity in tortillas produced from Mexican maize landrace processed by nixtamalization and lime extrusion cooking. Plant Foods Human Nutr. 2017, 72, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Gujral, H.S.; Sigh, B. Antioxidant activity of barley as affected by extrusion cooking. Food Chem. 2012, 131, 1406–1413. [Google Scholar] [CrossRef]
- Sarka, E.; Slukova, M.; Smrckova, P. New food compositions to increase the content of phenolic compounds in extrudates. Czech J. Food Sci. 2020, 38, 347–358. [Google Scholar] [CrossRef]
- Esposito, F.; Arlotti, G.; Bonifati, A.M.; Napolitano, A.; Vitale, D.; Fogliano, V. Antioxidant activity and dietary fibre in durum wheat bran by-products. Food Res. Int. 2005, 38, 1167–1173. [Google Scholar] [CrossRef]
- Apea-Bah, F.B.; Beta, T. Bound phenolic constituents as co-passengers of dietary fibre. In Cereal Grain-Based Functional Foods: Carbohydrate and Phytochemical Components; Beta, T., Camire, M.E., Eds.; RSC Publishing: London, UK, 2018; pp. 278–304. [Google Scholar]
- Zeng, Z.; Liu, C.; Luo, S.; Chen, J.; Gong, E. The profile and bioaccessibility of phenolic compounds in cereals influenced by improved extrusion cooking treatment. PLoS ONE 2016, 11, e0161086. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, A.; Oniszczuk, A.; Oniszczuk, T.; Kocir, S.; Wojtunik, K.; Mitrus, M.; Kocira, A.; Widelski, J.; Skalicka-Wozniak, K. Application of Moldavian dragonhead (Dracocephalum moldavica L.) leaves addition as a functional component of nutritionally valuable corn snacks. J. Food Sci. Technol. 2017, 54, 3218–3229. [Google Scholar] [CrossRef]
- Tian, R.-R.; Pan, Q.-H.; Zhan, J.-C.; Li, J.M.; Wan, S.-B.; Zhang, Q.-H.; Huang, W.-D. Comparison of phenolic acids and flavan-3-ols during wine fermentation of grapes with different harvest times. Molecules 2009, 14, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Zago, E.; Lecomte, J.; Barouh, N.; Aouf, C.; Carré, P.; Fine, F.; Villeneuve, P. Influence of rapeseed meal treatments on its total phenolic content and composition in sinapine, sinapic acid and canolol. Ind. Crops Prod. 2015, 76, 1061–1070. [Google Scholar] [CrossRef]
- Chen, C. Sinapic acid and its derivatives as medicine in oxidative stress-induced diseases and aging. Oxid. Med. Cell. Longev. 2016. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Khan, S.A.; Chi, J.; Wei, Z.; Zhang, Y.; Deng, Y.; Liu, L.; Zhang, M. Different effects of extrusion on the phenolic profiles and antioxidant activity in milled fractions of brown rice. LWT Food Sci. Technol. 2018, 88, 64–70. [Google Scholar] [CrossRef]
- Sun, L.; Bai, X.; Hang, Y. Effect of different cooking methods on total phenolic contents and antioxidant activities of four Boletus mushrooms. J. Food Sci. Technol. 2014, 51, 3362–3368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kędzierska-Matysek, M.; Stryjecka, M.; Teter, A.; Skałecki, P.; Domaradzki, P.; Florek, M. Relationships between the content of phenolic compounds and the antioxidant activity of Polish honey varieties as a tool for botanical discrimination. Molecules 2021, 26, 1810. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Kim, M.-C.; Um, J.-Y.; Hong, S.-H. The beneficial effect of vanillic acid on ulcerative colitis. Molecules 2010, 15, 7208–7217. [Google Scholar] [CrossRef]
- Gong, E.S.; Luo, S.; Li, T.; Liu, C.; Zhang, G.; Chen, J.; Zeng, Z.; Liu, R.H. Phytochemical profiles and antioxidant activity of processed brown rice products. Food Chem. 2017, 232, 67–78. [Google Scholar] [CrossRef]
- Anon. Showing all Foods in Which the Polyphenol Caffeic Acid is Found. Available online: http://phenol-explorer.eu/contents/polyphenol/457 (accessed on 25 June 2021).
