Sugars Replacement as a Strategy to Control the Formation of α-Dicarbonyl and Furanic Compounds during Cookie Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Chemicals
2.2. Production of Cookies
2.3. Determination of the Estimated Optimal Baking Time (EOBT)
2.4. Determination of the Moisture Content, pH, and the Total Titratable Acidity
2.5. Dicarbonyl Compounds and HMF Extraction
2.6. HMF Analysis
2.7. Determination of Dicarbonyl Compounds (3-DG, GO, and MGO)
2.8. Determination of Aromatic Compounds
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effect of Sugars on Optimal Baking Time and Chemical Properties of Cookies
3.2. Effect of Sugar Substitution on Aroma Volatile Compounds of Cookies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amaya-Farfan, J.; Rodriguez-Amaya, D.B. Chapter 6–The Maillard reactions. In Chemical Changes During Processing and Storage of Foods; Rodriguez-Amaya, D.B., Amaya-Farfan, J., Eds.; Academic Press: London, UK, 2021. [Google Scholar] [CrossRef]
- Wang, S.; Xu, H.; Luan, H.; Cai, J. Brief introduction of food processing methods and chemical hazards formed during thermal processing. In Chemical Hazards in Thermally-Processed Foods; Wang, S., Ed.; Springer Nature Singapore Pte. Ltd.: Singapore, 2019; pp. 1–17. [Google Scholar] [CrossRef]
- Fujioka, K.; Shibamoto, T. Formation of genotoxic dicarbonyl compounds in dietary oils upon oxidation. Lipids 2004, 39, 481–486. [Google Scholar] [CrossRef]
- Marceau, E.; Yaylayan, V.A. Profiling of α-dicarbonyl content of commercial honeys from different botanical origins: Identification of 3,4-dideoxyglucoson-3-ene (3,4-DGE) and related compounds. J. Agric. Food Chem. 2009, 57, 10837–10844. [Google Scholar] [CrossRef] [PubMed]
- Arena, E.; Ballistreri, G.; Fallico, B. Kinetics of 3-Deoxy-D-Erythro-Hexos-2-Ulose in Unifloral Honeys. J. Food Sci. 2011, 76, C1044–C1049. [Google Scholar] [CrossRef]
- Arena, E.; Ballistreri, G.; Tomaselli, F.; Fallico, B. Survey of 1,2-Dicarbonyl Compounds in Commercial Honey of Different Floral Origin. J. Food Sci. 2011, 76, C1203–C1210. [Google Scholar] [CrossRef]
- Degen, J.; Hellwig, M.; Henle, T. 1,2-Dicarbonyl Compounds in Commonly Consumed Foods. J. Agric. Food Chem. 2012, 60, 7071–7079. [Google Scholar] [CrossRef]
- Hellwig, M.; Gensberger-Reigl, S.; Henle, T.; Pischetsrieder, M. Food-derived 1,2-dicarbonyl compounds and their role in diseases. Semin. Cancer Biol. 2018, 49, 1–8. [Google Scholar] [CrossRef]
- Arribas-Lorenzo, G.; Morales, F.J. Analysis, Distribution, and Dietary Exposure of Glyoxal and Methylglyoxal in Cookies and Their Relationship with Other Heat-Induced Contaminants. J. Agric. Food Chem. 2010, 58, 2966–2972. [Google Scholar] [CrossRef] [Green Version]
- Maasen, K.; Scheijen, J.L.; Opperhuizen, A.; Stehouwer, C.D.; Van Greevenbroek, M.M.; Schalkwijk, C.G. Quantification of dicarbonyl compounds in commonly consumed foods and drinks; presentation of a food composition database for dicarbonyls. Food Chem. 2021, 339, 128063. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ho, C.-T. Flavour chemistry of methylglyoxal and glyoxal. Chem. Soc. Rev. 2012, 41, 4140–4149. [Google Scholar] [CrossRef]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Efukushima, A.; Ehasyashi-Nishino, M.; Enishino, K. Effect of methylglyoxal on multidrug-resistant Pseudomonas aeruginosa. Front. Microbiol. 2014, 5, 180. [Google Scholar] [CrossRef]
- Brighina, S.; Restuccia, C.; Arena, E.; Palmeri, R.; Fallico, B. Antibacterial activity of 1,2-dicarbonyl compounds and the influence of the in vitro assay system. Food Chem. 2020, 311, 125905. [Google Scholar] [CrossRef]
- Wang, J.; Chang, T. Methylglyoxal Content in Drinking Coffee as a Cytotoxic Factor. J. Food Sci. 2010, 75, H167–H171. [Google Scholar] [CrossRef]
- Amoroso, A.; Maga, G.; Daglia, M. Cytotoxicity of α-dicarbonyl compounds submitted to in vitro simulated digestion process. Food Chem. 2013, 140, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Brighina, S.; Turrado, C.P.; Restuccia, C.; Walton, G.; Fallico, B.; Oruna-Concha, M.J.; Arena, E. Detrimental effect on the gut microbiota of 1,2-dicarbonyl compounds after in vitro gastro-intestinal and fermentative digestion. Food Chem. 2021, 341, 128237. [Google Scholar] [CrossRef] [PubMed]
- McCance, D.R.; Dyer, D.G.; Dunn, J.A.; Bailie, K.E.; Thorpe, S.R.; Baynes, J.W.; Lyons, T.J. Maillard reaction products and their relation to complications in insulin-dependent diabetes mellitus. J. Clin. Investig. 1993, 91, 2470–2478. [Google Scholar] [CrossRef] [Green Version]
- Vitek, M.P.; Bhattacharya, K.; Glendening, J.M.; Stopa, E.; Vlassara, H.; Bucala, R.; Manogue, K.; Cerami, A. Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1994, 91, 4766–4770. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N.; Thornalley, P. Advanced glycation endproducts: What is their relevance to diabetic complications? Diabetes Obes. Metab. 2007, 9, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Šebeková, K.; Somoza, V. Dietary advanced glycation endproducts (AGEs) and their health effects—PRO. Mol. Nutr. Food Res. 2007, 51, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Mogol, B.A.; Gökmen, V. Thermal process contaminants: Acrylamide, chloropropanols and furan. Curr. Opin. Food Sci. 2016, 7, 86–92. [Google Scholar] [CrossRef]
- Crews, C.; Castle, L. A review of the occurrence, formation and analysis of furan in heat-processed foods. Trends Food Sci. Technol. 2007, 18, 365–372. [Google Scholar] [CrossRef]
- Condurso, C.; Cincotta, F.; Verzera, A. Determination of furan and furan derivatives in baby food. Food Chem. 2018, 250, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risks for public health related to the presence of furan and methylfurans in food. EFSA J. 2017, 15, e05005. [Google Scholar] [CrossRef] [PubMed]
- Okaru, A.O.; Lachenmeier, D.W. The Food and Beverage Occurrence of Furfuryl Alcohol and Myrcene—Two Emerging Potential Human Carcinogens? Toxics 2017, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- IARC International Agency for Research on Cancer. Furan. In IARC Monographs; World Health Organization: Lyon, France, 1995; Volume 63, pp. 393–407. [Google Scholar]