- Sarıdaş, M.A. Seasonal variation of strawberry fruit quality in widely grown cultivars under Mediterranean climate condition. J. Food Compos. Anal. 2021, 97, 10373. [Google Scholar] [CrossRef]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Kumar, C.S. Syringic acid (SA)—A review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef]
- Dey, G.; Chakraborty, M.; Mitra, A. Profiling C6–C3 and C6–C1 phenolic metabolites in Cocos nucifera. J. Plant Physiol. 2005, 162, 375–381. [Google Scholar] [CrossRef]
- Lohani, U.C.; Muthukumarappan, K. Effect of extrusion processing parameters on antioxidant, textural and functional properties of hydrodynamic cavitated corn flour, sorghum flour and apple pomace-based extrudates. J. Food Proc. Eng. 2017, 40, e12424. [Google Scholar] [CrossRef]
- Thakur, S.; Singh, N.; Kaur, A.; Singh, B. Effect of extrusion on physicochemical properties, digestibility, and phenolic profiles of grit fractions obtained from dry milling of normal and waxy corn. J. Food Sci. 2017, 82, 1101–1109. [Google Scholar] [CrossRef]
- Gumul, D.; Areczuk, A.; Ziobro, R.; Ivanisova, E.; Zieba, T. The influence of freeze-dried red and purple potatoes on content of bioactive polyphenolic compounds and quality properties of extruded maize snacks. Qual. Assur. Saf. Crop. 2018, 10, 51–60. [Google Scholar] [CrossRef]
- Gumul, D.; Ziobro, R.; Korus, J.; Kruczek, M.; Arvay, J. Characteristics of extruded cereal snacks enriched by an addition of freeze-dried red and purple potatoes. J. Food Process Eng. 2018, 41, e12927. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Rattan, B.; Singh, J.P.; Kaur, A.; Singh, B. Effect of chickpea and spinach on extrusion behavior of corn grit. J. Food Sci. Technol. 2019, 56, 2257–2266. [Google Scholar] [CrossRef]
- Zeng, Z.; Huang, K.; McClements, D.J.; Hu, X.; Luo, S.; Liu, C. Phenolics, antioxidant activity, and in vitro starch digestibility of extruded brown rice influenced by Choerospondias axillaris fruit peels addition. Starch Stärke 2019, 71, 1800346. [Google Scholar] [CrossRef]
- Albarracín, M.; Dyner, L.; Giacomino, M.S.; Weisstaub, A.; Zuleta, A.; Drago, S.R. Modification of nutritional properties of whole rice flours (Oryza sativa L.) by soaking, germination, and extrusion. J. Food Biochem. 2019, 43, e12854. [Google Scholar] [CrossRef]
- Tepsongkroh, B.; Jangchud, K.; Jangchud, A.; Charunuch, C.; Prinyawiwatkul, W. Healthy brown rice-based extrudates containing straw mushrooms: Effect of feed moisture and mushroom powder contents. J. Food Process. Preserv. 2019, 43, e14089. [Google Scholar] [CrossRef]
- Oliveira, A.R.; Ribeiro, A.E.C.; Oliveira, R.; Caroline, M.D.S.A.; Soares, M.S., Jr.; Caliari, M. Broken rice grains pregelatinized flours incorporated with lyophilized açaıí pulp and the effect of extrusion on their physicochemical properties. J. Food Sci. Technol. 2019, 56, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Guven, O.; Sensoy, I.; Senyuva, H.; Karakaya, S. Food processing and digestion: The effect of extrusion process on bioactive compounds in extrudates with artichoke leaf powder and resulting in vitro cynarin and cynaroside bioaccessibility. LWT Food Sci. Technol. 2018, 90, 232–237. [Google Scholar] [CrossRef]