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids. Scientific opinion on flavouring group evaluation 67, revision 1 (FGE.67Rev.1): Consideration of 40 furan-substituted aliphatic hydrocarbons, alcohols, aldehydes, ketones, carboxylic acids and related esters, sulfides, disulfides and ethers evaluated by JECFA at the 65th meeting (JECFA, 2006b) and re-evaluated at the 69th meeting (JECFA, 2009c). EFSA J. 2011, 9, 2315. [Google Scholar]
- Kim, J.-S.; Her, J.-Y.; Lee, K.-G. Formation and reduction of carcinogenic furan in various model systems containing food additives. Food Chem. 2015, 189, 108–113. [Google Scholar] [CrossRef]
- Belitz, H.B.; Grosch, W.; Schieberle, P. Food Chemistry, 3rd ed.; Springer: Berlin, Germany, 2004; pp. 258–282. [Google Scholar]
- Ramírez-Jiménez, A.; García-Villanova, B.; Guerra-Hernández, E. Hydroxymethylfurfural and methylfurfural content of selected bakery products. Food Res. Int. 2000, 33, 833–838. [Google Scholar] [CrossRef]
- Rada-Mendoza, M.; Olano, A.; Villamiel, M. Determination of hydroxymethylfurfural in commercial jams and in fruit-based infant foods. Food Chem. 2002, 79, 513–516. [Google Scholar] [CrossRef]
- Fallico, B.; Zappalà, M.; Arena, E.; Verzera, A. Effects of conditioning on HMF content in unifloral honeys. Food Chem. 2004, 85, 305–313. [Google Scholar] [CrossRef]
- Capuano, E.; Fogliano, V. Acrylamide and 5-hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT Food Sci. Technol. 2011, 44, 793–810. [Google Scholar] [CrossRef]
- Spina, A.; Brighina, S.; Muccilli, S.; Mazzaglia, A.; Rapisarda, P.; Fallico, B.; Arena, E. Partial Replacement of NaCl in Bread from Durum Wheat (Triticum turgidum L subsp. durum Desf.) with KCl and Yeast Extract: Evaluation of Quality Parameters During Long Storage. Food Bioprocess Technol. 2015, 8, 1089–1101. [Google Scholar] [CrossRef]
- Spina, A.; Brighina, S.; Muccilli, S.; Mazzaglia, A.; Fabroni, S.; Fallico, B.; Rapisarda, P.; Arena, E. Wholegrain Durum Wheat Bread Fortified with Citrus Fibers: Evaluation of Quality Parameters During Long Storage. Front. Nutr. 2019, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Arena, E.; Muccilli, S.; Mazzaglia, A.; Giannone, V.; Brighina, S.; Rapisarda, P.; Fallico, B.; Allegra, M.; Spina, A. Development of Durum Wheat Breads Low in Sodium Using a Natural Low-Sodium Sea Salt. Foods 2020, 9, 752. [Google Scholar] [CrossRef]
- Ramírez-Jiménez, A.; Guerra-Hernández, E.; García-Villanova, B. Browning Indicators in Bread. J. Agric. Food Chem. 2000, 48, 4176–4181. [Google Scholar] [CrossRef]
- Petisca, C.; Henriques, A.; Pérez-Palacios, T.; Pinho, O.; Ferreira, I. Assessment of hydroxymethylfurfural and furfural in commercial bakery products. J. Food Compos. Anal. 2014, 33, 20–25. [Google Scholar] [CrossRef]
- Abraham, K.; Gürtler, R.; Berg, K.; Heinemeyer, G.; Lampen, A.; Appel, K.E. Toxicology and risk assessment of 5-Hydroxymethylfurfural in food. Mol. Nutr. Food Res. 2011, 55, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Monien, B.H.; Engst, W.; Barknowitz, G.; Seidel, A.; Glatt, H. Mutagenicity of 5-Hydroxymethylfurfural in V79 Cells Expressing Human SULT1A1: Identification and Mass Spectrometric Quantification of DNA Adducts Formed. Chem. Res. Toxicol. 2012, 25, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Mastovska, K. Effectiveness of Ionizing Radiation in Reducing Furan and Acrylamide Levels in Foods. J. Agric. Food Chem. 2006, 54, 8266–8270. [Google Scholar] [CrossRef] [PubMed]
- Anese, M.; Bot, F.; Suman, M. Furan and 5-hydroxymethylfurfural removal from high- and low-moisture foods. LWT Food Sci. Technol. 2014, 56, 529–532. [Google Scholar] [CrossRef]
- Manley, D.J.R. Biscuit, Cracker, and Cookie Recipes for the Food Industry, 1st ed.; Duncan, J.R., Ed.; CRC Press: Boca Raton, FL, USA, 2001; p. 208. [Google Scholar]
- Lefebvre, D.; Gabriel, V.; Vayssier, Y.; Fontagné-Faucher, C. Simultaneous HPLC Determination of Sugars, Organic Acids and Ethanol in Sourdough Process. LWT Food Sci. Technol. 2002, 35, 407–414. [Google Scholar] [CrossRef]
- Weigel, K.U.; Opitz, T.; Henle, T. Studies on the occurrence and formation of 1,2-dicarbonyls in honey. Eur. Food Res. Technol. 2004, 218, 147–151. [Google Scholar] [CrossRef]
- Cincotta, F.; Tripodi, G.; Merlino, M.; Verzera, A.; Condurso, C. Variety and shelf-life of coffee packaged in capsules. LWT 2020, 118, 108718. [Google Scholar] [CrossRef]
- Ureta, M.; Olivera, D.F.; Salvadori, V.O. Quality Attributes of Muffins: Effect of Baking Operative Conditions. Food Bioprocess Technol. 2014, 7, 463–470. [Google Scholar] [CrossRef]
- Ureta, M.M.; Olivera, D.F.; Salvadori, V.O. Baking of muffins: Kinetics of crust color development and optimal baking time. Food Bioprocess Technol. 2014, 7, 3208–3216. [Google Scholar] [CrossRef]
- Ameur, L.; Mathieu, O.; Lalanne, V.; Trystram, G.; Birlouezaragon, I. Comparison of the effects of sucrose and hexose on furfural formation and browning in cookies baked at different temperatures. Food Chem. 2007, 101, 1407–1416. [Google Scholar] [CrossRef]
- Ameur, L.A.; Trystram, G.; Birlouez-Aragon, I. Accumulation of 5-hydroxymethyl-2-furfural in cookies during the backing process: Validation of an extraction method. Food Chem. 2006, 98, 790–796. [Google Scholar] [CrossRef]
- Kowalski, S.; Lukasiewicz, M.; Juszczak, L.; Kutyła-Kupidura, E.M. Dynamics of 5-hydroxymethylfurfural formation in shortbreads during thermal processing. Czech J. Food Sci. 2013, 31, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Gökmen, V.; Açar, O.; Köksel, H.; Acar, J. Effects of dough formula and baking conditions on acrylamide and hydroxymethylfurfural formation in cookies. Food Chem. 2007, 104, 1136–1142. [Google Scholar] [CrossRef]
- Courel, M.; Ait-Ameur, L.; Capuano, E.; Fogliano, V.; Morales, F.J.; Courtois, F.; Birlouez-Aragon, I. Effects of Formulation and Baking Conditions on Neo-formed Contaminants in Model Cookies. Czech J. Food Sci. 2009, 27, S93–S95. [Google Scholar] [CrossRef] [Green Version]
- Wells-Knecht, K.J.; Zyzak, D.V.; Litchfield, J.E.; Thorpe, S.R.; Baynes, J.W. Mechanism of autoxidative glycosylation: Identification of Glyoxal and Arabinose as Intermediates in the Autoxidative Modification of Proteins by Glucose. Biochemistry 1995, 34, 3702–3709. [Google Scholar] [CrossRef]