- Ramos-Enríquez, J.R.; Ramírez-Wong, B.; Robles-Sánchez, R.M.; Robles-Zepeda, R.E.; González-Aguilar, G.A.; Gutiérrez-Dorado, R. Effect of extrusion conditions and the optimization of phenolic compound content and antioxidant activity of wheat bran using response surface methodology. Plant Foods Human Nutr. 2018, 73, 228–234. [Google Scholar] [CrossRef]
- Dlamini, N.R.; Taylor, J.R.N.; Rooney, L.W. The effect of sorghum type and processing on the antioxidant properties of African sorghum-based foods. Food Chem. 2007, 105, 1412–1419. [Google Scholar] [CrossRef]
- Adarkwah-Yiadom, M.; Duodu, K.G. Effect of extrusion cooking and simulated in vitro gastrointestinal digestion on condensed tannins and radical scavenging activity of type II and type III whole grain sorghum. Int. J. Food Sci. Technol. 2017, 52, 2282–2294. [Google Scholar] [CrossRef]
- Azad, O.K.; Jeong, D.I.; Adnan, M.; Salitxay, T.; Heo, J.W.; Naznin, M.T.; Lim, J.D.; Cho, D.H.; Park, B.J.; Park, C.H. Effect of different processing methods on the accumulation of the phenolic compounds and antioxidant profile of broomcorn millet (Panicum miliaceum L.) flour. Foods 2019, 8, 230. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Jin, Z.; Jin, T. Effects of extrusion conditions on chemical properties of extruded white ginseng root hair. J. Sci. Food Agric. 2019, 99, 3186–3191. [Google Scholar] [CrossRef] [PubMed]
- Adebowale, A.A.; Kareem, S.T.; Sobukola, O.P.; Adebisi, M.A.; Obadina, A.O.; Kajihausa, O.E.; Adegunwa, M.O.; Sanni, L.O.; Keith, T. Mineral and antinutrient content of high quality cassava-tigernut composite flour extruded snack. J. Food Process. Preserv. 2017, 41, e13125. [Google Scholar] [CrossRef] [Green Version]
- Jan, R.; Saxena, D.C.; Singh, S. Effect of extrusion variables on antioxidant activity, total phenolic content and dietary fibre content of gluten-free extrudate from germinated Chenopodium (Chenopodium album) flour. Int. J. Food Sci. Technol. 2017, 52, 2623–2630. [Google Scholar] [CrossRef]
- Salgado, N.; Giraldo, G.I.; Orrego, C.E. Influence of the extrusion operating conditions on the antioxidant, hardness and color properties of extruded mango. LWT Food Sci. Technol. 2017, 86, 209–218. [Google Scholar] [CrossRef]
- Pico, J.; Xu, K.; Guo, M.; Mohamedshah, Z.; Ferruzzi, M.G.; Martinez, M.M. Manufacturing the ultimate green banana flour: Impact of drying and extrusion on phenolic profile and starch bioaccessibility. Food Chem. 2019, 297, 124990. [Google Scholar] [CrossRef]
- Rathod, R.P.; Annapure, U.S. Antioxidant activity and polyphenolic compound stability of lentil-orange peel powder blend in an extrusion process. J. Food Sci. Technol. 2017, 54, 954–963. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Ryu, G. Effects of green tea contents on the quality and antioxidant properties of textured vegetable protein by extrusion-cooking. Food Sci. Biotech. 2019, 28, 67–74. [Google Scholar] [CrossRef]
- Espinoza-Moreno, R.J.; Reyes-Moreno, C.; Milán-Carrillo, J.; López-Valenzuela, J.A.; Paredes-López, O.; Gutiérrez-Dorado, R. Healthy ready-to-eat expanded snack with high nutritional and antioxidant value produced from whole amarantin transgenic maize and black common bean. Plant Foods Human Nutr. 2016, 71, 218–224. [Google Scholar] [CrossRef]
- Ortak, M.; Caltinoglu, C.; Sensoy, I.; Karakaya, S.; Mert, B. Changes in functional properties and in vitro bioaccessibilities of β-carotene and lutein after extrusion processing. J. Food Sci. Technol. 2017, 54, 3543–3551. [Google Scholar] [CrossRef]
- Kasprzak, K.; Oniszczuk, T.; Wójtowicz, A.; Waksmundzka-Hajnos, M.; Olech, M.; Nowak, R.; Polak, R.; Oniszczuk, A. Phenolic acid content and antioxidant properties of extruded corn snacks enriched with kale. J. Anal. Methods Chem. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Oniszczuk, A.; Kasprzak, K.; Wójtowicz, A.; Oniszczuk, T.; Olech, M. The impact of processing parameters on the content of phenolic compounds in new gluten-free precooked buckwheat pasta. Molecules 2019, 24, 1262. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Ying, D.; Guo, B.; Cheng, L.J.; May, B.; Bird, T.; Sanguansri, L.; Cao, Y.; Augustin, M. Extrusion of apple pomace increases antioxidant activity upon in vitro digestion. Food Funct. 2019, 10, 951–963. [Google Scholar] [CrossRef]
- Bouasla, A.; Wójtowicz, A.; Zidoune, M.N.; Olech, M.; Nowak, R.; Mitrus, M.; Oniszczuk, A. Gluten-free precooked rice-yellow pea pasta: Effect of extrusion-cooking conditions on phenolic acids composition, selected properties and microstructure. J. Food Sci. 2016, 81, C1070–C1079. [Google Scholar] [CrossRef] [PubMed]
- Ververidis, F.; Trantas, E.; Douglas, C.; Vollmer, G.; Kretzschmar, G.; Panopoulos, N. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: Chemical diversity, impacts on plant biology and human health. Biotechnol. J. 2007, 2, 1214–1234. [Google Scholar] [CrossRef] [PubMed]
- Sluková, M.; Pipek, P.; Čurda, L.; Hinková, A.; Rajchl, A.; Hrádková, I. Výroba Potravin a Nutriční Hodnota; VŠCHT Praha: Prague, Czech Republic, 2016. [Google Scholar]
- Di Matteo, V.; Pierucci, M.; Di Giovanni, G.; Esposito, E. Prevention and therapy of neurodegenerative disorders: Role of nutritional antioxidants. In Oxidative Stress and Neurodegenerative Disorders; Qureshi, G.A., Parvez, S.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 621–661. [Google Scholar] [CrossRef]
- Arribas, C.; Pereira, E.; Barros, L.; Alves, M.J.; Calhelha, R.C.; Guillamon, E.; Pedrosa, M.M.; Ferreira, I.C.F.R. Healthy novel gluten-free formulations based on beans, carob fruit and rice: Extrusion effect on organic acids, tocopherols, phenolic compounds and bioactivity. Food Chem. 2019, 292, 304–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsanova-Savova, S.; Ribarova, F. Flavonols and flavones in some Bulgarian plant foods. Pol. J. Food Nutr. Sci. 2013, 63, 173–177. [Google Scholar] [CrossRef] [Green Version]
- Pastor-Villaescusa, B.; Rodriguez, E.S.; Rangel-Huerta, O.D. Polyphenols in obesity and metabolic syndrome. In Obesity. Oxidative Stress and Dietary Antioxidants; del Moral, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 213–239. [Google Scholar] [CrossRef]