- Weenen, H. Reactive intermediates and carbohydrate fragmentation in Maillard chemistry. Food Chem. 1998, 62, 393–401. [Google Scholar] [CrossRef]
- Pico, J.; Martínez, M.M.; Bernal, J.; Gomez, M. Evolution of volatile compounds in gluten-free bread: From dough to crumb. Food Chem. 2017, 227, 179–186. [Google Scholar] [CrossRef]
- Garvey, E.C.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Optimisation of HS-SPME Parameters for the Analysis of Volatile Compounds in Baked Confectionery Products. Food Anal. Methods 2020, 13, 1314–1327. [Google Scholar] [CrossRef]
- Garvey, E.; O’Sullivan, M.; Kerry, J.; Milner, L.; Gallagher, E.; Kilcawley, K.N. Characterising the sensory quality and volatile aroma profile of clean-label sucrose reduced sponge cakes. Food Chem. 2021, 342, 128124. [Google Scholar] [CrossRef]
- Yu, M.; He, S.; Tang, M.; Zhang, Z.; Zhu, Y.; Sun, H. Antioxidant activity and sensory characteristics of Maillard reaction products derived from different peptide fractions of soybean meal hydrolysate. Food Chem. 2018, 243, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Xu, Y.; Ren, F.; Zhang, H. Characteristics and antioxidant activity of Maillard reaction products from α-lactalbumin and 2′-fucosyllactose. Food Chem. 2020, 316, 126341. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Zheng, L.; Cui, C.; Yang, B.; Ren, J.; Zhao, M. Characterization of antioxidant activity and volatile compounds of Maillard reaction products derived from different peptide fractions of peanut hydrolysate. Food Res. Int. 2011, 44, 3250–3258. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, H.; Wang, W.; Jiao, W.; Chen, W.; Zhong, Q.; Yun, Y.-H.; Chen, W. Characterization of Volatile Profiles and Marker Substances by HS-SPME/GC-MS during the Concentration of Coconut Jam. Foods 2020, 9, 347. [Google Scholar] [CrossRef] [Green Version]
- Maire, M.; Rega, B.; Cuvelier, M.-E.; Soto, P.; Giampaoli, P. Lipid oxidation in baked products: Impact of formula and process on the generation of volatile compounds. Food Chem. 2013, 141, 3510–3518. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Erichsen, H.; Soerensen, J.; Petersen, M.A.; Skibsted, L.H. Optimising water activity for storage of high lipid and high protein infant formula milk powder using multivariate analysis. Int. Dairy J. 2019, 93, 92–98. [Google Scholar] [CrossRef]
- Huang, J.; Ren, J.; Tao, G.; Chen, Y.; Yao, S.; Han, D.; Qiu, R. Maize bran feruloylated oligosaccharides inhibited AGEs formation in glucose/amino acids and glucose/BSA models. Food Res. Int. 2019, 122, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Dueñas-Sánchez, R.; Pérez, A.G.; Codón, A.C.; Benítez, T.; Rincón, A.M. Overproduction of 2-phenylethanol by industrial yeasts to improve organoleptic properties of bakers’ products. Int. J. Food Microbiol. 2014, 180, 7–12. [Google Scholar] [CrossRef]