- Ciudad-Mulero, M.; Barros, L.; Fernandes, Â.; Berrios, J.D.J.; Cámara, M.; Morales, P.; Fernández-Ruiz, V.; Ferreira, I.C.F.R. Bioactive compounds and antioxidant capacity of extruded snack-type products developed from novel formulations of lentil and nutritional yeast flours. Food Funct. 2018, 9, 819–829. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, A.C.; Bento, C.; Jesus, F.; Alves, G.; Silva, L.R. sweet cherry phenolic compounds: Identification, characterization, and health benefits. In Studies in Natural Products Chemistry; Rahman, A.-U., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 31–78. [Google Scholar]
- Chen, Y.M.; Ho, S.C. Fruit, vegetables, and bone health. In Bioactive Foods in Promoting Health—Fruits and Vegetables; Watson, R.R.P., Victor, R., Eds.; Academic Press: Cambridge, MA, USA, 2010; pp. 173–194. [Google Scholar]
- Xiao, H.; Ho, C.-T. Bioactive food components nutraceuticals and toxicants. In Fennema’s Food Chemistry, 5th ed.; Damodaran, S., Parkin, K.L., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 865–903. [Google Scholar]
- Cardoso, L.D.; Pinheiro, S.S.; de Carvalho, C.W.P.; Queiroz, V.A.V.; de Menezes, C.B.; Moreira, A.V.B.; de Barros, F.A.R.; Awika, J.M.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Phenolic compounds profile in sorghum processed by extrusion cooking and dry heat in a conventional oven. J. Cereal Sci. 2015, 65, 220–226. [Google Scholar] [CrossRef]
- Banerjee, S.; Li, Y.; Wang, Z.; Sarkar, F.H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008, 269, 226–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Source | TPC Content in Input Raw Material (mg/100 g DM) | TPC Content in Output Extrudates (mg/100 g DM) | Retention (%) | Type of Extruder | Reference |
|---|---|---|---|---|---|
| corn grit | - | 132 1, 34 2 | - | single-screw | [45] 1, [46] 2 |
| corn grit | - | 41 | - | co-rotating twin-screw | [47] |
| corn grit | 126–165 1, 233–244 2 | 111–128 1+, 186–204 2+ | 74–87 1, 80–84 2 | single-screw; nixtamalization | [23] 1, [24] 2 |
| brown rice flour | 132 1, 253 2, 93 3 | 129 1, 178 2, 94 3 | 98 1, 70 2, 101 3 | single-screw | [38] 1, [48] 2, [49] 3 |
| brown rice flour | 178 1 | 77 1, 47–50 2, 46 3 | 43 1 | twin-screw | [38] 1, [50] 2, [29] 3 |
| flour from polished rice | 57 | 26 | 46 | co-rotating twin-screw | [38] |
| flour from broken rice | 44 | 30 | 68 | single-screw | [51] |
| flour from glutinous rice | 36 | 7–21 | 20–57 | co-rotating twin-screw | [18] |
| flour from glutinous rice + α-amylase | 36 | 12–31 | 34–85 | co-rotating twin-screw | [18] |
| extruded soaked rice | 98 | 99 | 101 | single-screw | [49] |
| extruded germinated rice | 141 | 135 | 96 | single-screw | [49] |
| rice bran | 756 | 811 | 107 | co-rotating twin-screw | [38] |
| wheat flour | 340 | 220 | 65 | co-rotating twin-screw | [52] |
| ground wheat | 266 | 210 | 79 | single-screw | [29] |
| wheat bran, var. Kronstad | 238 | 248–384 | 104–161 | single-screw | [53] |
| wholemeal sorghum flour, vars. Macia, NK 283, Gadam El Haman, PAN 8629r | 270–2450 1; 2335–2834 2 | 180–670 1; 580–1765 2 | 30–67 1; 24–62 2 | co-rotating twin-screw | [54] 1, [55] 2 |
| decorticated sorghum flour, vars. Macia and NK 283 | 200–850 | 230–410 | 38–170 | co-rotating twin-screw | [54] |
| oat flour | 158 | 146 | 92 | single-screw | [29] |
| chickpea | - | 60 | - | co-rotating twin-screw | [47] |
| soybean | 540 | 652–660 | 120–122 | twin-screw | [56] |
| white ginseng root hair | 278 | 292–553 | 105–199 | twin-screw | [57] |