- Pico, J.; Bernal, J.; Gomez, M. Wheat bread aroma compounds in crumb and crust: A review. Food Res. Int. 2015, 75, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Pico, J.; Tapia, J.A.; Bernal, J.; Gomez, M. Comparison of different extraction methodologies for the analysis of volatile compounds in gluten-free flours and corn starch by GC/QTOF. Food Chem. 2018, 267, 303–312. [Google Scholar] [CrossRef]
- Liu, J.; Wan, P.; Xie, C.; Chen, D.-W. Key aroma-active compounds in brown sugar and their influence on sweetness. Food Chem. 2021, 345, 128826. [Google Scholar] [CrossRef]
- Srivastava, R.; Bousquières, J.; Cepeda-Vázquez, M.; Roux, S.; Bonazzi, C.; Rega, B. Kinetic study of furan and furfural generation during baking of cake models. Food Chem. 2018, 267, 329–336. [Google Scholar] [CrossRef] [PubMed]
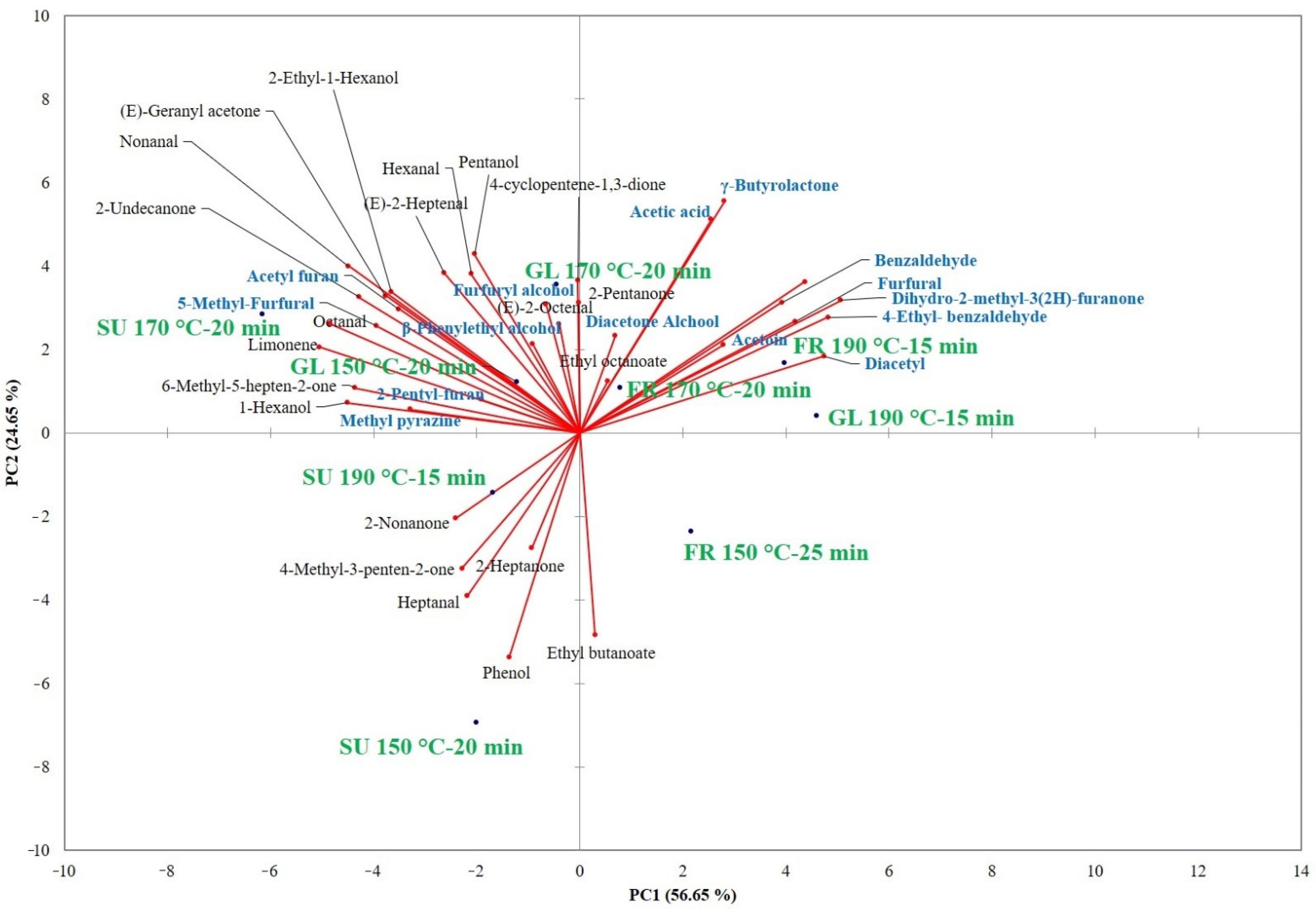
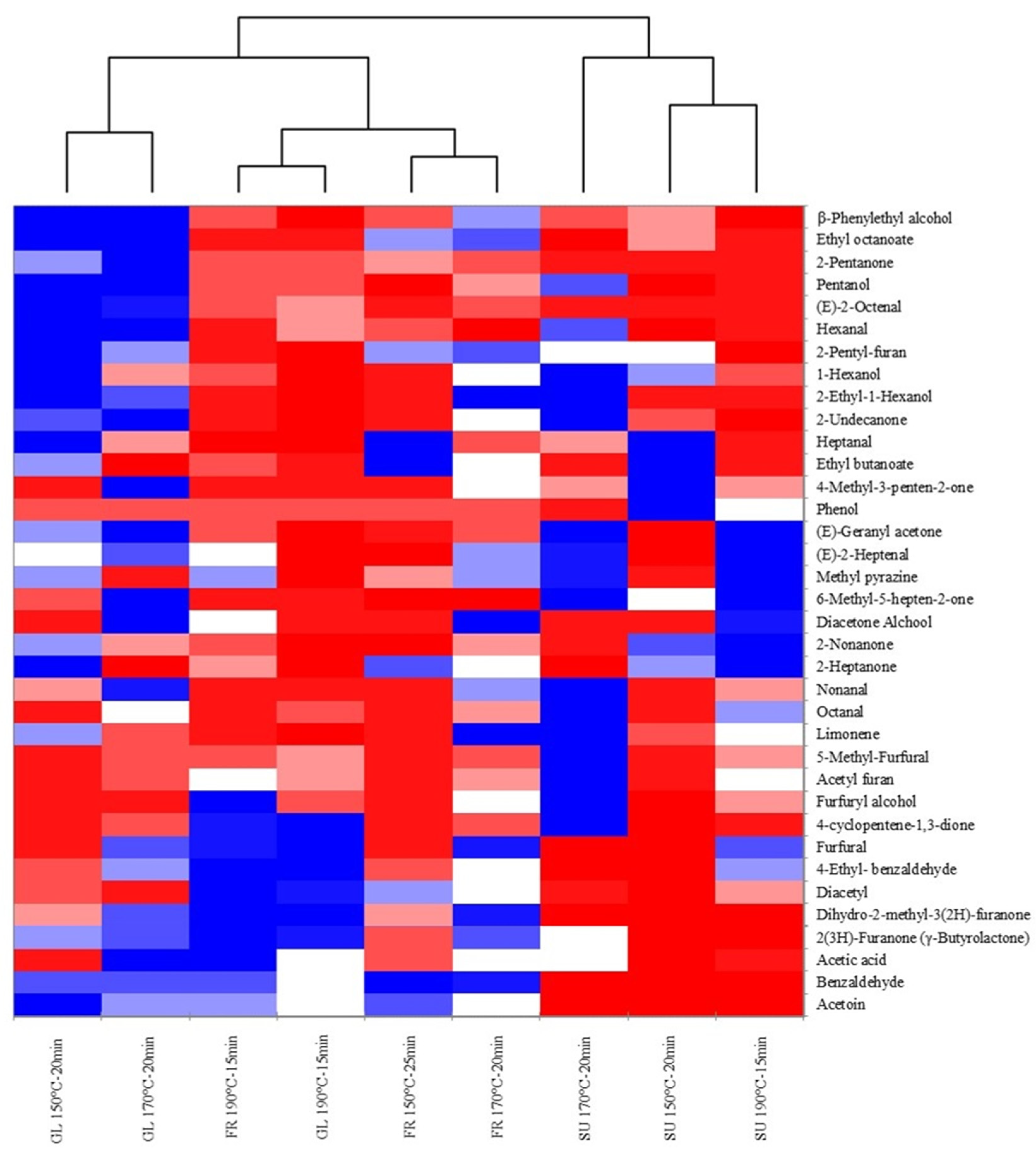
| SU Cookies | FR Cookies | GL Cookies | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature | Time 1 | Frequency | EOBT | Time 1 | Frequency | EOBT | Time 1 | Frequency | EOBT |
| 150 | 25 | 100% | 25 | 20 25 | 10% 90% | 24.5 | 20 25 | 75% 25% | 21.3 |
| 170 | 20 | 100% | 20 | 15 20 | 10% 90% | 19.5 | 15 20 | 30% 70% | 18.5 |
| 190 | 15 20 | 90% 10% | 15.5 | 15 | 100% | 15 | 10 15 | 20% 80% | 14 |
| Parameters | SU Cookies | FR Cookies | GL Cookies | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 150 °C 25 min | 170 °C 20 min | 190 °C 15 min | 150 °C 25 min | 170 °C 20 min | 190 °C 15 min | 150 °C 20 min | 170 °C 20 min | 190 °C 15 min | |
| Moisture 1 | 2.0 ± 0.3c | 2.3 ± 0.4c | 4.2 ± 0.7b | 6.1 ± 0.5b | 6.2 ± 0.3a | 6.3 ± 0.3a | 7.9 ± 0.6a | 4.9 ± 0.2b | 5.5 ± 0.6a |
| pH | 7.1 ± 0.02a | 7.2 ± 0.08a | 7.2 ± 0.04a | 6.5 ± 0.02b | 6.4 ± 0.12b | 6.4 ± 0.10b | 6.9 ± 0.13b | 6.5 ± 0.16b | 6.6 ± 0.09b |
| TTA 2 | 1.7 ± 0.1b | 1.7 ± 0.1b | 1.6 ± 0.1b | 4.0 ± 0.0a | 4.4 ± 0.4a | 4.0 ± 0.3a | 2.6 ± 0.1b | 3.7 ± 0.1a | 3.7 ± 0.1a |
| HMF 3 | 5.1 ± 0.9b | 5.2 ± 1.5c | 8.3± 0.4b | 10.4 ± 1.6a | 74.9 ± 5.3a | 22.2 ± 7.1a | 4.5 ± 1.0b | 14.9 ± 1.9b | 23.5 ± 3.2a |
| 3-DG 3 | 6.7 ± 1.7c | 6.1 ± 1.3c | 5.3 ± 1.3c | 1038.8 ± 133.0b | 1162.3 ± 188.5b | 960.9 ± 119.4b | 2113.9 ± 384.6a | 3517.9 ± 206.0a | 3250.3 ± 54.7a |
| GO 3 | 10.7 ± 0.4b | 10.3 ± 0.6a | 10.7 ± 0.4a | 14.5 ± 1.8a | 16.0 ± 2.3a | 14.1 ± 1.5a | 14.3 ± 3.2a | 15.4 ± 4.0a | 15.6 ± 4.1a |
| MGO 3 | 8.4 ± 0.3c | 8.3 ± 0.4c | 8.40 ± 0.3c | 33.5 ± 0.9a | 39.8 ± 2.1a | 37.5 ± 2.1a | 11.8 ± 0.8b | 17.3 ± 1.3b | 18.7 ± 0.8b |
| SU Cookies | FR Cookies | GL Cookies | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compounds | LRI | Odour Description | Tentative Origin | 150 °C 25 min | 170 °C 20 min | 190 °C 15 min | 150 °C 25 min | 170 °C 20 min | 190 °C 15 min | 150 °C 20 min | 170 °C 20 min | 190 °C 15 min |
| Esters | ||||||||||||
| Ethyl butanoate | 1037 | Fruity, sweet, apple | LO | 11.72a | 4.25c | 3.54c | 13.50a | 6.38b | 4.61c | 7.45b | 7.09b | 3.89c |
| Ethyl octanoate | 1437 | Fruity, wine, waxy | LO | 18.83a | 12.78b | 13.50b | 23.10a | 24.52a | 16.34b | 27.36a | 19.19a | 13.14b |
| Total | 30.55 | 17.03 | 17.04 | 36.60 | 30.90 | 20.95 | 34.81 | 26.28 | 17.03 | |||
| Ketones | ||||||||||||
| 2-Pentanone | 980 | Sweet, fruity, banana | LO | 17.76c | 23.10c | 21.67c | 57.24b | 51.55b | 45.86bc | 113.42a | 80.00b | 31.99c |
| Diacetyl | 1062 | Buttery, sweet, creamy | MR | nd c | 2.47b | 4.25b | 6.38b | 8.87a | 12.43a | 3.18b | 2.12b | 7.45a |
| 2-Heptanone | 1168 | Fruity, spicy, sweet, herbal | LO | 222.24b | 245.36b | 292.66a | 235.05b | 216.55b | 192.02b | 285.19a | 129.07c | 107.73c |
| Acetoin | 1290 | Sweet, buttery, creamy, dairy | MR | 13.14c | 10.65c | 9.94c | 37.32b | 30.57b | 32.34b | 56.88a | 33.77b | 29.14b |
| 2-Nonanone | 1394 | Roasty, cake crust, sweet | LO | 92.09a | 110.94a | 112.00a | 70.04b | 82.84b | 78.93b | 87.11b | 81.77b | 68.26b |
| 4-cyclopentene-1,3-dione | 1598 | MR | nd c | 14.56b | 10.65b | 4.25b | 13.85b | 33.05a | 7.45b | 12.43b | 40.52a | |
| 2-Undecanone | 1605 | Waxy, fruity, creamy, fatty | LO | 19.19a | 17.76a | 15.27a | 17.76a | 20.96a | 17.05a | 23.10a | 22.74a | 14.92a |
| Total | 364.42 | 424.84 | 466.44 | 428.04 | 425.19 | 411.68 | 576.33 | 361.90 | 300.01 | |||
| Aldheydes | ||||||||||||
| Hexanal | 1089 | Green, fatty, leafy | LO | 84.26b | 86.75b | 106.31b | 112.36b | 93.15b | 99.20b | 200.20a | 195.93a | 118.05b |
| Heptanal | 1189 | Fatty, oily | LO | 81.77a | 37.32b | 22.03c | 63.64a | 33.41b | 17.05c | 65.06a | 36.97b | 16.70c |
| Octanal | 1294 | Aldehydic, waxy, citrus | LO | 10.30b | 11.72b | 18.83a | 7.45b | 8.16b | 8.52b | 10.30b | 9.58b | 10.65b |
| (E)-2-Heptenal | 1329 | Pungent, green, vegetable | LO | 2.47b | 12.43a | 15.27a | 0.34b | 6.74b | 9.58a | 8.87a | 11.01a | 4.25b |
| Nonanal | 1400 | Bready, cake crust | LO | 33.05a | 44.79a | 55.10a | 36.61a | 38.74a | 33.41a | 56.53a | 44.79a | 40.88a |
| (E)-2-Octenal | 1425 | Damp, earthy | LO | 6.38c | 10.65c | 9.23c | 8.52c | 11.36c | 11.01c | 65.42a | 37.32b | 18.83c |
| Benzaldehyde | 1532 | Sweet | MR/LO | 18.12b | 26.30b | 28.43b | 57.95a | 56.17a | 53.68a | 52.26a | 51.90a | 44.08a |
| 4-Ethyl-benzaldehyde | 1729 | Almond, sweet | MR | 6.74b | 7.45b | 12.78a | 10.30a | 11.72a | 15.63a | 9.94a | 12.78a | 14.92a |
| Total | 243.09 | 237.41 | 267.98 | 297.17 | 259.45 | 248.08 | 468.58 | 400.28 | 268.36 | |||
| Alcohols | ||||||||||||
| Pentanol | 1242 | Fusel oil, sweet balsamic | LO | 11.01b | 12.07b | 12.43b | 11.01b | 13.50b | 13.14b | 19.90a | 17.05a | 12.78b |
| 1-Hexanol | 1355 | Ethereal, fusel oil, fruity | LO | 12.43b | 11.36b | 10.65b | 9.58b | 11.72b | 9.94b | 17.76a | 11.01b | 5.32b |
| Diacetone alcohol | 1368 | MR | 3.54d | 85.69c | 161.43b | 22.39d | 207.66a | 205.89a | 3.54d | 213.71a | 224.73a | |
| 2-Ethyl-1-Hexanol | 1489 | Citrus, fresh, floral, oily | LO | 14.92a | 14.56a | 15.27a | 15.63a | 14.92a | 14.21a | 20.96a | 19.90a | 13.14a |
| β-Phenylethyl alcohol | 1919 | Rose, honey-like | MR | 9.23b | 7.81b | 4.96c | 7.81b | 10.30b | 8.52b | 15.27a | 14.21a | 6.03b |
| Phenol | 2003 | Phenolic, plastic | 8.52a | 9.94a | 11.36a | 3.89b | 6.03b | 4.96b | 4.61b | 6.03b | 5.32b | |
| Total | 59.65 | 141.43 | 216.10 | 70.31 | 264.13 | 256.66 | 82.04 | 281.91 | 267.32 | |||
| Terpenes | ||||||||||||
| Limonene | 1183 | Citrus, lemon | 10.30b | 12.07a | 12.78a | 6.74c | 8.16c | 6.03c | 14.21a | 10.30b | 5.67c | |
| C13-Norisoprenoids | ||||||||||||