| cassava flour | 570 | 370 | 64 | single-screw | [58] |
| germinated Chenopodium flour | 565 | 57–81 | 10–14 | twin-screw | [59] |
| dried mango | - | 267–510 | - | single-screw | [60] |
| tigernut flour | 1130 | 1000 | 88 | single-screw | [58] |
| millet | 295 | 455 | 154 | twin-screw | [56] |
| oven dried green banana flour | 338 | 1196 | 356 | co-rotating twin-screw | [61] |
| lentil flour and orange peels powder | 4685 | 3285–4038 | 70–86 | co-rotating twin-screw | [62] |
| soy protein isolate/wheat gluten/corn starch/green tea | 3847–5749 | 1522–3160 | 39–55 | co-rotating twin-screw | [63] |
| rice flour/goji berries | 123–402 | 51–741 | 41–184 | twin-screw | [4] |
| genetically modified corn (line 1041/1.7k) and bean grits (var. Jamapa Black) | 140 | 244 | 174 | single-screw | [64] |
| soy protein isolate/wheat gluten/corn starch | 2747 | 985 | 36 | co-rotating twin-screw | [63] |
| broken rice/açaí | 188–500 | 104–279 | 51–56 | single-screw | [51] |
| corn grits and carrot | 1820 | 950–1030 | 52–57 | co-rotating twin-screw | [65] |
| Source | Total Content of Phenolic Acids in Input Raw Material (mg/100 g DM) | Total Content of Phenolic Acids in Output Extrudates (mg/100 g DM) | Retention (%) | Type of Extruder | Reference |
|---|---|---|---|---|---|
| corn grit | - | 24 | - | single-screw | [45] |
| corn grit | 202–215 | 200–213 + | 99 | single-screw; nixtamalization | [24] |
| brown rice flour | 36 1, 40 2 | 43 1, 42 2 | 121 1, 106 2 | single-screw | [38] 1, [29] 2 |
| brown rice flour | 77 | 71 | 92 | twin-screw | [34] |
| rice bran | 217 | 222 | 102 | co-rotating twin-screw | [34] |
| flour from polished rice | 26 | 23 | 89 | co-rotating twin-screw | [34] |
| ground wheat | 60 | 63 | 104 | single-screw | [29] |
| oat flour | 27 | 40 | 145 | single-screw | [29] |
| Phenolic Acid | Source | Content in Input Raw Material (mg /100 g DM) | Content in Output Extrudates (mg /100 g DM) | Retention (%) | Reference |
|---|---|---|---|---|---|
| Gallic acid | rice bran | 7 | 6 | 91 | [34] |
| brown rice flour | 5 | 3 | 74 | [34] | |
| buckwheat | 0.3 | 0.2–0.3 | 60–98 | [67] | |
| apple pomace, cv. Pink Lady | 0.17 | Nd | - | [68] | |
| Vanillic acid | corn grit | 2–3 | 3 | 103–142 | [24] |
| rice bran | 23 | 45 | 199 | [34] | |
| brown rice flour | 23 1, 2 2, 0.3 3 | 26 1, 32 2, 0.4 3 | 111 1, 125 2, 118 3 | [34] 1, [38] 2, [29] 3 | |
| wheat flour | 0.6 | 0.7 | 105 | [29] | |
| oat flour | 0.7 | 1.1 | 150 | [29] | |
| buckwheat | 0.07 | 0.03–0.07 | 47–99 | [67] | |
| corn/kale, var. sabellica | - | 0.08–0.11 | - | [66] | |
| Syringic acid | corn grit | 0.9–1.6 | 1.5–2.2 | 137–167 | [24] |
| rice bran | 3.3 | 4.3 | 130 | [34] | |
| flour from polished rice | Nd | Nd | - | [34] | |
| brown rice flour | 1.1 1, 0.7 2, 0.4 3 | 1.0 1, 0.7 2, 0.4 3 | 88 1, 103 2, 100 3 | [34] 1, [38] 2, [29] 3 | |
| wheat flour | 0.2 | 0.5 | 196 | [29] | |
| oat flour | 0.6 | 1.1 | 173 | [29] | |
| Ferulic acid | corn grit | 168–186 | 167–179 | 96–99 | [24] |
| rice bran | 166 | 152 | 91 | [34] | |
| brown rice flour | 39 1, 44 2, 31 3 | 34 1, 53 2, 31 3 | 86 1, 119 2, 100 3 | [34] 1, [38] 2, [29] 3 | |
| wheat flour | 46 | 50 | 109 | [29] | |
| oat flour | 19 | 28 | 143 | [29] | |