| 4-Methyl-3-penten-2-one | 1129 | Musty, nutty chocolate | 20.25a | 7.09b | 5.67b | 1.05c | 6.03b | 9.58b | nd d | nd d | nd d | |
| 6-Methyl-5-hepten-2-one | 1340 | Fruity, musty apple | 9.23b | 13.85a | 12.78a | 4.25c | 2.83c | 5.32c | 7.09b | 7.81b | 6.38b | |
| (E)-Geranyl acetone | 1862 | Fresh, rose, leafy, floral | nd c | 19.90a | 23.81a | 5.67b | 7.81b | 7.09b | 14.21a | 19.19a | nd c | |
| Total | 29.48 | 40.84 | 42.26 | 10.97 | 16.67 | 21.99 | 21.3 | 27.00 | 6.38 | |||
| Acids | ||||||||||||
| Acetic acid | 1464 | Pungent, sour, vinegar | MR, C | 1.40c | 13.85b | 12.43b | 14.92b | 17.41b | 31.28a | 12.07b | 15.99b | 19.54b |
| Pyrazines | ||||||||||||
| 2-Methylpyrazine | 1269 | Roasted, burnt, sweet | MR/LO | 2.47c | 5.32b | 7.09a | 3.18b | 4.25b | 4.25b | 4.25b | 2.12c | nd d |
| Furanoic compounds | ||||||||||||
| 2-Pentyl-furan | 1221 | Floral, fruity, green, earthy | F, LO, MR | 56.00b | 56.99b | 22.30c | 62.93b | 56.49b | 36.67c | 94.65a | 61.94b | 27.75c |
| Dihydro-2-methyl-3(2H)-furanone | 1264 | Woody, bready | MR, CR | 4.96c | 6.38c | 6.74c | 13.50b | 20.61a | 21.67a | 13.50b | 18.83a | 25.23a |
| Furfural | 1468 | Spicy, bready | MR, R | 159.56c | 167.59c | 588.56a | 291.02b | 639.24a | 651.28a | 279.48b | 616.66a | 730.06a |
| Acetyl furan | 1509 | Bready, caramel | MR | 0.53c | 26.47b | 43.94a | 5.29c | 35.47a | 48.70a | 7.94c | 21.18b | 33.35a |
| 5-Methyl-Furfural | 1584 | Sweet, caramellic, bready | MR | 10.04d | 23.58c | 47.17a | 9.53d | 36.63b | 31.11b | 6.52d | 37.63b | 55.69a |
| Dihydro-2(3H)-Furanone (γ-Butyrolactone) | 1648 | Burnt, sweet cream | F, MR | 11.01c | 18.47b | 23.45b | 29.85b | 42.66a | 47.28a | 37.68a | 40.88a | 44.08a |
| Furfuryl alcohol | 1666 | Biscuit, cake crust, caramelised | LO, MR, CR | 17.41d | 102.85b | 120.78b | 74.37c | 152.95b | 310.13a | 31.12d | 53.80c | 87.55b |
| Total | 259.51 | 402.33 | 852.94 | 486.49 | 984.05 | 1146.84 | 470.89 | 850.92 | 1003.71 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cincotta, F.; Brighina, S.; Condurso, C.; Arena, E.; Verzera, A.; Fallico, B. Sugars Replacement as a Strategy to Control the Formation of α-Dicarbonyl and Furanic Compounds during Cookie Processing. Foods 2021, 10, 2101. https://doi.org/10.3390/foods10092101
Cincotta F, Brighina S, Condurso C, Arena E, Verzera A, Fallico B. Sugars Replacement as a Strategy to Control the Formation of α-Dicarbonyl and Furanic Compounds during Cookie Processing. Foods. 2021; 10(9):2101. https://doi.org/10.3390/foods10092101
Chicago/Turabian StyleCincotta, Fabrizio, Selina Brighina, Concetta Condurso, Elena Arena, Antonella Verzera, and Biagio Fallico. 2021. "Sugars Replacement as a Strategy to Control the Formation of α-Dicarbonyl and Furanic Compounds during Cookie Processing" Foods 10, no. 9: 2101. https://doi.org/10.3390/foods10092101
APA StyleCincotta, F., Brighina, S., Condurso, C., Arena, E., Verzera, A., & Fallico, B. (2021). Sugars Replacement as a Strategy to Control the Formation of α-Dicarbonyl and Furanic Compounds during Cookie Processing. Foods, 10(9), 2101. https://doi.org/10.3390/foods10092101