| corn/kale, var. sabellica | - | 0.2–4.6 | - | [66] | |
| corn flour/sorghum flour/apple pomace | 4–5 | 2.5–3.5 | 71–76 | [43] | |
| rice/pea | - | 1.2–3.4 | - | [69] | |
| apple pomace, cv. Pink Lady | 0.06 | 0.04–0.08 | 67–133 | [68] | |
| 4-OH-Benzoic acid | brown rice flour | 0.8 1, 0.4 2 | 1.0 1, 0.6 2 | 114 1, 146 2 | [38] 1, [29] 2 |
| wheat flour | 4.4 | 2.5 | 57 | [29] | |
| oat flour | 0.4 | 0.9 | 214 | [29] | |
| buckwheat | 0.02 | 0.01–0.03 | 62–137 | [67] | |
| rice/pea | - | 0.03–0.07 | - | [69] | |
| corn/kale, var. sabellica | - | 0.01- 0.03 | - | [66] | |
| corn flour/sorghum flour/apple pomace | 1.7–2.3 | 1.1–1.7 | 63–71 | [43] | |
| Rosmarinic acid | corn/dragonhead leaves | - | 287–2884 | - | [30] |
| 5-OH-Salicylic acid | buckwheat | 0.02 | 0.02–0.04 | 131–248 | [67] |
| Sinapic acid | corn grit | 2–4 | 7–9 | 209–275 | [24] |
| corn/kale, var. sabellica | - | 0.6–1.3 | - | [66] | |
| brown rice flour | 4 1, 0.5 2 | 7 1, 2.2 2 | 197 1, 405 2 | [38] 1, [29] 2 | |
| wheat flour | 1.0 | 1.8 | 186 | [29] | |
| oat flour | 0.7 | 1.9 | 262 | [29] | |
| Chlorogenic acid | rice bran | 11 | 8 | 70 | [34] |
| brown rice flour | 3.3 1, 1.8 2, 0.73 3 | 2.6 1, 1.9 2, 1.0 3 | 79 1, 105 2, 134 3 | [34] 1, [38] 2, [29] 3 | |
| wheat flour | 0.7 | 0.9 | 121 | [29] | |
| oat flour | 1.9 | 1.9 | 100 | [29] | |
| apple pomace, cv. Pink Lady | 3 | 5–6 | 149–163 | [68] | |
| corn flour/sorghum flour/apple pomace | 0.3–0.8 | 0.05–0.3 | 15–38 | [43] | |
| corn/red potato, var. Magenta Love | - | 3–45 | - | [46] | |
| corn/purple potato, var. Blue Star | - | Nd–11 | - | [46] | |
| Caffeic acid | rice bran | 4 | 3 | 89 | [34] |
| flour from polished rice | 0.14 | 0.15 | 107 | [34] | |
| brown rice flour | 1.1 1, 0.09 2, 0.103 3 | 0.7 1, 0.05 2, 0.26 3 | 62 1, 59 2, 266 3 | [34] 1, [38] 2, [29] 3 | |
| wheat flour | 3.2 | 2.6 | 81 | [29] | |
| oat flour | 1.6 | 2.5 | 158 | [29] | |
| buckwheat | Nd | Nd–0.03 | 47–99 | [67] | |
| apple pomace, cv. Pink Lady | 0.07 | 0.11–0.12 | 143–171 | [68] | |
| corn flour/sorghum flour/apple pomace | 5.0–5.7 | 4.2–4.9 | 84–86 | [43] | |
| p-Coumaric acid | corn grit | 16–21 1 | 18–21 1, Nd 2 | 118–137 1 | [24] 1, [46] 2 |
| rice bran | 4.0 | 4.2 | 103 | [34] | |
| flour from polished rice | 2.8 | 0.8 | 31 | [34] | |
| brown rice flour | 5 1, 12 2, 6 3 | 4 1, 14 2, 6 3 | 79 1, 115 2, 97 3 | [34] 1, [38] 2, [29] 3 | |
| wheat flour | 4 | 4 | 90 | [29] | |
| oat flour | 2.1 | 1.9 | 92 | [29] | |
| buckwheat | 0.03 | 0.04–0.10 | 122–322 | [67] | |
| corn/kale, var. sabellica | - | 0.13–0.17 | - | [66] | |
| apple pomace, cv. Pink Lady | 0.06 | 0.14–0.21 | 233–350 | [68] | |
| corn flour/sorghum flour/apple pomace | 1.0–1.5 | 0.5–0.9 | 46–63 | [43] | |
| corn/red potato, var. Magenta Love | - | Nd–0.4 | - | [46] | |
| rice/pea | - | 0.03–0.08 | - | [69] | |
| Protocatechuic acid | corn flour/sorghum flour/apple pomace | 2–5 | 0.2–1.6 | 10–29 | [43] |
| rice/pea | - | Nd–0.04 | - | [69] | |
| buckwheat | 0.4 | 0.2–0.3 | 47–75 | [67] | |
| Salicylic acid | buckwheat | 0.25 | 0.04–0.11 | 18–46 | [67] |
| corn/kale, var. sabellica | - | 0.02–0.04 | - | [66] | |
| corn flour/sorghum flour/apple pomace | 5–7 | 3–5 | 67–74 | [43] | |
| rice/pea | - | 0.03–0.08 | - | [69] | |
| 3-OH-Cinnamic acid | corn/kale, var. sabellica | - | 0.02 | - | [66] |
| Cynarin and cynaroside | wheat/dried artichoke | 1.8–5.4 | 0.6–1.3 | 25–34 | [52] |
| wheat flour | Nd | Nd | - | [52] | |
| Diferulic acid | brown rice flour | 7 | 8 | 118 | [38] |
| Source | TFC in Input Raw Material (mg/100 g DM) | TFC in Output Extrudates (mg/100 g DM) | Retention (%) | Type of Extruder | Reference |
|---|---|---|---|---|---|
| rice bran | 612 | 642 | 104 | co-rotating twin-screw | [34] |
| polished rice | 57 | 34 | 60 | co-rotating twin-screw | [34] |
| brown rice | 171 | 119 | 70 | co-rotating twin-screw | [34] |
| corn | - | 35 | - | single-screw | [45] |
| corn/purple potato (var. Blue Star) | - | 47–99 | - | single-screw | [45] |
| corn/red potato (var. Magenta Love) | - | 85–206 | - | single-screw | [45] |
| lupin concentrate/lupin isolate/carrageenan | 79 | 77 | 97 | twin-screw | [7] |
| lupin concentrate/lupin isolate/carrageenan/semolina | 79–142 | 77–183 | 120–129 | twin-screw | [7] |
| millet | 183 | 219 | 120 | twin-screw | [56] |
| Source | Total Flavonols in Input Raw Material (mg/100 g DM) | Total Flavonols in Output Extrudates (mg/100 g DM) | Retention (%) | Type of Extruder | Reference |
|---|---|---|---|---|---|
| apple pomace, cv. Pink Lady | 21 | 26–28 | 122–133 | co-rotating twin-screw | [68] |
| lentil flour | 15 | 2 | 13 | co-rotating twin-screw | [76] |
| corn | - | 17 | - | single-screw | [45] |
| corn + purple potato (var. Blue Star) | - | 18–24 | - | single-screw | [45] |
| corn + red potato (var. Magenta Love) | - | 17–33 | - | single-screw | [45] |
| Flavonol | Source | Flavonol Content in Input Raw Material (mg/100 g DM) | Flavonol Content in Output Extrudates (mg/100 g DM) | Retention (%) | Reference |
|---|---|---|---|---|---|
| Catechin hexoside | lentil flour with yeast | 33–66 | 2–6 | 5–9 | [76] |
| Kaempferol-O-desoxyhexoside-O-hexoside-O-rutinoside | lentil flour with yeast | 1–3 | 0.4–0.6 | 13–34 | [76] |
| Quercetin | apple pomace, cv. Pink Lady | 0.04 | 0.5–1.3 | 1125–3200 | [68] |
| Quercetin-3-O-glucoside | lentil flour with yeast | 4–5 | 0.4–0.6 | 8–11 | [76] |
| Quercetin-3-O-glucoside | apple pomace, cv. Pink Lady | 4 | 5–6 | 133–147 | [68] |
| Quercetin-O-hexoside | lentil flour with yeast | 3–4 | 0.3–0.5 | 10–12 | [76] |
| Quercetin-O-pentoside | lentil flour with yeast | 5–6 | 0.5–0.8 | 8–12 | [76] |
| Quercetin-O-pentoside | rice, var. Montsianell/beans, vars. NRVP 20064637 and # TOV 002319/carob fruit, vars. Negreta and Roja | Nd–22 | Nd–3.4 | 0–31 | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šárka, E.; Sluková, M.; Henke, S. Changes in Phenolics during Cooking Extrusion: A Review. Foods 2021, 10, 2100. https://doi.org/10.3390/foods10092100
Šárka E, Sluková M, Henke S. Changes in Phenolics during Cooking Extrusion: A Review. Foods. 2021; 10(9):2100. https://doi.org/10.3390/foods10092100
Chicago/Turabian StyleŠárka, Evžen, Marcela Sluková, and Svatopluk Henke. 2021. "Changes in Phenolics during Cooking Extrusion: A Review" Foods 10, no. 9: 2100. https://doi.org/10.3390/foods10092100
APA StyleŠárka, E., Sluková, M., & Henke, S. (2021). Changes in Phenolics during Cooking Extrusion: A Review. Foods, 10(9), 2100. https://doi.org/10.3390/foods10092100